Clinical Possibility of Caenorhabditis elegans as a Novel Evaluation Tool for Esophageal Cancer Patients Receiving Chemotherapy: A Prospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Eligibility Criteria
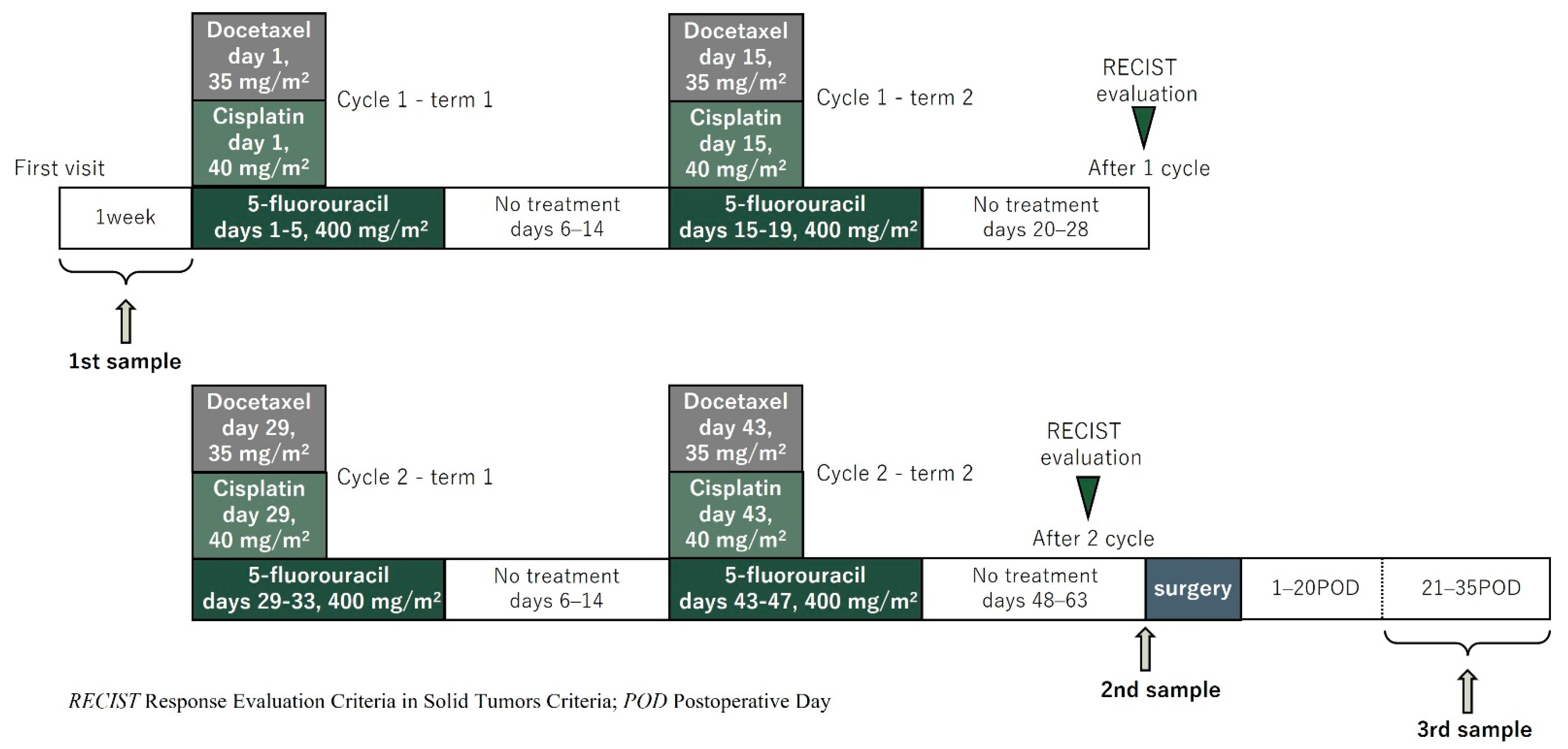
2.2. Treatment Strategy and Evaluation of Chemotherapy
2.3. Study Endpoints
2.4. Measurement Method of N-NOSE and Index Reduction Scores
2.5. Statistical Analyses
2.6. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: Part 1. Esophagus 2023, 20, 343–372. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: Part 2. Esophagus 2023, 20, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Kato, H.; Igaki, H.; Shinoda, M.; Ozawa, S.; Shimizu, H.; Nakamura, T.; Yabusaki, H.; Aoyama, N.; Kurita, A.; et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann. Surg. Oncol. 2012, 19, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yoshida, K.; Yamada, A.; Tanahashi, T.; Okumura, N.; Matsuhashi, N.; Yamaguchi, K.; Miyazaki, T. Phase II trial of biweekly docetaxel, cisplatin, and 5-fluorouracil chemotherapy for advanced esophageal squamous cell carcinoma. Cancer Chemother. Pharmacol. 2016, 77, 1143–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hipp, J.; Kuvendjiska, J.; Hillebrecht, H.C.; Timme-Bronsert, S.; Fichtner-Feigl, S.; Hoeppner, J.; Diener, M.K. Pathological complete response in multimodal treatment of esophageal cancer: A retrospective cohort study. Dis. Esophagus 2022, 36, doac095. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Boshier, P.; Myridakis, A.; Belluomo, I.; Hanna, G.B. Urinary Volatile Organic Compound Analysis for the Diagnosis of Cancer: A Systematic Literature Review and Quality Assessment. Metabolites 2020, 11, 17. [Google Scholar] [CrossRef]
- Tivey, A.; Church, M.; Rothwell, D.; Dive, C.; Cook, N. Circulating tumour DNA—looking beyond the blood. Nat. Rev. Clin. Oncol. 2022, 19, 600–612. [Google Scholar] [CrossRef]
- Shumilov, E.; Flach, J.; Pabst, T.; Fiedler, M.; Angelillo-Scherrer, A.; Trumper, L.; Joncourt, R.; Kohlmann, A.; Bacher, U. Genetic alterations crossing the borders of distinct hematopoetic lineages and solid tumors: Diagnostic challenges in the era of high-throughput sequencing in hemato-oncology. Crit. Rev. Oncol. Hematol. 2018, 126, 64–79. [Google Scholar] [CrossRef]
- Saalberg, Y.; Wolff, M. VOC breath biomarkers in lung cancer. Clin. Chim. Acta 2016, 459, 5–9. [Google Scholar] [CrossRef]
