Clinical Outcomes in AYAs (Adolescents and Young Adults) Treated with Proton Therapy for Uveal Melanoma: A Comparative Matching Study with Elder Adults
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design: Patients
2.2. Treatment and Follow-Up
2.3. Propensity Score Matching (PSM) and Statistical Analysis
3. Results
3.1. Matching the AYAs with Elder Adults, Using PSM
3.2. Comparing Clinical Outcomes between the AYAs and Elder Adults
3.2.1. Ocular Follow-Up and Local Tumor Control
3.2.2. Metastasis Incidence
3.2.3. Overall Survival (OS) and Relative Survival (RS)
4. Discussion
4.1. Demographic, Patient, and Tumor Characteristics
4.2. Ocular Follow-Up and Local Tumor Control
4.3. Metastasis Incidence
4.4. Overall Survival (OS) and Relative Survival (RS)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrari, A.; Stark, D.; Peccatori, F.A.; Fern, L.; Laurence, V.; Gaspar, N.; Bozovic-Spasojevic, I.; Smith, O.; De Munter, J.; Derwich, K.; et al. Adolescents and young adults (AYA) with cancer: A position paper from the AYA Working Group of the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOPE). ESMO Open 2021, 6, 100096. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, A.; Bergin, C.; Schalenbourg, A.; Goitein, G.; Zografos, L. Proton therapy for uveal melanoma in 43 juvenile patients: Long-term results. Ophthalmology 2014, 121, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Pogrzebielski, A.; Orlowska-Heitzman, J.; Romanowska-Dixon, B. Uveal melanoma in young patients. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 1646–1649. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, R.T.; Cassoux, N.; Desjardins, L.; Damato, B.; Konstantinidis, L.; Coupland, S.E.; Heimann, H.; Petrovic, A.; Zografos, L.; Schalenbourg, A.; et al. The Pediatric Choroidal and Ciliary Body Melanoma Study: A Survey by the European Ophthalmic Oncology Group. Ophthalmology 2016, 123, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Egger, E.; Zografos, L.; Schalenbourg, A.; Beati, D.; Bohringer, T.; Chamot, L.; Goitein, G. Eye retention after proton beam radiotherapy for uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 867–880. [Google Scholar] [CrossRef]
- Egger, E.; Schalenbourg, A.; Zografos, L.; Bercher, L.; Boehringer, T.; Chamot, L.; Goitein, G. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 138–147. [Google Scholar] [CrossRef]
- Fry, M.V.; Augsburger, J.J.; Correa, Z.M. Clinical Features, Metastasis, and Survival in Patients Younger than 21 Years with Posterior Uveal Melanoma. JAMA Ophthalmol. 2019, 137, 75–81. [Google Scholar] [CrossRef]
- Saloustros, E.; Stark, D.P.; Michailidou, K.; Mountzios, G.; Brugieres, L.; Peccatori, F.A.; Jezdic, S.; Essiaf, S.; Douillard, J.Y.; Bielack, S. The care of adolescents and young adults with cancer: Results of the ESMO/SIOPE survey. ESMO Open 2017, 2, e000252. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. In Matched Sampling for Causal Effects; Cambridge University Press: Cambridge, UK, 2006; pp. 170–184. [Google Scholar]
- Normand, S.T.; Landrum, M.B.; Guadagnoli, E.; Ayanian, J.Z.; Ryan, T.J.; Cleary, P.D.; McNeil, B.J. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: A matched analysis using propensity scores. J. Clin. Epidemiol. 2001, 54, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Kalbfleisch, J.D.; Prentice, R.L. The Statistical Analysis of Failure Time Data; John Wiley & Sons: Hoboken, NJ, USA, 1980. [Google Scholar]
- Zhou, B.; Fine, J.; Latouche, A.; Labopin, M. Competing risks regression for clustered data. Biostatistics 2012, 13, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Latouche, A. Competing Risks Regression for Stratified and Clustered Data. R Package Version 1.1. 2013. Available online: https://CRANR-projectorg/package=crrSC (accessed on 9 May 2022).
