Enhanced Therapeutic Effects of 177Lu-DOTA-M5A in Combination with Heat Shock Protein 90 Inhibitor Onalespib in Colorectal Cancer Xenografts
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Maintenance
2.2. M5A Monoclonal Antibody, Radiolabeling, and Onalespib
2.3. In Vivo Colorectal Xenograft Models
2.4. Radiolabeled M5A Biodistribution Studies
2.4.1. Xenograft Characterizations
2.4.2. 177Lu-DOTA-M5A Biodistribution Studies
2.4.3. SPECT/CT Imaging
2.4.4. Molecular Assessment and Short-Term Toxicity via Ex Vivo Immunohistochemistry
2.5. Therapy Regime, Tumor Growth, and Survival
2.6. Tumor Growth Rate Analysis
2.7. Statistical Analysis
3. Results
3.1. In Vivo Biodistribution Studies of Radiolabeled M5A
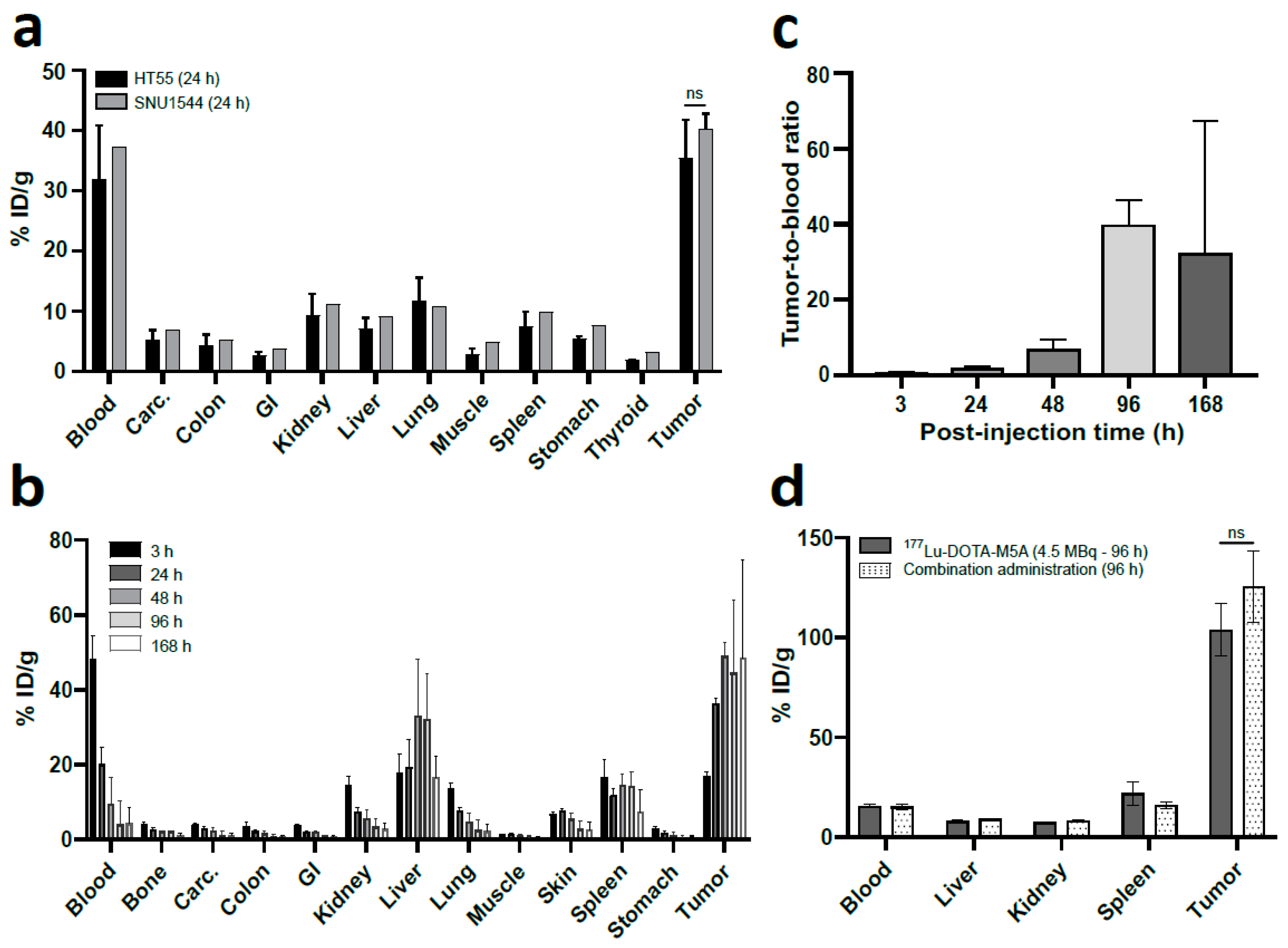
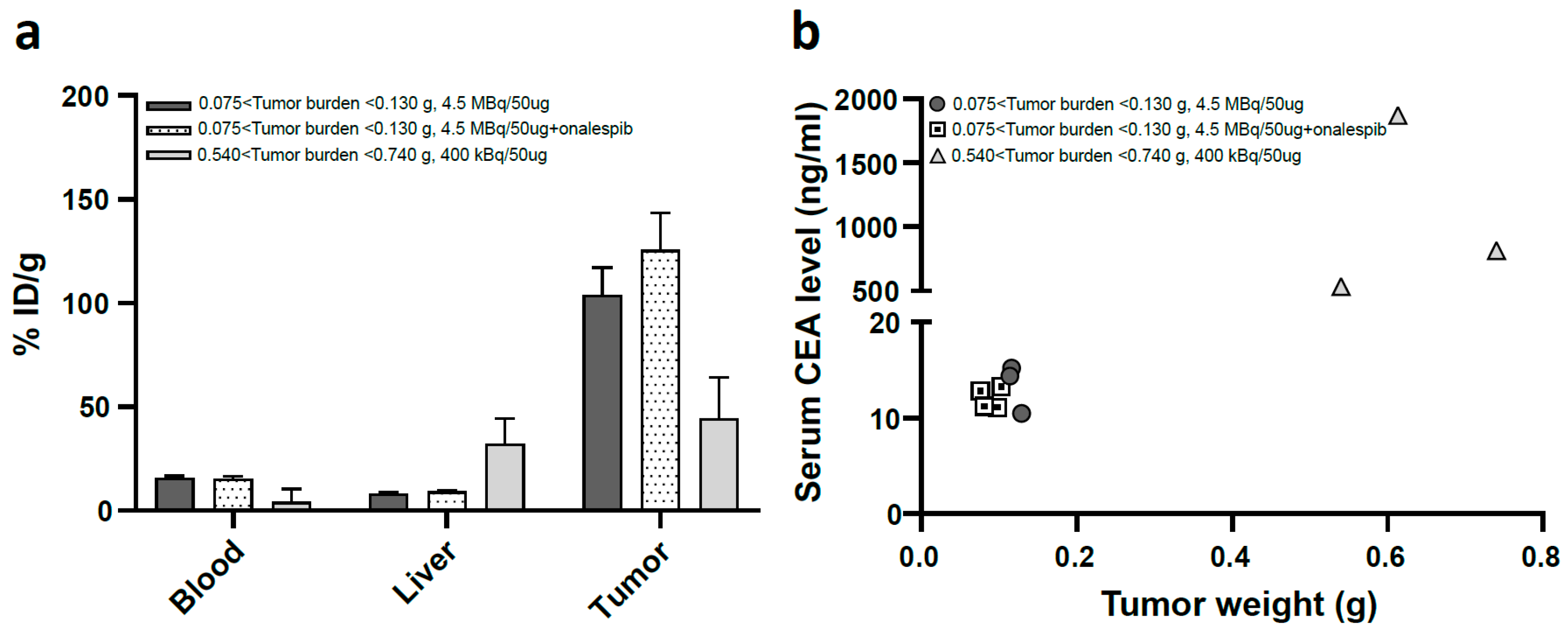
3.1.1. HT55 Is a Suitable Xenograft Model
3.1.2. In Vivo Tumor Targeting was Confirmed
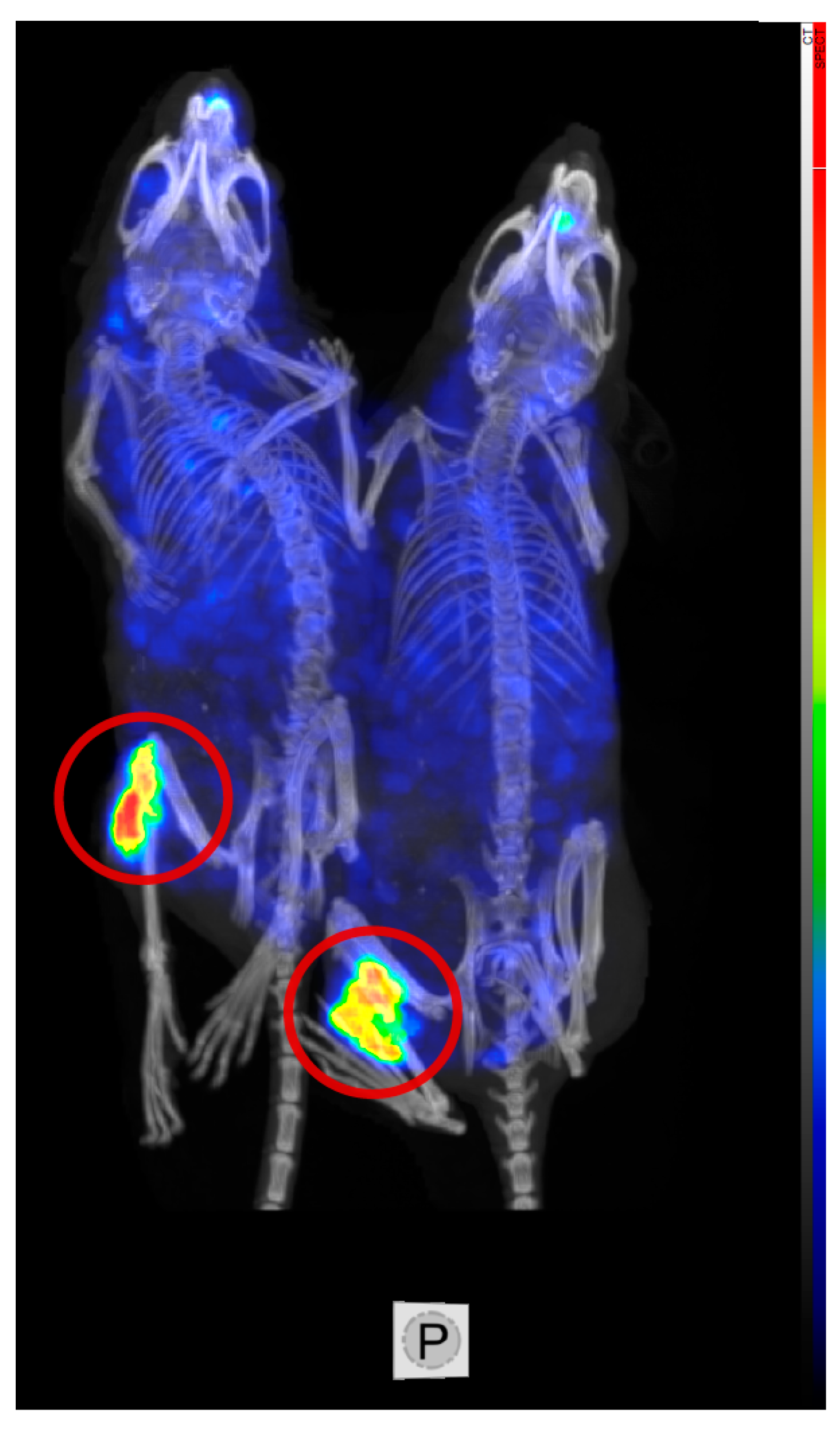
3.1.3. Low Uptake in Organs at Risk, SPECT/CT Imaging
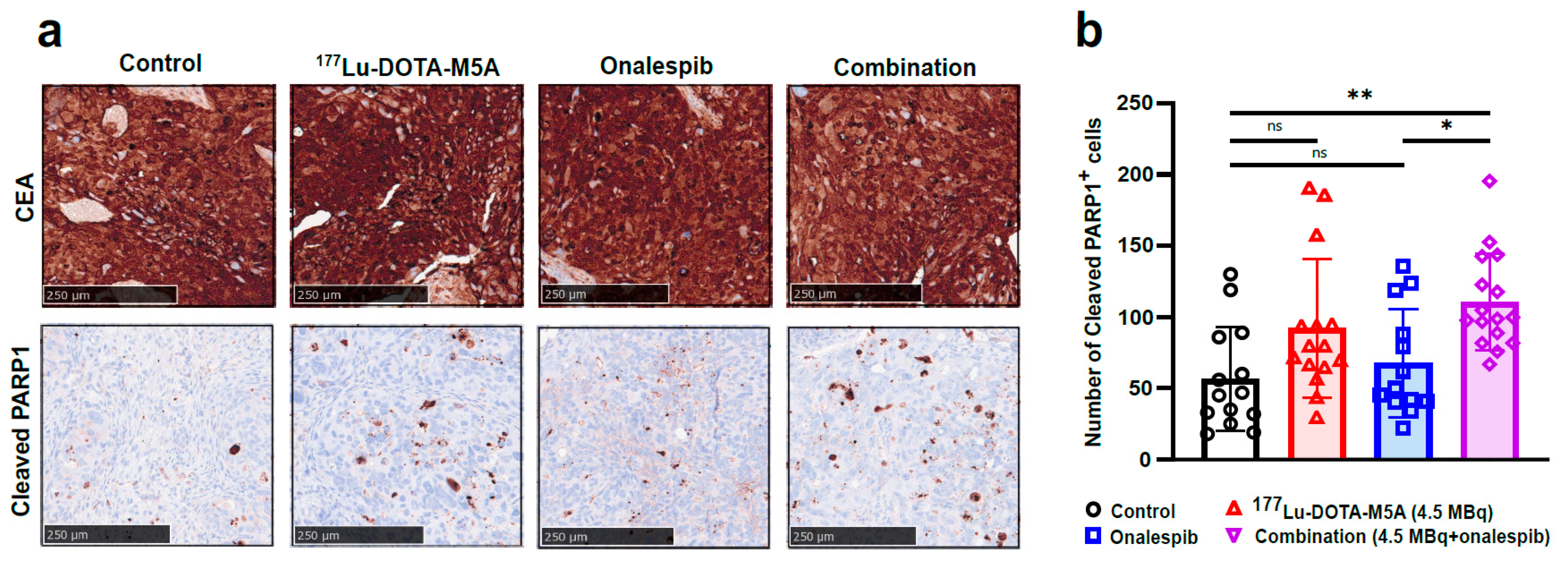
3.1.4. Therapeutic Effects at Molecular Level and No Detected Short-Term Toxicity
3.2. In Vivo Therapy Study
3.2.1. Combination Therapy Decreased Tumor Growth Rate
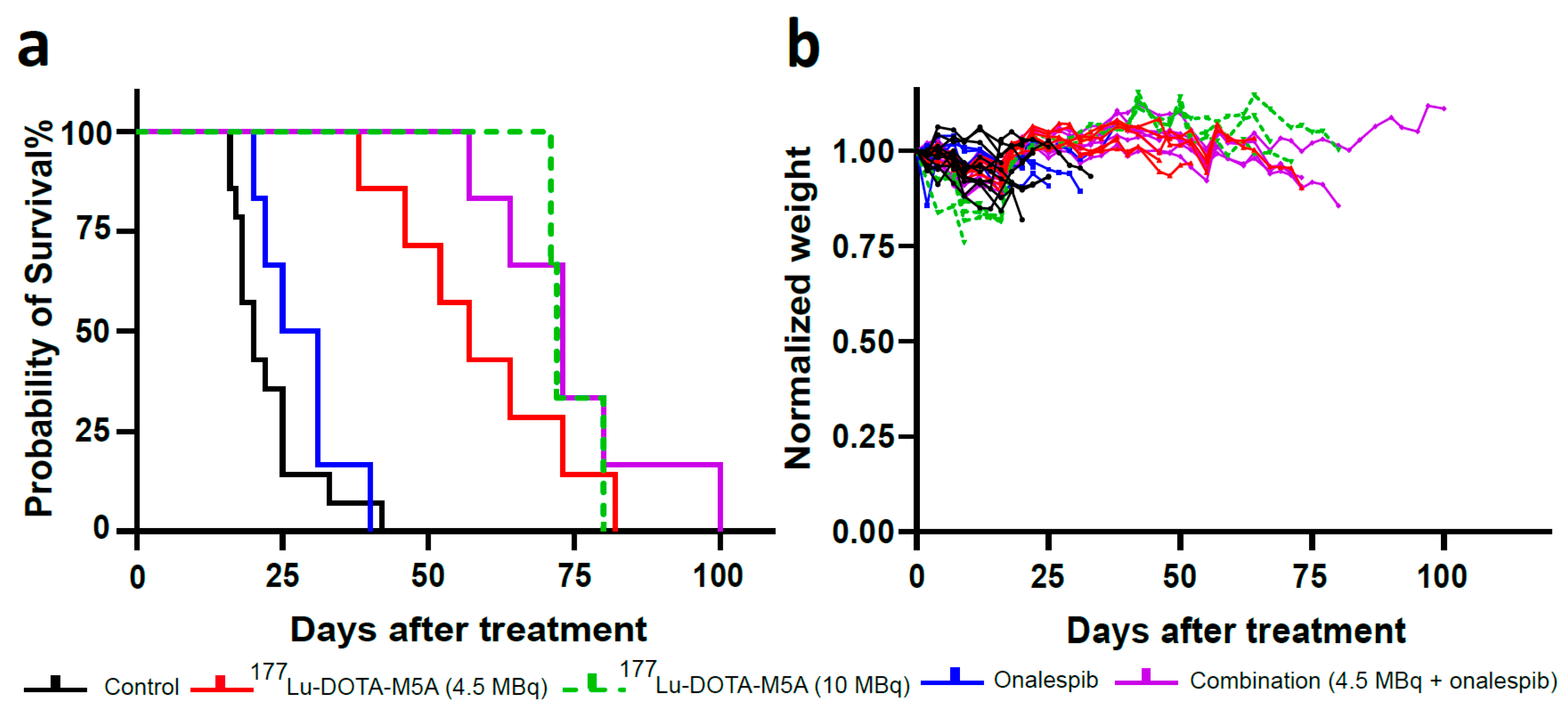
3.2.2. Combination Therapy Increased the Median Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Carcinoembryonic Antigen as a Marker for Colorectal Cancer: Is It Clinically Useful? Clin. Chem. 2001, 47, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Björkman, K.; Jalkanen, S.; Salmi, M.; Mustonen, H.; Kaprio, T.; Kekki, H.; Pettersson, K.; Böckelman, C.; Haglund, C. A prognostic model for colorectal cancer based on CEA and a 48-multiplex serum biomarker panel. Sci. Rep. 2021, 11, 4287. [Google Scholar] [CrossRef]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef]
- Öbrink, B. CEA adhesion molecules: Multifunctional proteins with signal-regulatory properties. Curr. Opin. Cell Biol. 1997, 9, 616–626. [Google Scholar] [CrossRef]
- Tchoupa, A.K.; Schuhmacher, T.; Hauck, C.R. Signaling by epithelial members of the CEACAM family–mucosal docking sites for pathogenic bacteria. Cell Commun. Signal. 2014, 12, 27. [Google Scholar] [CrossRef]
- Muenzner, P.; Rohde, M.; Kneitz, S.; Hauck, C.R. CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J. Cell Biol. 2005, 170, 825–836. [Google Scholar] [CrossRef]
- Ordoñez, C.; Screaton, R.A.; Ilantzis, C.; Stanners, C.P. Human Carcinoembryonic Antigen Functions as a General Inhibitor of Anoikis1. Cancer Res. 2000, 60, 3419–3424. [Google Scholar]
