Advances in the Radiological Evaluation of and Theranostics for Glioblastoma
Abstract
:Simple Summary
Abstract
1. Introduction
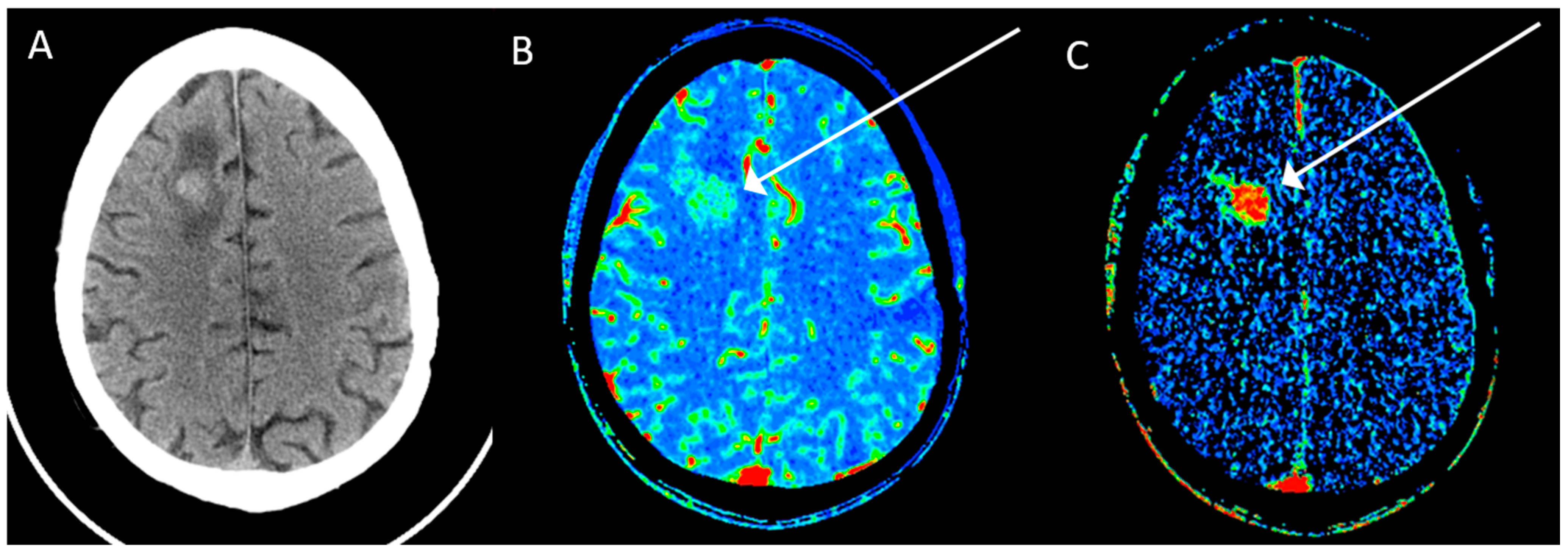
2. Computed Tomography (CT)
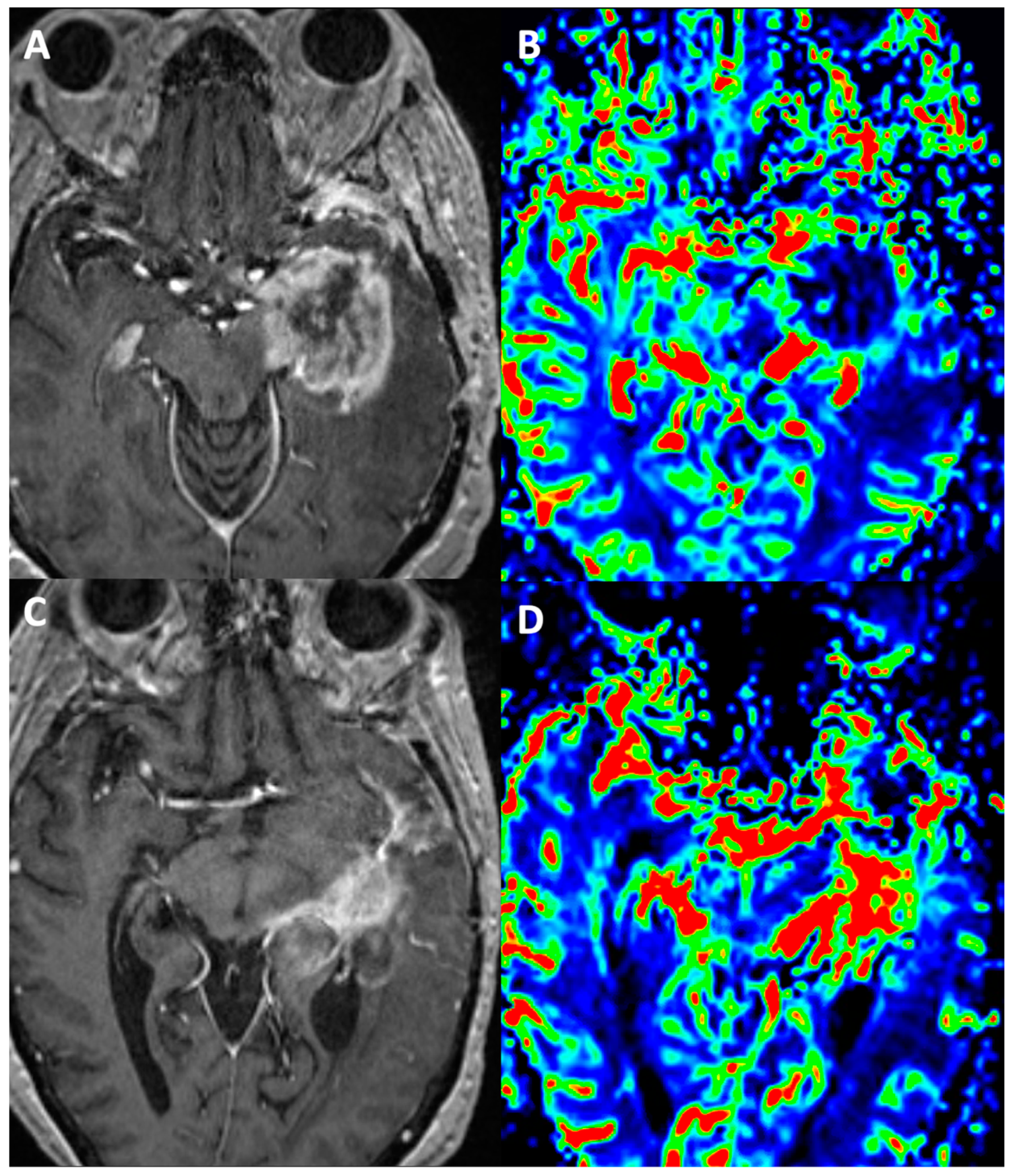
3. Magnetic Resonance Imaging (MRI)
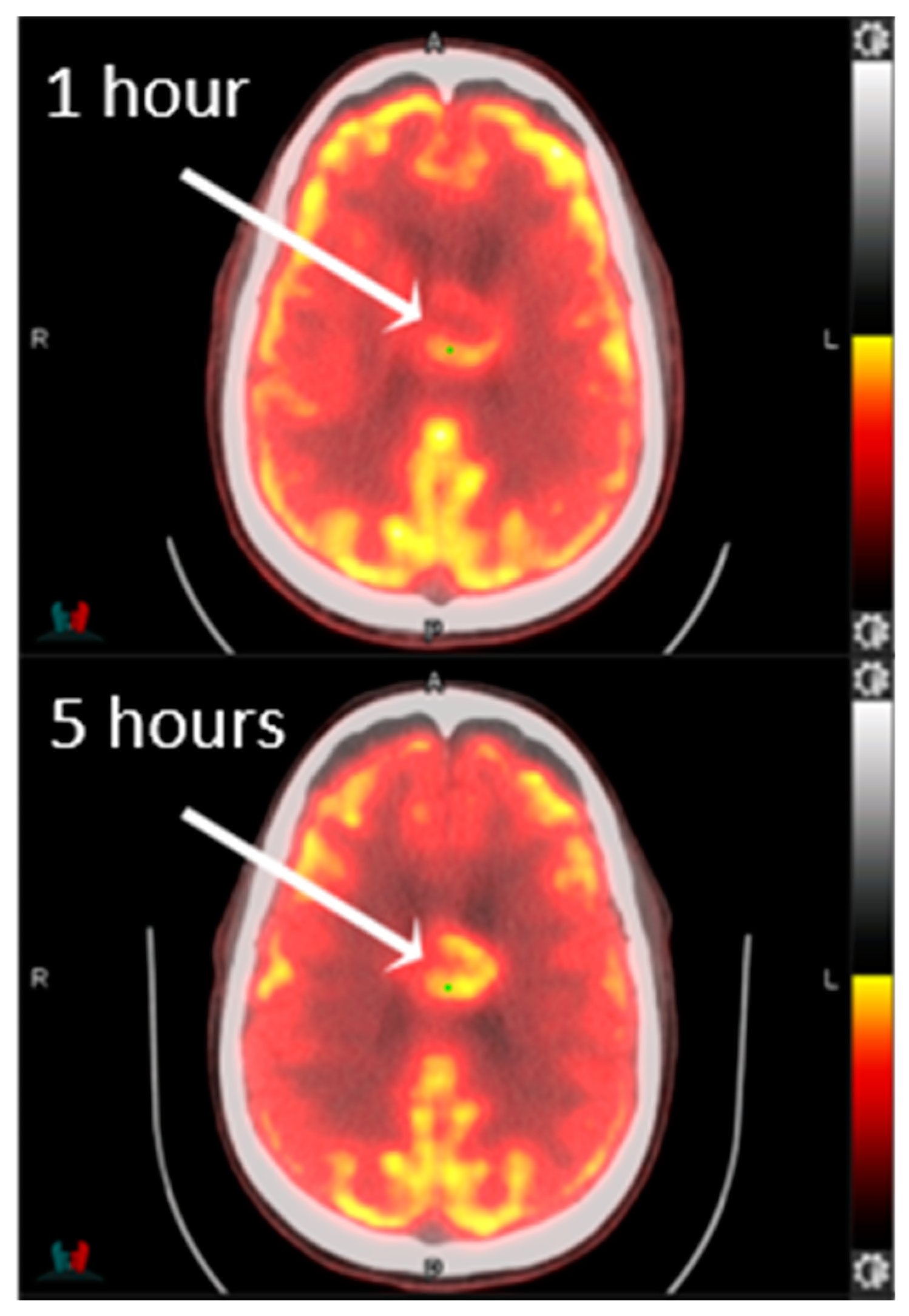
4. Positron Emission Tomography (PET)
5. Theranostics
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoyanov, G.S.; Lyutfi, E.; Georgieva, R.; Georgiev, R.; Dzhenkov, D.L.; Petkova, L.; Ivanov, B.D.; Kaprelyan, A.; Ghenev, P. Reclassification of Glioblastoma Multiforme According to the 2021 World Health Organization Classification of Central Nervous System Tumors: A Single Institution Report and Practical Significance. Cureus 2022, 14, e21822. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito, S.; D’Amico, D.; Eugenin, E. Novel approaches for glioblastoma treatment: Focus on tumor heterogeneity, treatment resistance, and computational tools. Cancer Rep. 2019, 2, e1220. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.L.V.; Gomes, I.N.F.; Carloni, A.C.; Rosa, M.N.; da Silva, L.S.; Evangelista, A.F.; Reis, R.M.; Silva, V.A.O. Role of glioblastoma stem cells in cancer therapeutic resistance: A perspective on antineoplastic agents from natural sources and chemical derivatives. Stem Cell Res. Ther. 2021, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Kajiwara, Y.; Takayasu, T.; Kolakshyapati, M.; Ishifuro, M.; Amatya, V.J.; Takeshima, Y.; Sugiyama, K.; Kurisu, K.; Yamasaki, F. Perfusion Computed Tomography Parameters Are Useful for Differentiating Glioblastoma, Lymphoma, and Metastasis. World Neurosurg. 2018, 119, e890–e897. [Google Scholar] [CrossRef]
- Shankar, J.J.; Woulfe, J.; Silva, V.D.; Nguyen, T.B. Evaluation of perfusion CT in grading and prognostication of high-grade gliomas at diagnosis: A pilot study. Am. J. Roentgenol. 2013, 200, W504–W509. [Google Scholar] [CrossRef] [PubMed]
- Skogen, K.; Ganeshan, B.; Good, C.; Critchley, G.; Miles, K. Measurements of heterogeneity in gliomas on computed tomography relationship to tumour grade. J. Neurooncol. 2013, 111, 213–219. [Google Scholar] [CrossRef]
- Marginean, L.; Stefan, P.A.; Lebovici, A.; Opincariu, I.; Csutak, C.; Lupean, R.A.; Coroian, P.A.; Suciu, B.A. CT in the Differentiation of Gliomas from Brain Metastases: The Radiomics Analysis of the Peritumoral Zone. Brain Sci. 2022, 12, 109. [Google Scholar] [CrossRef]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef]
- Pope, W.B.; Brandal, G. Conventional and advanced magnetic resonance imaging in patients with high-grade glioma. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 239–253. [Google Scholar] [CrossRef]
