Innate Immune System in the Context of Radiation Therapy for Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
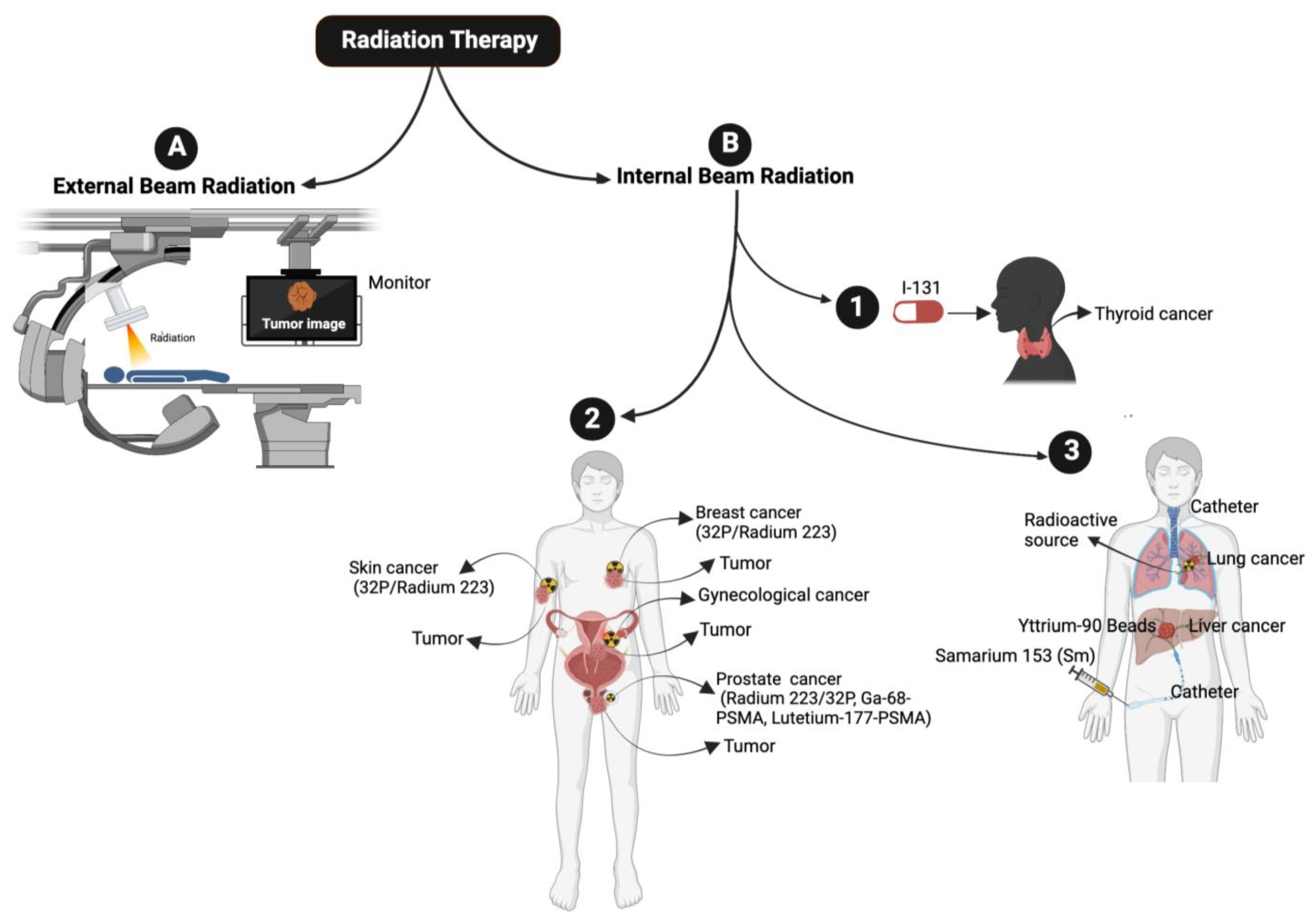
2. Radiation Therapy
2.1. External Beam Radiation Therapy
2.2. Internal Beam Radiation or Brachytherapy
3. Non-Ionizing Radiation Induces Oxidative Stress
4. Innate Immunity
Role of RT in Priming the Innate Immune Response
5. Mechanisms of Radiation-Induced Innate Immune Cell Activation
5.1. Dendritic Cells
5.2. Natural Killer Cells
5.3. Macrophages
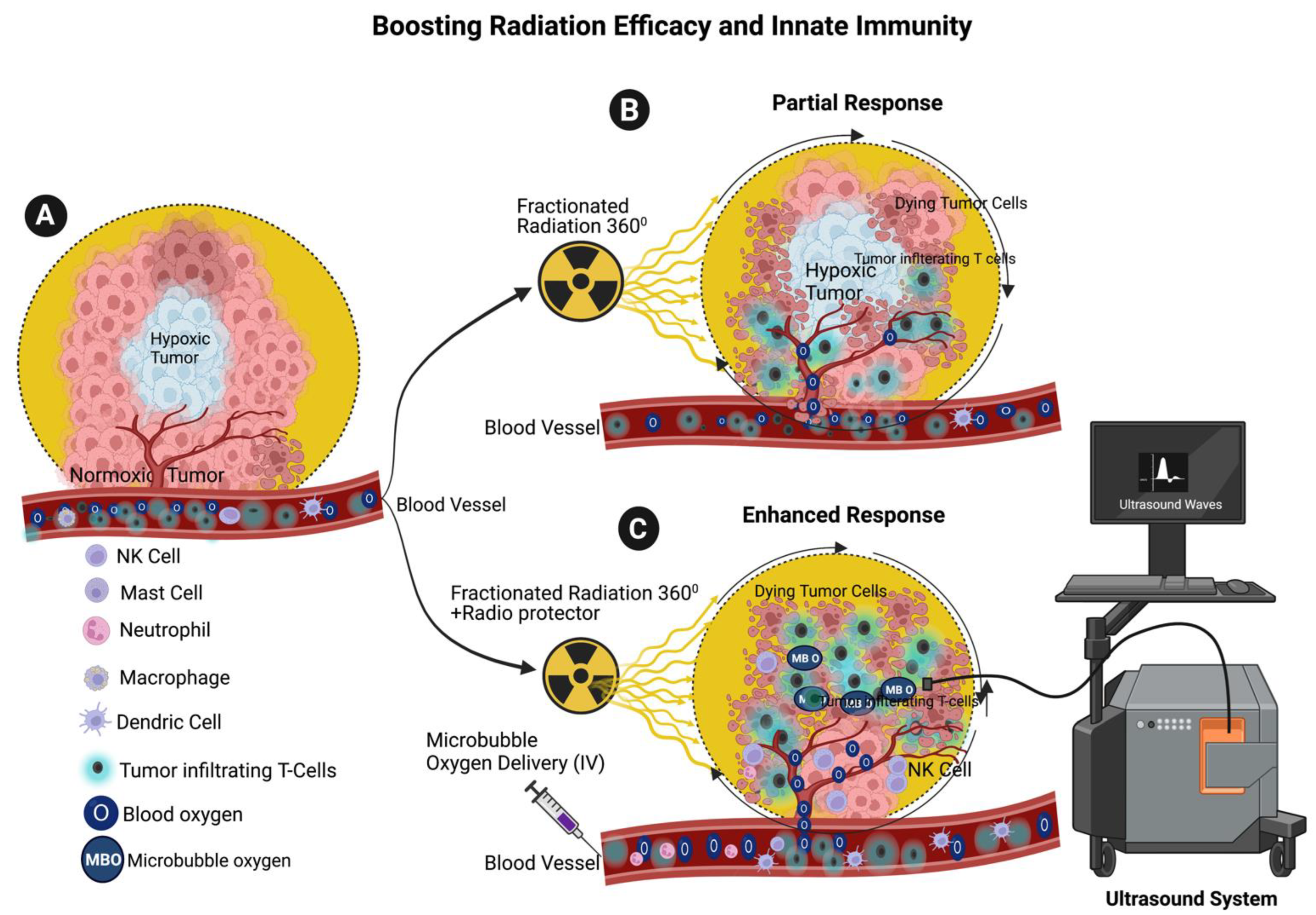
6. Enhancing the Radiotherapy Efficacy through Microbubble Oxygen Delivery
7. Enhancing Radiation Efficiency and Maintaining Innate Immune System through the Combination of Microbubble Oxygen Delivery and Radioprotection with RT
8. Potential Role of Endogenous Radioprotectors in DNA Damage and Immune Response to Radiation
9. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Buatti, J.M.; Rivero, L.R.; Jorgensen, T.J. Radiation-induced DNA single-strand breaks in freshly isolated human leukocytes. Radiat. Res. 1992, 132, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Mahaney, B.L.; Meek, K.; Lees-Miller, S.P. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem. J. 2009, 417, 639–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrego-Soto, G.; Ortiz-Lopez, R.; Rojas-Martinez, A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet. Mol. Biol. 2015, 38, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.X.; Zhou, P.K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Dennstadt, F.; Treffers, T.; Iseli, T.; Panje, C.; Putora, P.M. Creation of clinical algorithms for decision-making in oncology: An example with dose prescription in radiation oncology. BMC Med. Inform. Decis. Mak. 2021, 21, 212. [Google Scholar] [CrossRef]
- Leech, M.; Katz, M.S.; Kazmierska, J.; McCrossin, J.; Turner, S. Empowering patients in decision-making in radiation oncology—Can we do better? Mol. Oncol. 2020, 14, 1442–1460. [Google Scholar] [CrossRef] [Green Version]
- Akeem, S.; Lukman, O.; Eltahir, K.; Fatai, O.; Abiola, B.; Khadijat, O. Bone Marrow and Peripheral Blood Cells Toxicity of a Single 2.0 Gy Cobalt(60) Ionizing Radiation: An Animal Model. Ethiop. J. Health Sci. 2019, 29, 195–202. [Google Scholar] [CrossRef]
- Scott, D.; Lyons, C.Y. Homogeneous sensitivity of human peripheral blood lymphocytes to radiation-induced chromosome damage. Nature 1979, 278, 756–758. [Google Scholar] [CrossRef]
- Marin, A.; Martin, M.; Linan, O.; Alvarenga, F.; Lopez, M.; Fernandez, L.; Buchser, D.; Cerezo, L. Bystander effects and radiotherapy. Rep. Pract. Oncol. Radiother. 2015, 20, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Majeed, H.; Gupta, V. Adverse Effects of Radiation Therapy. In Treasure Island; StatPearls: Orlando, FL, USA, 2022. [Google Scholar]
- Maani, E.V.; Maani, C.V. Radiation Therapy. In Treasure Island; StatPearls: Orlando, FL, USA, 2022. [Google Scholar]
- Podder, T.K.; Fredman, E.T.; Ellis, R.J. Advances in Radiotherapy for Prostate Cancer Treatment. Adv. Exp. Med. Biol. 2018, 1096, 31–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meaney, C.; Kohandel, M.; Novruzi, A. Temporal optimization of radiation therapy to heterogeneous tumour populations and cancer stem cells. J. Math. Biol. 2022, 85, 51. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Kirkpatrick, D.R.; Smith, S.; Smith, T.K.; Pearson, T.; Kailasam, A.; Herrmann, K.Z.; Schubert, J.; Agrawal, D.K. Radioprotective agents to prevent cellular damage due to ionizing radiation. J. Transl. Med. 2017, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Sanz, A.G.; Garcia, G.; Sanche, L. Radiation Damage to DNA: The Indirect Effect of Low Energy Electrons. J. Phys. Chem. Lett. 2013, 4, 820–825. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, E.; Orlando, T.M.; Sanche, L. Biomolecular damage induced by ionizing radiation: The direct and indirect effects of low-energy electrons on DNA. Annu. Rev. Phys. Chem. 2015, 66, 379–398. [Google Scholar] [CrossRef]
- Mirzayans, R.; Waters, R.; Paterson, M.C. Induction and repair of DNA strand breaks and 1-beta-D-arabinofuranosylcytosine-detectable sites in 40-75 kVp X-irradiated compared to 60Co gamma-irradiated human cell lines. Radiat. Res. 1988, 114, 168–185. [Google Scholar] [CrossRef]
- Hanson, W.R.; Grdina, D.J. Radiation-induced DNA single-strand breaks in the intestinal mucosal cells of mice treated with the radioprotectors WR-2721 or 16-16 dimethyl prostaglandin E2. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1987, 52, 67–76. [Google Scholar] [CrossRef]
- Ghardi, M.; Moreels, M.; Chatelain, B.; Chatelain, C.; Baatout, S. Radiation-induced double strand breaks and subsequent apoptotic DNA fragmentation in human peripheral blood mononuclear cells. Int. J. Mol. Med. 2012, 29, 769–780. [Google Scholar] [CrossRef]
- Khozooei, S.; Lettau, K.; Barletta, F.; Jost, T.; Rebholz, S.; Veerappan, S.; Franz-Wachtel, M.; Macek, B.; Iliakis, G.; Distel, L.V.; et al. Fisetin induces DNA double-strand break and interferes with the repair of radiation-induced damage to radiosensitize triple negative breast cancer cells. J. Exp. Clin. Cancer Res. 2022, 41, 256. [Google Scholar] [CrossRef]
