Prostate Region-Wise Imaging Biomarker Profiles for Risk Stratification and Biochemical Recurrence Prediction
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Risk Stratification and Biochemical Recurrence
2.3. MRI Acquisition
- MRI sequences necessary for the subsequent analysis were present and complete (T2w and DWI or T2w, DWI, and DCE)
- Optimal contrast to noise ratio was verified on the whole sequences
- Full coverage within the field of view (FOV) and centered on the prostate
- Absence of artifacts that may affect the analysis (i.e., patient movement)
2.4. Automated Segmentation
2.5. Imaging Biomarkers
2.5.1. Texture Analysis
- Shape features: The quantitative description of the region of interest (ROI)s’ geometric properties, such as surface area, total volume, diameter, elongation, sphericity, and surface-to-volume ratio.
- First-order statistics (histogram-based features): These describe the distribution of voxel intensities within the image ROI through commonly used conventional metrics (e.g., energy, entropy, mean, interquartile range, skewness, kurtosis, and uniformity).
- Second-order statistics (textural features): These are obtained from secondary matrices that include statistical inter-relationships between neighboring voxels, such as:
- Gray-level Co-occurrence Matrix (GLCM): The spatial distribution of gray-level intensities within a 3D image.
- Gray-level Run-length Matrix (GLRLM): The number of contiguous voxels that have the same gray-level value. This characterizes the gray-level run lengths of different gray-level intensities in any direction.
- Gray-level Size-zone Matrix (GLSZM): This quantifies gray-level zones, i.e., the number of connected voxels that share the same gray-level intensity, in a 3D image.
- Neighboring Gray-Tone Difference Matrix (NGTMD): This quantifies the difference between a gray value and the average gray value of its neighbors within a distance δ.