- Leon Ruiz, M. A Novel Case of Solitary Cerebral Toxoplasmosis Mimicking Glioblastoma as the First Presentation of HIV. J. Clin. Neurol. 2016, 12, 248–250. [Google Scholar] [CrossRef]
- Bull, J.G.; Saunders, D.E.; Clark, C.A. Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur. Radiol. 2012, 22, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Shindo, T.; Fukukura, Y.; Umanodan, T.; Takumi, K.; Hakamada, H.; Nakajo, M.; Umanodan, A.; Ideue, J.; Kamimura, K.; Yoshiura, T. Histogram Analysis of Apparent Diffusion Coefficient in Differentiating Pancreatic Adenocarcinoma and Neuroendocrine Tumor. Medicine 2016, 95, e2574. [Google Scholar] [CrossRef]
- Horvath-Rizea, D.; Surov, A.; Hoffmann, K.T.; Garnov, N.; Vorkel, C.; Kohlhof-Meinecke, P.; Ganslandt, O.; Bazner, H.; Gihr, G.A.; Kalman, M.; et al. The value of whole lesion ADC histogram profiling to differentiate between morphologically indistinguishable ring enhancing lesions-comparison of glioblastomas and brain abscesses. Oncotarget 2018, 9, 18148–18159. [Google Scholar] [CrossRef] [PubMed]
- Gihr, G.; Horvath-Rizea, D.; Hekeler, E.; Ganslandt, O.; Henkes, H.; Hoffmann, K.T.; Scherlach, C.; Schob, S. Diffusion weighted imaging in high-grade gliomas: A histogram-based analysis of apparent diffusion coefficient profile. PLoS ONE 2021, 16, e0249878. [Google Scholar] [CrossRef]
- Mair, D.B.; Ames, H.M.; Li, R. Mechanisms of invasion and motility of high-grade gliomas in the brain. Mol. Biol. Cell 2018, 29, 2509–2515. [Google Scholar] [CrossRef]
- Jin, Y.; Randall, J.W.; Elhalawani, H.; Feghali, K.A.A.; Elliott, A.M.; Anderson, B.M.; Lacerda, L.; Tran, B.L.; Mohamed, A.S.; Brock, K.K.; et al. Detection of Glioblastoma Subclinical Recurrence Using Serial Diffusion Tensor Imaging. Cancers 2020, 12, 568. [Google Scholar] [CrossRef]
- Toh, C.H.; Castillo, M.; Wong, A.M.; Wei, K.C.; Wong, H.F.; Ng, S.H.; Wan, Y.L. Primary cerebral lymphoma and glioblastoma multiforme: Differences in diffusion characteristics evaluated with diffusion tensor imaging. Am. J. Neuroradiol. 2008, 29, 471–475. [Google Scholar] [CrossRef]
- Kinoshita, M.; Hashimoto, N.; Goto, T.; Kagawa, N.; Kishima, H.; Izumoto, S.; Tanaka, H.; Fujita, N.; Yoshimine, T. Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage 2008, 43, 29–35. [Google Scholar] [CrossRef]
- Abdel Razek, A.A.K.; El-Serougy, L.; Abdelsalam, M.; Gaballa, G.; Talaat, M. Differentiation of Primary Central Nervous System Lymphoma From Glioblastoma: Quantitative Analysis Using Arterial Spin Labeling and Diffusion Tensor Imaging. World Neurosurg. 2019, 123, e303–e309. [Google Scholar] [CrossRef]