- Kinashi, Y.; Yokomizo, N.; Takahashi, S. DNA Double-Strand Breaks Induced byFractionated Neutron Beam Irradiation for Boron Neutron Capture Therapy. Anticancer. Res. 2017, 37, 1681–1685. [Google Scholar] [CrossRef]
- Vignard, J.; Mirey, G.; Salles, B. Ionizing-radiation induced DNA double-strand breaks: A direct and indirect lighting up. Radiother. Oncol. 2013, 108, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Palma, D.A.; Verbakel, W.F.; Otto, K.; Senan, S. New developments in arc radiation therapy: A review. Cancer Treat. Rev. 2010, 36, 393–399. [Google Scholar] [CrossRef]
- Hernandez, V.; Angerud, A.; Bogaert, E.; Hussein, M.; Lemire, M.; Garcia-Miguel, J.; Saez, J. Challenges in modeling the Agility multileaf collimator in treatment planning systems and current needs for improvement. Med. Phys. 2022, 49, 7404–7416. [Google Scholar] [CrossRef]
- Matsuda, R.; Hasegawa, M.; Tamamoto, T.; Inooka, N.; Nikimoto, M.; Ochi, T.; Miyasaka, T.; Hontsu, S.; Yamaki, K.; Miura, S.; et al. Long-Term Survival after Linac-Based Stereotactic Radiosurgery and Radiotherapy with a Micro-Multileaf Collimator for Brain Metastasis. Curr. Oncol. 2022, 29, 6068–6076. [Google Scholar] [CrossRef]
- Matsuda, R.; Hasegawa, M.; Tamamoto, T.; Ochi, T.; Miyasaka, T.; Inooka, N.; Hontsu, S.; Miura, S.; Takeshima, Y.; Tamura, K.; et al. Linac-based stereotactic radiosurgery and fractionated stereotactic radiotherapy with a micro-multileaf collimator for brain metastasis in the primary motor cortex. J. Radiat. Res. 2022, 63, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Moskvin, V.; Cheng, C.W.; Das, I.J. Pitfalls of tungsten multileaf collimator in proton beam therapy. Med. Phys. 2011, 38, 6395–6406. [Google Scholar] [CrossRef] [PubMed]
- Shirato, H.; Onimaru, R.; Ishikawa, M.; Kaneko, J.; Takeshima, T.; Mochizuki, K.; Shimizu, S.; Umegaki, K. Real-time 4-D radiotherapy for lung cancer. Cancer Sci. 2012, 103, 1–6. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Duma, M.N.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; et al. Heart-sparing radiotherapy techniques in breast cancer patients: A recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO). Strahlenther. Onkol. 2019, 195, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H. Abscopal effect of stereotactic radiotherapy combined with anti-PD-1/PD-L1 immunotherapy: Mechanisms, clinical efficacy, and issues. Cancer Commun. 2020, 40, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Poppe, M.M.; Hua, C.H.; Marcus, K.J.; Esiashvili, N. Acute lymphoblastic leukemia. Pediatr. Blood Cancer 2021, 68 (Suppl. S2), e28371. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Moroni, M.; Sanjak, J.; MacMillan, L.; Hritzo, B.; Martello, S.; Bylicky, M.; May, J.; Coleman, C.N.; Aryankalayil, M.J. Whole blood gene expression within days after total-body irradiation predicts long term survival in Gottingen minipigs. Sci. Rep. 2021, 11, 15873. [Google Scholar] [CrossRef] [PubMed]
- Koksal, M.; Baumert, J.; Jazmati, D.; Schoroth, F.; Garbe, S.; Koch, D.; Scafa, D.; Sarria, G.R.; Leitzen, C.; Massoth, G.; et al. Whole body irradiation with intensity-modulated helical tomotherapy prior to haematopoietic stem cell transplantation: Analysis of organs at risk by dose and its effect on blood kinetics. J. Cancer Res. Clin. Oncol. 2023, 149, 7007–7015. [Google Scholar] [CrossRef] [PubMed]
- LaRiviere, M.J.; Santos, P.M.G.; Hill-Kayser, C.E.; Metz, J.M. Proton Therapy. Hematol. Oncol. Clin. N. Am. 2019, 33, 989–1009. [Google Scholar] [CrossRef]
- Wei, S.; Lin, H.; Shi, C.; Xiong, W.; Chen, C.C.; Huang, S.; Press, R.H.; Hasan, S.; Chhabra, A.M.; Choi, J.I.; et al. Use of single-energy proton pencil beam scanning Bragg peak for intensity-modulated proton therapy FLASH treatment planning in liver-hypofractionated radiation therapy. Med. Phys. 2022, 49, 6560–6574. [Google Scholar] [CrossRef]
- Mollerberg, M.L.; Langegard, U.; Johansson, B.; Ohlsson-Nevo, E.; Fransson, P.; Ahlberg, K.; Witt-Nystrom, P.; Sjovall, K. Evaluation of skin reactions during proton beam radiotherapy—Patient-reported versus clinician-reported. Tech. Innov. Patient Support Radiat. Oncol. 2021, 19, 11–17. [Google Scholar] [CrossRef]
- Palma, G.; Monti, S.; Conson, M.; Xu, T.; Hahn, S.; Durante, M.; Mohan, R.; Liao, Z.; Cella, L. NTCP Models for Severe Radiation Induced Dermatitis after IMRT or Proton Therapy for Thoracic Cancer Patients. Front. Oncol. 2020, 10, 344. [Google Scholar] [CrossRef] [Green Version]
- Boehle, A.; Katic, K.; Konig, I.R.; Robrahn-Nitschke, I.; Brandenburg, B. Comparison of outcome endpoints in intermediate- and high-risk prostate cancer after combined-modality radiotherapy. Brachytherapy 2020, 19, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaorsky, N.G.; Davis, B.J.; Nguyen, P.L.; Showalter, T.N.; Hoskin, P.J.; Yoshioka, Y.; Morton, G.C.; Horwitz, E.M. The evolution of brachytherapy for prostate cancer. Nat. Rev. Urol. 2017, 14, 415–439. [Google Scholar] [CrossRef]
- Jungels, C.; Karfis, I. 131I-metaiodobenzylguanidine and peptide receptor radionuclide therapy in pheochromocytoma and paraganglioma. Curr. Opin. Oncol. 2021, 33, 33–39. [Google Scholar] [CrossRef]
- Middleton, S.M.; White, M.E.; Denson, K.W. The assay of porcine factor VIII. Thromb. Haemost. 1982, 48, 114. [Google Scholar] [CrossRef]