- Jung, S.H. Rank Tests for Matched Survival Data. Lifetime Data Anal. 1999, 5, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Wilmoth, J.R.; Shkolnikov, V.M.; Glei, D.; Jasilionis, D.; Jdanov, D.; Boe, C.; Riffe, T.; Grigoriev, P.; Winant, C. Data Resource Profile: The Human Mortality Database (HMD). Int. J. Epidemiol. 2015, 44, 1549–1556. [Google Scholar] [CrossRef]
- Cho, H.H.N.; Mariotto, A.B.; Cronin, K.A. Estimating Relative Survival for Cancer Patients from the SEER Program Using Expected Rates Based on Ederer I Versus Ederer II Method; Surveillance Research Program; Edited by #2011-01 TR; National Cancer Institute: Bethesda, MD, USA, 2011. Available online: http://surveillance.cancer.gov/reports/tech2011.01.pdf (accessed on 9 May 2022).
- R Core Team: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 January 2022).
- Lewis, D.R.; Seibel, N.L.; Smith, A.W.; Stedman, M.R. Adolescent and young adult cancer survival. J. Natl. Cancer Inst. Monogr. 2014, 2014, 228–235. [Google Scholar] [CrossRef]
- Sender, L.; Zabokrtsky, K.B. Adolescent and young adult patients with cancer: A milieu of unique features. Nat. Rev. Clin. Oncol. 2015, 12, 465–480. [Google Scholar] [CrossRef]
- Singh, A.D.; Topham, A. Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology 2003, 110, 956–961. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Mashayekhi, A.; Shields, J.A. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8033 cases. Retina 2012, 32, 1363–1372. [Google Scholar] [CrossRef]
- Kodjikian, L.; Nguyen, K.; Lumbroso, L.; Gauthier-Villars, M.; Chauvel, P.; Plauchu, H.; Sterkers, M.; Devouassoux, M.; Grange, J.D. Familial uveal melanoma: A report on two families and a review of the literature. Acta Ophthalmol. Scand 2003, 81, 389–395. [Google Scholar] [CrossRef]
- Krohn, J.; Sundal, K.V.; Froystein, T. Topography and clinical features of iris melanoma. BMC Ophthalmol. 2022, 22, 6. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L.; Mashayekhi, A.; Ganesh, A.; Furuta, M.; Shields, J.A. Influence of age on prognosis of young patients with uveal melanoma: A matched retrospective cohort study. Eur. J. Ophthalmol. 2013, 23, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Shields, C.L.; Shields, J.A.; Sato, T. Uveal melanoma in young patients. Arch. Ophthalmol. 2000, 118, 918–923. [Google Scholar] [PubMed]