- Pons-Belda, O.D.; Fernandez-Uriarte, A.; Diamandis, E.P. Multi Cancer Early Detection by Using Circulating Tumor DNA-The Galleri Test. Reply to Klein et al. The Promise of Multicancer Early Detection. Comment on “Pons-Belda et al. Can Circulating Tumor DNA Support a Successful Screening Test for Early Cancer Detection? The Grail Paradigm. Diagnostics 2021, 11, 2171”. Diagnostics 2022, 12, 1244. [Google Scholar] [CrossRef]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef]
- Woollam, M.; Teli, M.; Angarita-Rivera, P.; Liu, S.; Siegel, A.P.; Yokota, H.; Agarwal, M. Detection of Volatile Organic Compounds (VOCs) in Urine via Gas Chromatography-Mass Spectrometry QTOF to Differentiate Between Localized and Metastatic Models of Breast Cancer. Sci. Rep. 2019, 9, 2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallafre-Muro, C.; Llambrich, M.; Cumeras, R.; Pardo, A.; Brezmes, J.; Marco, S.; Guma, J. Comprehensive Volatilome and Metabolome Signatures of Colorectal Cancer in Urine: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 2534. [Google Scholar] [CrossRef]
- Bargmann, C.I. Comparative chemosensation from receptors to ecology. Nature 2006, 444, 295–301. [Google Scholar] [CrossRef]
- Hirotsu, T.; Sonoda, H.; Uozumi, T.; Shinden, Y.; Mimori, K.; Maehara, Y.; Ueda, N.; Hamakawa, M. A highly accurate inclusive cancer screening test using Caenorhabditis elegans scent detection. PLoS ONE 2015, 10, e0118699. [Google Scholar] [CrossRef] [Green Version]
- Bargmann, C.I.; Hartwieg, E.; Horvitz, H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 1993, 74, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, T.; Saeki, S.; Yamamoto, M.; Iino, Y. The Ras-MAPK pathway is important for olfaction in Caenorhabditis elegans. Nature 2000, 404, 289–293. [Google Scholar] [CrossRef]
- Kusumoto, H.; Tashiro, K.; Shimaoka, S.; Tsukasa, K.; Baba, Y.; Furukawa, S.; Furukawa, J.; Suenaga, T.; Kitazono, M.; Tanaka, S.; et al. Behavioural Response Alteration in Caenorhabditis elegans to Urine After Surgical Removal of Cancer: Nematode-NOSE (N-NOSE) for Postoperative Evaluation. Biomark. Cancer 2019, 11, 1179299X19896551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, A.; Konno, M.; Ozaki, M.; Kawamoto, K.; Chijimatsu, R.; Kondo, N.; Hirotsu, T.; Ishii, H. Scent test using Caenorhabditis elegans to screen for early-stage pancreatic cancer. Oncotarget 2021, 12, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Inaba, S.; Shimozono, N.; Yabuki, H.; Enomoto, M.; Morishita, M.; Hirotsu, T.; di Luccio, E. Accuracy evaluation of the C. elegans cancer test (N-NOSE) using a new combined method. Cancer Treat. Res. Commun. 