- Prado-Wohlwend, S.; Del Olmo-Garcia, M.I.; Bello-Arques, P.; Merino-Torres, J.F. Response to targeted radionuclide therapy with [(131)I]MIBG AND [(177)Lu]Lu-DOTA-TATE according to adrenal vs. extra-adrenal primary location in metastatic paragangliomas and pheochromocytomas: A systematic review. Front. Endocrinol. 2022, 13, 957172. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, R.; Mastrangelo, P.A.; Nardelli, A.; Mainenti, P.P.; Colasurdo, A.P.; Landriscina, M.; Guglielmi, G.; Storto, G. Radium-223 for the treatment of bone metastases in castration-resistant prostate cancer: When and why. Tumori 2019, 105, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraju, V.S.; Kumar, R.; Mittal, B.R.; Sharma, V.; Singh, H.; Nada, R.; Bal, A.; Rohilla, M.; Singh, H.; Rana, S.S. Differentiating benign and malignant pancreatic masses: Ga-68 PSMA PET/CT as a new diagnostic avenue. Eur. Radiol. 2021, 31, 2199–2208. [Google Scholar] [CrossRef]
- Masters, S.C.; Hofling, A.A.; Gorovets, A.; Marzella, L. FDA Approves Ga 68 PSMA-11 for Prostate Cancer Imaging. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Prive, B.M.; Peters, S.M.B.; Muselaers, C.H.J.; van Oort, I.M.; Janssen, M.J.R.; Sedelaar, J.P.M.; Konijnenberg, M.W.; Zamecnik, P.; Uijen, M.J.M.; Schilham, M.G.M.; et al. Lutetium-177-PSMA-617 in Low-Volume Hormone-Sensitive Metastatic Prostate Cancer: A Prospective Pilot Study. Clin. Cancer Res. 2021, 27, 3595–3601. [Google Scholar] [CrossRef]
- Haynes, D. The integrity of research published by Stephen E. Breuning. Bull. Med. Libr. Assoc. 1988, 76, 272. [Google Scholar]
- Farhanghi, M.; Holmes, R.A.; Volkert, W.A.; Logan, K.W.; Singh, A. Samarium-153-EDTMP: Pharmacokinetic, toxicity and pain response using an escalating dose schedule in treatment of metastatic bone cancer. J. Nucl. Med. 1992, 33, 1451–1458. [Google Scholar]
- Hoskin, P.; Sartor, O.; O’Sullivan, J.M.; Johannessen, D.C.; Helle, S.I.; Logue, J.; Bottomley, D.; Nilsson, S.; Vogelzang, N.J.; Fang, F.; et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: A prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014, 15, 1397–1406. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Parikh, S.; Murray, L.; Kenning, L.; Bottomley, D.; Din, O.; Dixit, S.; Ferguson, C.; Handforth, C.; Joseph, L.; Mokhtar, D.; et al. Real-world Outcomes and Factors Predicting Survival and Completion of Radium 223 in Metastatic Castrate-resistant Prostate Cancer. Clin. Oncol. 2018, 30, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yao, K.; Wang, K.J.; Lu, D.Q.; He, J.L.; Xu, L.H.; Sun, W.J. [Blocking 1800 MHz mobile phone radiation-induced reactive oxygen species production and DNA damage in lens epithelial cells by noise magnetic fields]. Zhejiang Da Xue Xue Bao Yi Xue Ban 2008, 37, 34–38. [Google Scholar] [CrossRef]
- Yao, K.; Wu, W.; Wang, K.; Ni, S.; Ye, P.; Yu, Y.; Ye, J.; Sun, L. Electromagnetic noise inhibits radiofrequency radiation-induced DNA damage and reactive oxygen species increase in human lens epithelial cells. Mol. Vis. 2008, 14, 964–969. [Google Scholar]
- Yao, K.; Wu, W.; Yu, Y.; Zeng, Q.; He, J.; Lu, D.; Wang, K. Effect of superposed electromagnetic noise on DNA damage of lens epithelial cells induced by microwave radiation. Invest. Ophthalmol. Vis. Sci. 2008, 49, 2009–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zmyslony, M.; Politanski, P.; Rajkowska, E.; Szymczak, W.; Jajte, J. Acute exposure to 930 MHz CW electromagnetic radiation in vitro affects reactive oxygen species level in rat lymphocytes treated by iron ions. Bioelectromagnetics 2004, 25, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Singh, N.P. Magnetic-field-induced DNA strand breaks in brain cells of the rat. Environ. Health Perspect. 2004, 112, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Oktem, F.; Ozguner, F.; Mollaoglu, H.; Koyu, A.; Uz, E. Oxidative damage in the kidney induced by 900-MHz-emitted mobile phone: Protection by melatonin. Arch. Med. Res. 2005, 36, 350–355. [Google Scholar] [CrossRef]
- Tkalec, M.; Malaric, K.; Pevalek-Kozlina, B. Exposure to radiofrequency radiation induces oxidative stress in duckweed Lemna minor L. Sci. Total Environ. 2007, 388, 78–89. [Google Scholar] [CrossRef]
- Friedman, J.; Kraus, S.; Hauptman, Y.; Schiff, Y.; Seger, R. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem. J. 2007, 405, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Sepehrimanesh, M.; Kazemipour, N.; Saeb, M.; Nazifi, S.; Davis, D.L. Proteomic analysis of continuous 900-MHz radiofrequency electromagnetic field exposure in testicular tissue: A rat model of human cell phone exposure. Environ. Sci. Pollut. Res. Int. 2017, 24, 13666–13673. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, M.K.; Polen, M.R.; Hiatt, R.A. The risk of miscarriage and birth defects among women who use visual display terminals during pregnancy. Am. J. Ind. Med. 1988, 13, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, Z.; Somosy, Z.; Kubinyi, G.; Bakos, J.; Hudak, A.; Surjan, A.; Thuroczy, G. Effect of whole-body 1800MHz GSM-like microwave exposure on testicular steroidogenesis and histology in mice. Reprod. Toxicol. 2006, 22, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ozguner, M.; Koyu, A.; Cesur, G.; Ural, M.; Ozguner, F.; Gokcimen, A.; Delibas, N. Biological and morphological effects on the reproductive organ of rats after exposure to electromagnetic field. Saudi Med. J. 2005, 26, 405–410. [Google Scholar]
