Preoperative Radiotherapy with a Simultaneous Integrated Boost Compared to Chemoradiotherapy for cT3-4 Rectal Cancer: Long-Term Results of a Multicenter Randomized Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Pre-Reatment Evaluation
2.3. Random Assignment and Treatment
2.4. Surgical Procedure and Pathological Evaluation
2.5. Adjuvant Chemotherapy
2.6. Toxicity Monitoring and Follow-Up
2.7. Endpoints
2.8. Quality Assurance
2.9. Statistical Analysis
3. Results
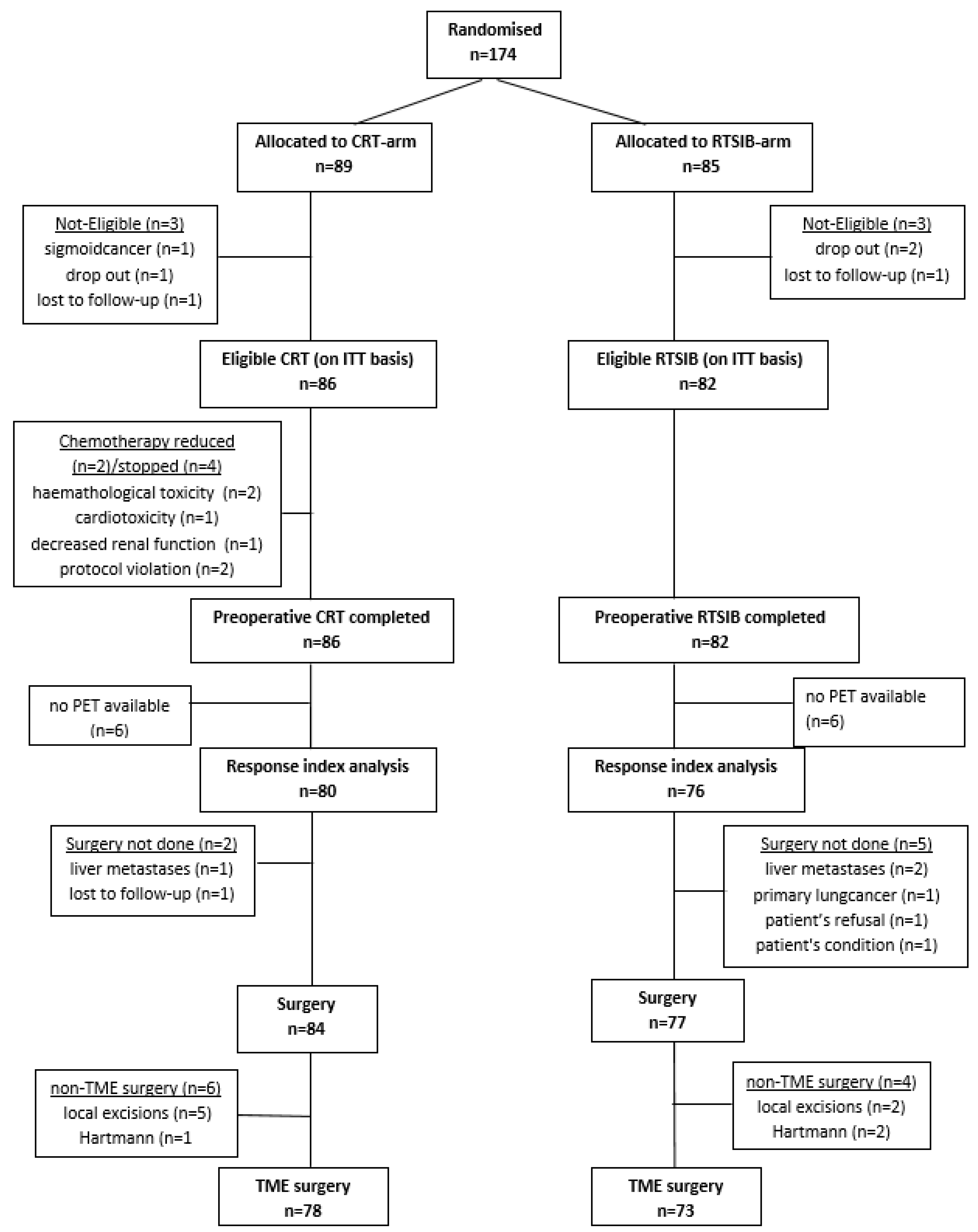
3.1. Patient Population
3.2. Treatment Compliance and Acute Toxicity
3.3. Surgery
3.4. Primary Endpoint
3.5. Secondary Endpoints
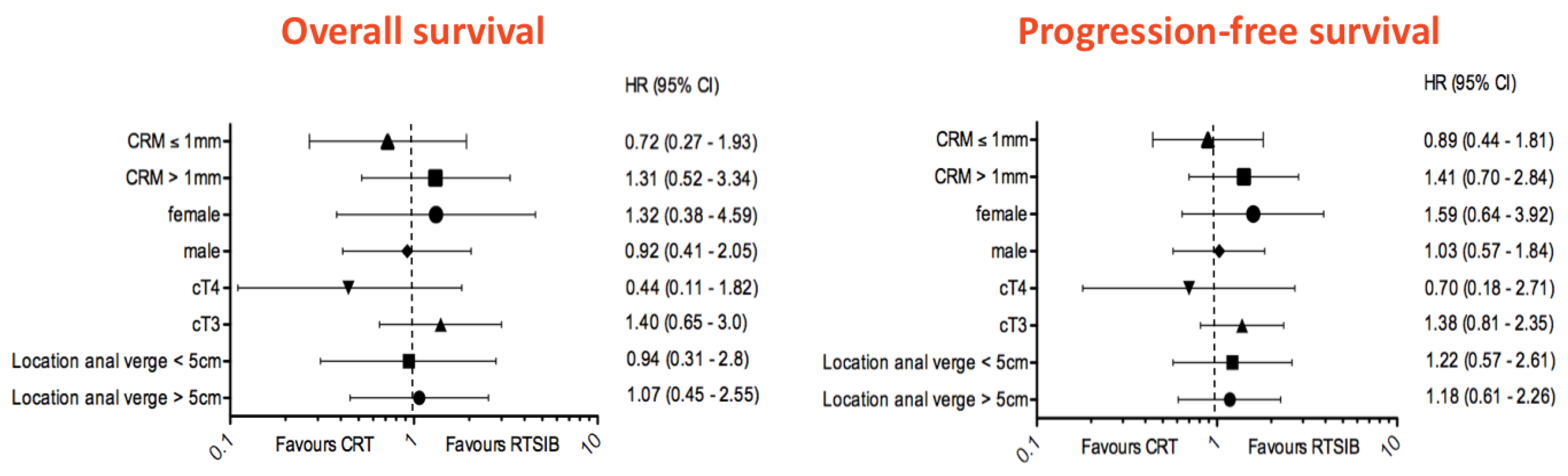
3.6. Survival Data and Late Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bosset, J.F.; Collette, L.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Ollier, J.C. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006, 355, 1114–1123. [Google Scholar] [CrossRef]
- Gérard, J.P.; Conroy, T.; Bonnetain, F.; Bouché, O.; Chapet, O.; Closon-Dejardin, M.T.; Untereiner, M.; Leduc, B.; Francois, E.; Maurel, J.; et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J. Clin. Oncol. 2006, 24, 4620–4625. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- De Ridder, M.; Tournel, K.; Van Nieuwenhove, Y.; Engels, B.; Hoorens, A.; Everaert, H.; Op de Beeck, B.; Vinh-Hung, V.; De Grève, J.; Delvaux, G.; et al. Phase II study of preoperative helical tomotherapy for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 728–734. [Google Scholar] [CrossRef]
- Engels, B.; Tournel, K.; Everaert, H.; Hoorens, A.; Sermeus, A.; Christian, N.; Storme, G.; Verellen, D.; De Ridder, M. Phase II study of preoperative helical tomotherapy with a simultaneous integrated boost for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 142–148. [Google Scholar] [CrossRef]
- Engels, B.; Platteaux, N.; Van den Begin, R.; Gevaert, T.; Sermeus, A.; Storme, G.; Verellen, D.; De Ridder, M. Preoperative intensity-modulated and image-guided radiotherapy with a simultaneous integrated boost in locally advanced rectal cancer: Report on late toxicity and outcome. Radiother. Oncol. 2014, 110, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Engels, B.; De Ridder, M.; Tournel, K.; Sermeus, A.; De Coninck, P.; Verellen, D.; Storme, G.A. Preoperative helical tomotherapy and megavoltage computed tomography for rectal cancer: Impact on the irradiated volume of small bowel. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: Results of the MERCURY study. Radiology 2007, 243, 132–139. [CrossRef] [PubMed]