2021, 27, 100370. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoshida, K.; Sanada, Y.; Osada, S.; Yamaguchi, K.; Takahashi, T. Biweekly docetaxel, cisplatin, and 5-fluorouracil (DCF) chemotherapy for advanced esophageal squamous cell carcinoma: A phase I dose-escalation study. Cancer Chemother. Pharmacol. 2010, 66, 1159–1165. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Tanaka, Y.; Imai, T.; Okumura, N.; Matsuhashi, N.; Takahashi, T.; Shimokawa, T.; Yoshida, K. Serum diamine oxidase activity derived from response to chemotherapy affects adverse events and serum amino acid levels. Support Care Cancer 2022, 30, 9369–9377. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Pineros, M.; Van Eycken, E.; Weir, H.K.; Gospodarowicz, M. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Yoshida, K.; Hirotsu, T.; Tagawa, T.; Oda, S.; Wakabayashi, T.; Iino, Y.; Ishihara, T. Odour concentration-dependent olfactory preference change in C. elegans. Nat. Commun. 2012, 3, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, E.; Wittig, A.; Basilico, F.; Rossi, R.; De Palma, A.; Di Silvestre, D.; Sauerwein, W.A.G.; Mauri, P.L. Urinary Proteomics Profiles Are Useful for Detection of Cancer Biomarkers and Changes Induced by Therapeutic Procedures. Molecules 2019, 24, 794. [Google Scholar] [CrossRef] [Green Version]
- Massihnia, D.; Pizzutilo, E.G.; Amatu, A.; Tosi, F.; Ghezzi, S.; Bencardino, K.; Di Masi, P.; Righetti, E.; Patelli, G.; Scaglione, F.; et al. Liquid biopsy for rectal cancer: A systematic review. Cancer Treat. Rev. 2019, 79, 101893. [Google Scholar] [CrossRef]
- Lukaszewicz-Zajac, M.; Mroczko, B.; Kozlowski, M.; Niklinski, J.; Laudanski, J.; Szmitkowski, M. Higher importance of interleukin 6 than classic tumor markers (carcinoembryonic antigen and squamous cell cancer antigen) in the diagnosis of esophageal cancer patients. Dis. Esophagus 2012, 25, 242–249. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Number of Patients (n = 30) |
|---|---|
| Age (median, range) | 72 (61–82) |
| Sex (Male/Female) | 24/6 |
| clinical T stage (2/3/4b) | 2/16/12 |
| clinical N stage (0/1/2/3) | 2/17/7/4 |