- Wang, W.; Steward, C.E.; Desmond, P.M. Diffusion tensor imaging in glioblastoma multiforme and brain metastases: The role of p, q, L, and fractional anisotropy. Am. J. Neuroradiol 2009, 30, 203–208. [Google Scholar] [CrossRef]
- Holly, K.S.; Barker, B.J.; Murcia, D.; Bennett, R.; Kalakoti, P.; Ledbetter, C.; Gonzalez-Toledo, E.; Nanda, A.; Sun, H. High-grade Gliomas Exhibit Higher Peritumoral Fractional Anisotropy and Lower Mean Diffusivity than Intracranial Metastases. Front. Surg. 2017, 4, 18. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, B. Differentiation among Glioblastomas, Primary Cerebral Lymphomas, and Solitary Brain Metastases Using Diffusion-Weighted Imaging and Diffusion Tensor Imaging: A PRISMA-Compliant Meta-analysis. ACS Chem. Neurosci. 2020, 11, 477–483. [Google Scholar] [CrossRef]
- Zakaria, H.; Haider, S.; Lee, I. Automated Whole Brain Tractography Affects Preoperative Surgical Decision Making. Cureus 2017, 9, e1656. [Google Scholar] [CrossRef]
- Wang, L.; Wei, L.; Wang, J.; Li, N.; Gao, Y.; Ma, H.; Qu, X.; Zhang, M. Evaluation of perfusion MRI value for tumor progression assessment after glioma radiotherapy: A systematic review and meta-analysis. Medicine 2020, 99, e23766. [Google Scholar] [CrossRef]
- Yun, J.; Yun, S.; Park, J.E.; Cheong, E.N.; Park, S.Y.; Kim, N.; Kim, H.S. Deep Learning of Time-Signal Intensity Curves from Dynamic Susceptibility Contrast Imaging Enables Tissue Labeling and Prediction of Survival in Glioblastoma. Am. J. Neuroradiol 2023, 44, 543–552. [Google Scholar] [CrossRef]
- Pons-Escoda, A.; Smits, M. Dynamic-susceptibility-contrast perfusion-weighted-imaging (DSC-PWI) in brain tumors: A brief up-to-date overview for clinical neuroradiologists. Eur. Radiol. 2023. [Google Scholar] [CrossRef]
- Kazda, T.; Bulik, M.; Pospisil, P.; Lakomy, R.; Smrcka, M.; Slampa, P.; Jancalek, R. Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: Single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. Neuroimage Clin. 2016, 11, 316–321. [Google Scholar] [CrossRef]
- Galijasevic, M.; Steiger, R.; Mangesius, S.; Mangesius, J.; Kerschbaumer, J.; Freyschlag, C.F.; Gruber, N.; Janjic, T.; Gizewski, E.R.; Grams, A.E. Magnetic Resonance Spectroscopy in Diagnosis and Follow-Up of Gliomas: State-of-the-Art. Cancers 2022, 14, 3197. [Google Scholar] [CrossRef]
- Aseel, A.; McCarthy, P.; Mohammed, A. Brain magnetic resonance spectroscopy to differentiate recurrent neoplasm from radiation necrosis: A systematic review and meta-analysis. J. Neuroimaging 2023, 33, 189–201. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Andronesi, O.C.; Barker, P.B.; Bizzi, A.; Bogner, W.; Henning, A.; Nelson, S.J.; Posse, S.; Shungu, D.C.; Soher, B.J. Advanced magnetic resonance spectroscopic neuroimaging: Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4309. [Google Scholar] [CrossRef]