- Vavvas, D.; Kim, I.; Lane, A.M.; Chaglassian, A.; Mukai, S.; Gragoudas, E. Posterior uveal melanoma in young patients treated with proton beam therapy. Retina 2010, 30, 1267–1271. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Arepalli, S.; Atalay, H.T.; Manjandavida, F.P.; Pieretti, G.; Shields, J.A. Uveal melanoma in children and teenagers. Saudi J. Ophthalmol. 2013, 27, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, R.T.; Kivela, T. Uveal melanoma among Finnish children and young adults. J. AAPOS 2014, 18, 61–66. [Google Scholar] [CrossRef]
- Lane, A.M.; Kim, I.K.; Gragoudas, E.S. Long-term Risk of Melanoma-Related Mortality for Patients with Uveal Melanoma Treated with Proton Beam Therapy. JAMA Ophthalmol. 2015, 133, 792–796. [Google Scholar] [CrossRef] [PubMed]


| Before Matching | After Matching | ||||||
|---|---|---|---|---|---|---|---|
| AYAs | Elder Adults | p-Value | AYAs | Elder Adults | SMD | p-Value | |
| Patients, n | 272 | 1979 | 270 | 270 | |||
| Age in years, | |||||||
| Mean ± SD | 32.5 ± 5.4 | 61.1 ± 10.9 | 32.5 ± 5.4 | 60.5 ± 10.8 | |||
| Median [IQR] | 34 [29–37] | 61 [53–69] | <0.001 a | 34 [29–37] | 61 [52–69] | ||
| Range | 16–39 | 40–91 | 16–39 | 40–91 | |||
| Sex, n (%) * | 0.056 b | 0.053 | |||||
| Male | 112 (41.2) | 941 (47.5) | 111 (41.1) | 104 (38.5) | |||
| Female | 160 (58.8) | 1038 (52.5) | 159 (58.8) | 166 (61.5) | |||
| Country of residence, n (%) * | 0.507 b | 0.009 | |||||
| Switzerland | 58 (21.3) | 465 (23.5) | 58 (21.5) | 59 (21.9) | |||
| Bordering country | 140 (51.5) | 1034 (52.2) | 139 (51.5) | 138 (51.1) | |||
| Other | 74 (27.2) | 480 (24.3) | 73 (27.0) | 73 (27.0) | |||
| Systemic and ocular history, n (%) | |||||||
| Familial uveal melanoma * | 6 (2.2) | 34 (1.7) | 0.621 c | 6 (2.2) | 8 (3.0) | 0.047 | |
| Other known tumors * | 10 (3.7) | 179 (9.0) | 0.004 b | 10 (3.7) | 8 (3.0) | 0.041 | |
| Ocular melanocytosis | 7 (2.6) | 47 (2.4) | 1.000 b | 7 (2.6) | 3 (1.1) | ||
| Patient presented with symptoms | 238 (87.5) | 1596 (80.6) | 0.008 b | 236 (87.4) | 224 (83.0) | 0.15 e | |
| Documented tumor growth (i.e., treatment after an observation period) | 53 (19.5) | 317 (16.0) | 0.174 b | 52 | 53 | 0.91 e | |
| Eye laterality | |||||||
| Right eye, n (%) | 136 (50.0) | 984 (49.7) | 0.983 b | 135 (50) | 124 (45.9) | ||
| BCVA (Snellen) in the affected eye | |||||||
| ≥0.1 (20/200), n (%) | 251 (92.3) | 1759 (88.9) | 0.111 b | 249 (92.2) | 242 (89.6) | 0.30 e | |
| ≥0.6 (20/33), n (%) | 162 (59.6) | 1120 (56.6) | 0.389 b | 160 (59.3) | 155 (57.4) | 0.65 e | |
| Proton therapy | |||||||
| Year of PT, median [IQR] * | 2001 [1999–2004] | 2002 [1999–2005] | 0.014 a | 2001 [1999–2004] | 2001 [1999–2004] | 0.035 | |
| More than 3 months delay between diagnosis and treatment, n (%) | 5 (1.8) | 48 (2.4) | 0.700 b | ||||
| Tumor location | |||||||
| Origin within the iris, n (%) * | 11 (4.0) | 28 (1.4) | 0.005 c | 9 (3.3) | 11 (4.1) | 0.051 | |
