Prognostic Values of Tissue and Serum Angiogenic Growth Factors Depend on the Phenotypic Subtypes of Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Serum Markers
2.3. Preparation of Tissue Samples
2.4. Immunohistochemistry
2.5. Scoring of Samples
2.6. Statistical Analyses
3. Results
3.1. Correlations between Clinicopathological Variables and Serum Angiogenic Growth Factor Concentrations
3.2. Associations between Clinicopathological Variables and Tissue Expressions of Angiogenic Growth Factors
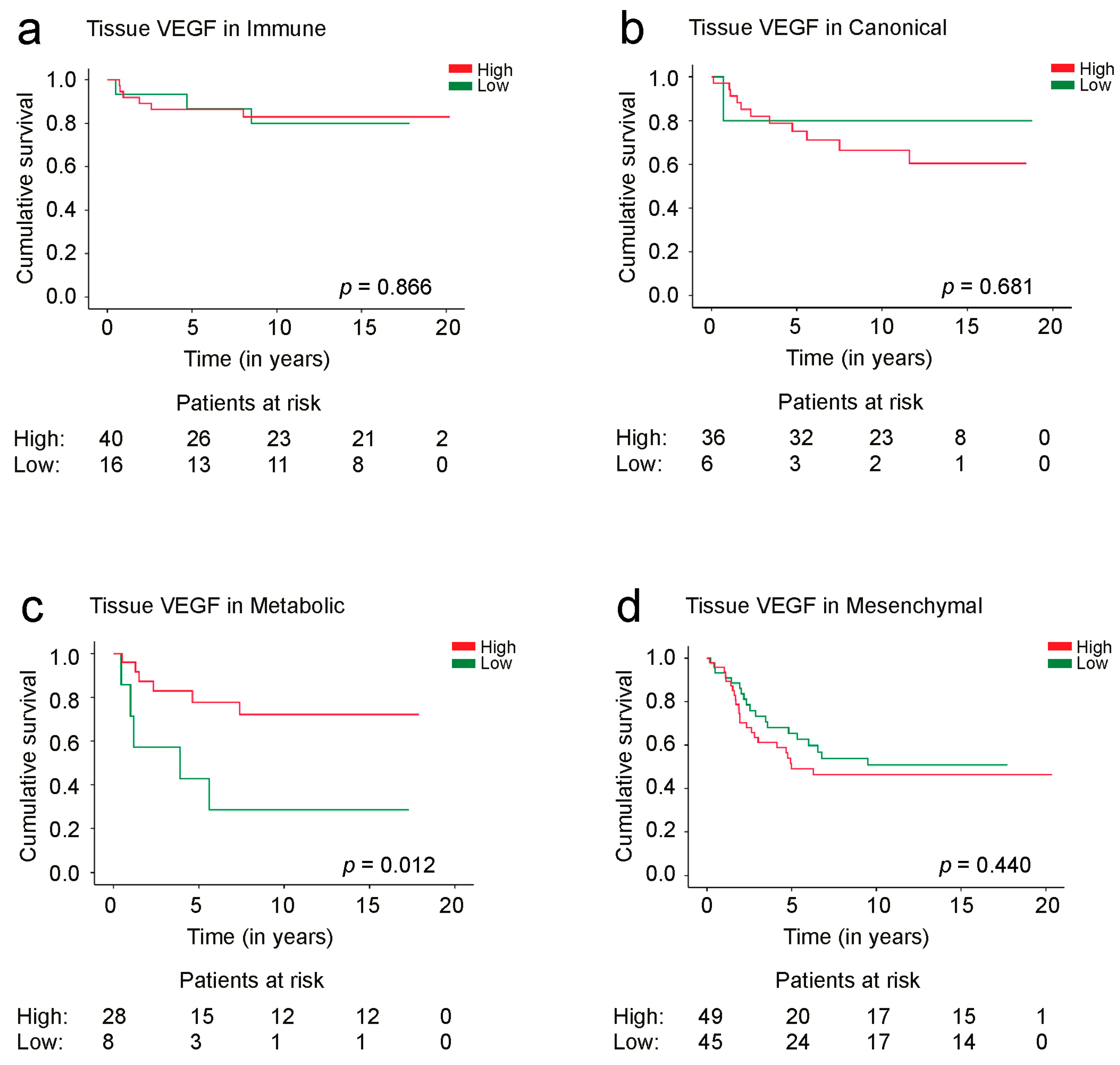
3.3. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Douaiher, J.; Ravipati, A.; Grams, B.; Chowdhury, S.; Alatise, O.; Are, C. Colorectal cancer—Global burden, trends, and geographical variations. J. Surg. Oncol. 2017, 115, 619–630. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Review Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar]
- Hosaka, K.; Yang, Y.; Seki, T.; Fischer, C.; Dubey, O.; Fredlund, E.; Hartman, J.; Religa, P.; Morikawa, H.; Ishii, Y.; et al. Pericyte–fibroblast transition promotes tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, E5618–E5627. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Ferrara, N. VEGF as a Therapeutic Target in Cancer. Oncology 2005, 69, 11–16. [Google Scholar] [CrossRef]
- Mohamed, S.Y.; Mohammed, H.L.; Ibrahim, H.M.; Mohamed, E.M.; Salah, M. Role of VEGF, CD105, and CD31 in the Prognosis of Colorectal Cancer Cases. J. Gastrointest. Cancer 2019, 50, 23–34. [Google Scholar] [CrossRef]
- Beştaş, R.; Kaplan, M.A.; Işikdoğan, A. The Correlation between Serum VEGF Levels and Known Prognostic Risk Factors in Colorectal Carcinoma. Hepatogastroenterology 2014, 61, 267–271. [Google Scholar]
- Liu, Z.; Zhang, Y.; Niu, Y.; Li, K.; Liu, X.; Chen, H.; Gao, C. A Systematic Review and Meta-Analysis of Diagnostic and Prognostic Serum Biomarkers of Colorectal Cancer. PLoS ONE 2014, 9, e103910. [Google Scholar] [CrossRef]
- Gonzalez, F.-J.; Quesada, A.-R.; Sevilla, I.; Baca, J.-J.; Medina, M.-A.; Amores, J.; Diaz, J.M.; Rius-Diaz, F.; Marques, E.; Alba, E. Prognostic value of serum angiogenic activity in colorectal cancer patients. J. Cell. Mol. Med. 2007, 11, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Heer, K.; Lee, P.W.; Duthie, G.S.; MacDonald, A.W.; Greenman, J.; Kerin, M.J.; Monson, J.R. Preoperative Serum Vascular Endothelial Growth Factor Can Predict Stage in Colrectal Cancer. Clin. Cancer Res. 1998, 4, 1279–1285. [Google Scholar]