- Gray-level Dependence Matrix (GLDM): This quantifies the number of connected voxels within a distance δ that are dependent on the center voxel.
2.5.2. Diffusion Parameters
2.5.3. Perfusion Parameters
- Ktrans_mean, Ktrans_Std, Ktrans_median, Ktrans_p25, Ktrans_p75
- kep_mean, kep_Std, kep_median, kep_p25, Kep_p75
- ve_mean, ve_std, ve_median, ve_p25, Ve_p75
2.5.4. Weighted Biomarkers Averaging for the Whole Prostate
2.6. Statistical Analysis
2.6.1. Univariate Analysis
2.6.2. Multivariate Analysis
3. Results
3.1. Clinical Characteristics
3.2. Imaging Biomarker Profiles to Define Patient Stratification Risk
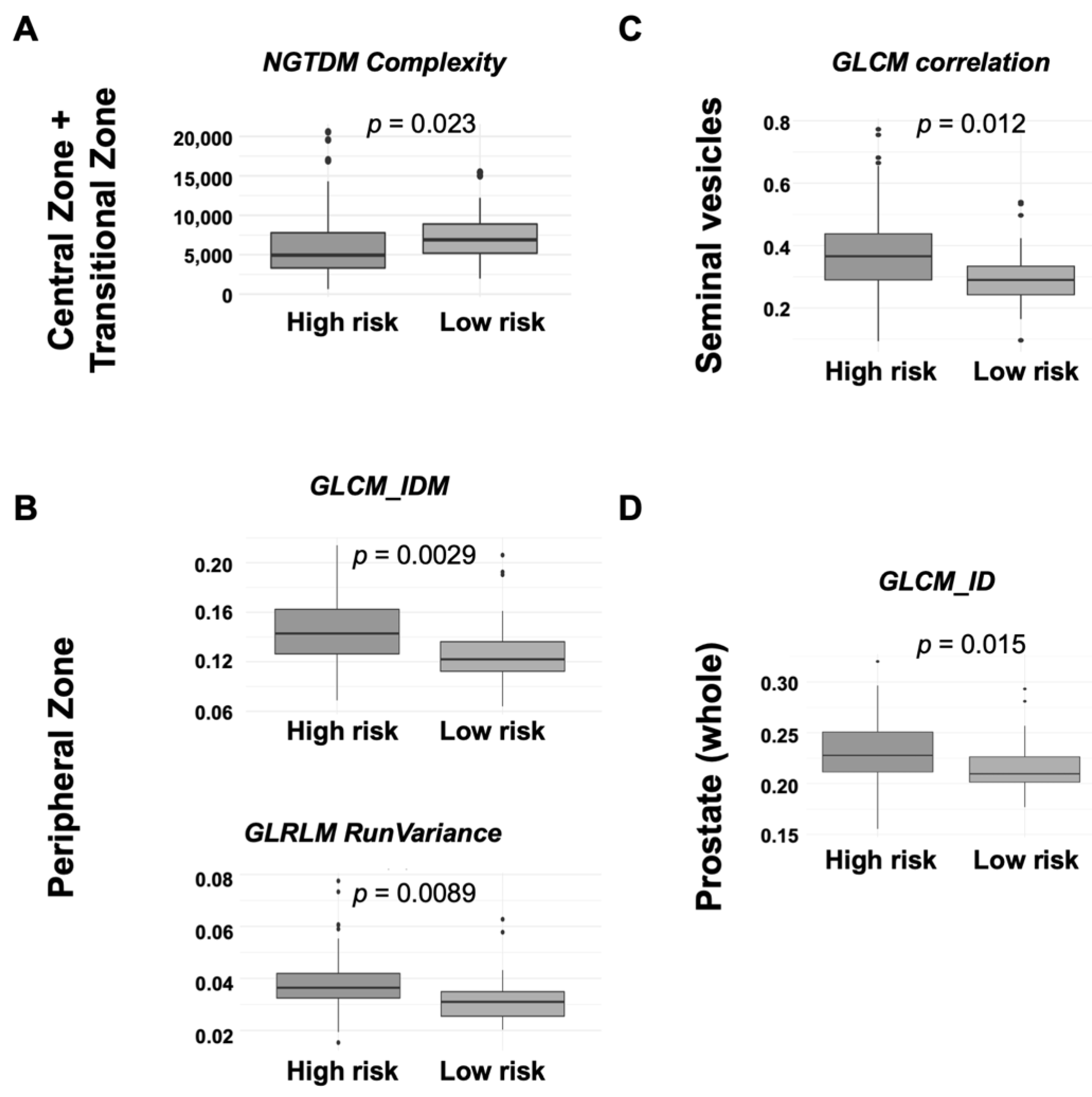
3.2.1. Central Zone and Transitional Zone
3.2.2. Peripheral Zone
3.2.3. Seminal Vesicles
3.2.4. Whole Prostate Gland
3.3. Imaging Biomarker Profiles to Define Biochemical Relapse
3.3.1. Central Zone and Transitional Zone
3.3.2. Peripheral Zone
3.3.3. Seminal Vesicles
3.3.4. Whole Prostate Gland
3.4. Imaging Biomarker Profiles to Define Biochemical Relapse in High/Unfavorable-Intermediate Risk Patients, Exploratory Analysis
3.4.1. Central Zone and Transitional Zone
3.4.2. Peripheral Zone
3.4.3. Seminal Vesicles
3.4.4. Whole Prostate Gland
3.5. Predictive Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crocetto, F.; Buonerba, C.; Caputo, V.; Ferro, M.; Persico, F.; Trama, F.; Iliano, E.; Rapisarda, S.; Bada, M.; Facchini, G.; et al. Urologic malignancies: Advances in the analysis and interpretation of clinical findings. Future Sci. OA 2021, 7, Fso674. [Google Scholar] [CrossRef] [PubMed]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors—A Systematic Review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Tomorrow. Available online: https://gco.iarc.fr/ (accessed on 12 August 2023).