- Calcabrini, C.; Mancini, U.; De Bellis, R.; Diaz, A.R.; Martinelli, M.; Cucchiarini, L.; Sestili, P.; Stocchi, V.; Potenza, L. Effect of extremely low-frequency electromagnetic fields on antioxidant activity in the human keratinocyte cell line NCTC 2544. Biotechnol. Appl. Biochem. 2017, 64, 415–422. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, S.K.; Devaraj, S.; Yang, T.; Jialal, I. Alpha-tocopherol decreases superoxide anion release in human monocytes under hyperglycemic conditions via inhibition of protein kinase C-alpha. Diabetes 2002, 51, 3049–3054. [Google Scholar] [CrossRef] [Green Version]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [Green Version]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef]
- Jang, J.H.; Shin, H.W.; Lee, J.M.; Lee, H.W.; Kim, E.C.; Park, S.H. An Overview of Pathogen Recognition Receptors for Innate Immunity in Dental Pulp. Mediat. Inflamm. 2015, 2015, 794143. [Google Scholar] [CrossRef] [Green Version]
- Shertzer, H.G.; Sainsbury, M. Intrinsic acute toxicity and hepatic enzyme inducing properties of the chemoprotectants indole-3-carbinol and 5,10-dihydroindeno [1,2-b]indole in mice. Food Chem. Toxicol. 1991, 29, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Boutrot, F.; Zipfel, C. Function, Discovery, and Exploitation of Plant Pattern Recognition Receptors for Broad-Spectrum Disease Resistance. Annu. Rev. Phytopathol. 2017, 55, 257–286. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [Green Version]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef]
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal 2015, 23, 1329–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Carroll, P.W.; Cahn, M.A.; Auston, I.; Selden, C.R. Information needs in public health and health policy: Results of recent studies. J. Urban Health 1998, 75, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Aymeric, L.; Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Martins, I.; Kroemer, G.; Smyth, M.J.; Zitvogel, L. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010, 70, 855–858. [Google Scholar] [CrossRef] [Green Version]
- Lam, K.C.; Araya, R.E.; Huang, A.; Chen, Q.; Di Modica, M.; Rodrigues, R.R.; Lopes, A.; Johnson, S.B.; Schwarz, B.; Bohrnsen, E.; et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 2021, 184, 5338–5356.e21. [Google Scholar] [CrossRef]
- Price, J.D.; Tarbell, K.V. The Role of Dendritic Cell Subsets and Innate Immunity in the Pathogenesis of Type 1 Diabetes and Other Autoimmune Diseases. Front. Immunol. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallahan, D.E.; Spriggs, D.R.; Beckett, M.A.; Kufe, D.W.; Weichselbaum, R.R. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc. Natl. Acad. Sci. USA 1989, 86, 10104–10107. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Chiang, C.S.; Tsao, C.Y.; Lin, P.Y.; McBride, W.H.; Wu, C.J. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int. J. Radiat. Biol. 1999, 75, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Tanaka, I.; Nemoto, K.; Tsuneoka, K.; Cheeramakara, C.; Yoshida, K.; Ohtsu, H. Immediate-early, transient induction of the interleukin-1 beta gene in mouse spleen macrophages by ionizing radiation. J. Radiat. Res. 1995, 36, 112–124. [Google Scholar] [CrossRef]
- Nemoto, K.; Ishihara, H.; Tanaka, I.; Suzuki, G.; Tsuneoka, K.; Yoshida, K.; Ohtsu, H. Expression of IL-1 beta mRNA in mice after whole body X-irradiation. J. Radiat. Res. 1995, 36, 125–133. [Google Scholar] [CrossRef]
- McBride, W.H.; Chiang, C.S.; Olson, J.L.; Wang, C.C.; Hong, J.H.; Pajonk, F.; Dougherty, G.J.; Iwamoto, K.S.; Pervan, M.; Liao, Y.P. A sense of danger from radiation. Radiat. Res. 2004, 162, 1–19. [Google Scholar] [CrossRef]
- Porkolab, V.; Chabrol, E.; Varga, N.; Ordanini, S.; Sutkeviciu Te, I.; Thepaut, M.; Garcia-Jimenez, M.J.; Girard, E.; Nieto, P.M.; Bernardi, A.; et al. Rational-Differential Design of Highly Specific Glycomimetic Ligands: Targeting DC-SIGN and Excluding Langerin Recognition. ACS Chem. Biol. 2018, 13, 600–608. [Google Scholar] [CrossRef]
- Dar, T.B.; Henson, R.M.; Shiao, S.L. Targeting Innate Immunity to Enhance the Efficacy of Radiation Therapy. Front. Immunol. 2018, 9, 3077. [Google Scholar] [CrossRef] [Green Version]
- Vatner, R.E.; Janssen, E.M. STING, DCs and the link between innate and adaptive tumor immunity. Mol. Immunol. 2019, 110, 13–23. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, X.; Zeng, Z.; Li, J.; Luo, Y.; Sun, W.; Gong, Y.; Zhang, J.; Wu, Q.; Xie, C. Immunomodulation of NK Cells by Ionizing Radiation. Front. Oncol. 2020, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Crinier, A.; Narni-Mancinelli, E.; Ugolini, S.; Vivier, E. SnapShot: Natural Killer Cells. Cell 2020, 180, 1280–1280.e1. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Wennerberg, E.; Vanpouille-Box, C.; Bornstein, S.; Yamazaki, T.; Demaria, S.; Galluzzi, L. Immune recognition of irradiated cancer cells. Immunol. Rev. 2017, 280, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Ina, Y.; Sakai, K. Activation of immunological network by chronic low-dose-rate irradiation in wild-type mouse strains: Analysis of immune cell populations and surface molecules. Int. J. Radiat. Biol. 2005, 81, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Lacoste-Collin, L.; Jozan, S.; Cances-Lauwers, V.; Pipy, B.; Gasset, G.; Caratero, C.; Courtade-Saidi, M. Effect of continuous irradiation with a very low dose of gamma rays on life span and the immune system in SJL mice prone to B-cell lymphoma. Radiat. Res. 2007, 168, 725–732. [Google Scholar] [CrossRef]