| Anterior tumor margin within the * | <0.001 b | 0.048 | |||||
| iris, n (%) | 33 (12.1) | 117 (5.9) | 31 (11.5) | 33 (12.2) | |||
| ciliary body, n (%) | 66 (24.3) | 518 (26.2) | 66 (24.4) | 67 (24.8) | |||
| anterior choroid, n (%) | 58 (21.3) | 574 (29.0) | 58 (21.5) | 61 (22.6) | |||
| posterior choroid, n (%) | 115 (42.3) | 770 (38.9) | 115 (42.6) | 109 (40.4) | |||
| Tumor dimensions in mm, mean ± SD | |||||||
| LTD * | 16.2 ± 4.2 | 16.3 ± 4.2 | 0.641 d | 16.2 ± 4.2 | 16.4 ± 4.3 | 0.047 | |
| STD * | 13.9 ± 3.7 | 14.0 ± 3.8 | 0.762 d | 13.9 ± 3.7 | 13.9 ± 3.8 | 0.016 | |
| Tumor thickness * | 6.0 ± 2.8 | 6.2 ± 2.8 | 0.493 d | 6.1 ± 2.8 | 6.2 ± 2.9 | 0.047 | |
| Extra-scleral extension, n (%) * | 9 (3.3) | 103 (5.2) | 0.230 b | 9 (3.3) | 13 (4.8) | 0.075 | |
| TNM classification, n (%) * | 0.981 b | 0.059 | |||||
| T1 | 21 (7.7) | 147 (7.4) | 21 (7.8) | 19 (7.0) | |||
| T2 | 84 (30.9) | 591 (29.9) | 82 (30.4) | 78 (28.9) | |||
| T3 | 79 (29.0) | 589 (29.8) | 79 (29.3) | 78 (28.9) | |||
| T4 | 88 (32.4) | 652 (32.9) | 88 (32.6) | 95 (35.2) | |||
| Other tumor characteristics, n (%) | |||||||
| Disruption of Bruch’s membrane | 43 (15.8) | 413 (20.9) | 0.062 b | 43 (15.9) | 61 (22.6) | 0.02 e | |
| Disruption of the retina | 18 (6.6) | 240 (12.1) | 0.010 b | 18 (6.7) | 25 (9.3) | 0.26 e | |
| Pigment dispersion | 28 (10.3) | 238 (12.0) | 0.466 b | 28 (10.4) | 20 (7.4) | 0.21 e | |
| Hemorrhage | 22 (8.1) | 311 (15.7) | 0.158 b | 22 (8.2) | 34 (12.6) | 0.10 e | |
| Invasion of the optic disc | 28 (10.3) | 164 (8.3) | 0.320 b | 28 (10.4) | 23 (8.5) | 0.46 e | |
| Invasion of the macula | 48 (17.6) | 253 (12.8) | 0.034 b | 48 (17.8) | 41 (15.2) | 0.41 e | |
| Categorical distance between tumor and optic disc, n (%) | 0.069 b | ||||||
| Touching the optic disc | 58 (21.3) | 338 (17.1) | 58 (21.5) | 46 (17.0) | |||
| Not touching but ≤3.5 mm from optic disc | 60 (22.1) | 548 (27.7) | 60 (22.2) | 75 (27.8) | |||
| >3.5 mm from optic disc | 154 (56.6) | 1093 (55.2) | 152 (56.3) | 149 (55.2) | |||
| Categorical distance between tumor and fovea, n (%) | 0.302 b | ||||||
| Subfoveal | 72 (26.5) | 442 (22.3) | 72 (26.7) | 69 (25.6) | |||
| Not subfoveal but ≤3.5 mm from fovea | 70 (25.7) | 553 (27.9) | 70 (25.9) | 60 (22.1) | |||
| >3.5 mm from fovea | 130 (47.8) | 984 (49.7) | 128 (47.4) | 141 (52.2) | |||
| AYAs | Elder Adults | p Value | |
|---|---|---|---|
| Patients, n | 270 | 270 | |
| Ocular Follow-up | |||
| Median ocular follow-up in years (range) | 4.9 (0–20.8) | 3.7 (0–16.0) | |
| Mean ocular follow-up in years (SD) | 5.7 (4.7) | 4.5 (4.0) | 0.0016 |