- Chan, C.H.; Camacho-Leal, P.; Stanners, C.P. Colorectal hyperplasia and dysplasia due to human carcinoembryonic antigen (CEA) family member expression in transgenic mice. PLoS ONE 2007, 2, e1353. [Google Scholar] [CrossRef]
- Chan, C.H.F.; Cook, D.; Stanners, C.P. Increased colon tumor susceptibility in azoxymethane treated CEABAC transgenic mice. Carcinogenesis 2006, 27, 1909–1916. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Chu, D.Z.; Williams, L.E.; Liu, A.; Zhan, J.; Yamauchi, D.M.; Wilczynski, S.; Wu, A.M.; Yazaki, P.J.; Shively, J.E.; et al. A Phase I Trial of 90Y-DOTA-Anti-CEA Chimeric T84.66 (cT84.66) Radioimmunotherapy in Patients with Metastatic CEA-Producing Malignancies. Cancer Biother. Radiopharm. 2006, 21, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, R.; van der Graaf, W.T.A.; Franssen, G.; Sharkey, R.M.; Goldenberg, D.M.; McBride, W.J.; Rossi, E.A.; Eek, A.; Oyen, W.J.G.; Boerman, O.C. Pretargeted 177Lu Radioimmunotherapy of Carcinoembryonic Antigen–Expressing Human Colonic Tumors in Mice. J. Nucl. Med. 2010, 51, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, D.; Yazaki, P.; Yamauchi, D.; Simpson, J.; Frankel, P.H.; Bading, J.; Colcher, D.; Poku, K.; Chen, Y.; Lim, D.; et al. Phase I Study of Yttrium-90 Radiolabeled M5A Anti-Carcinoembryonic Antigen Humanized Antibody in Patients with Advanced Carcinoembryonic Antigen Producing Malignancies. Cancer Biother. Radiopharm. 2020, 35, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Clarke, P.; Szalai, G.; Shively, J.E.; Williams, L.E.; Shyr, Y.; Shi, E.; Primus, F.J. Targeting and Therapy of Carcinoembryonic Antigen-expressing Tumors in Transgenic Mice with an Antibody-Interleukin 2 Fusion Protein. Cancer Res. 2000, 60, 4475–4484. [Google Scholar]
- Schmidt, M.M.; Thurber, G.M.; Wittrup, K.D. Kinetics of anti-carcinoembryonic antigen antibody internalization: Effects of affinity, bivalency, and stability. Cancer Immunol. Immunother. 2008, 57, 1879–1890. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.-W. The Roles of Carcinoembryonic Antigen in Liver Metastasis and Therapeutic Approaches. Gastroenterol. Res. Pract. 2017, 2017, e7521987. [Google Scholar] [CrossRef]
- Kujawski, M.; Sherman, M.; Hui, S.; Zuro, D.; Lee, W.-H.; Yazaki, P.; Sherman, A.; Szpikowska, B.; Chea, J.; Lasiewski, D.; et al. Potent immunomodulatory effects of an anti-CEA-IL-2 immunocytokine on tumor therapy and effects of stereotactic radiation. OncoImmunology 2020, 9, 1724052. [Google Scholar] [CrossRef]
