Extraintestinal Cancers in Inflammatory Bowel Disease: A Literature Review
Abstract
:Simple Summary
Abstract
1. Introduction
- The association between IBD and EICs.
- The safety of immunomodulators and biological drugs, considering their possible association with the risk of developing EICs.
- Therapy management with immunomodulators and biologic agents in patients with IBD and prior or current EICs.
2. Materials and Methods
- Articles that estimated the association between IBD and EICs.
- Articles that evaluated the safety of immunomodulators and biological drugs.
- Articles that evaluated the therapy management with immunomodulators and biological drugs in patients with IBD and prior or current diagnosis of EICs.
3. Results
3.1. Association between IBD and EICs
3.1.1. Solid-Organ Tumor
3.1.2. Hematological Malignancies
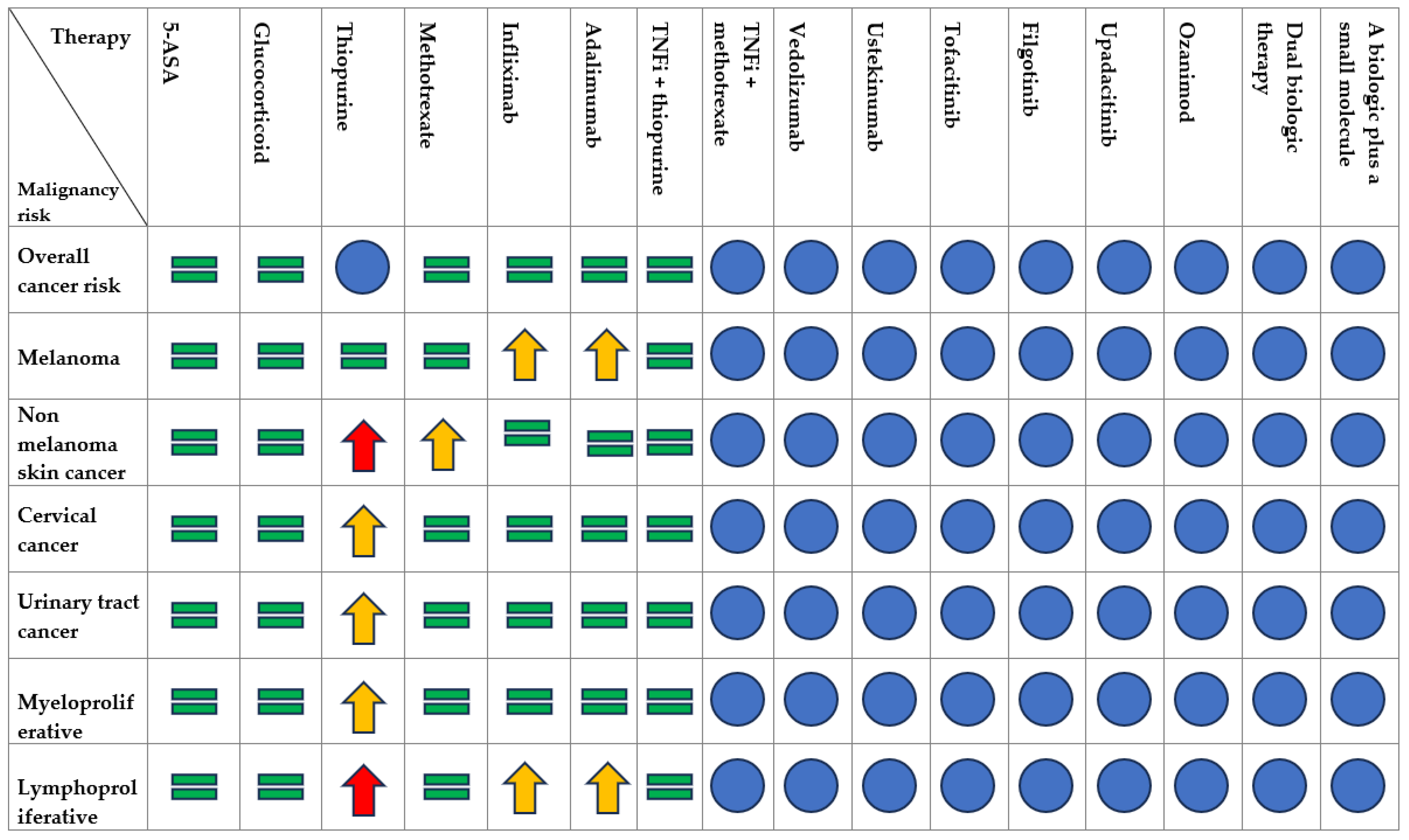
3.2. Safety of Immunomodulators and Biologic Agents
3.2.1. Thiopurines
3.2.2. Methotrexate
3.2.3. TNFα Inhibitors
3.2.4. Combination Therapy of TNFi with Immunomodulators
3.2.5. Anti-Integrins
3.2.6. Anti-IL12/IL23
3.2.7. JAK Inhibitors
3.2.8. SP1-Receptor Modulators
3.2.9. Dual-Targeted Therapy (DTT)
3.3. Therapy Management with Immunomodulators and Biologic Agents in Patients with IBD and Prior or Current History of EICs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current State of the Art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory Bowel Disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Actis, G.C.; Pellicano, R.; Fagoonee, S.; Ribaldone, D.G. History f Inflammatory Bowel Diseases. J. Clin. Med. 2019, 8, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barberio, B.; Massimi, D.; Cazzagon, N.; Zingone, F.; Ford, A.C.; Savarino, E.V. Prevalence of Primary Sclerosing Cholangitis in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2021, 161, 1865–1877. [Google Scholar] [CrossRef]
- Giordani, A.S.; Candelora, A.; Fiacca, M.; Cheng, C.; Barberio, B.; Baritussio, A.; Marcolongo, R.; Iliceto, S.; Carturan, E.; De Gaspari, M.; et al. Myocarditis and Inflammatory Bowel Diseases: A Single-Center Experience and a Systematic Literature Review. Int. J. Cardiol. 2023, 376, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.; Lichtiger, S.; Yajnik, V. Inflammatory Bowel Disease and Cancer: The Role of Inflammation, Immunosuppression, and Cancer Treatment. World J. Gastrointest. Oncol. 2016, 22, 4794–4801. [Google Scholar] [CrossRef] [PubMed]
- Marabotto, E.; Kayali, S.; Buccilli, S.; Levo, F.; Bodini, G.; Giannini, E.G.; Savarino, V.; Savarino, E.V. Colorectal Cancer in Inflammatory Bowel Diseases: Epidemiology and Prevention: A Review. Cancers 2022, 14, 4254. [Google Scholar] [CrossRef]
- Festa, S.; Zerboni, G.; Derikx, L.A.A.P.; Ribaldone, D.G.; Dragoni, G.; Buskens, C.; van Dijkum, E.N.; Pugliese, D.; Panzuto, F.; Krela-Kaźmierczak, I.; et al. Gastroenteropancreatic Neuroendocrine Neoplasms in Patients with Inflammatory Bowel Disease: An ECCO CONFER Multicentre Case Series. J. Crohns Colitis 2022, 16, 940–945. [Google Scholar] [CrossRef]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Al Sulais, E.; Axelrad, J.E.; Balendran, K.; Burisch, J.; de Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohns Colitis 2022, 17, 827–854. [Google Scholar] [CrossRef]
- Garg, S.K.; Loftus, E.V. Risk of Cancer in Inflammatory Bowel Disease: Going up, Going down, or Still the Same? Curr. Opin. Gastroenterol. 2016, 32, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Vavricka, S.; König, A.O.; Beaugerie, L.; Scharl, M.; Swiss IBDnet, An Official Working Group of the Swiss Society of Gastroenterology. Malignancies in Inflammatory Bowel Disease. Digestion 2020, 101 (Suppl. S1), 136–145. [Google Scholar] [CrossRef] [PubMed]
- Laredo, V.; García-Mateo, S.; Martínez-Domínguez, S.J.; López de la Cruz, J.; Gargallo-Puyuelo, C.J.; Gomollón, F. Risk of Cancer in Patients with Inflammatory Bowel Diseases and Keys for Patient Management. Cancers 2023, 15, 871. [Google Scholar] [CrossRef] [PubMed]
- Mala, A.; Foteinogiannopoulou, K.; Koutroubakis, I.E. Solid Extraintestinal Malignancies in Patients with Inflammatory Bowel Disease. World J. Gastrointest. Oncol. 2021, 13, 1956–1980. [Google Scholar] [CrossRef]
- Pedersen, N.; Duricova, D.; Elkjaer, M.; Gamborg, M.; Munkholm, P.; Jess, T. Risk of Extra-Intestinal Cancer in Inflammatory Bowel Disease: Meta-Analysis of Population-Based Cohort Studies. Am. J. Gastroenterol. 2010, 105, 1480–1487. [Google Scholar] [CrossRef]
- Lo, B.; Zhao, M.; Vind, I.; Burisch, J. The Risk of Extraintestinal Cancer in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Population-Based Cohort Studies. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, 19, 1117–1138.e19. [Google Scholar] [CrossRef]