- Tourinho-Barbosa, R.; Srougi, V.; Nunes-Silva, I.; Baghdadi, M.; Rembeyo, G.; Eiffel, S.S.; Barret, E.; Rozet, F.; Galiano, M.; Cathelineau, X.; et al. Biochemical recurrence after radical prostatectomy: What does it mean? Int. Braz. J. Urol. 2018, 44, 14–21. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Daskivich, T.J.; Wood, L.N.; Skarecky, D.; Ahlering, T.; Freedland, S. Limitations of the National Comprehensive Cancer Network® (NCCN®) Guidelines for Prediction of Limited Life Expectancy in Men with Prostate Cancer. J. Urol. 2017, 197, 356–362. [Google Scholar] [CrossRef]
- Björeland, U.; Nyholm, T.; Jonsson, J.; Skorpil, M.; Blomqvist, L.; Strandberg, S.; Riklund, K.; Beckman, L.; Thellenberg-Karlsson, C. Impact of neoadjuvant androgen deprivation therapy on magnetic resonance imaging features in prostate cancer before radiotherapy. Phys. Imaging Radiat. Oncol. 2021, 17, 117–123. [Google Scholar] [CrossRef]
- Daniel, M.; Kuess, P.; Andrzejewski, P.; Nyholm, T.; Helbich, T.; Polanec, S.; Dragschitz, F.; Goldner, G.; Georg, D.; Baltzer, P. Impact of androgen deprivation therapy on apparent diffusion coefficient and T2w MRI for histogram and texture analysis with respect to focal radiotherapy of prostate cancer. Strahlenther. Onkol. 2019, 195, 402–411. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Shiradkar, R.; Ghose, S.; Jambor, I.; Taimen, P.; Ettala, O.; Purysko, A.S.; Madabhushi, A. Radiomic features from pretreatment biparametric MRI predict prostate cancer biochemical recurrence: Preliminary findings. J. Magn. Reson. Imaging 2018, 48, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Fehr, D.; Veeraraghavan, H.; Wibmer, A.; Gondo, T.; Matsumoto, K.; Vargas, H.A.; Sala, E.; Hricak, H.; Deasy, J.O. Automatic classification of prostate cancer Gleason scores from multiparametric magnetic resonance images. Proc. Natl. Acad. Sci. USA 2015, 112, E6265–E6273. [Google Scholar] [CrossRef]
- Ginsburg, S.B.; Viswanath, S.E.; Bloch, B.N.; Rofsky, N.M.; Genega, E.M.; Lenkinski, R.E.; Madabhushi, A. Novel PCA-VIP scheme for ranking MRI protocols and identifying computer-extracted MRI measurements associated with central gland and peripheral zone prostate tumors. J. Magn. Reson. Imaging 2015, 41, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Lemaître, G.; Martí, R.; Freixenet, J.; Vilanova, J.C.; Walker, P.M.; Meriaudeau, F. Computer-Aided Detection and diagnosis for prostate cancer based on mono and multi-parametric MRI: A review. Comput. Biol. Med. 2015, 60, 8–31. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Debats, O.; Barentsz, J.; Karssemeijer, N.; Huisman, H. Computer-aided detection of prostate cancer in MRI. IEEE Trans. Med. Imaging 2014, 33, 1083–1092. [Google Scholar] [CrossRef]
- Viswanath, S.E.; Bloch, N.B.; Chappelow, J.C.; Toth, R.; Rofsky, N.M.; Genega, E.M.; Lenkinski, R.E.; Madabhushi, A. Central gland and peripheral zone prostate tumors have significantly different quantitative imaging signatures on 3 Tesla endorectal, in vivo T2-weighted MR imagery. J. Magn. Reson. Imaging 2012, 36, 213–224. [Google Scholar] [CrossRef]
- Gnep, K.; Fargeas, A.; Gutiérrez-Carvajal, R.E.; Commandeur, F.; Mathieu, R.; Ospina, J.D.; Rolland, Y.; Rohou, T.; Vincendeau, S.; Hatt, M.; et al. Haralick textural features on T2-weighted MRI are associated with biochemical recurrence following radiotherapy for peripheral zone prostate cancer. J. Magn. Reson. Imaging 2017, 45, 103–117. [Google Scholar] [CrossRef]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- American College of Radiology. PI-RADS v2.1 Guidelines. Available online: https://www.acr.org/-/media/ACR/Files/RADS/Pi-RADS/PIRADS-v2-1.pdf (accessed on 12 August 2023).