- Cahan, B.; Leong, L.; Wagman, L.; Yamauchi, D.; Shibata, S.; Wilzcynski, S.; Williams, L.E.; Yazaki, P.; Colcher, D.; Frankel, P.; et al. Phase I/II Trial of Anticarcinoembryonic Antigen Radioimmunotherapy, Gemcitabine, and Hepatic Arterial Infusion of Fluorodeoxyuridine Postresection of Liver Metastasis for Colorectal Carcinoma. Cancer Biother. Radiopharm. 2017, 32, 258–265. [Google Scholar] [CrossRef]
- Cha, S.E.; Kujawski, M.; Yazaki, P.J.; Brown, C.; Shively, J.E. Tumor regression and immunity in combination therapy with anti-CEA chimeric antigen receptor T cells and anti-CEA-IL2 immunocytokine. OncoImmunology 2021, 10, 1899469. [Google Scholar] [CrossRef]
- Duda, R.B.; Beatty, J.D.; Sheibani, K.; Williams, L.E.; Paxton, R.J.; Beatty, B.G.; Shively, J.E.; Vlahos, W.G.; Werner, J.L.; Kemeny, M.M.; et al. Imaging of Human Colorectal Adenocarcinoma with Indium-Labeled Anticarcinoembryonic Antigen Monoclonal Antibody. Arch. Surg. 1986, 121, 1315–1319. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Shibata, S.; Williams, L.E.; Kwok, C.S.; Liu, A.; Chu, D.Z.; Yamauchi, D.M.; Wilczynski, S.; Ikle, D.N.; Wu, A.M.; et al. A Phase I Trial of 90Y-Anti-Carcinoembryonic Antigen Chimeric T84.66 Radioimmunotherapy with 5-Fluorouracil in Patients with Metastatic Colorectal Cancer. Clin. Cancer Res. 2003, 9, 5842–5852. [Google Scholar]
- Shibata, S.; Raubitschek, A.; Leong, L.; Koczywas, M.; Williams, L.; Zhan, J.; Wong, J.Y.C. A Phase I Study of a Combination of Yttrium-90–Labeled Anti–Carcinoembryonic Antigen (CEA) Antibody and Gemcitabine in Patients with CEA-Producing Advanced Malignancies. Clin. Cancer Res. 2009, 15, 2935–2941. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, J.Y.; Williams, L.E.; Yamauchi, D.M.; Odom-Maryon, T.; Esteban, J.M.; Neumaier, M.; Wu, A.M.; Johnson, D.K.; Primus, F.J.; Shively, J.E. Initial experience evaluating 90yttrium-radiolabeled anti-carcinoembryonic antigen chimeric T84.66 in a phase I radioimmunotherapy trial. Cancer Res. 1995, 55 (Suppl. S23), 5929s–5934s. [Google Scholar] [PubMed]
- Yazaki, P.J.; Sherman, M.A.; Shively, J.E.; Ikle, D.; Williams, L.E.; Wong, J.Y.C.; Colcher, D.; Wu, A.M.; Raubitschek, A.A. Humanization of the anti-CEA T84.66 antibody based on crystal structure data. Protein Eng. Des. Sel. 2004, 17, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.; Dodd, G.D. Chapter 86—Interventional Radiology in the Cirrhotic Liver. In Textbook of Gastrointestinal Radiology, 3rd ed.; Gore, R.M., Levine, M.S., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2008; pp. 1553–1575. ISBN 978-1-4160-2332-6. [Google Scholar]