- Ekbom, A.; Helmick, C.; Zack, M.; Adami, H.O. Extracolonic Malignancies in Inflammatory Bowel Disease. Cancer 1991, 67, 2015–2019. [Google Scholar] [CrossRef]
- Persson, P.G.; Karlén, P.; Bernell, O.; Leijonmarck, C.E.; Broström, O.; Ahlbom, A.; Hellers, G. Crohn’s Disease and Cancer: A Population-Based Cohort Study. Gastroenterology 1994, 107, 1675–1679. [Google Scholar] [CrossRef]
- Mellemkjaer, L.; Olsen, J.H.; Frisch, M.; Johansen, C.; Gridley, G.; McLaughlin, J.K. Cancer in Patients with Ulcerative Colitis. Int. J. Cancer 1995, 60, 330–333. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Kliewer, E.; Wajda, A. Cancer Risk in Patients with Inflammatory Bowel Disease: A Population-Based Study. Cancer 2001, 91, 854–862. [Google Scholar] [CrossRef]
- Jess, T.; Winther, K.V.; Munkholm, P.; Langholz, E.; Binder, V. Intestinal and Extra-Intestinal Cancer in Crohn’s Disease: Follow-up of a Population-Based Cohort in Copenhagen County, Denmark. Aliment. Pharmacol. Ther. 2004, 19, 287–293. [Google Scholar] [CrossRef]
- Bhatia, J.; Bratcher, J.; Korelitz, B.; Vakher, K.; Mannor, S.; Shevchuk, M.; Panagopoulos, G.; Ofer, A.; Tamas, E.; Kotsali, P.; et al. Abnormalities of Uterine Cervix in Women with Inflammatory Bowel Disease. World J. Gastroenterol. WJG 2006, 12, 6167–6171. [Google Scholar] [CrossRef]
- Hemminki, K.; Li, X.; Sundquist, J.; Sundquist, K. Cancer Risks in Ulcerative Colitis Patients. Int. J. Cancer 2008, 123, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Li, X.; Sundquist, J.; Sundquist, K. Cancer Risks in Crohn Disease Patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009, 20, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Demers, A.A.; Nugent, Z.; Mahmud, S.M.; Kliewer, E.V.; Bernstein, C.N. Risk of Cervical Abnormalities in Women with Inflammatory Bowel Disease: A Population-Based Nested Case-Control Study. Gastroenterology 2009, 136, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, R.; Jepsen, P.; Vilstrup, H.; Ekbom, A.; Sørensen, H.T. Incidence and Prognosis of Cholangiocarcinoma in Danish Patients with and without Inflammatory Bowel Disease: A National Cohort Study, 1978–2003. Eur. J. Epidemiol. 2009, 24, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.W.; Critchley, J.; Chee, N.; Beez, T.; Gailer, R.E.; Williams, A.R.; Shand, A.G.; Arnott, I.D.R.; Satsangi, J. Lack of Association between Cervical Dysplasia and IBD: A Large Case-Control Study. Inflamm. Bowel Dis. 2009, 15, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Long, M.D.; Martin, C.F.; Pipkin, C.A.; Herfarth, H.H.; Sandler, R.S.; Kappelman, M.D. Risk of Melanoma and Nonmelanoma Skin Cancer among Patients with Inflammatory Bowel Disease. Gastroenterology 2012, 143, 390–399.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jussila, A.; Virta, L.J.; Pukkala, E.; Färkkilä, M.A. Malignancies in Patients with Inflammatory Bowel Disease: A Nationwide Register Study in Finland. Scand. J. Gastroenterol. 2013, 48, 1405–1413. [Google Scholar] [CrossRef]
- Jess, T.; Horváth-Puhó, E.; Fallingborg, J.; Rasmussen, H.H.; Jacobsen, B.A. Cancer Risk in Inflammatory Bowel Disease According to Patient Phenotype and Treatment: A Danish Population-Based Cohort Study. Am. J. Gastroenterol. 2013, 108, 1869–1876. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Farkas, D.K.; Long, M.D.; Erichsen, R.; Sandler, R.S.; Sørensen, H.T.; Baron, J.A. Risk of Cancer in Patients with Inflammatory Bowel Diseases: A Nationwide Population-Based Cohort Study with 30 Years of Follow-up Evaluation. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2014, 12, 265–273.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rungoe, C.; Simonsen, J.; Riis, L.; Frisch, M.; Langholz, E.; Jess, T. Inflammatory Bowel Disease and Cervical Neoplasia: A Population-Based Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2015, 13, 693–700.e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Glynn, R.J.; Giovannucci, E.; Hernández-Díaz, S.; Liu, J.; Feldman, S.; Karlson, E.W.; Schneeweiss, S.; Solomon, D.H. Risk of High-Grade Cervical Dysplasia and Cervical Cancer in Women with Systemic Inflammatory Diseases: A Population-Based Cohort Study. Ann. Rheum. Dis. 2015, 74, 1360–1367. [Google Scholar] [CrossRef]
- Van den Heuvel, T.R.A.; Wintjens, D.S.J.; Jeuring, S.F.G.; Wassink, M.H.H.; Romberg-Camps, M.J.L.; Oostenbrug, L.E.; Sanduleanu, S.; Hameeteman, W.H.; Zeegers, M.P.; Masclee, A.A.; et al. Inflammatory Bowel Disease, Cancer and Medication: Cancer Risk in the Dutch Population-Based IBDSL Cohort. Int. J. Cancer 2016, 139, 1270–1280. [Google Scholar] [CrossRef]
- Wilson, J.C.; Furlano, R.I.; Jick, S.S.; Meier, C.R. A Population-Based Study Examining the Risk of Malignancy in Patients Diagnosed with Inflammatory Bowel Disease. J. Gastroenterol. 2016, 51, 1050–1062. [Google Scholar] [CrossRef]