- Radiopaedia. Diffusion-Weighted Imaging. Available online: https://radiopaedia.org/articles/diffusion-weighted-imaging-2?lang=us (accessed on 12 August 2023).
- Radiopaedia. Dynamic Contrast Enhanced MR Perfusion (DCE). Available online: https://radiopaedia.org/articles/dynamic-contrast-enhanced-dce-mr-perfusion-1?lang=us (accessed on 12 August 2023).
- Sourbron, S.P.; Buckley, D.L. On the scope and interpretation of the Tofts models for DCE-MRI. Magn. Reson. Med. 2011, 66, 735–745. [Google Scholar] [CrossRef]
- Chang, E.; Joel, M.Z.; Chang, H.Y.; Du, J.; Khanna, O.; Omuro, A.; Chiang, V.; Aneja, S. Comparison of radiomic feature aggregation methods for patients with multiple tumors. Sci. Rep. 2021, 11, 9758. [Google Scholar] [CrossRef]
- Pyradiomics. Radiomic Features. Available online: https://pyradiomics.readthedocs.io/en/latest/features.html#module-radiomics.ngtdm (accessed on 12 August 2023).
- Flores-Téllez, T.; Baena, E. Experimental challenges to modeling prostate cancer heterogeneity. Cancer Lett. 2022, 524, 194–205. [Google Scholar] [CrossRef]
- Tolkach, Y.; Kristiansen, G. The Heterogeneity of Prostate Cancer: A Practical Approach. Pathobiology 2018, 85, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Davnall, F.; Yip, C.S.P.; Ljungqvist, G.; Selmi, M.; Ng, F.; Sanghera, B.; Ganeshan, B.; Miles, K.A.; Cook, G.J.; Goh, V. Assessment of tumor heterogeneity: An emerging imaging tool for clinical practice? Insights Imaging 2012, 3, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.S.; Sharma, P.; de Riese, W.T.W. MRI determined prostate volume and the incidence of prostate cancer on MRI-fusion biopsy: A systemic review of reported data for the last 20 years. Int. Urol. Nephrol. 2022, 54, 3047–3054. [Google Scholar] [CrossRef]
- Al-Khalil, S.; Ibilibor, C.; Cammack, J.T.; de Riese, W. Association of prostate volume with incidence and aggressiveness of prostate cancer. Res. Rep. Urol. 2016, 8, 201–205. [Google Scholar] [CrossRef]
- Buie, J.; De Riese, W.; Sharma, P. Smaller Prostate Volume is Associated with Adverse Pathological Features and Biochemical Recurrence after Radical Prostatectomy. Urol. Sci. 2019, 30, 255–261. [Google Scholar] [CrossRef]
- Just, N. Improving tumour heterogeneity MRI assessment with histograms. Br. J. Cancer 2014, 111, 2205–2213. [Google Scholar] [CrossRef]
- Le Bihan, D. Apparent diffusion coefficient and beyond: What diffusion MR imaging can tell us about tissue structure. Radiology 2013, 268, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef] [PubMed]