- Leather, T.; Jenkinson, M.D.; Das, K.; Poptani, H. Magnetic Resonance Spectroscopy for Detection of 2-Hydroxyglutarate as a Biomarker for IDH Mutation in Gliomas. Metabolites 2017, 7, 29. [Google Scholar] [CrossRef]
- Hangel, G.; Lazen, P.; Sharma, S.; Hristoska, B.; Cadrien, C.; Furtner, J.; Rausch, I.; Lipka, A.; Niess, E.; Hingerl, L.; et al. 7T HR FID-MRSI Compared to Amino Acid PET: Glutamine and Glycine as Promising Biomarkers in Brain Tumors. Cancers 2022, 14, 2163. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, L.; Verma, G.; Hangel, G.; Neal, A.; Moffat, B.A.; Stockmann, J.P.; Andronesi, O.C.; Balchandani, P.; Hadjipanayis, C.G. Application of 7T MRS to High-Grade Gliomas. Am. J. Neuroradiol 2022, 43, 1378–1395. [Google Scholar] [CrossRef] [PubMed]
- Kogan, F.; Hariharan, H.; Reddy, R. Chemical Exchange Saturation Transfer (CEST) Imaging: Description of Technique and Potential Clinical Applications. Curr. Radiol. Rep. 2013, 1, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Paech, D.; Windschuh, J.; Oberhollenzer, J.; Dreher, C.; Sahm, F.; Meissner, J.E.; Goerke, S.; Schuenke, P.; Zaiss, M.; Regnery, S.; et al. Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multipool CEST MRI at 7.0 T. Neuro. Oncol. 2018, 20, 1661–1671. [Google Scholar] [CrossRef]
- Jiang, S.; Zou, T.; Eberhart, C.G.; Villalobos, M.A.V.; Heo, H.Y.; Zhang, Y.; Wang, Y.; Wang, X.; Yu, H.; Du, Y.; et al. Predicting IDH mutation status in grade II gliomas using amide proton transfer-weighted (APTw) MRI. Magn. Reson. Med. 2017, 78, 1100–1109. [Google Scholar] [CrossRef]
- Xu, X.; Sehgal, A.A.; Yadav, N.N.; Laterra, J.; Blair, L.; Blakeley, J.; Seidemo, A.; Coughlin, J.M.; Pomper, M.G.; Knutsson, L.; et al. d-glucose weighted chemical exchange saturation transfer (glucoCEST)-based dynamic glucose enhanced (DGE) MRI at 3T: Early experience in healthy volunteers and brain tumor patients. Magn. Reson. Med. 2020, 84, 247–262. [Google Scholar] [CrossRef]
- Bender, B.; Herz, K.; Deshmane, A.; Richter, V.; Tabatabai, G.; Schittenhelm, J.; Skardelly, M.; Scheffler, K.; Ernemann, U.; Kim, M.; et al. GLINT: GlucoCEST in neoplastic tumors at 3 T-clinical results of GlucoCEST in gliomas. Magn. Reson. Mater. Phys. Biol. Med. 2021, 35, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lingl, J.P.; Wunderlich, A.; Goerke, S.; Paech, D.; Ladd, M.E.; Liebig, P.; Pala, A.; Kim, S.Y.; Braun, M.; Schmitz, B.L.; et al. The Value of APTw CEST MRI in Routine Clinical Assessment of Human Brain Tumor Patients at 3T. Diagnostics 2022, 12, 490. [Google Scholar] [CrossRef]