- Madanchi, M.; Zeitz, J.; Barthel, C.; Samaras, P.; Scharl, S.; Sulz, M.C.; Biedermann, L.; Frei, P.; Vavricka, S.R.; Rogler, G.; et al. Malignancies in Patients with Inflammatory Bowel Disease: A Single-Centre Experience. Digestion 2016, 94, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wadhwa, V.; Lopez, R.; Shen, B. Crohn’s Disease Is Associated with the Risk for Thyroid Cancer. Inflamm. Bowel Dis. 2016, 22, 2902–2906. [Google Scholar] [CrossRef] [PubMed]
- Hovde, Ø.; Høivik, M.L.; Henriksen, M.; Solberg, I.C.; Småstuen, M.C.; Moum, B.A. Malignancies in Patients with Inflammatory Bowel Disease: Results from 20 Years of Follow-up in the IBSEN Study. J. Crohns Colitis 2017, 11, 571–577. [Google Scholar] [CrossRef]
- So, J.; Tang, W.; Leung, W.K.; Li, M.; Lo, F.H.; Wong, M.T.L.; Sze, A.S.F.; Leung, C.M.; Tsang, S.W.C.; Shan, E.H.S.; et al. Cancer Risk in 2621 Chinese Patients with Inflammatory Bowel Disease: A Population-Based Cohort Study. Inflamm. Bowel Dis. 2017, 23, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Han, M.; Park, S.; Kim, W.H.; Cheon, J.H. Cancer Risk in the Early Stages of Inflammatory Bowel Disease in Korean Patients: A Nationwide Population-Based Study. J. Crohns Colitis 2017, 11, 954–962. [Google Scholar] [CrossRef] [Green Version]
- Mosher, C.A.; Brown, G.R.; Weideman, R.A.; Crook, T.W.; Cipher, D.J.; Spechler, S.J.; Feagins, L.A. Incidence of Colorectal Cancer and Extracolonic Cancers in Veteran Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 617–623. [Google Scholar] [CrossRef]
- Loo, S.Y.; Vutcovici, M.; Bitton, A.; Lakatos, P.L.; Azoulay, L.; Suissa, S.; Brassard, P. Risk of Malignant Cancers in Inflammatory Bowel Disease. J. Crohns Colitis 2019, 13, 1302–1310. [Google Scholar] [CrossRef]
- Burns, J.A.; Weiner, A.B.; Catalona, W.J.; Li, E.V.; Schaeffer, E.M.; Hanauer, S.B.; Strong, S.; Burns, J.; Hussain, M.H.A.; Kundu, S.D. Inflammatory Bowel Disease and the Risk of Prostate Cancer. Eur. Urol. 2019, 75, 846–852. [Google Scholar] [CrossRef]
- Taborelli, M.; Sozzi, M.; Del Zotto, S.; Toffolutti, F.; Montico, M.; Zanier, L.; Serraino, D. Risk of Intestinal and Extra-Intestinal Cancers in Patients with Inflammatory Bowel Diseases: A Population-Based Cohort Study in Northeastern Italy. PLoS ONE 2020, 15, e0235142. [Google Scholar] [CrossRef]
- Everhov, Å.H.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Ludvigsson, J.F.; Sørensen, H.T.; Olén, O. Inflammatory Bowel Disease and Pancreatic Cancer: A Scandinavian Register-Based Cohort Study 1969–2017. Aliment. Pharmacol. Ther. 2020, 52, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Yang, H.; Zhang, M.; Qian, J. The Incidence Rate and Risk Factors of Malignancy in Elderly-Onset Inflammatory Bowel Disease: A Chinese Cohort Study From 1998 to 2020. Front. Oncol. 2021, 11, 788980. [Google Scholar] [CrossRef] [PubMed]
- Goetgebuer, R.L.; Kreijne, J.E.; Aitken, C.A.; Dijkstra, G.; Hoentjen, F.; de Boer, N.K.; Oldenburg, B.; van der Meulen, A.E.; Ponsioen, C.I.J.; Pierik, M.J.; et al. Increased Risk of High-Grade Cervical Neoplasia in Women with Inflammatory Bowel Disease: A Case-Controlled Cohort Study. J. Crohns Colitis 2021, 15, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; D’Arcy, M.; Barnes, E.L.; Freedman, N.D.; Engels, E.A.; Song, M. Associations of Inflammatory Bowel Disease and Subsequent Cancers in a Population-Based Study of Older Adults in the United States. JNCI Cancer Spectr. 2022, 6, pkab096. [Google Scholar] [CrossRef]
- Huai, J.-P.; Ding, J.; Ye, X.-H.; Chen, Y.-P. Inflammatory Bowel Disease and Risk of Cholangiocarcinoma: Evidence from a Meta-Analysis of Population-Based Studies. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 3477–3482. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, P.J.; Crothers, H.; Mytton, J.; Bosch, S.; Iqbal, T.; Ferguson, J.; Hirschfield, G.M. Effects of Primary Sclerosing Cholangitis on Risks of Cancer and Death in People with Inflammatory Bowel Disease, Based on Sex, Race, and Age. Gastroenterology 2020, 159, 915–928. [Google Scholar] [CrossRef]
- Yuan, F.; Pfeiffer, R.M.; Julián-Serrano, S.; Arjani, S.; Barrett, M.J.; Koshiol, J.; Stolzenberg-Solomon, R.Z. Autoimmune Conditions and Pancreatic Cancer Risk in Older American Adults. Int. J. Cancer 2023, 152, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chang, L.; Chang, H.M.; Chang, F. Intestinal and Extraintestinal Cancers Associated with Inflammatory Bowel Disease. Clin. Colorectal Cancer 2018, 17, e29–e37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.-G.; Yu, Q.; Jiang, X.; Mei, Y.-Z.; Ding, Y.-B.; Wang, M. Association between Inflammatory Bowel Disease and Risk of Incident Prostate Cancer: A Systematic Review and Meta-Analysis of Cohort Studies. Int. J. Colorectal Dis. 2023, 38, 168. [Google Scholar] [CrossRef]