- Tournel, K.; De Ridder, M.; Engels, B.; Bijdekerke, P.; Fierens, Y.; Duchateau, M.; Linthout, N.; Reynders, T.; Verellen, D.; Storme, G. Assessment of intrafractional movement and internal motion in radiotherapy of rectal cancer using megavoltage computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 934–939. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; van Krieken, J.H. The role of pathologists in the quality control of diagnosis and treatment of rectal cancer-an overview. Eur. J. Cancer 2002, 38, 964–972. [Google Scholar] [CrossRef]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Colorectal Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Zijdenbos, A.P.; Dawant, B.M.; Margolin, R.A.; Palmer, A.C. Morphometric analysis of white matter lesions in MR images: Method and validation. IEEE Trans. Med. Imaging 1994, 13, 716–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepper, J.E.; Wang, A.Z. Improving local control in rectal cancer: Radiation sensitizers or radiation dose? J. Clin. Oncol. 2010, 28, 1623–1624. [Google Scholar] [CrossRef]
- Gérard, J.P.; Azria, D.; Gourgou-Bourgade, S.; Martel-Laffay, I.; Hennequin, C.; Etienne, P.L.; Vendrely, V.; François, E.; de La Roche, G.; Bouché, O.; et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J. Clin. Oncol. 2010, 28, 1638–1644. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Colangelo, L.H.; Beart, R.W.; Petrelli, N.J.; Allegra, C.J.; Sharif, S.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: Surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J. Clin. Oncol. 2014, 32, 1927–1934. [Google Scholar] [CrossRef] [Green Version]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.J.; Stein, A.; Van Cutsem, E.; Price, T.; Hofheinz, R.D.; Nordlinger, B.; Daisne, J.F.; Janssens, J.; Brenner, B.; Reinel, H.; et al. Pre- and Postoperative Capecitabine Without or With Oxaliplatin in Locally Advanced Rectal Cancer: PETACC 6 Trial by EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD. J. Clin. Oncol. 2021, 39, 17–29. [Google Scholar] [CrossRef]
- Valentini, V.; Gambacorta, M.A.; Cellini, F.; Aristei, C.; Coco, C.; Barbaro, B.; Alfieri, S.; D’Ugo, D.; Persiani, R.; Deodato, F.; et al. The INTERACT Trial: Long-term results of a randomised trial on preoperative capecitabine-based radiochemotherapy intensified by concomitant boost or oxaliplatin, for cT2 (distal)-cT3 rectal cancer. Radiother. Oncol. 2019, 134, 110–118. [Google Scholar] [CrossRef]