- Sartoretti, E.; Sartoretti, T.; Wyss, M.; Reischauer, C.; van Smoorenburg, L.; Binkert, C.A.; Sartoretti-Schefer, S.; Mannil, M. Amide proton transfer weighted (APTw) imaging based radiomics allows for the differentiation of gliomas from metastases. Sci. Rep. 2021, 11, 5506. [Google Scholar] [CrossRef]
- Zhuo, Z.; Qu, L.; Zhang, P.; Duan, Y.; Cheng, D.; Xu, X.; Sun, T.; Ding, J.; Xie, C.; Liu, X.; et al. Prediction of H3K27M-mutant brainstem glioma by amide proton transfer-weighted imaging and its derived radiomics. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4426–4436. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.R.; Hudson, A.L.; Khong, P.; Parkinson, J.F.; Dwight, T.; Ikin, R.J.; Zhu, Y.; Cheng, Z.J.; Vafaee, F.; Chen, J.; et al. Intratumoral heterogeneity identified at the epigenetic, genetic and transcriptional level in glioblastoma. Sci. Rep. 2016, 6, 22477. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Miyake, M.; Takahashi, M.; Hamamoto, R. Observing deep radiomics for the classification of glioma grades. Sci. Rep. 2021, 11, 10942. [Google Scholar] [CrossRef]
- Calabrese, E.; Rudie, J.D.; Rauschecker, A.M.; Villanueva-Meyer, J.E.; Clarke, J.L.; Solomon, D.A.; Cha, S. Combining radiomics and deep convolutional neural network features from preoperative MRI for predicting clinically relevant genetic biomarkers in glioblastoma. Neuro-Oncol. Adv. 2022, 4, vdac060. [Google Scholar] [CrossRef]
- He, J.; Ren, J.; Niu, G.; Liu, A.; Wu, Q.; Xie, S.; Ma, X.; Li, B.; Wang, P.; Shen, J.; et al. Multiparametric MR radiomics in brain glioma: Models comparation to predict biomarker status. BMC Med. Imaging 2022, 22, 137. [Google Scholar] [CrossRef]
- Halilibrahimoglu, H.; Polat, K.; Keskin, S.; Genc, O.; Aslan, O.; Ozturk-Isik, E.; Yakicier, C.; Danyeli, A.E.; Pamir, M.N.; Ozduman, K.; et al. Associating IDH and TERT Mutations in Glioma with Diffusion Anisotropy in Normal-Appearing White Matter. Am. J. Neuroradiol. 2023, 44, 553–561. [Google Scholar] [CrossRef]
- Gao, M.; Lin, Y.; Liu, X.; Zhao, Z.; Zhu, Z.; Zhang, H.; Ban, Y.; Bie, Y.; He, X.; Sun, X.; et al. TERT Mutation Is Accompanied by Neutrophil Infiltration and Contributes to Poor Survival in Isocitrate Dehydrogenase Wild-Type Glioma. Front. Cell Dev. Biol. 2021, 9, 654407. [Google Scholar] [CrossRef]
- Bae, S.; Choi, Y.S.; Ahn, S.S.; Chang, J.H.; Kang, S.G.; Kim, E.H.; Kim, S.H.; Lee, S.K. Radiomic MRI Phenotyping of Glioblastoma: Improving Survival Prediction. Radiology 2018, 289, 797–806. [Google Scholar] [CrossRef]