- Cheddani, H.; Dauchet, L.; Fumery, M.; Charpentier, C.; Marie Bouvier, A.; Dupas, J.-L.; Pariente, B.; Peyrin-Biroulet, L.; Savoye, G.; Gower-Rousseau, C. Cancer in Elderly Onset Inflammatory Bowel Disease: A Population-Based Study. Am. J. Gastroenterol. 2016, 111, 1428–1436. [Google Scholar] [CrossRef]
- Karlén, P.; Löfberg, R.; Broström, O.; Leijonmarck, C.E.; Hellers, G.; Persson, P.G. Increased Risk of Cancer in Ulcerative Colitis: A Population-Based Cohort Study. Am. J. Gastroenterol. 1999, 94, 1047–1052. [Google Scholar] [CrossRef]
- Palli, D.; Trallori, G.; Bagnoli, S.; Saieva, C.; Tarantino, O.; Ceroti, M.; d’Albasio, G.; Pacini, F.; Amorosi, A.; Masala, G. Hodgkin’s Disease Risk Is Increased in Patients with Ulcerative Colitis. Gastroenterology 2000, 119, 647–653. [Google Scholar] [CrossRef]
- Wang, L.-H.; Yang, Y.-J.; Cheng, W.-C.; Wang, W.-M.; Lin, S.-H.; Shieh, C.-C. Higher Risk for Hematological Malignancies in Inflammatory Bowel Disease: A Nationwide Population-Based Study in Taiwan. Am. J. Gastroenterol. 2016, 111, 1313–1319. [Google Scholar] [CrossRef]
- Winther, K.V.; Jess, T.; Langholz, E.; Munkholm, P.; Binder, V. Long-Term Risk of Cancer in Ulcerative Colitis: A Population-Based Cohort Study from Copenhagen County. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2004, 2, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Singh, S.; Harmsen, W.S.; Edakkanambeth Varayil, J.; Tremaine, W.J.; Loftus, E.V. Effect of Medications on Risk of Cancer in Patients with Inflammatory Bowel Diseases: A Population-Based Cohort Study from Olmsted County, Minnesota. Mayo Clin. Proc. 2015, 90, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Olén, O.; Smedby, K.E.; Erichsen, R.; Pedersen, L.; Halfvarson, J.; Hallqvist-Everhov, Å.; Bryder, N.; SWIBREG Study Group; Askling, J.; Ekbom, A.; et al. Increasing Risk of Lymphoma Over Time in Crohn’s Disease but Not in Ulcerative Colitis: A Scandinavian Cohort Study. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Yu, J.; Refsum, E.; Wieszczy, P.; Helsingen, L.M.; Perrin, V.; Högdén, A.; Løberg, M.; Blom, J.; Bretthauer, M.; Adami, H.-O.; et al. Risk of Malignant Lymphomas in Patients with Inflammatory Bowel Disease: A Population-Based Cohort Study. BMJ Open Gastroenterol. 2023, 10, e001037. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Bilker, W.B.; Brensinger, C.; Deren, J.J.; Vaughn, D.J.; Strom, B.L. Inflammatory Bowel Disease Is Not Associated with an Increased Risk of Lymphoma. Gastroenterology 2001, 121, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Broséus, J.; Feugier, P.; Thieblemont, C.; Beaugerie, L.; Danese, S.; Arnone, D.; Ndiaye, N.C.; Kokten, T.; Houlgatte, R.; et al. Characteristics of Lymphoma in Patients with Inflammatory Bowel Disease: A Systematic Review. J. Crohns Colitis 2021, 15, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.F.; Diddoro, A.; Iodice, A.; Severi, C.; Castagneto-Gissey, L.; Casella, G. Incidence of Lymphomas in Inflammatory Bowel Disease: Report of an Emblematic Case, Systematic Review, and Meta-Analysis. Front. Med. 2023, 10, 1172634. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.G.; West, J.; Card, T.R. Risk of Cancer in Inflammatory Bowel Disease Treated with Azathioprine: A UK Population-Based Case-Control Study. Am. J. Gastroenterol. 2010, 105, 1604–1609. [Google Scholar] [CrossRef]
- Guerra, I.; Bujanda, L.; Mañosa, M.; Pérez-Martínez, I.; Casanova, M.J.; de la Peña, L.; de Benito, M.; Rivero, M.; Varela, P.; Bernal, L.; et al. Clinical Presentation, Management, and Evolution of Lymphomas in Patients with Inflammatory Bowel Disease: An ENEIDA Registry Study. Cancers 2023, 15, 750. [Google Scholar] [CrossRef]
- Zanelli, M.; Sanguedolce, F.; Palicelli, A.; Zizzo, M.; Martino, G.; Caprera, C.; Fragliasso, V.; Soriano, A.; Valle, L.; Ricci, S.; et al. EBV-Driven Lymphoproliferative Disorders and Lymphomas of the Gastrointestinal Tract: A Spectrum of Entities with a Common Denominator (Part 1). Cancers 2021, 13, 4578. [Google Scholar] [CrossRef]
- Severyns, T.; Kirchgesner, J.; Lambert, J.; Thieblemont, C.; Amiot, A.; Abitbol, V.; Treton, X.; Cazals-Hatem, D.; Malamut, G.; Coppo, P.; et al. Prognosis of Lymphoma in Patients with Known Inflammatory Bowel Disease: A French Multicentre Cohort Study. J. Crohns Colitis 2020, 14, 1222–1230. [Google Scholar] [CrossRef]