- Mohajershojai, T.; Jha, P.; Boström, A.; Frejd, F.Y.; Yazaki, P.J.; Nestor, M. In Vitro Characterization of 177Lu-DOTA-M5A Anti-Carcinoembryonic Antigen Humanized Antibody and HSP90 Inhibition for Potentiated Radioimmunotherapy of Colorectal Cancer. Front. Oncol. 2022, 12, 849338. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 2003, 228, 111–133. [Google Scholar] [CrossRef]
- Miyata, Y.; Nakamoto, H.; Neckers, L. The therapeutic target Hsp90 and cancer hallmarks. Curr. Pharm. Des. 2013, 19, 347–365. [Google Scholar] [CrossRef]
- Wang, J.; Cui, S.; Zhang, X.; Wu, Y.; Tang, H. High Expression of Heat Shock Protein 90 Is Associated with Tumor Aggressiveness and Poor Prognosis in Patients with Advanced Gastric Cancer. PLoS ONE 2013, 8, e62876. [Google Scholar] [CrossRef]
- Ren, X.; Li, T.; Zhang, W.; Yang, X. Targeting Heat-Shock Protein 90 in Cancer: An Update on Combination Therapy. Cells 2022, 11, 2556. [Google Scholar] [CrossRef]
- Koga, F.; Kihara, K.; Neckers, L. Inhibition of Cancer Invasion and Metastasis by Targeting the Molecular Chaperone Heat-shock Protein 90. Anticancer. Res. 2009, 29, 797–807. [Google Scholar] [PubMed]
- Zhang, S.; Guo, S.; Li, Z.; Li, D.; Zhan, Q. High expression of HSP90 is associated with poor prognosis in patients with colorectal cancer. PeerJ 2019, 7, e7946. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Pick, E.; Kluger, Y.; Gould-Rothberg, B.; Lazova, R.; Camp, R.L.; Rimm, D.L.; Kluger, H.M. HSP90 as a marker of progression in melanoma. Ann. Oncol. 2008, 19, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Žáčková, M.; Moučková, D.; Lopotová, T.; Ondráčková, Z.; Klamová, H.; Moravcová, J. Hsp90—A Potential prognostic marker in CML. Blood Cells Mol. Dis. 2013, 50, 184–189. [Google Scholar] [CrossRef]
- Liu, K.; Kang, M.; Li, J.; Qin, W.; Wang, R. Prognostic value of the mRNA expression of members of the HSP90 family in non-small cell lung cancer. Exp. Ther. Med. 2019, 17, 2657–2665. [Google Scholar] [CrossRef]
- Lin, T.; Qiu, Y.; Peng, W.; Peng, L. Heat Shock Protein 90 Family Isoforms as Prognostic Biomarkers and Their Correlations with Immune Infiltration in Breast Cancer. BioMed Res. Int. 2020, 2020, e2148253. [Google Scholar] [CrossRef]