- Zarcone, D.; Tilden, A.B.; Lane, V.G.; Grossi, C.E. Radiation sensitivity of resting and activated nonspecific cytotoxic cells of T lineage and NK lineage. Blood 1989, 73, 1615–1621. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Kong, Q.; Wang, G.; Jin, H.; Zhou, L.; Yu, D.; Niu, C.; Han, W.; Li, W.; Cui, J. Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer Biother. Radiopharm. 2014, 29, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Cheda, A.; Wrembel-Wargocka, J.; Lisiak, E.; Nowosielska, E.M.; Marciniak, M.; Janiak, M.K. Single low doses of X rays inhibit the development of experimental tumor metastases and trigger the activities of NK cells in mice. Radiat. Res. 2004, 161, 335–340. [Google Scholar] [CrossRef]
- Hashimoto, S.; Shirato, H.; Hosokawa, M.; Nishioka, T.; Kuramitsu, Y.; Matushita, K.; Kobayashi, M.; Miyasaka, K. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat. Res. 1999, 151, 717–724. [Google Scholar] [CrossRef]
- Chini, C.C.; Boos, M.D.; Dick, C.J.; Schoon, R.A.; Leibson, P.J. Regulation of p38 mitogen-activated protein kinase during NK cell activation. Eur. J. Immunol. 2000, 30, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Ames, E.; Canter, R.J.; Grossenbacher, S.K.; Mac, S.; Smith, R.C.; Monjazeb, A.M.; Chen, M.; Murphy, W.J. Enhanced targeting of stem-like solid tumor cells with radiation and natural killer cells. Oncoimmunology 2015, 4, e1036212. [Google Scholar] [CrossRef] [Green Version]
- Terme, M.; Ullrich, E.; Delahaye, N.F.; Chaput, N.; Zitvogel, L. Natural killer cell-directed therapies: Moving from unexpected results to successful strategies. Nat. Immunol. 2008, 9, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.R.; Kim, T.D.; Choi, I. Understanding of molecular mechanisms in natural killer cell therapy. Exp. Mol. Med. 2015, 47, e141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg, C.; Taieb, J.; Terme, M.; Maruyama, K.; Flament, C.; Angevin, E.; Zitvogel, L. [NK cell-based immunotherapy: New prospects and involvement of dendritic cells]. Bull. Cancer 2003, 90, 699–705. [Google Scholar]
- Walle, T.; Kraske, J.A.; Liao, B.; Lenoir, B.; Timke, C.; von Bohlen Und Halbach, E.; Tran, F.; Griebel, P.; Albrecht, D.; Ahmed, A.; et al. Radiotherapy orchestrates natural killer cell dependent antitumor immune responses through CXCL8. Sci. Adv. 2022, 8, eabh4050. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef]
- Gomez, V.; Mustapha, R.; Ng, K.; Ng, T. Radiation therapy and the innate immune response: Clinical implications for immunotherapy approaches. Br. J. Clin. Pharmacol. 2020, 86, 1726–1735. [Google Scholar] [CrossRef]
- Shi, X.; Shiao, S.L. The role of macrophage phenotype in regulating the response to radiation therapy. Transl. Res. 2018, 191, 64–80. [Google Scholar] [CrossRef]
- Meziani, L.; Mondini, M.; Petit, B.; Boissonnas, A.; Thomas de Montpreville, V.; Mercier, O.; Vozenin, M.C.; Deutsch, E. CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur. Respir. J. 2018, 51, 1702120. [Google Scholar] [CrossRef] [Green Version]
- Travis, E.L. The sequence of histological changes in mouse lungs after single doses of x-rays. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 345–347. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [Green Version]
- Aminin, D.; Wang, Y.M. Macrophages as a “weapon” in anticancer cellular immunotherapy. Kaohsiung J. Med. Sci. 2021, 37, 749–758. [Google Scholar] [CrossRef]
- Mills, C.D.; Lenz, L.L.; Harris, R.A. A Breakthrough: Macrophage-Directed Cancer Immunotherapy. Cancer Res. 2016, 76, 513–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salah, A.; Li, Y.; Wang, H.; Qi, N.; Wu, Y. Macrophages as a Double-Edged Weapon: The Use of Macrophages in Cancer Immunotherapy and Understanding the Cross-Talk between Macrophages and Cancer. DNA Cell Biol. 2021, 40, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wu, X.; Zhang, J.; Fang, Y.; Pan, Y.; Shu, Y.; Ma, P. The evolving landscape of N(6)-methyladenosine modification in the tumor microenvironment. Mol. Ther. 2021, 29, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Park, Y.; Ni, X.; Li, H.; Zahnow, C.A.; Gabrielson, E.; Pan, F.; Semenza, G.L. Chemotherapy induces enrichment of CD47(+)/CD73(+)/PDL1(+) immune evasive triple-negative breast cancer cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1239–E1248. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Lin, Q.; Yun, Z. Cellular and molecular mechanisms underlying oxygen-dependent radiosensitivity. Radiat. Res. 2015, 183, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.J.; Chu, S.W.; Liao, E.C.; Fan, C.H.; Chan, H.L.; Wei, K.C.; Yeh, C.K. Normalization of Tumor Vasculature by Oxygen Microbubbles with Ultrasound. Theranostics 2019, 9, 7370–7383. [Google Scholar] [CrossRef]