- Manetta, R.; Palumbo, P.; Gianneramo, C.; Bruno, F.; Arrigoni, F.; Natella, R.; Maggialetti, N.; Agostini, A.; Giovagnoni, A.; Di Cesare, E.; et al. Correlation between ADC values and Gleason score in evaluation of prostate cancer: Multicentre experience and review of the literature. Gland. Surg. 2019, 8, S216–S222. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, S.B.; Algohary, A.; Pahwa, S.; Gulani, V.; Ponsky, L.; Aronen, H.J.; Boström, P.J.; Böhm, M.; Haynes, A.M.; Brenner, P.; et al. Radiomic features for prostate cancer detection on MRI differ between the transition and peripheral zones: Preliminary findings from a multi-institutional study. J. Magn. Reson. Imaging 2017, 46, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Sinnott, J.A.; Rider, J.R.; Carlsson, J.; Gerke, T.; Tyekucheva, S.; Penney, K.L.; Sesso, H.D.; Loda, M.; Fall, K.; Stampfer, M.J.; et al. Molecular differences in transition zone and peripheral zone prostate tumors. Carcinogenesis 2015, 36, 632–638. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, 6, 610–621. [Google Scholar] [CrossRef]
- Shu, X.; Liu, Y.; Qiao, X.; Ai, G.; Liu, L.; Liao, J.; Deng, Z.; He, X. Radiomic-based machine learning model for the accurate prediction of prostate cancer risk stratification. Br. J. Radiol. 2023, 96, 20220238. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.; Chen, F.; Hwang, D.; Palmer, S.L.; De Castro Abreu, A.L.; Ukimura, O.; Aron, M.; Aron, M.; Gill, I.; Duddalwar, V.; et al. Objective risk stratification of prostate cancer using machine learning and radiomics applied to multiparametric magnetic resonance images. Sci. Rep. 2019, 9, 1570. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, J.; Xue, C.; Poon, D.M.C.; Yang, B.; Yu, S.K.; Cheung, K.Y. A pilot study of MRI radiomics for high-risk prostate cancer stratification in 1.5 T MR-guided radiotherapy. Magn. Reson. Med. 2023, 89, 2088–2099. [Google Scholar] [CrossRef]
- Wolf, A.M.; Wender, R.C.; Etzioni, R.B.; Thompson, I.M.; D’Amico, A.V.; Volk, R.J.; Brooks, D.D.; Dash, C.; Guessous, I.; Andrews, K.; et al. American Cancer Society guideline for the early detection of prostate cancer: Update 2010. CA Cancer J. Clin. 2010, 60, 70–98. [Google Scholar] [CrossRef]
- Koo, K.M.; Mainwaring, P.N.; Tomlins, S.A.; Trau, M. Merging new-age biomarkers and nanodiagnostics for precision prostate cancer management. Nat. Rev. Urol. 2019, 16, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Prensner, J.R.; Rubin, M.A.; Wei, J.T.; Chinnaiyan, A.M. Beyond PSA: The next generation of prostate cancer biomarkers. Sci. Transl. Med. 2012, 4, 127rv123. [Google Scholar] [CrossRef] [PubMed]