- Akl, M.R.; Nagpal, P.; Ayoub, N.M.; Tai, B.; Prabhu, S.A.; Capac, C.M.; Gliksman, M.; Goy, A.; Suh, K.S. Molecular and clinical significance of fibroblast growth factor 2 (FGF2/bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget 2016, 7, 44735–44762. [Google Scholar]
- George, M.; Tutton, M.; Abulafi, A.; Eccles, S.; Swift, R. Plasma basic fibroblast growth factor levels in colorectal cancer: A clinically useful assay? Clin. Exp. Metastasis 2002, 19, 735–738. [Google Scholar]
- Jibiki, N.; Saito, N.; Kameoka, S.; Kobayashi, M. Clinical Significance of Fibroblast Growth Factor (FGF) Expression in Colorectal Cancer. Int. Surg. 2014, 99, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Elagoz, S.; Egilmez, R.; Koyuncu, A.; Muslehiddinoglu, A.; Arici, S. The intratumoral microvessel density and expression of bFGF and nm23-H1 in colorectal cancer. Pathol. Oncol. Res. 2006, 12, 21–27. [Google Scholar] [CrossRef]
- Fredriksson, L.; Li, H.; Eriksson, U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine Growth Factor. Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef]
- Gianni-Barrera, R.; Butschkau, A.; Uccelli, A.; Certelli, A.; Valente, P.; Bartolomeo, M.; Groppa, E.; Burger, M.G.; Hlushchuk, R.; Heberer, M.; et al. PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis 2018, 21, 883–900. [Google Scholar] [CrossRef] [Green Version]
- Belizon, A.; Balik, E.; Horst, P.K.; Kumara, H.M.C.S.; Nasar, A.; Whelan, R.L. Platelet-Derived Growth Factor (Subtype BB) Is Elevated in Patients with Colorectal Carcinoma. Dis. Colon Rectum 2009, 52, 1166–1171. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tanaka, F.; Yoshikawa, Y.; Mimori, K.; Inoue, H.; Yanaga, K.; Mori, M. PDGF-BB is a Novel Prognostic Factor in Colorectal Cancer. Ann. Surg. Oncol. 2008, 15, 2129–2136. [Google Scholar] [CrossRef]
- Roepman, P.; Schlicker, A.; Tabernero, J.; Majewski, I.; Tian, S.; Moreno, V.; Snel, M.H.; Chresta, C.M.; Rosenberg, R.; Nitsche, U.; et al. Colorectal cancer intrinsic subtypes predict chemotherapy benefit, deficient mismatch repair and epithelial-to-mesenchymal transition. Int. J. Cancer 2014, 134, 552–562. [Google Scholar]