- Spiegelberg, D.; Dascalu, A.; Mortensen, A.C.; Abramenkovs, A.; Kuku, G.; Nestor, M.; Stenerlöw, B. The novel HSP90 inhibitor AT13387 potentiates radiation effects in squamous cell carcinoma and adenocarcinoma cells. Oncotarget 2015, 6, 35652–35666. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, R.; Ascenzi, P.; di Masi, A. Hsp90: A New Player in DNA Repair? Biomolecules 2015, 5, 2589–2618. [Google Scholar] [CrossRef]
- Spiegelberg, D.; Abramenkovs, A.; Mortensen, A.C.L.; Lundsten, S.; Nestor, M.; Stenerlöw, B. The HSP90 inhibitor Onalespib exerts synergistic anti-cancer effects when combined with radiotherapy: An in vitro and in vivo approach. Sci. Rep. 2020, 10, 5923. [Google Scholar] [CrossRef]
- Slovin, S.; Hussain, S.; Saad, F.; Garcia, J.; Picus, J.; Ferraldeschi, R.; Crespo, M.; Flohr, P.; Riisnaes, R.; Lin, C.; et al. Pharmacodynamic and Clinical Results from a Phase I/II Study of the HSP90 Inhibitor Onalespib in Combination with Abiraterone Acetate in Prostate Cancer. Clin. Cancer Res. 2019, 25, 4624–4633. [Google Scholar] [CrossRef]
- Lundsten, S.; Spiegelberg, D.; Stenerlöw, B.; Nestor, M. The HSP90 inhibitor onalespib potentiates 177Lu-DOTATATE therapy in neuroendocrine tumor cells. Int. J. Oncol. 2019, 55, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.F.; Sanger, C. Properties of a cell line from human adenocarcinoma of the rectum. Br. J. Cancer 1977, 35, 785–794. [Google Scholar] [CrossRef]
- Ku, J.-L.; Shin, Y.-K.; Kim, D.-W.; Kim, K.-H.; Choi, J.-S.; Hong, S.-H.; Jeon, Y.-K.; Kim, S.-H.; Kim, H.-S.; Park, J.-H.; et al. Establishment and characterization of 13 human colorectal carcinoma cell lines: Mutations of genes and expressions of drug-sensitivity genes and cancer stem cell markers. Carcinogenesis 2010, 31, 1003–1009. [Google Scholar] [CrossRef]
- Medico, E.; Russo, M.; Picco, G.; Cancelliere, C.; Valtorta, E.; Corti, G.; Buscarino, M.; Isella, C.; Lamba, S.; Martinoglio, B.; et al. The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets. Nat. Commun. 2015, 6, 7002. [Google Scholar] [CrossRef] [PubMed]
- Bacac, M.; Fauti, T.; Sam, J.; Colombetti, S.; Weinzierl, T.; Ouaret, D.; Bodmer, W.; Lehmann, S.; Hofer, T.; Hosse, R.J.; et al. A Novel Carcinoembryonic Antigen T-Cell Bispecific Antibody (CEA TCB) for the Treatment of Solid Tumors. Clin. Cancer Res. 2016, 22, 3286–3297. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.R.; Raubitschek, A.; Shively, J.E. A facile, water-soluble method for modification of proteins with DOTA. Use of elevated temperature and optimized pH to achieve high specific activity and high chelate stability in radiolabeled immunoconjugates. Bioconjug. Chem. 1994, 5, 565–576. [Google Scholar] [CrossRef]