- Saltman, A.; Zegar, J.; Haj-Hamed, M.; Verma, S.; Sidana, A. Prostate cancer biomarkers and multiparametric MRI: Is there a role for both in prostate cancer management? Ther. Adv. Urol. 2021, 13, 1756287221997186. [Google Scholar] [CrossRef] [PubMed]
- Couture, F.; Wang, L.; Dufour, F.; Chabot-Maheux, K.; Ekindi Ndongo, N.; Sabbagh, R.; Day, R. PACE4-altCT isoform of proprotein convertase PACE4 as tissue and plasmatic biomarker for prostate cancer. Sci. Rep. 2022, 12, 6066. [Google Scholar] [CrossRef]
- Zhou, K.; Arslanturk, S.; Craig, D.B.; Heath, E.; Draghici, S. Discovery of primary prostate cancer biomarkers using cross cancer learning. Sci. Rep. 2021, 11, 10433. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Joniau, S.; Mason, M.; Matveev, V.; Schmid, H.P.; van der Kwast, T.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Actas Urológicas Españolas (Engl. Ed.) 2011, 35, 565–579. [Google Scholar]
- Bittencourt, L.K.; Hausmann, D.; Sabaneeff, N.; Gasparetto, E.L.; Barentsz, J.O. Multiparametric magnetic resonance imaging of the prostate: Current concepts. Radiol. Bras. 2014, 47, 292–300. [Google Scholar] [CrossRef]
- Pecoraro, M.; Messina, E.; Bicchetti, M.; Carnicelli, G.; Del Monte, M.; Iorio, B.; La Torre, G.; Catalano, C.; Panebianco, V. The future direction of imaging in prostate cancer: MRI with or without contrast injection. Andrology 2021, 9, 1429–1443. [Google Scholar] [CrossRef]
- Wang, Y.; Tadimalla, S.; Rai, R.; Goodwin, J.; Foster, S.; Liney, G.; Holloway, L.; Haworth, A. Quantitative MRI: Defining repeatability, reproducibility and accuracy for prostate cancer imaging biomarker development. Magn. Reson. Imaging 2021, 77, 169–179. [Google Scholar] [CrossRef]
- Calace, F.P.; Napolitano, L.; Arcaniolo, D.; Stizzo, M.; Barone, B.; Crocetto, F.; Olivetta, M.; Amicuzi, U.; Cirillo, L.; Rubinacci, A.; et al. Micro-Ultrasound in the Diagnosis and Staging of Prostate and Bladder Cancer: A Comprehensive Review. Medicina 2022, 58, 1624. [Google Scholar] [CrossRef]
- Bayrak, E.A.; Kirci, P. Chapter 11—Big data analytics and radiomics to discover diagnostics on different cancer types. In Big Data Analytics for Healthcare; Keikhosrokiani, P., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 125–138. [Google Scholar] [CrossRef]
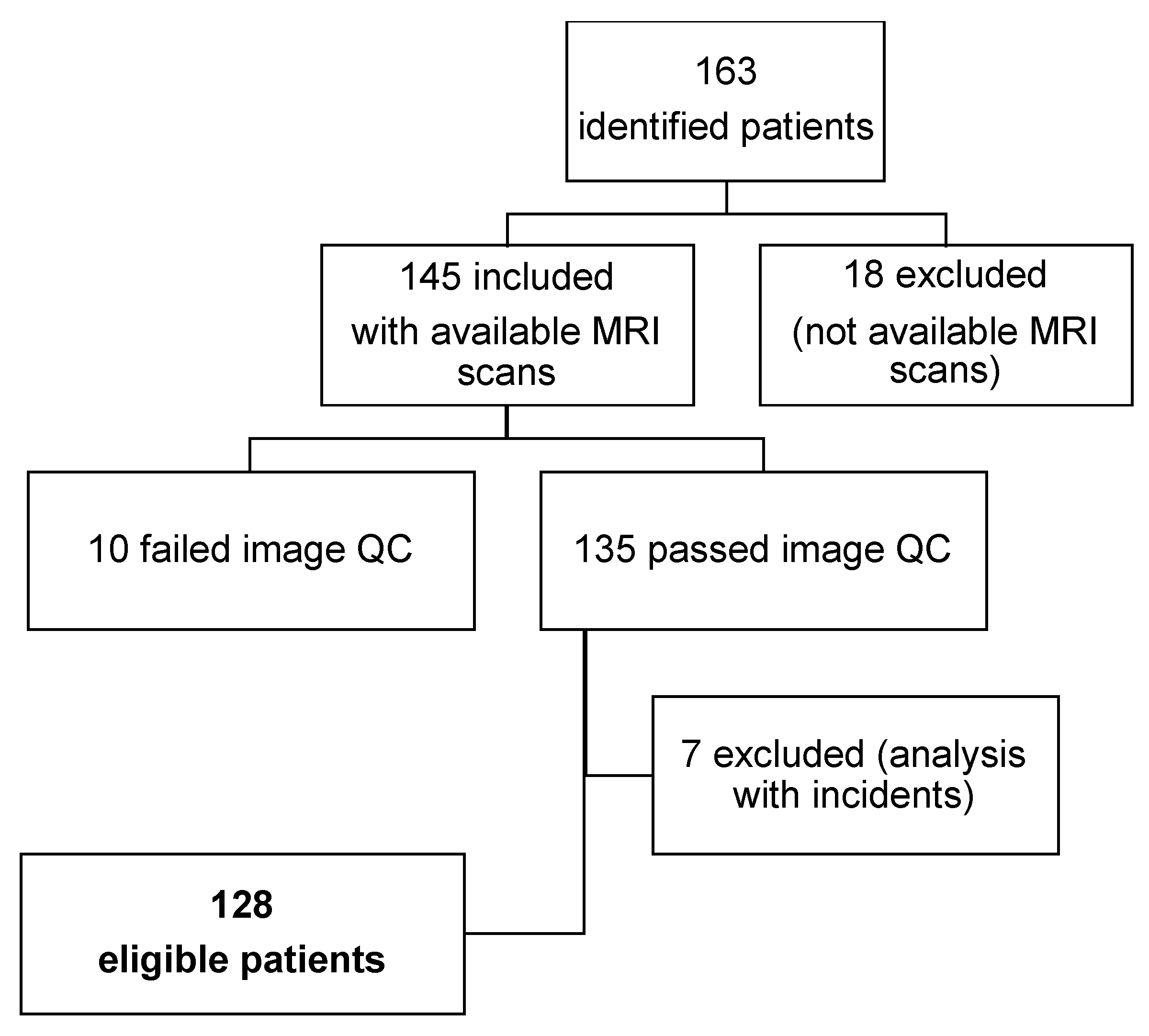
| Characteristic | Patients (N = 128) n (%) |