- Budinska, E.; Popovici, V.; Tejpar, S.; D’Ario, G.; Lapique, N.; Sikora, K.O.; Di Narzo, A.F.; Yan, P.; Hodgson, J.G.; Weinrich, S.; et al. Gene expression patterns unveil a new level of molecular heterogeneity in colorectal cancer. J. Pathol. 2013, 231, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, A.; Beran, G.; Chresta, C.M.; McWalter, G.; Pritchard, A.; Weston, S.; Runswick, S.; Davenport, S.; Heathcote, K.; Castro, D.A.; et al. Subtypes of primary colorectal tumors correlate with response to targeted treatment in colorectal cell lines. BMC Med. Genom. 2012, 5, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadanandam, A.; Lyssiotis, C.A.; Homicsko, K.; Collisson, E.A.; Gibb, W.J.; Wullschleger, S.; Ostos, L.C.G.; Lannon, W.A.; Grötzinger, C.; Del Rio, M.; et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat. Med. 2013, 19, 619–625. [Google Scholar] [CrossRef] [Green Version]
- De Sousa EMelo, F.; Wang, X.; Jansen, M.; Fessler, E.; Trinh, A.; De Rooij, L.P.; De Jong, J.H.; De Boer, O.J.; Van Leersum, R.; Bijlsma, M.F.; et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat. Med. 2013, 19, 614–618. [Google Scholar] [CrossRef]
- Marisa, L.; de Reyniès, A.; Duval, A.; Selves, J.; Gaub, M.P.; Vescovo, L.; Etienne-Grimaldi, M.-C.; Schiappa, R.; Guenot, D.; Ayadi, M.; et al. Gene Expression Classification of Colon Cancer into Molecular Subtypes: Characterization, Validation, and Prognostic Value. PLoS Med. 2013, 10, e1001453. [Google Scholar] [CrossRef] [Green Version]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- de la Chapelle, A.; Hampel, H. Clinical Relevance of Microsatellite Instability in Colorectal Cancer. J. Clin. Oncol. 2010, 28, 3380–3387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, M.; Katoh, M. Molecular genetics and targeted therapy of WNT-related human diseases (Review). Int. J. Mol. Med. 2017, 40, 587–606. [Google Scholar] [PubMed] [Green Version]
- Hagland, H.R.; Berg, M.; Jolma, I.W.; Carlsen, A.; Søreide, K. Molecular Pathways and Cellular Metabolism in Colorectal Cancer. Dig. Surg. 2013, 30, 12–25. [Google Scholar] [CrossRef]