- Sultan, K.; Korelitz, B.I.; Present, D.; Katz, S.; Sunday, S.; Shapira, I. Prognosis of Lymphoma in Patients Following Treatment with 6-Mercaptopurine/Azathioprine for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2012, 18, 1855–1858. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Brousse, N.; Bouvier, A.M.; Colombel, J.F.; Lémann, M.; Cosnes, J.; Hébuterne, X.; Cortot, A.; Bouhnik, Y.; Gendre, J.P.; et al. Lymphoproliferative Disorders in Patients Receiving Thiopurines for Inflammatory Bowel Disease: A Prospective Observational Cohort Study. Lancet 2009, 374, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.G.; Orchard, T.R.; Robinson, E.M.; Jewell, D.P. Long-Term Risk of Malignancy after Treatment of Inflammatory Bowel Disease with Azathioprine. Aliment. Pharmacol. Ther. 2002, 16, 1225–1232. [Google Scholar] [CrossRef]
- Gómez-García, M.; Cabello-Tapia, M.J.; Sánchez-Capilla, A.D.; De Teresa-Galván, J.; Redondo-Cerezo, E. Thiopurines Related Malignancies in Inflammatory Bowel Disease: Local Experience in Granada, Spain. World J. Gastroenterol. 2013, 19, 4877–4886. [Google Scholar] [CrossRef]
- Algaba, A.; Guerra, I.; Marín-Jiménez, I.; Quintanilla, E.; López-Serrano, P.; García-Sánchez, M.C.; Casis, B.; Taxonera, C.; Moral, I.; Chaparro, M.; et al. Incidence, Management, and Course of Cancer in Patients with Inflammatory Bowel Disease. J. Crohns Colitis 2015, 9, 326–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biancone, L.; Onali, S.; Petruzziello, C.; Calabrese, E.; Pallone, F. Cancer and Immunomodulators in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2015, 21, 674–698. [Google Scholar] [CrossRef]
- Axelrad, J.; Bernheim, O.; Colombel, J.-F.; Malerba, S.; Ananthakrishnan, A.; Yajnik, V.; Hoffman, G.; Agrawal, M.; Lukin, D.; Desai, A.; et al. Risk of New or Recurrent Cancer in Patients with Inflammatory Bowel Disease and Previous Cancer Exposed to Immunosuppressive and Anti-Tumor Necrosis Factor Agents. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2016, 14, 58–64. [Google Scholar] [CrossRef]
- Chaparro, M.; Ramas, M.; Benítez, J.M.; López-García, A.; Juan, A.; Guardiola, J.; Mínguez, M.; Calvet, X.; Márquez, L.; Fernández Salazar, L.I.; et al. Extracolonic Cancer in Inflammatory Bowel Disease: Data from the GETECCU Eneida Registry. Am. J. Gastroenterol. 2017, 112, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, B.; Svanström, H.; Schmiegelow, K.; Jess, T.; Hviid, A. Use of Azathioprine and the Risk of Cancer in Inflammatory Bowel Disease. Am. J. Epidemiol. 2013, 177, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Carrat, F.; Colombel, J.-F.; Bouvier, A.-M.; Sokol, H.; Babouri, A.; Carbonnel, F.; Laharie, D.; Faucheron, J.-L.; Simon, T.; et al. Risk of New or Recurrent Cancer under Immunosuppressive Therapy in Patients with IBD and Previous Cancer. Gut 2014, 63, 1416–1423. [Google Scholar] [CrossRef]
- Beigel, F.; Steinborn, A.; Schnitzler, F.; Tillack, C.; Breiteneicher, S.; John, J.M.; Van Steen, K.; Laubender, R.; Göke, B.; Seiderer, J.; et al. Risk of Malignancies in Patients with Inflammatory Bowel Disease Treated with Thiopurines or Anti-TNF Alpha Antibodies. Pharmacoepidemiol. Drug Saf. 2014, 23, 735–744. [Google Scholar] [CrossRef]
- Scharl, S.; Barthel, C.; Rossel, J.-B.; Biedermann, L.; Misselwitz, B.; Schoepfer, A.M.; Straumann, A.; Vavricka, S.R.; Rogler, G.; Scharl, M.; et al. Malignancies in Inflammatory Bowel Disease: Frequency, Incidence and Risk Factors-Results from the Swiss IBD Cohort Study. Am. J. Gastroenterol. 2019, 114, 116–126. [Google Scholar] [CrossRef]
- Phillips, F.; Verstockt, B.; Ribaldone, D.G.; Guerra, I.; Teich, N.; Katsanos, K.; Filip, R.; Molnar, T.; Karmiris, K.; ECCO CONFER Investigators. Diagnosis and Outcome of Extranodal Primary Intestinal Lymphoma in Inflammatory Bowel Disease: An ECCO CONFER Case Series. J. Crohns Colitis 2022, 16, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Beaugerie, L.; Maynadié, M.; Laharie, D.; Dupas, J.-L.; Flourié, B.; Lerebours, E.; Peyrin-Biroulet, L.; Allez, M.; Simon, T.; et al. Excess Primary Intestinal Lymphoproliferative Disorders in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2012, 18, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Holubar, S.D.; Dozois, E.J.; Loftus, E.V., Jr.; Teh, S.H.; Benavente, L.A.; Harmsen, S.W.; Wolff, B.G.; Cima, R.R.; Larson, D.W. Primary Intestinal Lymphoma in Patients with Inflammatory Bowel Disease: A Descriptive Series from the Prebiologic Therapy Era. Inflamm. Bowel Dis. 2011, 17, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, N.A.; Hall, P.A.; Williams, G.T.; Codling, B.W.; Jones, E.L.; Levison, D.A.; Morson, B.C. Primary malignant lymphoma of the large intestine complicating chronic inflammatory bowel disease. Histopathology 1989, 15, 325–337. [Google Scholar] [CrossRef]