|---|---|
| ECOG PS | |
| 0 | 2 (1.56) |
| 1 | 73 (57.03) |
| 2 | 53 (41.41) |
| ISUP | |
| 1 | 38 (29.69) |
| 2 | 34 (26.56) |
| 3 | 23 (17.97) |
| 4 | 16 (12.50) |
| 5 | 17 (13.28) |
| c(N) from TNM stage | |
| cN0 | 113 |
| cN1 | 15 |
| Perineural invasion | |
| No | 114 (89.06) |
| Yes | 10 (7.81) |
| Unknown | 4 (3.13) |
| Risk stratification | |
| Low | 6 (4.69) |
| Favorable intermediate | 26 (20.31) |
| Unfavorable intermediate | 31 (24.22) |
| High | 65 (50.78) |
| Biochemical relapse (10 years from diagnosis) | |
| Yes | 20 (15.63) |
| No | 108 (84.38) |
| Type | CZ + TZ | PZ | Seminal Vesicles | Whole Prostate | |
|---|---|---|---|---|---|
| Texture analysis | |||||
| 10percentile | First-order | – | – | 0.0161 | – |
| Median | First-order | – | – | 0.0031 | – |
| Skewness | First-order | – | – | 0.0036 | 0.0118 |
| Flatness | Shape 2D | – | 0.030 | – | – |
| Major Axis Length | Shape 2D | – | 0.002 | 0.0159 | 0.0057 |
| Minor Axis Length | Shape 2D | – | – | 0.0261 | |
| Maximum 2D Diameter | Shape 2D | – | – | 0.0031 | |
| Maximum 3D Diameter | Shape 3D | – | 0.024 | – | – |
| Surface Volume Ratio | Shape 3D | – | – | 0.0141 | – |
| GLCM_Inverse Difference | Second-order | – | – | – | 0.0079 |
| GLCM_IMC1 | Second-order | – | – | 0.0079 | – |
| GLCM_IMC2 | Second-order | – | – | 0.0334 | – |
| GLCM_Cluster Shade | Second-order | – | – | 0.0166 | – |
| GLCM_Inverse Variance | Second-order | – | – | 0.0460 | – |
| GLCM_Maximum Probability | Second-order | – | – | 0.0004 | – |
| GLSZM_LAE | High-order | 0.0249 | – | – | 0.0109 |
| GLSZM_LAHGLE | High-order | – | – | 0.0048 | – |
| GLSZM_LALGLE | High-order | – | – | – | 0.0025 |
| GLRLM_SRE | High-order | 0.0311 | – | – | |
| GLDM_DE | Second-order | – | – | 0.0086 | – |
| GLDM_LDLGLE | Second-order | – | – | 0.0001 | 0.0047 |
| NGTDM_Strength | High-order | – | – | 0.0009 | – |
| Diffusion biomarkers | |||||
| ADC_mean | – | 0.0292 | – | – | – |
| Performance | Sen. | Spe. | Acc. | AUC | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictive Models | |||||||||||
| I | I + C | I | I + C | I | R + C | I | I + C | I | I + C | ||
| Risk groups | |||||||||||
| CZ + TZ | 0.408 | 0.542 | 0.690 | 0.604 | 0.561 | 0.574 | 0.610 | 0.651 | 0.603 | 0.318 | |
| PZ | 0.667 | 0.563 | 0.631 | 0.717 | 0.648 | 0.644 | 0.670 | 0.685 | 0.025 | 0.190 | |
| Seminal vesicles | 0.673 | 0.708 | 0.741 | 0.736 | 0.710 | 0.723 | 0.797 | 0.784 | 0.005 | 0.035 | |
| Whole prostate | 0.551 | 0.604 | 0.724 | 0.679 | 0.645 | 0.644 | 0.659 | 0.693 | 0.524 | 0.386 | |