- Roseweir, A.K.; McMillan, D.C.; Horgan, P.G.; Edwards, J. Colorectal cancer subtypes: Translation to routine clinical pathology. Cancer Treat. Rev. 2017, 57, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kasurinen, J.; Beilmann-Lehtonen, I.; Kaprio, T.; Hagström, J.; Haglund, C.; Böckelman, C. Phenotypic subtypes predict outcomes in colorectal cancer. Acta Oncol. 2023, 62, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Björkman, K.; Jalkanen, S.; Salmi, M.; Mustonen, H.; Kaprio, T.; Kekki, H.; Pettersson, K.; Böckelman, C.; Haglund, C. A prognostic model for colorectal cancer based on CEA and a 48-multiplex serum biomarker panel. Sci. Rep. 2021, 11, 4287. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-P.; Bai, Y.; Jiang, J.-Q.; Wu, J.-L. Effects of laparoscopic radical surgery in the treatment of colorectal cancer and correlations of VEGF and TGF-β1 with prognosis. Am. J. Transl. Res. 2021, 13, 12887–12896. [Google Scholar] [PubMed]
- Ishigami, S.-I.; Arii, S.; Furutani, M.; Niwano, M.; Harada, T.; Mizumoto, M.; Mori, A.; Onodera, H.; Imamura, M. Predictive value of vascular endothelial growth factor (VEGF) in metastasis and prognosis of human colorectal cancer. Br. J. Cancer 1998, 78, 1379–1384. [Google Scholar] [CrossRef] [Green Version]
- Braicu, C.; Tudoran, O.; Balacescu, L.; Catana, C.; Neagoe, E.; Berindan-Neagoe, I.; Ionescu, C. The significance of pdgf expression in serum of colorectal carcinoma patients—Correlation with dukes clasification. can pdgf become a potential biomarker? Chirurgia 2013, 108, 849–854. [Google Scholar]
- Unterleuthner, D.; Neuhold, P.; Schwarz, K.; Janker, L.; Neuditschko, B.; Nivarthi, H.; Crncec, I.; Kramer, N.; Unger, C.; Hengstschläger, M.; et al. Faculty Opinions recommendation of Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis 2020, 23, 159–177. [Google Scholar] [CrossRef] [Green Version]
- Kasurinen, J.; Hagström, J.; Kaprio, T.; Beilmann-Lehtonen, I.; Haglund, C.; Böckelman, C. Tumor-associated CD3- and CD8-positive immune cells in colorectal cancer: The additional prognostic value of CD8+-to-CD3+ ratio remains debatable. Tumor Biol. 2022, 44, 37–52. [Google Scholar] [CrossRef]