- Modugno, L.; Giannerini, S. The Wild Bootstrap for Multilevel Models. Commun. Stat.-Theory Methods 2015, 44, 4812–4825. [Google Scholar] [CrossRef]
- Loy, A.; Steele, S.; Korobova, J. lmeresampler: Bootstrap Methods for Nested Linear Mixed-Effects Models. Published online 29 April 2022. Available online: https://CRAN.R-project.org/package=lmeresampler (accessed on 14 December 2022).
- Li, Z.; Zhang, D.; Pang, X.; Yan, S.; Lei, M.; Cheng, X.; Song, Q.; Cai, L.; Wang, Z.; You, D. Association Between Serum Carcinoembryonic Antigen Levels at Different Perioperative Time Points and Colorectal Cancer Outcomes. Front. Oncol. 2021, 11, 722883. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2021.722883 (accessed on 25 December 2022). [CrossRef]
- Song, H.; Sgouros, G. Radioimmunotherapy of Solid Tumors: Searching for the Right Target. Curr. Drug Deliv. 2011, 8, 26–44. [Google Scholar] [CrossRef]
- Sahlmann, C.-O.; Homayounfar, K.; Niessner, M.; Dyczkowski, J.; Conradi, L.-C.; Braulke, F.; Meller, B.; Beißbarth, T.; Ghadimi, B.M.; Meller, J.; et al. Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: Safety, feasibility, and long-term efficacy results of a prospective phase 2 study. Cancer 2017, 123, 638–649. [Google Scholar] [CrossRef]
- Lin, B.; Du, H.; Fan, J.; Huang, D.; Gao, F.; Li, J.; Zhang, Y.; Feng, G.; Dai, T.; Du, X. Radioimmunotherapy Combined with Low-Intensity Ultrasound and Microbubbles: A Potential Novel Strategy for Treatment of Solid Tumors. Front. Oncol. 2021, 11, 750741. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2021.750741 (accessed on 21 November 2022). [CrossRef]
- Jain, M.; Venkatraman, G.; Batra, S.K. Optimization of Radioimmunotherapy of Solid Tumors: Biological Impediments and Their Modulation. Clin. Cancer Res. 2007, 13, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Kryeziu, K.; Bruun, J.; Guren, T.K.; Sveen, A.; Lothe, R.A. Combination therapies with HSP90 inhibitors against colorectal cancer. Biochim. Biophys. Acta 2019, 1871, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Lundsten, S.; Spiegelberg, D.; Raval, N.R.; Nestor, M. The radiosensitizer Onalespib increases complete remission in 177Lu-DOTATATE-treated mice bearing neuroendocrine tumor xenografts. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Wu, A.M.; Kenanova, V.E.; Olafsen, T.; Yazaki, P.J. Numerical Comparison of Iodine-Based and Indium-Based Antibody Biodistributions. Cancer Biother. Radiopharm. 2014, 29, 91–98. [Google Scholar] [CrossRef]