- Gidwani, M.; Chang, K.; Patel, J.B.; Hoebel, K.V.; Ahmed, S.R.; Singh, P.; Fuller, C.D.; Kalpathy-Cramer, J. Inconsistent Partitioning and Unproductive Feature Associations Yield Idealized Radiomic Models. Radiology 2023, 307, e220715. [Google Scholar] [CrossRef]
- Verger, A.; Langen, K.J. PET Imaging in Glioblastoma: Use in Clinical Practice. In Glioblastoma; De Vleeschouwer, S., Ed.; Exon Publications: Brisbane, Australia, 2017. [Google Scholar]
- Shooli, H.; Dadgar, H.; Wang, Y.J.; Vafaee, M.S.; Kashuk, S.R.; Nemati, R.; Jafari, E.; Nabipour, I.; Gholamrezanezhad, A.; Assadi, M.; et al. An update on PET-based molecular imaging in neuro-oncology: Challenges and implementation for a precision medicine approach in cancer care. Quant. Imaging Med. Surg. 2019, 9, 1597–1610. [Google Scholar] [CrossRef]
- Johnson, J.M.; Chen, M.M.; Rohren, E.M.; Prabhu, S.; Chasen, B.; Mawlawi, O.; Liu, H.L.; Gule-Monroe, M.K. Delayed FDG PET Provides Superior Glioblastoma Conspicuity Compared to Conventional Image Timing. Front. Neurol. 2021, 12, 740280. [Google Scholar] [CrossRef]
- Drake, L.R.; Hillmer, A.T.; Cai, Z. Approaches to PET Imaging of Glioblastoma. Molecules 2020, 25, 568. [Google Scholar] [CrossRef]
- Soni, N.; Ora, M.; Jena, A.; Rana, P.; Mangla, R.; Ellika, S.; Almast, J.; Puri, S.; Meyers, S.P. Amino Acid Tracer PET MRI in Glioma Management: What a Neuroradiologist Needs to Know. Am. J. Neuroradiol. 2023, 44, 236–246. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Xie, P.; Li, W.; Li, X.; Ma, L. Comparison of magnetic resonance spectroscopy and positron emission tomography in detection of tumor recurrence in posttreatment of glioma: A diagnostic meta-analysis. Asia-Pac. J. Clin. Oncol. 2015, 11, 97–105. [Google Scholar] [CrossRef]
- Hotta, M.; Minamimoto, R.; Miwa, K. 11C-methionine-PET for differentiating recurrent brain tumor from radiation necrosis: Radiomics approach with random forest classifier. Sci. Rep. 2019, 9, 15666. [Google Scholar] [CrossRef]
- Somme, F.; Bender, L.; Namer, I.J.; Noel, G.; Bund, C. Usefulness of (18)F-FDOPA PET for the management of primary brain tumors: A systematic review of the literature. Cancer Imaging 2020, 20, 70. [Google Scholar] [CrossRef]
- De Marco, R.; Pesaresi, A.; Bianconi, A.; Zotta, M.; Deandreis, D.; Morana, G.; Zeppa, P.; Melcarne, A.; Garbossa, D.; Cofano, F. A Systematic Review of Amino Acid PET Imaging in Adult-Type High-Grade Glioma Surgery: A Neurosurgeon’s Perspective. Cancers 2022, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Ora, M.; Mohindra, N.; Menda, Y.; Bathla, G. Diagnostic Performance of PET and Perfusion-Weighted Imaging in Differentiating Tumor Recurrence or Progression from Radiation Necrosis in Posttreatment Gliomas: A Review of Literature. Am. J. Neuroradiol. 2020, 41, 1550–1557. [Google Scholar] [CrossRef]