- Caspi, O.; Polliack, A.; Klar, R.; Yehuda, D.B. The Association of Inflammatory Bowel Disease and Leukemia—Coincidence or Not? Leuk. Lymphoma 1995, 17, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Askling, J.; Brandt, L.; Lapidus, A.; Karlén, P.; Björkholm, M.; Löfberg, R.; Ekbom, A. Risk of Haematopoietic Cancer in Patients with Inflammatory Bowel Disease. Gut 2005, 54, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenstein, A.J.; Gennuso, R.; Sachar, D.B.; Heimann, T.; Smith, H.; Janowitz, H.D.; Aufses, A.H. Extraintestinal Cancers in Inflammatory Bowel Disease. Cancer 1985, 56, 2914–2921. [Google Scholar] [CrossRef] [PubMed]
- Mir Madjlessi, S.H.; Farmer, R.G.; Weick, J.K. Inflammatory Bowel Disease and Leukemia. A Report of Seven Cases of Leukemia in Ulcerative Colitis and Crohn’s Disease and Review of the Literature. Dig. Dis. Sci. 1986, 31, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.A.; Pfeiffer, R.M.; Landgren, O.; Gadalla, S.; Berndt, S.I.; Engels, E.A. Risks of Myeloid Malignancies in Patients with Autoimmune Conditions. Br. J. Cancer 2009, 100, 822–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangolo, A.; Sagireddy, S.; Desrochers, P.; Laabidi, I.; Nagesh, V.K.; Jarri, A.; Sekhon, I.; Laabidi, Y.; Muralidhar, D.; Singh, A.; et al. Association between Multiple Myeloma and Ulcerative Colitis: A Cross-Sectional Analysis. Diseases 2023, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Zurba, Y.; Gros, B.; Shehab, M. Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review. Biomedicines 2023, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourrier, A.; Carrat, F.; Colombel, J.-F.; Bouvier, A.-M.; Abitbol, V.; Marteau, P.; Cosnes, J.; Simon, T.; Peyrin-Biroulet, L.; Beaugerie, L. Excess Risk of Urinary Tract Cancers in Patients Receiving Thiopurines for Inflammatory Bowel Disease: A Prospective Observational Cohort Study. Aliment. Pharmacol. Amp Ther. 2015, 43, 252–261. [Google Scholar] [CrossRef]
- Sawchik, J.; Hamdani, J.; Vanhaeverbeek, M. Randomized Clinical Trials and Observational Studies in the Assessment of Drug Safety. Rev. Epidemiol. Sante Publique 2018, 66, 217–225. [Google Scholar] [CrossRef]
- Bezzio, C.; Vernero, M.; Ribaldone, D.G.; Alimenti, E.; Manes, G.; Saibeni, S. Cancer Risk in Patients Treated with the JAK Inhibitor Tofacitinib: Systematic Review and Meta-Analysis. Cancers 2023, 15, 2197. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Orlando, A.; Papi, C.; Festa, S.; Pugliese, D.; Bonovas, S.; Pansieri, C.; Piovani, D.; Fiorino, G.; Fantini, M.C.; et al. Use of Biologics and Small Molecule Drugs for the Management of Moderate to Severe Ulcerative Colitis: IG-IBD Clinical Guidelines Based on the GRADE Methodology. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2022, 54, 440–451. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Papi, C.; Orlando, A.; Festa, S.; Pugliese, D.; Bonovas, S.; Pansieri, C.; Piovani, D.; Fiorino, G.; Fantini, M.C.; et al. Use of Biologics for the Management of Crohn’s Disease: IG-IBD Clinical Guidelines Based on the GRADE Methodology. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2023, 55, 442–453. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Rahier, J.-F.; Kirchgesner, J.; Abitbol, V.; Shaji, S.; Armuzzi, A.; Karmiris, K.; Gisbert, J.P.; Bossuyt, P.; Helwig, U.; et al. I-CARE, a European Prospective Cohort Study Assessing Safety and Effectiveness of Biologics in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2023, 21, 771–788.e10. [Google Scholar] [CrossRef]
- Lemaitre, M.; Kirchgesner, J.; Rudnichi, A.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Association Between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients with Inflammatory Bowel Disease. JAMA 2017, 318, 1679–1686. [Google Scholar] [CrossRef]
- Khan, N.; Patel, D.; Trivedi, C.; Kavani, H.; Pernes, T.; Medvedeva, E.; Lewis, J.; Xie, D.; Yang, Y.-X. Incidence of Acute Myeloid Leukemia and Myelodysplastic Syndrome in Patients with Inflammatory Bowel Disease and the Impact of Thiopurines on Their Risk. Off. J. Am. Coll. Gastroenterol. ACG 2021, 116, 741. [Google Scholar] [CrossRef]
- Kobayashi, T.; Uda, A.; Udagawa, E.; Hibi, T. Lack of Increased Risk of Lymphoma by Thiopurines or Biologics in Japanese Patients with Inflammatory Bowel Disease: A Large-Scale Administrative Database Analysis. J. Crohns Colitis 2020, 14, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh Ardabili, A.; Jeuring, S.; Mujagic, Z.; Oostenbrug, L.; Romberg-Camps, M.; Jonkers, D.; van Bodegraven, A.; Pierik, M. Classic Drugs in the Time of New Drugs: Real-World, Long-Term Outcomes of Thiopurine Monotherapy in 1016 Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2022, 56, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Herrinton, L.J.; Liu, L.; Weng, X.; Lewis, J.D.; Hutfless, S.; Allison, J.E. Role of Thiopurine and Anti-TNF Therapy in Lymphoma in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2011, 106, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.M.; Almukhtar, R.M.; Loftus, E.V.; Lichtenstein, G.R.; Khan, N. Risk of Melanoma and Non-Melanoma Skin Cancer in Ulcerative Colitis Patients Treated with Thiopurines: A Nationwide Retrospective Cohort. Am. J. Gastroenterol. 2014, 109, 1781–1793. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Khosrotehrani, K.; Carrat, F.; Bouvier, A.-M.; Chevaux, J.-B.; Simon, T.; Carbonnel, F.; Colombel, J.-F.; Dupas, J.-L.; Godeberge, P.; et al. Increased Risk for Nonmelanoma Skin Cancers in Patients Who Receive Thiopurines for Inflammatory Bowel Disease. Gastroenterology 2011, 141, 1621–1628.e5. [Google Scholar] [CrossRef] [PubMed]