| Patients with ≥6 months of ocular follow-up, n (%) | 235 (87.0) | 217 (80.4) | 0.034 |
| Patients with ≥5 years of ocular follow-up, n (%) | 132 (48.9) | 113 (41.9) | 0.084 |
| Patients with useful vision (BCVA ≥ 0.1 (20/200) 5 years after treatment, n (%) | 74 (56.0) | 59 (52.2) | 0.724 |
| Patients with good vision (BCVA ≥ 0.6 (20/33) 5 years after treatment, n (%) | 47 (35.6) | 36 (31.9) | 0.102 |
| Ocular complications at last visit, n | |||
| Rubeosis | 34 | 38 | |
| Pupillary seclusion | 15 | 18 | |
| Cataract | 96 | 166 | |
| Hemorrhage | 59 | 61 | |
| Actinic maculopathy | 79 | 80 | 0.358 |
| Actinic neuropathy | 36 | 41 | 0.296 |
| Local recurrence, n (%) | 9 (3.3) | 5 (1.6) | 0.960 |
| Enucleation, n (%) | 15 (5.6) | 9 (3.3) | |
| Systemic Follow-up | |||
| Median follow-up to last info in years (range) | 5.1 (0–20.8) | 4.0 (0–16.0) | |
| Mean follow-up to last info in years (SD) | 5.9 (4.6) | 4.6 (4.0) | 0.0006 |
| Metastasis discovery, n (%) | 33 (12.2) | 19 (7.0) | 0.214 |
| Median time to metastasis discovery in years (range) | 2.5 (0–9.3) | 2.5 (0.6–11.8) | |
| Mean time to metastasis discovery in years (SD) | 3.5 (2.5) | 3.7 (2.9) | |
| Alive at 5 years, n (%) | 138 (51.1) | 119 (44.1) | |
| Dead at 5 years, n (%) | 8 (3.0) | 6 (2.2) | |
| Lost to follow-up at 5 years, n (%) | 124 (45.9) | 145 (53.7) | 0.058 |
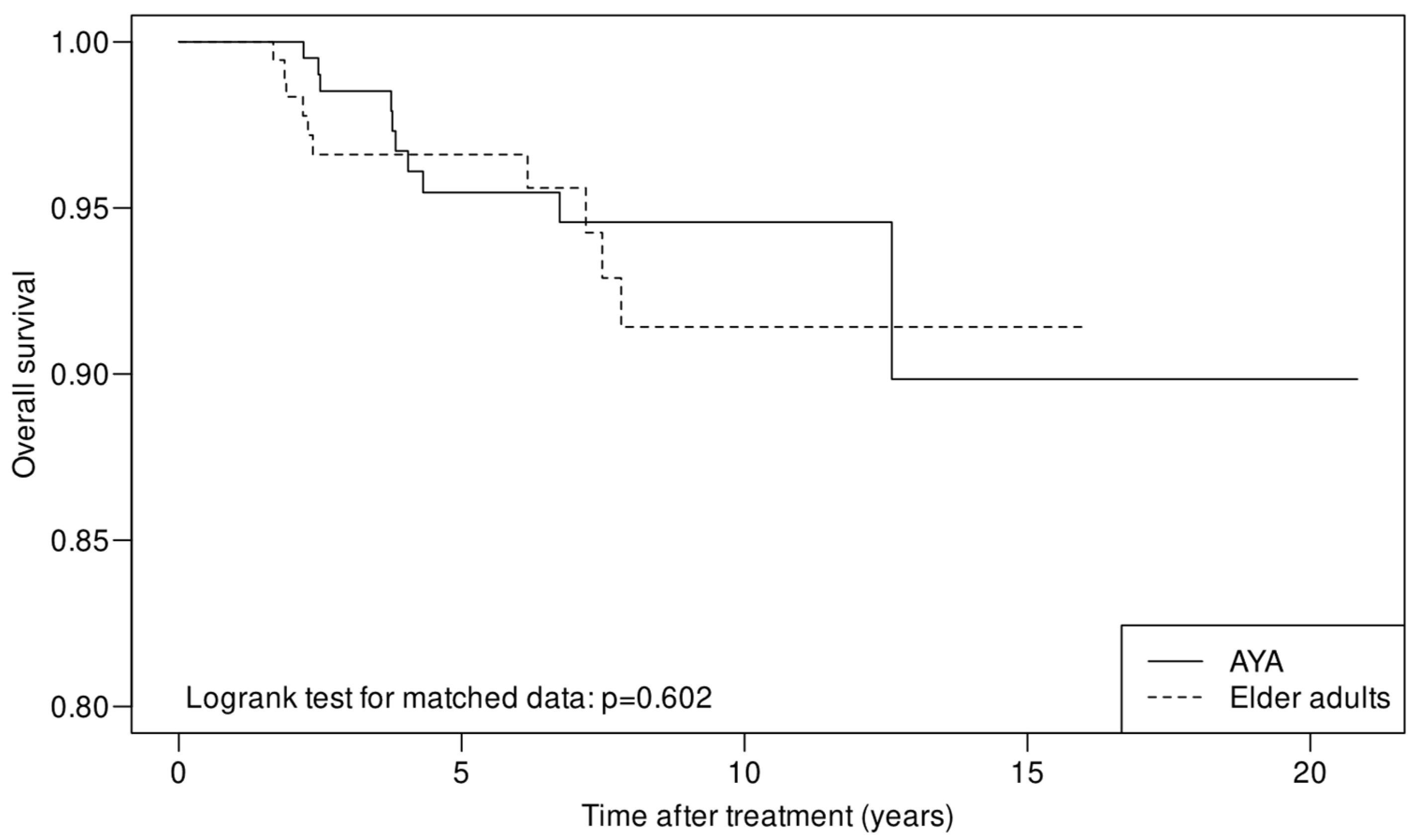
| 5-year OS in % (95% CI) (according to cumulative survival) | 95.5% (92.4–98.6%) | 96.6% (94.0–99.3%) | |
| 10-year OS in % (95% CI) (according to cumulative survival) | 94.6% (91.1–98.2%) | 91.4% (86.0–97.2%) | |
| Confirmed death, n (%) | 10 (3.7) | 10 (3.7) | 0.82 |
| First Author [Ref] Publication Year | Age Category in Years | Patients, n | Mean Age in Years (Range) | Mean Follow-Up in Years (Range) | Metastasis Incidence at 10 Years | Overall/Relative Survival at | Comments | |
|---|---|---|---|---|---|---|---|---|
| 5 Years | 10 Years | |||||||
| Singh AD [27] 2000 | ≤20 | 63 | 16 (3–20) | 4.3 a (1–25.6) | 23% b | 95% | 89% | Overall survival at 15 years: 77% |
| Pogrzebielski A [4] 2006 | ≤20 | 11 | 17.9 (12–20) | 5.1 (2.5–10.4) | 0% | 100% | n/a | |
| Vavvas D [28] 2010 | ≤20 | 17 | 18.1 (11–20) | 16.4 (4.7–25) | 0% c | 100% c | 100% c | Controls >20 matched for largest tumor diameter, pigmentation of tumor, presence of symptoms, eye color, and tumor location. |
| >20 | 51 | 57 a (26–81) | 16.7 a (3–25) | n/a | 98% b,c | 92% b,c | ||
| Shields CL [29] 2013 | ≤20 | 122 | 15 (3–20) | n/a | 0.088 | n/a | n/a | Metastasis incidence at 20 years: 20.2% |