- Campbell, I. Liver: Metabolic functions. Anaesth. Intensive Care Med. 2006, 7, 51–54. [Google Scholar] [CrossRef]
- Topdagi, O.; Timuroglu, A. Evaluation of the Relationship between Carcinoembryonic Antigen and TNM Stage in Colorectal Cancer. Eurasian J. Med. 2018, 50, 96–98. [Google Scholar] [CrossRef]
- Auclin, E.; Taieb, J.; Lepage, C.; Aparicio, T.; Faroux, R.; Mini, E.; Folprecht, G.; Salazar, R.; Benetkiewicz, M.; Banzi, M.; et al. Carcinoembryonic Antigen Levels and Survival in Stage III Colon Cancer: Post hoc Analysis of the MOSAIC and PETACC-8 Trials. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1153–1161. [Google Scholar] [CrossRef]
- Ravi, K.S.; N.B, P.; Kishore, S.; Kaur, S.; Mehta, V.; Krishnan, A.S. Karyoanomalic frequency assay during radiation therapy–A promising marker in the prognosis of oral and oropharyngeal carcinoma. J. Family Med. Prim. Care 2021, 10, 4548–4552. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Chu, D.Z.; Yamauchi, D.M.; Williams, L.E.; Liu, A.; Wilczynski, S.; Wu, A.M.; Shively, J.E.; Doroshow, J.H.; Raubitschek, A.A. A phase I radioimmunotherapy trial evaluating 90yttrium-labeled anti-carcinoembryonic antigen (CEA) chimeric T84.66 in patients with metastatic CEA-producing malignancies. Clin. Cancer Res. 2000, 6, 3855–3863. [Google Scholar]
- Ding, Y.; Xuan, W.; Chen, C.; Chen, Z.; Yang, Z.; Zuo, Y.; Ren, S. Differences in carcinoembryonic antigen levels between colon and rectal cancer. Mol. Clin. Oncol. 2014, 2, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Boxer, G.M.; Begent, R.H.J.; Kelly, A.M.B.; Southall, P.J.; Blair, S.B.; Theodorou, N.A.; Dawson, P.M.; Ledermann, J.A. Factors influencing variability of localisation of antibodies to carcinoembryonic antigen (CEA) in patients with colorectal carcinoma–implications for radioimmunotherapy. Br. J. Cancer 1992, 65, 825–831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xenaki, K.T.; Oliveira, S.; van Bergen en Henegouwen, P.M.P. Antibody or Antibody Fragments: Implications for Molecular Imaging and Targeted Therapy of Solid Tumors. Front. Immunol. 2017, 8, 1287. [Google Scholar] [CrossRef]
- Sharkey, R.M.; Goldenberg, D.M. Cancer radioimmunotherapy. Immunotherapy 2011, 3, 349–370. [Google Scholar] [CrossRef]