- Herrmann, K.; Czernin, J.; Cloughesy, T.; Lai, A.; Pomykala, K.L.; Benz, M.R.; Buck, A.K.; Phelps, M.E.; Chen, W. Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol. 2014, 16, 603–609. [Google Scholar] [CrossRef]
- Albano, D.; Tomasini, D.; Bonu, M.; Giubbini, R.; Bertagna, F. (18)F-Fluciclovine ((18)F-FACBC) PET/CT or PET/MRI in gliomas/glioblastomas. Ann. Nucl. Med. 2020, 34, 81–86. [Google Scholar] [CrossRef]
- Najjar, A.M.; Johnson, J.M.; Schellingerhout, D. The Emerging Role of Amino Acid PET in Neuro-Oncology. Bioengineering 2018, 5, 104. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Hill, R.; Pilkington, G.J.; Madureira, P.A. The Role of Hypoxia in Glioblastoma Invasion. Cells 2017, 6, 45. [Google Scholar] [CrossRef]
- Olivier, C.; Oliver, L.; Lalier, L.; Vallette, F.M. Drug Resistance in Glioblastoma: The Two Faces of Oxidative Stress. Front. Mol. Biosci. 2021, 7, 620677. [Google Scholar] [CrossRef]
- Reeves, K.M.; Song, P.N.; Angermeier, A.; Della Manna, D.; Li, Y.; Wang, J.; Yang, E.S.; Sorace, A.G.; Larimer, B.M. (18)F-FMISO PET Imaging Identifies Hypoxia and Immunosuppressive Tumor Microenvironments and Guides Targeted Evofosfamide Therapy in Tumors Refractory to PD-1 and CTLA-4 Inhibition. Clin. Cancer Res. 2021, 28, 327–337. [Google Scholar] [CrossRef]
- Stokes, A.M.; Hart, C.P.; Quarles, C.C. Hypoxia Imaging With PET Correlates With Antitumor Activity of the Hypoxia-Activated Prodrug Evofosfamide (TH-302) in Rodent Glioma Models. Tomography 2016, 2, 229–237. [Google Scholar] [CrossRef]
- Won, W.J.; Deshane, J.S.; Leavenworth, J.W.; Oliva, C.R.; Griguer, C.E. Metabolic and functional reprogramming of myeloid-derived suppressor cells and their therapeutic control in glioblastoma. Cell Stress 2019, 3, 47–65. [Google Scholar] [CrossRef]
- Foster, A.; Nigam, S.; Tatum, D.S.; Raphael, I.; Xu, J.; Kumar, R.; Plakseychuk, E.; Latoche, J.D.; Vincze, S.; Li, B.; et al. Novel theranostic agent for PET imaging and targeted radiopharmaceutical therapy of tumour-infiltrating immune cells in glioma. EBioMedicine 2021, 71, 103571. [Google Scholar] [CrossRef]
- Chen, K.T.; Wei, K.C.; Liu, H.L. Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Front. Pharmacol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Arif, W.M.; Elsinga, P.H.; Gasca-Salas, C.; Versluis, M.; Martinez-Fernandez, R.; Dierckx, R.; Borra, R.J.H.; Luurtsema, G. Focused ultrasound for opening blood-brain barrier and drug delivery monitored with positron emission tomography. J. Control. Release 2020, 324, 303–316. [Google Scholar] [CrossRef]