- Appelt, A.L.; Pløen, J.; Vogelius, I.R.; Bentzen, S.M.; Jakobsen, A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Burbach, J.P.; den Harder, A.M.; Intven, M.; van Vulpen, M.; Verkooijen, H.M.; Reerink, O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: A systematic review and meta-analysis. Radiother. Oncol. 2014, 113, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Everaert, H.; Hoorens, A.; Vanhove, C.; Sermeus, A.; Ceulemans, G.; Engels, B.; Vermeersch, M.; Verellen, D.; Urbain, D.; Storme, G.; et al. Prediction of response to neoadjuvant radiotherapy in patients with locally advanced rectal cancer by means of sequential 18FDG-PET. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 91–96. [Google Scholar] [CrossRef]
- Capirci, C.; Rubello, D.; Pasini, F.; Galeotti, F.; Bianchini, E.; Del Favero, G.; Panzavolta, R.; Crepaldi, G.; Rampin, L.; Facci, E.; et al. The role of dual-time combined 18-fluorodeoxyglucose positron emission tomography and computed tomography in the staging and restaging workup of locally advanced rectal cancer, treated with preoperative chemoradiation therapy and radical surgery. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Glynne-Jones, R.; Appelt, A.; Beets-Tan, R.; Beets, G.; Haustermans, K.; Marijnen, C.; Minsky, B.D.; Ludmir, E.; Quirke, P.; et al. Outcome measures in multimodal rectal cancer trials. Lancet Oncol. 2020, 21, e252–e264. [Google Scholar] [CrossRef] [PubMed]
- Aschele, C.; Cionini, L.; Lonardi, S.; Pinto, C.; Cordio, S.; Rosati, G.; Artale, S.; Tagliagambe, A.; Ambrosini, G.; Rosetti, P.; et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J. Clin. Oncol. 2011, 29, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Couwenberg, A.M.; Burbach, J.P.M.; Berbee, M.; Lacle, M.M.; Arensman, R.; Raicu, M.G.; Wessels, F.J.; Verdult, J.; Roodhart, J.; Reerink, O.; et al. Efficacy of Dose-Escalated Chemoradiation on Complete Tumor Response in Patients with Locally Advanced Rectal Cancer (RECTAL-BOOST): A Phase 2 Randomized Controlled Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1008–1018. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, F.; Gu, W.; Lian, P.; Sheng, W.; Xu, J.; Cai, G.; Shi, D.; Cai, S.; Zhang, Z. Concomitant boost IMRT-based neoadjuvant chemoradiotherapy for clinical stage II/III rectal adenocarcinoma: Results of a phase II study. Radiat. Oncol. 2014, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Picardi, V.; Macchia, G.; Guido, A.; Giaccherini, L.; Deodato, F.; Farioli, A.; Cilla, S.; Compagnone, G.; Ardizzoni, A.; Cuicchi, D.; et al. Preoperative Chemoradiation With VMAT-SIB in Rectal Cancer: A Phase II Study. Clin. Colorectal Cancer 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Li, J.L.; Ji, J.F.; Cai, Y.; Li, X.F.; Li, Y.H.; Wu, H.; Xu, B.; Dou, F.Y.; Li, Z.Y.; Bu, Z.D.; et al. Preoperative concomitant boost intensity-modulated radiotherapy with oral capecitabine in locally advanced mid-low rectal cancer: A phase II trial. Radiother. Oncol. 2012, 102, 4–9. [Google Scholar] [CrossRef]