- Kwan, J.J.; Kaya, M.; Borden, M.A.; Dayton, P.A. Theranostic oxygen delivery using ultrasound and microbubbles. Theranostics 2012, 2, 1174–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.; Song, R.; Lin, Q.; Zhang, Y.; Yang, Y.; Luo, M.; Zhong, Z.; Xu, X.; Lu, L.; Yao, S.; et al. A Robust Oxygen Microbubble Radiosensitizer for Iodine-125 Brachytherapy. Adv. Sci. 2021, 8, 2002567. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, I.K.; Ha, J.H.; Yeo, C.D.; Kang, H.H.; Kim, J.W.; Lee, S.H. Normobaric hyperoxia inhibits the progression of lung cancer by inducing apoptosis. Exp. Biol. Med. 2018, 243, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S. Naphthalimides in fluorescent imaging of tumor hypoxia—An up-to-date review. Bioorg. Chem. 2019, 88, 102979. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Boss, M.K.; Dewhirst, M.W. Imaging tumor hypoxia to advance radiation oncology. Antioxid. Redox Signal 2014, 21, 313–337. [Google Scholar] [CrossRef] [Green Version]
- Vaupel, P.; Kelleher, D.K.; Hockel, M. Oxygen status of malignant tumors: Pathogenesis of hypoxia and significance for tumor therapy. Semin. Oncol. 2001, 28, 29–35. [Google Scholar] [CrossRef]
- Baginska, J.; Viry, E.; Paggetti, J.; Medves, S.; Berchem, G.; Moussay, E.; Janji, B. The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front. Immunol. 2013, 4, 490. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, P.N.; Issa, M.M.; Stutzman, R.E.; Goldman, S.M. Gross hematuria and upper pole renal filling defect. Urology 1991, 37, 595–597. [Google Scholar] [CrossRef]
- Lee, C.T.; Mace, T.; Repasky, E.A. Hypoxia-driven immunosuppression: A new reason to use thermal therapy in the treatment of cancer? Int. J. Hyperthermia 2010, 26, 232–246. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Wang, P.; Zhang, K.; Geng, X.; Liu, Q.; Wang, X. Tumor targeting DVDMS-nanoliposomes for an enhanced sonodynamic therapy of gliomas. Biomater. Sci. 2019, 7, 985–994. [Google Scholar] [CrossRef]
- Bertolet, A.; Carabe, A. Proton monoenergetic arc therapy (PMAT) to enhance LETd within the target. Phys. Med. Biol. 2020, 65, 165006. [Google Scholar] [CrossRef]
- Fix, S.M.; Papadopoulou, V.; Velds, H.; Kasoji, S.K.; Rivera, J.N.; Borden, M.A.; Chang, S.; Dayton, P.A. Oxygen microbubbles improve radiotherapy tumor control in a rat fibrosarcoma model—A preliminary study. PLoS ONE 2018, 13, e0195667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomedicine 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Oronsky, B.T.; Knox, S.J.; Scicinski, J. Six degrees of separation: The oxygen effect in the development of radiosensitizers. Transl. Oncol. 2011, 4, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Nair, C.K.; Parida, D.K.; Nomura, T. Radioprotectors in radiotherapy. J. Radiat. Res. 2001, 42, 21–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wouters, B.G.; Brown, J.M. Cells at intermediate oxygen levels can be more important than the “hypoxic fraction” in determining tumor response to fractionated radiotherapy. Radiat. Res. 1997, 147, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Dasu, A.; Denekamp, J. New insights into factors influencing the clinically relevant oxygen enhancement ratio. Radiother. Oncol. 1998, 46, 269–277. [Google Scholar] [CrossRef]
- Espinoza, I.; Peschke, P.; Karger, C.P. A voxel-based multiscale model to simulate the radiation response of hypoxic tumors. Med. Phys. 2015, 42, 90–102. [Google Scholar] [CrossRef]
- Evans, S.M.; Jenkins, W.T.; Shapiro, M.; Koch, C.J. Evaluation of the concept of “hypoxic fraction” as a descriptor of tumor oxygenation status. Adv. Exp. Med. Biol. 1997, 411, 215–225. [Google Scholar] [CrossRef]
- Horsman, M.R.; Overgaard, J. The impact of hypoxia and its modification of the outcome of radiotherapy. J. Radiat. Res. 2016, 57 (Suppl. S1), i90–i98. [Google Scholar] [CrossRef] [Green Version]
- Stone, H.B.; Brown, J.M.; Phillips, T.L.; Sutherland, R.M. Oxygen in human tumors: Correlations between methods of measurement and response to therapy. Summary of a workshop held November 19–20, 1992, at the National Cancer Institute, Bethesda, Maryland. Radiat. Res. 1993, 136, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Dong, Y.; Lu, J.; Yang, F.; Zheng, Y.; Yang, H. Role of hypoxia in inhibiting dendritic cells by VEGF signaling in tumor microenvironments: Mechanism and application. Am. J. Cancer Res. 2021, 11, 3777–3793. [Google Scholar] [PubMed]
- Solocinski, K.; Padget, M.R.; Fabian, K.P.; Wolfson, B.; Cecchi, F.; Hembrough, T.; Benz, S.C.; Rabizadeh, S.; Soon-Shiong, P.; Schlom, J.; et al. Overcoming hypoxia-induced functional suppression of NK cells. J. Immunother. Cancer 2020, 8, e000246. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Wang, P.; Geng, X.; Zhu, L.; Li, M. Corrigendum: Low Lymphocyte Count Is Associated with Radiotherapy Parameters and Affects the Outcomes of Esophageal Squamous Cell Carcinoma Patients. Front. Oncol. 2020, 10, 630877. [Google Scholar] [CrossRef]
- Fridovich, I. The biology of oxygen radicals. Science 1978, 201, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [Green Version]
- Petkau, A.; Chelack, W.S.; Pleskach, S.D. Letter: Protection of post-irradiated mice by superoxide dismutase. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1976, 29, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Petkau, A.; Chelack, W.S.; Pleskach, S.D.; Meeker, B.E.; Brady, C.M. Radioprotection of mice by superoxide dismutase. Biochem. Biophys. Res. Commun. 1975, 65, 886–893. [Google Scholar] [CrossRef]