| Kaliki S [26] 2013 | Young ≤ 20 | 122 | 12 (3–20) | 5.25 (0–23) | 8% (18% c) | n/a | n/a | Controls >20 matched for gender, tumor and anterior margin location, largest basal diameter, thickness, and extraocular extension |
| Mid-adults 21–60 | 122 | n/a | 3 (0–24) | 26% (21% c) | n/a | n/a | ||
| Older adults > 60 | 122 | n/a | 2.8 (0–19) | 24% (33% c) | n/a | n/a | ||
| Petrovic A [3] 2014 | Young ≤ 20 | 43 | 17.3 (9–20) | 12.9 (0.5–28) | 11% | 93% | 93% | Controls >20 matched for anterior margin location, largest basal diameter, and thickness No metastasis before age 16 (n = 14) |
| Adults > 20 | 129 | 50.4 (29–81) | 6.6 (0.3–23.4) | 34% | 77% | 65% | ||
| Al-Jamal RT [30] 2014 | <25 | 18 | 19.3 (13–24) | 13.4 b (0.6–37) | 24% b,c | 76% c | 76% c | Overall survival at 15 years: 68% (i.e.: <18 y: 100%; 18–20 y: 80%; 21–24 y: 58%) |
| Al-Jamal RT [5] 2016 | <18 | 114 | 15.1 a (2–17) | 6.6 a (0–41) | n/a | 97% c | 91% c | No metastases before age 10 (n = 15) |
| 18–24 | 185 | 21.9 a (18–24) | 5.1 a (0–37) | n/a | 89% c | 78% c | ||
| Fry M [8] 2019 | ≤20 | 18 | 16.6 (4–20) | 8.5 (0.2–21.5) | 56% c | 69% c | 52% c | |
| Present study 2023 | AYA 15–39 | 270 | 32.5 (16–39) | 5.9 (0–20.8) | 19.70% | 96% | 95% | First study on AYAs with UM Propensity score matching |
| ≥40 | 270 | 60.5 (40–91) | 4.6 (0–16) | 12.70% | 97% | 91% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pica, A.; Weber, D.C.; Schweizer, C.; Chaouch, A.; Zografos, L.; Schalenbourg, A. Clinical Outcomes in AYAs (Adolescents and Young Adults) Treated with Proton Therapy for Uveal Melanoma: A Comparative Matching Study with Elder Adults. Cancers 2023, 15, 4652. https://doi.org/10.3390/cancers15184652
Pica A, Weber DC, Schweizer C, Chaouch A, Zografos L, Schalenbourg A. Clinical Outcomes in AYAs (Adolescents and Young Adults) Treated with Proton Therapy for Uveal Melanoma: A Comparative Matching Study with Elder Adults. Cancers. 2023; 15(18):4652. https://doi.org/10.3390/cancers15184652
Chicago/Turabian StylePica, Alessia, Damien C. Weber, Claude Schweizer, Aziz Chaouch, Leonidas Zografos, and Ann Schalenbourg. 2023. "Clinical Outcomes in AYAs (Adolescents and Young Adults) Treated with Proton Therapy for Uveal Melanoma: A Comparative Matching Study with Elder Adults" Cancers 15, no. 18: 4652. https://doi.org/10.3390/cancers15184652
APA StylePica, A., Weber, D. C., Schweizer, C., Chaouch, A., Zografos, L., & Schalenbourg, A. (2023). Clinical Outcomes in AYAs (Adolescents and Young Adults) Treated with Proton Therapy for Uveal Melanoma: A Comparative Matching Study with Elder Adults. Cancers, 15(18), 4652. https://doi.org/10.3390/cancers15184652