- Lupattelli, M.; Matrone, F.; Gambacorta, M.A.; Osti, M.; Macchia, G.; Palazzari, E.; Nicosia, L.; Navarria, F.; Chiloiro, G.; Valentini, V.; et al. Preoperative intensity-modulated radiotherapy with a simultaneous integrated boost combined with Capecitabine in locally advanced rectal cancer: Short-term results of a multicentric study. Radiat. Oncol. 2017, 12, 139. [Google Scholar] [CrossRef]
- Braendengen, M.; Tveit, K.M.; Berglund, A.; Birkemeyer, E.; Frykholm, G.; Påhlman, L.; Wiig, J.N.; Byström, P.; Bujko, K.; Glimelius, B. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J. Clin. Oncol. 2008, 26, 3687–3694. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Liersch, T.; Becker, H.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Graeven, U.; Arnold, D.; Lang-Welzenbach, M.; Raab, H.R.; et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012, 13, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Samuelian, J.M.; Callister, M.D.; Ashman, J.B.; Young-Fadok, T.M.; Borad, M.J.; Gunderson, L.L. Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.S.; Moughan, J.; Garofalo, M.C.; Bendell, J.; Berger, A.C.; Oldenburg, N.B.; Anne, P.R.; Perera, F.; Lee, R.J.; Jabbour, S.K.; et al. NRG Oncology Radiation Therapy Oncology Group 0822: A Phase 2 Study of Preoperative Chemoradiation Therapy Using Intensity Modulated Radiation Therapy in Combination With Capecitabine and Oxaliplatin for Patients With Locally Advanced Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.J.; Winter, K.; Meropol, N.J.; Anne, P.R.; Kachnic, L.; Rashid, A.; Watson, J.C.; Mitchell, E.; Pollock, J.; Lee, R.J.; et al. Radiation Therapy Oncology Group 0247: A randomized Phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1367–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef]

| CRT (n = 86) | RTSIB (n = 82) | p-Value | |||
|---|---|---|---|---|---|
| Characteristics | No. of Patients | % | No. of Patients | % | |
| Age, years Average Range | 66 48–83 | 65 37–87 | 0.67 | ||
| Sex | 0.63 | ||||
| Male | 55 | 64 | 56 | 68 | |
| Female | 31 | 36 | 26 | 32 | |
| ECOG Performance state | 0.27 | ||||
| 0 | 73 | 85 | 68 | 83 | |
| ≥1 | 13 | 15 | 14 | 17 | |
| Distance from anal verge | 0.85 | ||||
| <5 cm | 40 | 46 | 34 | 41 | |
| 5–10 cm | 42 | 49 | 44 | 54 | |
| >10 cm | 4 | 5 | 4 | 5 | |
| T stage | 0.35 | ||||
| T2 | 2 | 2 | 0 | 0 | |
| T3—CRM > 1 mm | 48 | 56 | 45 | 55 | |
| T3—CRM ≤ 1 mm | 31 | 36 | 28 | 34 | |
| T4 | 5 | 6 | 9 | 11 | |
| N stage | 0.16 | ||||
| node-negative | 9 | 10 | 15 | 18 | |
| node-positive | 77 | 90 | 67 | 82 | |
| Tumor biopsy | 0.93 | ||||
| Grade 1 adenocarcinoma | 8 | 9 | 7 | 8 | |
| Grade 2 adenocarcinoma | 36 | 42 | 39 | 48 | |