- Stockmann, C.; Doedens, A.; Weidemann, A.; Zhang, N.; Takeda, N.; Greenberg, J.I.; Cheresh, D.A.; Johnson, R. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 2008, 456, 814–818. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. Faculty Opinions recommendation of ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Berk, V.; Deniz, K.; Bozkurt, O.; Ozaslan, E.; Karaca, H.; Inanc, M.; Duran, A.O.; Ozkan, M. Predictive Significance of VEGF and HIF-1α Expression in Patients with Metastatic Colorectal Cancer Receiving Chemotherapy Combinations with Bevacizumab. Asian Pac. J. Cancer Prev. 2015, 16, 6149–6154. [Google Scholar] [CrossRef] [Green Version]
- Stintzing, S.; Wirapati, P.; Lenz, H.-J.; Neureiter, D.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Kaiser, F.; Al-Batran, S.; Heintges, T.; et al. Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann. Oncol. 2019, 30, 1796–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jawhar, N.M. Tissue Microarray: A rapidly evolving diagnostic and research tool. Ann. Saudi Med. 2009, 29, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
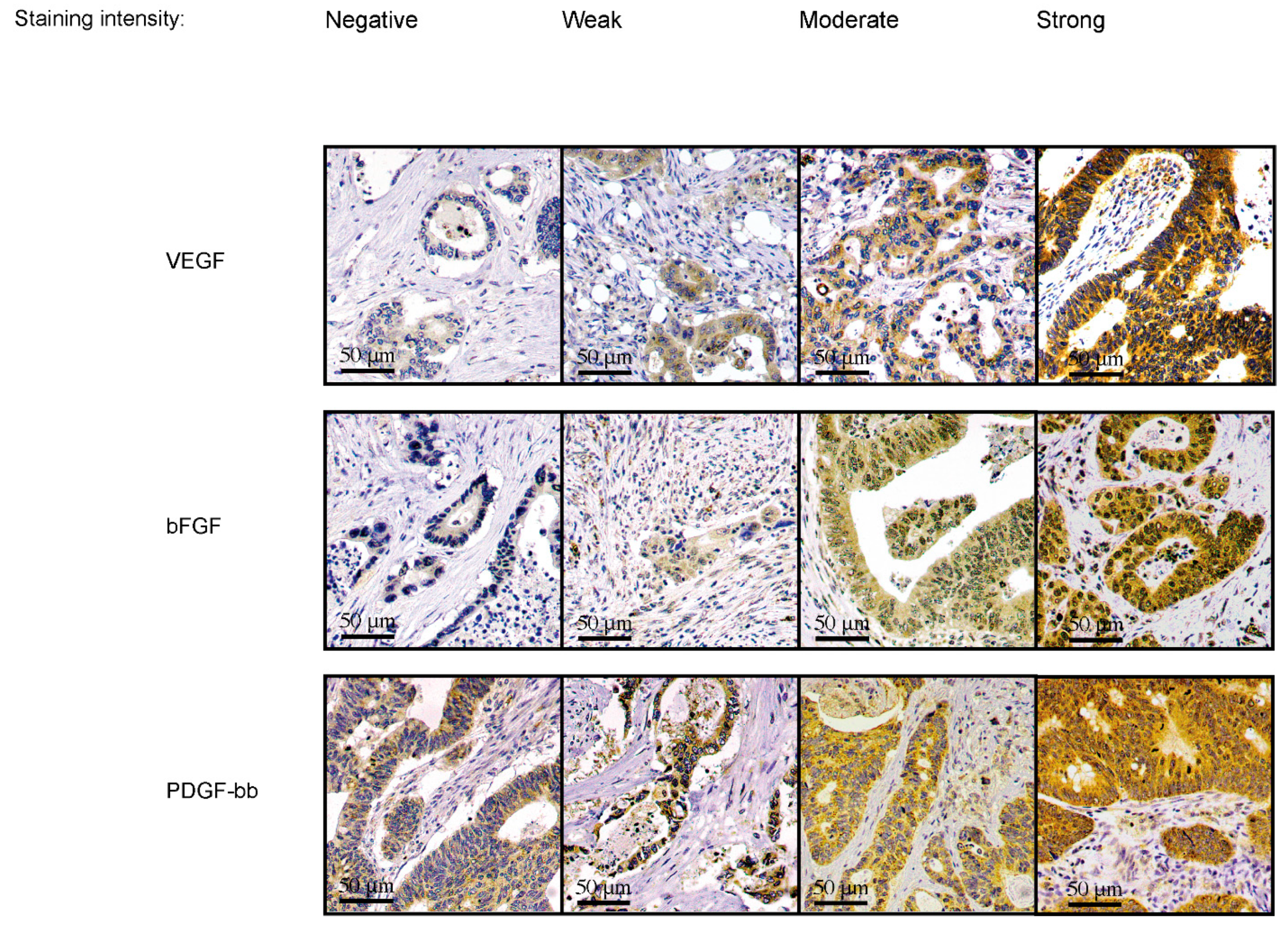

| Serum Angiogenic Growth Factor Concentrations | ||||||
|---|---|---|---|---|---|---|
| Clinicopathological Variable | VEGF | bFGF | PDGF-bb | |||
| rs | p Value | rs | p Value | rs | p Value | |
| Age | 0.041 | 0.465 | 0.088 | 0.116 | 0.309 | <0.001 |
| Gender | 0.027 | 0.632 | 0.014 | 0.808 | 0.047 | 0.402 |
| Stage (I–IV) | 0.039 | 0.494 | 0.053 | 0.347 | 0.039 | 0.490 |
| Tumor location | 0.072 | 0.200 | 0.066 | 0.239 | 0.054 | 0.336 |
| Phenotypic subtype | 0.088 | 0.181 | 0.036 | 0.583 | 0.117 | 0.076 |
| Tissue Expressions of Angiogenic Growth Factors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinicopathological Variable | VEGF | bFGF | PDGF-bb | ||||||
| Age | Low | High | p Value 1 | Low | High | p Value 1 | Low | High | p Value 1 |
| ≤66 | 51 (39.2%) | 79 (60.8%) | 0.466 | 46 (37.1%) | 78 (62.9%) | 0.670 | 33 (26.2%) | 93 (73.8%) | 0.772 |
| >66 | 50 (35.0%) | 93 (65.0%) | 47 (34.6%) | 89 (65.4%) | 35 (24.6%) | 107 (75.4%) | |||
| Gender | |||||||||
| Female | 56 (41.5%) | 79 (58.5%) | 0.129 | 48 (36.6%) | 83 (63.4%) | 0.768 | 36 (26.7%) | 99 (73.3%) | 0.624 |
| Male | 45 (32.6%) | 93 (67.4%) | 45 (34.9%) | 84 (65.1%) | 32 (24.1%) | 101 (75.9%) | |||
| Stage (I–IV) | |||||||||
| I | 9 (23.1%) | 30 (76.9%) | 0.130 | 12 (33.3%) | 24 (66.7%) | 0.756 | 7 (18.4%) | 31 (81.6%) | 0.132 |