- Guerra, D.B.; Oliveira, E.M.N.; Sonntag, A.R.; Sbaraine, P.; Fay, A.P.; Morrone, F.B.; Papaleo, R.M. Intercomparison of radiosensitization induced by gold and iron oxide nanoparticles in human glioblastoma cells irradiated by 6 MV photons. Sci. Rep. 2022, 12, 9602. [Google Scholar] [CrossRef]
- Norouzi, M. Gold Nanoparticles in Glioma Theranostics. Pharmacol. Res. 2020, 156, 104753. [Google Scholar] [CrossRef]
- Durand, M.; Chateau, A.; Jubreaux, J.; Devy, J.; Paquot, H.; Laurent, G.; Bazzi, R.; Roux, S.; Richet, N.; Reinhard-Ruch, A.; et al. Radiosensitization with Gadolinium Chelate-Coated Gold Nanoparticles Prevents Aggressiveness and Invasiveness in Glioblastoma. Int. J. Nanomed. 2023, 18, 243–261. [Google Scholar] [CrossRef]
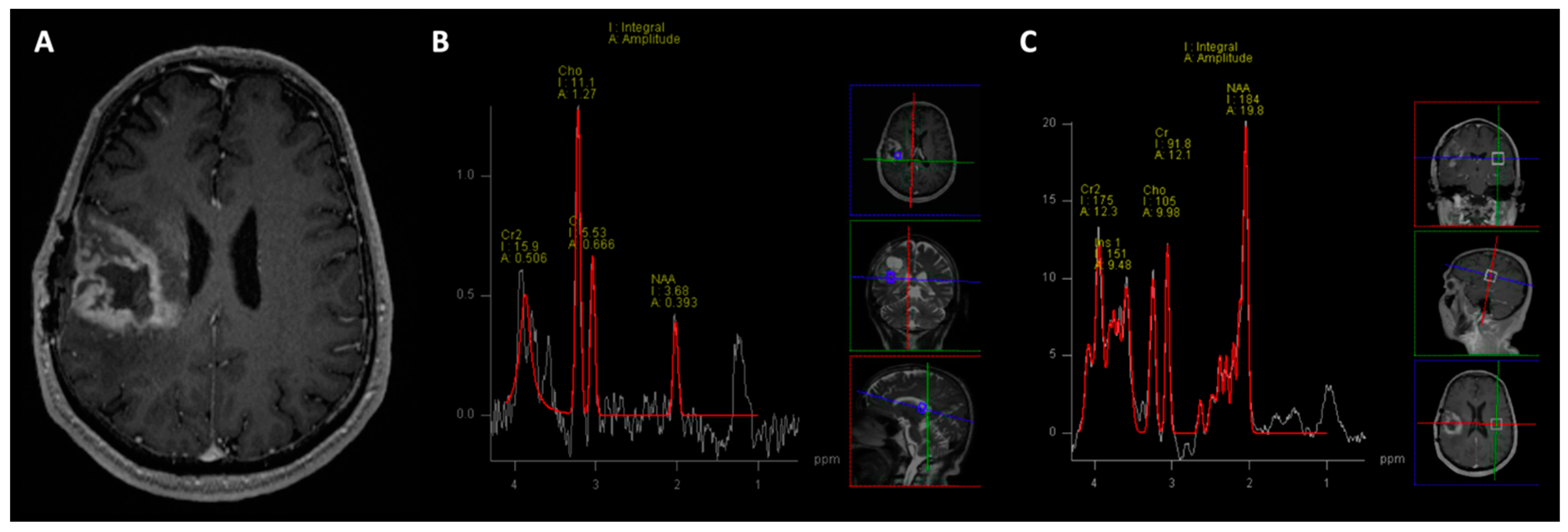
| Disease | Myoinositol | Creatine | Choline | NAA | Lipid-Lactate | Chol/Cr | Chol/NAA |
|---|---|---|---|---|---|---|---|
| GBM | ↓ | ↓ | ↑ | ↓ | ↑ ↑ | >2.5 | >2.2 |
| CVA | ↓ | ↓ | ↑ (Lactate) | ||||
| TMS | - | ↓ | ↑ | ↓ | ↑ (Lactate) | ↑ | ↑ |
| Oligodendroglioma | ↑ | ↓ | ↑ | ↓ | ↑↑ | ↑ | ↑ |
| Metastasis | - | - | ↑ | ↓ | ↑ | ↑ > 1.24 | ↑ > 1.11 |
| Radiotracer | Function |
|---|---|
| 18F-FDG | Glucose analog |
| [11C]Methionine ([11C]MET) | Amino acid preferentially utilized by gliomas vs. normal brain tissue; very short half-life. |
| [18F]L-fluoro-dihydroxyphenylalanine ([18F]FDOPA) | Amino acid similar to CMET but longer half-life. |
| [18F]Fluoromisoinodazole ([18F]FMISO) | Hypoxia-sensing agent, poor specificity. Low BBB penetration. |
| [18F]-fluorocyclobutane-1-carboxylic acid (18F-FACBC, Fluciclovine) | FDA-approved amino acid trace for prostate with performance similar to [11C]MET. |
| [18F]fluoroethyl-tyrosine ([18F]FET) | Actively transported; highly valuable when paired with MRI. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooper, G.W.; Ansari, S.; Johnson, J.M.; Ginat, D.T. Advances in the Radiological Evaluation of and Theranostics for Glioblastoma. Cancers 2023, 15, 4162. https://doi.org/10.3390/cancers15164162
Hooper GW, Ansari S, Johnson JM, Ginat DT. Advances in the Radiological Evaluation of and Theranostics for Glioblastoma. Cancers. 2023; 15(16):4162. https://doi.org/10.3390/cancers15164162
Chicago/Turabian StyleHooper, Grayson W., Shehbaz Ansari, Jason M. Johnson, and Daniel T. Ginat. 2023. "Advances in the Radiological Evaluation of and Theranostics for Glioblastoma" Cancers 15, no. 16: 4162. https://doi.org/10.3390/cancers15164162
APA StyleHooper, G. W., Ansari, S., Johnson, J. M., & Ginat, D. T. (2023). Advances in the Radiological Evaluation of and Theranostics for Glioblastoma. Cancers, 15(16), 4162. https://doi.org/10.3390/cancers15164162