| Grade 3 adenocarcinoma | 4 | 5 | 4 | 5 | |
| Grade not stated | 38 | 44 | 32 | 39 | |
| M1 | 0 | 0 | 2 | 2 | 0.15 |
| CRT (n = 86) | RTSIB (n = 82) | p-Value | ||||
|---|---|---|---|---|---|---|
| Grade | 2 | 3 | 2 | 3 | ||
| No. of patients | No. of patients | No. of patients | No. of patients | |||
| Gastrointestinal | 36 | 1 | 35 | 2 | 0.91 | (0.53) |
| Diarrhea | 17 | 0 | 17 | 1 | 0.88 | (0.30) |
| Enteritis (abdominal pain) | 18 | 0 | 10 | 0 | 0.13 | - |
| Proctitis | 13 | 1 | 18 | 1 | 0.25 | (0.97) |
| Urinary | 7 | 0 | 11 | 0 | 0.27 | - |
| Dysuria | 4 | 0 | 10 | 0 | 0.08 | - |
| Urinary frequency | 5 | 0 | 4 | 0 | 0.79 | - |
| Hematology | 3 | 1 | 2 | 0 | 0.69 | (0.33) |
| Anemia | 0 | 1 | 1 | 0 | 0.30 | (0.33) |
| Leucopenia | 3 | 1 | 0 | 0 | 0.09 | (0.33) |
| Thrombopenia | 0 | 0 | 1 | 0 | - | (0.30) |
| Other | ||||||
| Hand–foot syndrome * | 0 | 0 | 0 | 0 | - | - |
| Radiation dermatitis | 14 | 2 | 10 | 1 | 0.45 | (0.59) |
| Vaginal mucositis | 2 | 2 | 1 | 0 | 0.59 | (0.16) |
| Overall toxicity score | 43 | 5 | 46 | 3 | 0.43 | (0.51) |
| CRT (n = 84) | RTSIB (n = 77) | p-Value | |||
|---|---|---|---|---|---|
| Characteristics | No. of Patients | % | No. of Patients | % | |
| Surgery | 0.55 | ||||
| Abdominoperineal resection | 20 | 24 | 23 | 30 | |
| Anterior resection | 58 | 69 | 50 | 66 | |
| Hartmann’s resection | 1 | 1 | 2 | 2 | |
| Local excision | 5 | 6 | 2 | 2 | |
| Colostoma | 0.45 | ||||
| Permanent | 21 | 25 | 25 | 32 | |
| Protective | 55 | 65 | 43 | 56 | |
| None | 8 | 10 | 9 | 12 | |
| Leakage anastomosis | 0.37 | ||||
| Not present | 74 | 87 | 69 | 90 | |
| <30 days post surgery | 7 | 12 | 8 | 10 | |
| >30 days post surgery | 4 | 7 | 0 | 0 | |
| Complications | 22 | 26 | 14 | 18 | 0.22 |
| Pulmonary | 3 | 4 | 2 | 3 | |
| Urinary | 3 | 4 | 3 | 4 | |
| Fistula | 4 | 5 | 1 | 1 | |
| Stoma (prolapse/ necrosis) | 2 | 2 | 3 | 4 | |
| Abdominal wall hernia | 2 | 2 | 1 | 1 | |
| Perineal wound infection | 2 | 2 | 1 | 1 | |
| Ileus | 1 | 1 | 3 | 4 | |
| Peritonitis | 1 | 1 | 1 | 1 | |
| Heart failure | 1 | 1 | 0 | 0 | |
| Duration of hospital stay (in days) | |||||
| Median | 11 | 12 | |||
| Range | 4–77 | 4–43 | |||
| Post-operative mortality (<60 days) | 2 | 2 | 1 | 1 | 0.61 |
| CRT (n = 86) | RTSIB (n = 82) | p-Value | |||
|---|---|---|---|---|---|
| Characteristics | No. of Patients | % | No. of Patients | % | |
| Resection status | 0.93 | ||||
| R0 | 82 | 98 | 75 | 97 | |
| R1 | 2 | 2 | 2 | 3 | |
| R2 | 0 | 0 | 0 | 0 | |
| Dworak regression | 0.16 | ||||
| Grade 0 | 1 | 1 | 0 | 0 | |
| Grade 1 | 25 | 30 | 17 | 22 | |
| Grade 2 | 17 | 20 | 25 | 33 | |
| Grade 3 | 21 | 25 | 24 | 31 | |
| Grade 4 (pCR) | 20 | 24 | 11 | 14 | |