- Chu, Y.; Lan, R.S.; Huang, R.; Feng, H.; Kumar, R.; Dayal, S.; Chan, K.S.; Dai, D.F. Glutathione peroxidase-1 overexpression reduces oxidative stress, and improves pathology and proteome remodeling in the kidneys of old mice. Aging Cell 2020, 19, e13154. [Google Scholar] [CrossRef]
- Ketterer, B.; Meyer, D.J. Glutathione transferases: A possible role in the detoxication and repair of DNA and lipid hydroperoxides. Mutat. Res. 1989, 214, 33–40. [Google Scholar] [CrossRef]
- Blasi, A.; D’Alfonso, G. [Respiratory system and environmental noxiousness]. Arch. Monaldi Mal. Torace 1987, 42, 7–23. [Google Scholar]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliper, A.M.; Bozdaganyan, M.E.; Sarkisova, V.A.; Veviorsky, A.P.; Ozerov, I.V.; Orekhov, P.S.; Korzinkin, M.B.; Moskalev, A.; Zhavoronkov, A.; Osipov, A.N. Radioprotectors.org: An open database of known and predicted radioprotectors. Aging 2020, 12, 15741–15755. [Google Scholar] [CrossRef] [PubMed]
- Whitnall, M.H.; Inal, C.E.; Jackson, W.E., 3rd; Miner, V.L.; Villa, V.; Seed, T.M. In vivo radioprotection by 5-androstenediol: Stimulation of the innate immune system. Radiat. Res. 2001, 156, 283–293. [Google Scholar] [CrossRef]
- Singh, V.K.; Shafran, R.L.; Inal, C.E.; Jackson, W.E., 3rd; Whitnall, M.H. Effects of whole-body gamma irradiation and 5-androstenediol administration on serum G-CSF. Immunopharmacol. Immunotoxicol. 2005, 27, 521–534. [Google Scholar] [CrossRef]
- Whitnall, M.H.; Elliott, T.B.; Harding, R.A.; Inal, C.E.; Landauer, M.R.; Wilhelmsen, C.L.; McKinney, L.; Miner, V.L.; Jackson, W.E.r.; Loria, R.M.; et al. Androstenediol stimulates myelopoiesis and enhances resistance to infection in gamma-irradiated mice. Int. J. Immunopharmacol. 2000, 22, 1–14. [Google Scholar] [CrossRef]
- Whitnall, M.H.; Elliott, T.B.; Landauer, M.R.; Wilhelmsen, C.L.; McKinney, L.; Kumar, K.S.; Srinivasan, V.; Ledney, G.D.; Seed, T.M. Protection against gamma-irradiation with 5-androstenediol. Mil. Med. 2002, 167, 64–65. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Liu, W.; Fan, T.; Zhong, H.; Zhou, H.; Guo, W.; Zhu, X. 5-Androstenediol prevents radiation injury in mice by promoting NF-kappaB signaling and inhibiting AIM2 inflammasome activation. Biomed. Pharmacother. 2020, 121, 109597. [Google Scholar] [CrossRef]
- Fischer, N.; Seo, E.J.; Efferth, T. Prevention from radiation damage by natural products. Phytomedicine 2018, 47, 192–200. [Google Scholar] [CrossRef]
- Harapanhalli, R.S.; Narra, V.R.; Yaghmai, V.; Azure, M.T.; Goddu, S.M.; Howell, R.W.; Rao, D.V. Vitamins as radioprotectors in vivo. II. Protection by vitamin A and soybean oil against radiation damage caused by internal radionuclides. Radiat. Res. 1994, 139, 115–122. [Google Scholar] [CrossRef]
- Kazmierczak-Baranska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [Green Version]
- Narra, V.R.; Harapanhalli, R.S.; Howell, R.W.; Sastry, K.S.; Rao, D.V. Vitamins as radioprotectors in vivo. I. Protection by vitamin C against internal radionuclides in mouse testes: Implications to the mechanism of damage caused by the Auger effect. Radiat. Res. 1994, 137, 394–399. [Google Scholar] [CrossRef]
- Jang, G.Y.; Lee, J.W.; Kim, Y.S.; Lee, S.E.; Han, H.D.; Hong, K.J.; Kang, T.H.; Park, Y.M. Interactions between tumor-derived proteins and Toll-like receptors. Exp. Mol. Med. 2020, 52, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Rubin, S.J.S.; Bloom, M.S.; Robinson, W.H. B cell checkpoints in autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Sedor, G.; Ellsworth, P.; Scarborough, J.A.; Ahmed, K.A.; Oliver, D.E.; Eschrich, S.A.; Kattan, M.W.; Torres-Roca, J.F. Pan-cancer prediction of radiotherapy benefit using genomic-adjusted radiation dose (GARD): A cohort-based pooled analysis. Lancet Oncol. 2021, 22, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, T.; Kashihara, K. Radiotherapy with genomic-adjusted radiation dose. Lancet Oncol. 2021, 22, e468. [Google Scholar] [CrossRef]
- Moulder, J.E.; Dutreix, J.; Rockwell, S.; Siemann, D.W. Applicability of animal tumor data to cancer therapy in humans. Int. J. Radiat. Oncol. Biol. Phys. 1988, 14, 913–927. [Google Scholar] [CrossRef]
- Overgaard, J. Hypoxic radiosensitization: Adored and ignored. J. Clin. Oncol. 2007, 25, 4066–4074. [Google Scholar] [CrossRef] [Green Version]
- Rockwell, S.; Dobrucki, I.T.; Kim, E.Y.; Marrison, S.T.; Vu, V.T. Hypoxia and radiation therapy: Past history, ongoing research, and future promise. Curr. Mol. Med. 2009, 9, 442–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardman, P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol 2007, 19, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Horsman, M.R. Modification of Hypoxia-Induced Radioresistance in Tumors by the Use of Oxygen and Sensitizers. Semin. Radiat. Oncol. 1996, 6, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, G.; Hou, X.; Kala, S.; Qiu, Z.; Wong, K.F.; Cao, F.; Sun, L. Biogenic nanobubbles for effective oxygen delivery and enhanced photodynamic therapy of cancer. Acta Biomater. 2020, 108, 313–325. [Google Scholar] [CrossRef]