| II | 31 (35.2%) | 57 (64.8%) | 30 (34.1%) | 58 (65.9%) | 17 (19.8%) | 69 (80.2%) | |||
| III | 38 (40.0%) | 57 (60.0%) | 31 (34.4%) | 59 (65.6%) | 26 (28.0%) | 67 (72.0%) | |||
| IV | 22 (46.8%) | 25 (53.2%) | 18 (42.9%) | 24 (57.1%) | 17 (36.2%) | 30 (63.8%) | |||
| Tumor location | |||||||||
| Right colon | 25 (33.3%) | 50 (66.7%) | 0.659 | 23 (31.1%) | 51 (68.9%) | 0.012 | 17 (23.0%) | 57 (77.0%) | 0.806 |
| Left colon | 56 (39.4%) | 86 (60.6%) | 58 (43.9%) | 74 (56.1%) | 38 (27.0%) | 103 (73.0%) | |||
| Rectum | 20 (35.7%) | 36 (64.3%) | 12 (22.2%) | 42 (77.8%) | 13 (24.5%) | 40 (75.5%) | |||
| Phenotypic subtype | |||||||||
| Immune | 16 (28.6%) | 40 (71.4%) | <0.001 | 18 (32.7%) | 37 (67.3%) | 0.022 | 8 (14.5%) | 47 (85.5%) | <0.001 |
| Canonical | 6 (14.3%) | 36 (85.7%) | 7 (16.7%) | 35 (83.3%) | 5 (11.6%) | 38 (88.4%) | |||
| Metabolic | 8 (22.2%) | 28 (77.8%) | 15 (42.9%) | 20 (57.1%) | 6 (17.1%) | 29 (82.9%) | |||
| Mesenchymal | 45 (47.9%) | 49 (52.1%) | 39 (42.9%) | 52 (57.1%) | 35 (38.5%) | 56 (61.5%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasurinen, J.H.; Hagström, J.; Kaprio, T.; Jalkanen, S.; Salmi, M.; Böckelman, C.; Haglund, C. Prognostic Values of Tissue and Serum Angiogenic Growth Factors Depend on the Phenotypic Subtypes of Colorectal Cancer. Cancers 2023, 15, 3871. https://doi.org/10.3390/cancers15153871
Kasurinen JH, Hagström J, Kaprio T, Jalkanen S, Salmi M, Böckelman C, Haglund C. Prognostic Values of Tissue and Serum Angiogenic Growth Factors Depend on the Phenotypic Subtypes of Colorectal Cancer. Cancers. 2023; 15(15):3871. https://doi.org/10.3390/cancers15153871
Chicago/Turabian StyleKasurinen, Jussi Herman, Jaana Hagström, Tuomas Kaprio, Sirpa Jalkanen, Marko Salmi, Camilla Böckelman, and Caj Haglund. 2023. "Prognostic Values of Tissue and Serum Angiogenic Growth Factors Depend on the Phenotypic Subtypes of Colorectal Cancer" Cancers 15, no. 15: 3871. https://doi.org/10.3390/cancers15153871
APA StyleKasurinen, J. H., Hagström, J., Kaprio, T., Jalkanen, S., Salmi, M., Böckelman, C., & Haglund, C. (2023). Prognostic Values of Tissue and Serum Angiogenic Growth Factors Depend on the Phenotypic Subtypes of Colorectal Cancer. Cancers, 15(15), 3871. https://doi.org/10.3390/cancers15153871