| Pathological stage | 0.07 | ||||
| ypT0 | 20 | 24 | 11 | 14 | |
| ypTis | 0 | 0 | 1 | 1 | |
| ypT1 | 7 | 8 | 4 | 5 | |
| ypT2 | 16 | 19 | 30 | 39 | |
| ypT3 | 37 | 44 | 29 | 38 | |
| ypT4 | 4 | 5 | 2 | 3 | |
| Number of resected lymph nodes | |||||
| Median | 12 | 11 | |||
| Range | 2–25 | 1–55 | |||
| Nodal stage | 0.63 | ||||
| ypN0 | 62 | 74 | 55 | 71 | |
| ypN1 | 11 | 13 | 12 | 16 | |
| ypN2 | 6 | 7 | 8 | 10 | |
| No lymph node dissection | 5 | 6 | 2 | 3 | |
| CRT (n = 84) | RTSIB (n = 77) | |||
|---|---|---|---|---|
| Grade | 3 | 4–5 | 3 | 4–5 |
| No. of patients | No. of patients | No. of patients | No. of patients | |
| Gastrointestinal | 4 | 2 | 3 | 1 |
| Small bowel obstruction | 2 | 1 | 0 | 1 |
| Stricture anastomosis | 1 | 0 | 1 | 0 |
| Anal incontinence | 0 | 1 | 2 | 0 |
| Other | 1 | 0 | 1 | 0 |
| Urinary | 3 | 1 | 2 | 1 |
| Urinary incontinence | 2 | 0 | 0 | 0 |
| Retention | 1 | 0 | 1 | 0 |
| Fistula | 0 | 1 | 0 | 1 |
| Other | 0 | 0 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engels, B.; De Paoli, A.; Delmastro, E.; Munoz, F.; Vagge, S.; Norkus, D.; Everaert, H.; Tabaro, G.; Gariboldi, E.; Ricardi, U.; et al. Preoperative Radiotherapy with a Simultaneous Integrated Boost Compared to Chemoradiotherapy for cT3-4 Rectal Cancer: Long-Term Results of a Multicenter Randomized Study. Cancers 2023, 15, 3869. https://doi.org/10.3390/cancers15153869
Engels B, De Paoli A, Delmastro E, Munoz F, Vagge S, Norkus D, Everaert H, Tabaro G, Gariboldi E, Ricardi U, et al. Preoperative Radiotherapy with a Simultaneous Integrated Boost Compared to Chemoradiotherapy for cT3-4 Rectal Cancer: Long-Term Results of a Multicenter Randomized Study. Cancers. 2023; 15(15):3869. https://doi.org/10.3390/cancers15153869
Chicago/Turabian StyleEngels, Benedikt, Antonino De Paoli, Elena Delmastro, Fernando Munoz, Stefano Vagge, Darius Norkus, Hendrik Everaert, Gianna Tabaro, Elisabetta Gariboldi, Umberto Ricardi, and et al. 2023. "Preoperative Radiotherapy with a Simultaneous Integrated Boost Compared to Chemoradiotherapy for cT3-4 Rectal Cancer: Long-Term Results of a Multicenter Randomized Study" Cancers 15, no. 15: 3869. https://doi.org/10.3390/cancers15153869
APA StyleEngels, B., De Paoli, A., Delmastro, E., Munoz, F., Vagge, S., Norkus, D., Everaert, H., Tabaro, G., Gariboldi, E., Ricardi, U., Borsatti, E., Gabriele, P., Innocente, R., Palazzari, E., Dubaere, E., Mahé, M.-A., Van Laere, S., Gevaert, T., & De Ridder, M. (2023). Preoperative Radiotherapy with a Simultaneous Integrated Boost Compared to Chemoradiotherapy for cT3-4 Rectal Cancer: Long-Term Results of a Multicenter Randomized Study. Cancers, 15(15), 3869. https://doi.org/10.3390/cancers15153869