| clinical M stage (0/1) | 28/2 |
| Stage (II /III/IVa/IVb) | 3/12/10/5 |
| CR/PR/SD/PD | 3/20/6/1 |
| CR or PR (n = 23) | SD or PD (n = 7) | p-Value | |
|---|---|---|---|
| IRSI | 0.003 | −0.088 | 0.288 |
| [−0.055, 0.091] | [−0.101, −0.061] | ||
| IRS2 | −0.003 | −0.039 | 0.598 |
| [−0.044, 0.071] | [−0.158, 0.107] | ||
| IRS3 | 0.033 | −0.027 | 0.107 |
| [−0.026, 0.088] | [−0.114, −0.02] |
| CR | CR or PR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | 95%CI | Sensitivity | Specificity | Cutoff Point | AUC | 95%CI | Sensitivity | Specificity | Cutoff Point | |
| IRS 1 | 0.78 | 0.46–1 | 0.67 | 0.85 | 0.1 | 0.64 | 0.36–0.91 | 0.83 | 0.57 | −0.08 |
| IRS 2 | 0.51 | 0.06–0.96 | 0.67 | 0.67 | −0.04 | 0.57 | 0.28–0.86 | 0.57 | 0.71 | −0.01 |
| IRS 3 | 0.85 | 0.62–1 | 1 | 0.63 | 0.03 | 0.7 | 0.49–0.92 | 0.61 | 0.86 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Futamura, M.; Tanaka, Y.; Tsuchiya, H.; Fukada, M.; Higashi, T.; Yasufuku, I.; Asai, R.; Tajima, J.Y.; Kiyama, S.; et al. Clinical Possibility of Caenorhabditis elegans as a Novel Evaluation Tool for Esophageal Cancer Patients Receiving Chemotherapy: A Prospective Study. Cancers 2023, 15, 3870. https://doi.org/10.3390/cancers15153870
Sato Y, Futamura M, Tanaka Y, Tsuchiya H, Fukada M, Higashi T, Yasufuku I, Asai R, Tajima JY, Kiyama S, et al. Clinical Possibility of Caenorhabditis elegans as a Novel Evaluation Tool for Esophageal Cancer Patients Receiving Chemotherapy: A Prospective Study. Cancers. 2023; 15(15):3870. https://doi.org/10.3390/cancers15153870
Chicago/Turabian StyleSato, Yuta, Manabu Futamura, Yoshihiro Tanaka, Hiroshi Tsuchiya, Masahiro Fukada, Toshiya Higashi, Itaru Yasufuku, Ryuichi Asai, Jesse Yu Tajima, Shigeru Kiyama, and et al. 2023. "Clinical Possibility of Caenorhabditis elegans as a Novel Evaluation Tool for Esophageal Cancer Patients Receiving Chemotherapy: A Prospective Study" Cancers 15, no. 15: 3870. https://doi.org/10.3390/cancers15153870
APA StyleSato, Y., Futamura, M., Tanaka, Y., Tsuchiya, H., Fukada, M., Higashi, T., Yasufuku, I., Asai, R., Tajima, J. Y., Kiyama, S., Hatakeyama, H., Morishita, M., Hirotsu, T., Luccio, E. d., Ishihara, T., Matsuhashi, N., & Yoshida, K. (2023). Clinical Possibility of Caenorhabditis elegans as a Novel Evaluation Tool for Esophageal Cancer Patients Receiving Chemotherapy: A Prospective Study. Cancers, 15(15), 3870. https://doi.org/10.3390/cancers15153870