- Ariyaratnam, J.; Subramanian, V. Association Between Thiopurine Use and Nonmelanoma Skin Cancers in Patients with Inflammatory Bowel Disease: A Meta-Analysis. Am. J. Gastroenterol. 2014, 109, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J.W.; Pugliano-Mauro, M.A. Nonmelanoma Skin Cancer Risk in Patients with Inflammatory Bowel Disease Undergoing Thiopurine Therapy: A Systematic Review of the Literature. Dermatol. Surg. 2018, 44, 469–480. [Google Scholar] [CrossRef]
- Huang, S.-Z.; Liu, Z.-C.; Liao, W.-X.; Wei, J.-X.; Huang, X.-W.; Yang, C.; Xia, Y.-H.; Li, L.; Ye, C.; Dai, S.-X. Risk of Skin Cancers in Thiopurines-Treated and Thiopurines-Untreated Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2018, 34, 507–516. [Google Scholar] [CrossRef]
- Kotlyar, D.S.; Lewis, J.D.; Beaugerie, L.; Tierney, A.; Brensinger, C.M.; Gisbert, J.P.; Loftus, E.V.; Peyrin-Biroulet, L.; Blonski, W.C.; Domselaar, M.V.; et al. Risk of Lymphoma in Patients with Inflammatory Bowel Disease Treated with Azathioprine and 6-Mercaptopurine: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 847–858.e4. [Google Scholar] [CrossRef]
- Chupin, A.; Perduca, V.; Meyer, A.; Bellanger, C.; Carbonnel, F.; Dong, C. Systematic Review with Meta-Analysis: Comparative Risk of Lymphoma with Anti-Tumour Necrosis Factor Agents and/or Thiopurines in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2020, 52, 1289–1297. [Google Scholar] [CrossRef]
- Kandiel, A.; Fraser, A.G.; Korelitz, B.I.; Brensinger, C.; Lewis, J.D. Increased Risk of Lymphoma among Inflammatory Bowel Disease Patients Treated with Azathioprine and 6-Mercaptopurine. Gut 2005, 54, 1121–1125. [Google Scholar] [CrossRef] [Green Version]
- Siegel, C.A.; Marden, S.M.; Persing, S.M.; Larson, R.J.; Sands, B.E. Risk of Lymphoma Associated with Combination Anti–Tumor Necrosis Factor and Immunomodulator Therapy for the Treatment of Crohn’s Disease: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2009, 7, 874–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levhar, N.; Ungar, B.; Kopylov, U.; Fudim, E.; Yavzori, M.; Picard, O.; Amariglio, N.; Chowers, Y.; Shemer-Avni, Y.; Mao, R.; et al. Propagation of EBV-Driven Lymphomatous Transformation of Peripheral Blood B Cells by Immunomodulators and Biologics Used in the Treatment of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Biank, V.F.; Sheth, M.K.; Talano, J.; Margolis, D.; Simpson, P.; Kugathasan, S.; Stephens, M. Association of Crohn’s Disease, Thiopurines, and Primary Epstein-Barr Virus Infection with Hemophagocytic Lymphohistiocytosis. J. Pediatr. 2011, 159, 808–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brambilla, B.; Barbosa, A.M.; da Scholze, C.S.; Riva, F.; Freitas, L.; Balbinot, R.A.; Balbinot, S.; Soldera, J. Hemophagocytic Lymphohistiocytosis and Inflammatory Bowel Disease: Case Report and Systematic Review. Inflamm. Intest. Dis. 2020, 5, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zerón, P.; Bosch, X.; Pérez-de-Lis, M.; Pérez-Álvarez, R.; Fraile, G.; Gheitasi, H.; Retamozo, S.; Bové, A.; Monclús, E.; Escoda, O.; et al. Infection Is the Major Trigger of Hemophagocytic Syndrome in Adult Patients Treated with Biological Therapies. Semin. Arthritis Rheum. 2016, 45, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.F.; Zhang, J.; Xia, X.F.; Zhou, L.Y.; Liu, J.J.; Song, Z.Q.; Lv, Y.M.; Wang, A.Y.; Zhang, Y.P.; et al. Features of Patients with Inflammatory Bowel Diseases Who Develop Hemophagocytic Lymphohistiocytosis. Int. J. Color. Dis. 2016, 31, 1375–1376. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Abdelhay, A.; Khamis, A.; Mostafa, M.; Shehadah, A.; Mohamed, M.S.; Eltaher, B.; Malik, T. Hemophagocytic Lymphohistiocytosis in Inflammatory Bowel Disease: A Nationwide Analysis. Ann. Hematol. 2023, 102, 1705–1711. [Google Scholar] [CrossRef]
- De Francisco, R.; Castaño-García, A.; Martínez-González, S.; Pérez-Martínez, I.; González-Huerta, A.J.; Morais, L.R.; Fernández-García, M.S.; Jiménez, S.; Díaz-Coto, S.; Flórez-Díez, P.; et al. Impact of Epstein-Barr Virus Serological Status on Clinical Outcomes in Adult Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2018, 48, 723–730. [Google Scholar] [CrossRef]