- Do, K.; Speranza, G.; Chang, L.-C.; Polley, E.C.; Bishop, R.; Zhu, W.; Trepel, J.B.; Lee, S.; Lee, M.-J.; Kinders, R.J.; et al. Phase I study of the heat shock protein 90 (Hsp90) inhibitor onalespib (AT13387) administered on a daily for 2 consecutive days per week dosing schedule in patients with advanced solid tumors. Investig. New Drugs 2015, 33, 921–930. [Google Scholar] [CrossRef] [PubMed]

| Groups\Days | −6 | 1 | 2 | 3 | 4 | Termination of First/Last Mouse (Days after Treatment Start) |
|---|---|---|---|---|---|---|
| Control (no treatment) | TI * | - | - | - | - | 16/25 |
| Control (DOTA-M5A) | TI | - | X | - | - | 20/33 |
| Control (DMSO) | TI | X | X | X | X | 18/22 |
| 177Lu-DOTA-M5A (4.5 MBq) | TI | - | X | - | - | 38/82 |
| 177Lu-DOTA-M5A (10 MBq) | TI | - | X | - | - | 67/80 |
| Onalespib | TI | X | X | X | X | 20/40 |
| Combination (4.5 MBq of 177Lu-DOTA-M5A and onalespib) | TI | X | X | X | X | 57/100 |
| Treatment | Median Survival (days) | Maximum Survival (days) | Average Daily Tumor Growth Rate (%, 95% CI) |
|---|---|---|---|
| Control (no treatment) | 20 | 33 | 8.95 (7.67–10.14) |
| 177Lu-DOTA-M5A (4.5 MBq) | 55 | 73 | 3.63 (1.35–5.89) |
| 177Lu-DOTA-M5A (10 MBq) | 72 | >80 * | 2.6 (0.24–4.82) |
| Onalespib | 25 | 40 | 7.99 (4.95–10.87) |
| Combination (4.5 MBq of 177Lu-DOTA-M5A and onalespib) | 73 | >100 ** | 2.25 (−0.17–4.60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohajershojai, T.; Spangler, D.; Chopra, S.; Frejd, F.Y.; Yazaki, P.J.; Nestor, M. Enhanced Therapeutic Effects of 177Lu-DOTA-M5A in Combination with Heat Shock Protein 90 Inhibitor Onalespib in Colorectal Cancer Xenografts. Cancers 2023, 15, 4239. https://doi.org/10.3390/cancers15174239
Mohajershojai T, Spangler D, Chopra S, Frejd FY, Yazaki PJ, Nestor M. Enhanced Therapeutic Effects of 177Lu-DOTA-M5A in Combination with Heat Shock Protein 90 Inhibitor Onalespib in Colorectal Cancer Xenografts. Cancers. 2023; 15(17):4239. https://doi.org/10.3390/cancers15174239
Chicago/Turabian StyleMohajershojai, Tabassom, Douglas Spangler, Saloni Chopra, Fredrik Y. Frejd, Paul J. Yazaki, and Marika Nestor. 2023. "Enhanced Therapeutic Effects of 177Lu-DOTA-M5A in Combination with Heat Shock Protein 90 Inhibitor Onalespib in Colorectal Cancer Xenografts" Cancers 15, no. 17: 4239. https://doi.org/10.3390/cancers15174239
APA StyleMohajershojai, T., Spangler, D., Chopra, S., Frejd, F. Y., Yazaki, P. J., & Nestor, M. (2023). Enhanced Therapeutic Effects of 177Lu-DOTA-M5A in Combination with Heat Shock Protein 90 Inhibitor Onalespib in Colorectal Cancer Xenografts. Cancers, 15(17), 4239. https://doi.org/10.3390/cancers15174239