- Nizet, V.; Johnson, R.S. Interdependence of hypoxic and innate immune responses. Nat. Rev. Immunol. 2009, 9, 609–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaney, L.J.; Ciraku, L.; Oeffinger, B.E.; Wessner, C.E.; Liu, J.B.; Li, J.; Nam, K.; Forsberg, F.; Leeper, D.B.; O’Kane, P.; et al. Breast Cancer Brain Metastasis Response to Radiation after Microbubble Oxygen Delivery in a Murine Model. J. Ultrasound Med. 2019, 38, 3221–3228. [Google Scholar] [CrossRef]
- Lacerda, Q.; Tantawi, M.; Leeper, D.B.; Wheatley, M.A.; Eisenbrey, J.R. Emerging Applications of Ultrasound-Contrast Agents in Radiation Therapy. Ultrasound Med. Biol. 2021, 47, 1465–1474. [Google Scholar] [CrossRef]
- Reusser, T.D.; Song, K.H.; Ramirez, D.; Benninger, R.K.; Papadopoulou, V.; Borden, M.A. Phospholipid Oxygen Microbubbles for Image-Guided Therapy. Nanotheranostics 2020, 4, 83–90. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Cheadle, E.J.; Popple, A.L.; Poon, E.; Morrow, M.; Stewart, R.; Yusko, E.C.; Sanders, C.M.; Vignali, M.; Emerson, R.O.; et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations When Combined with PD-1 Blockade. Clin. Cancer Res. 2017, 23, 5514–5526. [Google Scholar] [CrossRef] [Green Version]
- Menon, H.; Chen, D.; Ramapriyan, R.; Verma, V.; Barsoumian, H.B.; Cushman, T.R.; Younes, A.I.; Cortez, M.A.; Erasmus, J.J.; de Groot, P.; et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J. Immunother. Cancer 2019, 7, 237. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, H.; Blackman, C.; Lesser, G.; Debinski, W.; Chan, M.; Sharma, S.; Watabe, K.; Lo, H.W.; Thomas, A.; Godwin, D.; et al. Use of non-ionizing electromagnetic fields for the treatment of cancer. Front. Biosci. 2018, 23, 284–297. [Google Scholar] [CrossRef] [Green Version]
- Sanie-Jahromi, F.; Saadat, I.; Saadat, M. Effects of extremely low frequency electromagnetic field and cisplatin on mRNA levels of some DNA repair genes. Life Sci. 2016, 166, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Shayeghan, M.; Forouzesh, F.; Madjid Ansari, A.; Javidi, M.A. DNMT1 and miRNAs: Possible epigenetics footprints in electromagnetic fields utilization in oncology. Med. Oncol. 2021, 38, 125. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.W.; Jimenez, H.; Pennison, M.J.; Brezovich, I.; Morgan, D.; Mudry, A.; Costa, F.P.; Barbault, A.; Pasche, B. Targeted treatment of cancer with radiofrequency electromagnetic fields amplitude-modulated at tumor-specific frequencies. Chin. J. Cancer 2013, 32, 573–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Trial Number | Study Title | Status | Cancer Type | Target Analysis |
|---|---|---|---|---|
| NCT02310594 | Anti-tumor immune response in patients with cancer-undergoing radiation therapy | Completed (2022) | Malignant neoplasm | Innate & adaptive immune cells and serum markers. |
| NCT01376674 | T-cell immunity during standard radiotherapy | Completed (2013) | Localized prostate cancer | Use of peripheral blood mononuclear cells |
| NCT01985958 | A pilot study to evaluate the anti-tumor immunity in metastatic carcinoma of the pancreas. | Completed (2020) | Metastatic pancreatic cancer | Neutrophil, Platelets, Hemoglobin and Bilirubin |
| NCT05076500 | Investigating the tumor immune response of radiotherapy | Recruiting currently | Cervical, rectal, Head and Neck cancer, nodal non-Hodgkin lymphoma | Immune signatures and immune phenotypes |
| NCT05035706 | Anti-Leukemia immune response after irradiation of extramedullary tumors. | Recruiting currently | Leukemia | Lymphocytes |
| NCT01777802 | Immune response in prostate, lung, breast and melanoma in response to SBRT and IMRT | Ongoing (Will be completed by 2023) | Melanoma, lung, prostate, and breast cancer | Change in circulating immune biomarkers and pro-inflammatory cytokine |
| NCT03383107 | Effect of radiotherapy variables on circulating effectors of immune response and local microbiome | Completed (2021) | Prostate and breast cancer | Immune change before and after RT in and correlating with microbiome. |
| NCT05371132 | Recruiting A study to evaluate CD8 PET imaging as a marker of immune response to stereotactic body radiation therapy (ELIXR) | Recruiting currently | Metastatic, malignant solid tumors | Monitor CD8+ T cells |
| NCT04624828 | Immune response in evaluation in oligo-recurrent and oligo-progressive prostate cancer treated with SBRT | Recruiting currently | Prostate cancer | Monitor the dynamics of monocytes, granulocyte and NK cells |
| NCT03331367 | Characterizing the immune response to prostate cancer | Completed (2020) | Prostate cancer | Immune markers from blood and urine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boopathi, E.; Den, R.B.; Thangavel, C. Innate Immune System in the Context of Radiation Therapy for Cancer. Cancers 2023, 15, 3972. https://doi.org/10.3390/cancers15153972
Boopathi E, Den RB, Thangavel C. Innate Immune System in the Context of Radiation Therapy for Cancer. Cancers. 2023; 15(15):3972. https://doi.org/10.3390/cancers15153972
Chicago/Turabian StyleBoopathi, Ettickan, Robert B. Den, and Chellappagounder Thangavel. 2023. "Innate Immune System in the Context of Radiation Therapy for Cancer" Cancers 15, no. 15: 3972. https://doi.org/10.3390/cancers15153972
APA StyleBoopathi, E., Den, R. B., & Thangavel, C. (2023). Innate Immune System in the Context of Radiation Therapy for Cancer. Cancers, 15(15), 3972. https://doi.org/10.3390/cancers15153972