| BCR | |||||||||||
| CZ + TZ | 1.00 | 0.961 | 0.00 | 0.176 | 0.813 | 0.850 | 0.644 | 0.841 | 0.518 | 0.020 | |
| PZ | 0.977 | 0.976 | 0.150 | 0.294 | 0.822 | 0.860 | 0.748 | 0.877 | 0.025 | 0.002 | |
| Seminal vesicles | 0.977 | 0.976 | 0.250 | 0.412 | 0.841 | 0.880 | 0.788 | 0.862 | 0.104 | 0.080 | |
| Whole prostate | 0.989 | 0.976 | 0.150 | 0.353 | 0.832 | 0.870 | 0.771 | 0.855 | 0.158 | 0.093 | |
| BCR (high/unf. IR) | |||||||||||
| CZ + TZ | 1.00 | 0.905 | 0.00 | 0.400 | 0.776 | 0.808 | 0.699 | 0.912 | 0.381 | 0.032 | |
| PZ | 0.978 | 0.980 | 0.308 | 0.600 | 0.828 | 0.915 | 0.716 | 0.951 | 0.097 | 0.001 | |
| Seminal vesicles | 0.981 | 0.976 | 0.308 | 0.600 | 0.846 | 0.904 | 0.756 | 0.898 | 0.246 | 0.042 | |
| Whole prostate | 0.978 | 0.980 | 0.231 | 0.600 | 0.810 | 0.915 | 0.725 | 0.920 | 0.447 | 0.017 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Iglesias, Á.; Morillo Macías, V.; Picó Peris, A.; Fuster-Matanzo, A.; Nogué Infante, A.; Muelas Soria, R.; Bellvís Bataller, F.; Domingo Pomar, M.; Casillas Meléndez, C.; Yébana Huertas, R.; et al. Prostate Region-Wise Imaging Biomarker Profiles for Risk Stratification and Biochemical Recurrence Prediction. Cancers 2023, 15, 4163. https://doi.org/10.3390/cancers15164163
Sánchez Iglesias Á, Morillo Macías V, Picó Peris A, Fuster-Matanzo A, Nogué Infante A, Muelas Soria R, Bellvís Bataller F, Domingo Pomar M, Casillas Meléndez C, Yébana Huertas R, et al. Prostate Region-Wise Imaging Biomarker Profiles for Risk Stratification and Biochemical Recurrence Prediction. Cancers. 2023; 15(16):4163. https://doi.org/10.3390/cancers15164163
Chicago/Turabian StyleSánchez Iglesias, Ángel, Virginia Morillo Macías, Alfonso Picó Peris, Almudena Fuster-Matanzo, Anna Nogué Infante, Rodrigo Muelas Soria, Fuensanta Bellvís Bataller, Marcos Domingo Pomar, Carlos Casillas Meléndez, Raúl Yébana Huertas, and et al. 2023. "Prostate Region-Wise Imaging Biomarker Profiles for Risk Stratification and Biochemical Recurrence Prediction" Cancers 15, no. 16: 4163. https://doi.org/10.3390/cancers15164163
APA StyleSánchez Iglesias, Á., Morillo Macías, V., Picó Peris, A., Fuster-Matanzo, A., Nogué Infante, A., Muelas Soria, R., Bellvís Bataller, F., Domingo Pomar, M., Casillas Meléndez, C., Yébana Huertas, R., & Ferrer Albiach, C. (2023). Prostate Region-Wise Imaging Biomarker Profiles for Risk Stratification and Biochemical Recurrence Prediction. Cancers, 15(16), 4163. https://doi.org/10.3390/cancers15164163