- Offman, J.; Opelz, G.; Doehler, B.; Cummins, D.; Halil, O.; Banner, N.R.; Burke, M.M.; Sullivan, D.; Macpherson, P.; Karran, P. Defective DNA Mismatch Repair in Acute Myeloid Leukemia/Myelodysplastic Syndrome after Organ Transplantation. Blood 2004, 104, 822–828. [Google Scholar] [CrossRef] [Green Version]
- Narous, M.; Nugent, Z.; Singh, H.; Bernstein, C.N. Risks of Melanoma and Nonmelanoma Skin Cancers Pre– and Post–Inflammatory Bowel Disease Diagnosis. Inflamm. Bowel Dis. 2022, 29, 1047–1056. [Google Scholar] [CrossRef]
- Kopylov, U.; Vutcovici, M.; Kezouh, A.; Seidman, E.; Bitton, A.; Afif, W. Risk of Lymphoma, Colorectal and Skin Cancer in Patients with IBD Treated with Immunomodulators and Biologics: A Quebec Claims Database Study. Inflamm. Bowel Dis. 2015, 21, 1847–1853. [Google Scholar] [CrossRef]
- Magro, F.; Peyrin-Biroulet, L.; Sokol, H.; Aldeger, X.; Costa, A.; Higgins, P.D.; Joyce, J.C.; Katsanos, K.H.; Lopez, A.; de Xaxars, T.M.; et al. Extra-Intestinal Malignancies in Inflammatory Bowel Disease: Results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J. Crohns Colitis 2014, 8, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Allegretti, J.R.; Barnes, E.L.; Cameron, A. Are Patients with Inflammatory Bowel Disease on Chronic Immunosuppressive Therapy at Increased Risk of Cervical High-Grade Dysplasia/Cancer? A Meta-Analysis. Inflamm. Bowel Dis. 2015, 21, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Sifuentes, H.; Kane, S. Monitoring for Extra-Intestinal Cancers in IBD. Curr. Gastroenterol. Rep. 2015, 17, 42. [Google Scholar] [CrossRef]
- Mann, S.; Jess, T.; Allin, K.; Elmahdi, R. Risk of Cervical Cancer in Inflammatory Bowel Disease: A Meta-Analysis of Population-Based Studies. Clin. Transl. Gastroenterol. 2022, 13, e00513. [Google Scholar] [CrossRef]
- Hazenberg, H.M.J.L.; de Boer, N.K.H.; Mulder, C.J.J.; Mom, S.H.; van Bodegraven, A.A.; Tack Md PhD, G.J. Neoplasia and Precursor Lesions of the Female Genital Tract in IBD: Epidemiology, Role of Immunosuppressants, and Clinical Implications. Inflamm. Bowel Dis. 2018, 24, 510–531. [Google Scholar] [CrossRef] [Green Version]
- Algaba, A.; Guerra, I.; Castaño, A.; de la Poza, G.; Castellano, V.M.; López, M.; Bermejo, F. Risk of Cancer, with Special Reference to Extra-Intestinal Malignancies, in Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2013, 19, 9359–9365. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Martini, G.; Armandi, A.; Rosso, C.; Vernero, M.; Bugianesi, E.; Astegiano, M.; Saracco, G.M.; Ribaldone, D.G. Risk Factors of Urothelial Cancer in Inflammatory Bowel Disease. J. Clin. Med. 2021, 10, 3257. [Google Scholar] [CrossRef] [PubMed]
- Masunaga, Y.; Ohno, K.; Ogawa, R.; Hashiguchi, M.; Echizen, H.; Ogata, H. Meta-Analysis of Risk of Malignancy with Immunosuppressive Drugs in Inflammatory Bowel Disease. Ann. Pharmacother. 2007, 41, 21–28. [Google Scholar] [CrossRef]
- Singh, H.; Nugent, Z.; Demers, A.A.; Bernstein, C.N. Increased Risk of Nonmelanoma Skin Cancers Among Individuals With Inflammatory Bowel Disease. Gastroenterology 2011, 141, 1612–1620. [Google Scholar] [CrossRef]
- Dugué, P.-A.; Rebolj, M.; Hallas, J.; Garred, P.; Lynge, E. Risk of Cervical Cancer in Women with Autoimmune Diseases, in Relation with Their Use of Immunosuppressants and Screening: Population-Based Cohort Study. Int. J. Cancer 2015, 136, E711–E719. [Google Scholar] [CrossRef]
- Polesie, S.; Gillstedt, M.; Schmidt, S.A.J.; Egeberg, A.; Pottegård, A.; Kristensen, K. Use of Methotrexate and Risk of Skin Cancer: A Nationwide Case-Control Study. Br. J. Cancer 2023, 128, 1311–1319. [Google Scholar] [CrossRef]
- Deepak, P.; Sifuentes, H.; Sherid, M.; Stobaugh, D.; Sadozai, Y.; Ehrenpreis, E.D. T-Cell Non-Hodgkin’s Lymphomas Reported to the FDA AERS with Tumor Necrosis Factor-Alpha (TNF-α) Inhibitors: Results of the REFURBISH Study. Am. J. Gastroenterol. 2013, 108, 99–105. [Google Scholar] [CrossRef]
- Muller, M.; D’Amico, F.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. TNF Inhibitors and Risk of Malignancy in Patients with Inflammatory Bowel Diseases: A Systematic Review. J. Crohns Colitis 2021, 15, 840–859. [Google Scholar] [CrossRef]
- Poullenot, F.; Seksik, P.; Beaugerie, L.; Amiot, A.; Nachury, M.; Abitbol, V.; Stefanescu, C.; Reenaers, C.; Fumery, M.; Pelletier, A.-L.; et al. Risk of Incident Cancer in Inflammatory Bowel Disease Patients Starting Anti-TNF Therapy While Having Recent Malignancy. Inflamm. Bowel Dis. 2016, 22, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Borren, N.Z.; Ananthakrishnan, A.N. Safety of Biologic Therapy in Older Patients with Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1736–1743.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyboe Andersen, N.; Pasternak, B.; Basit, S.; Andersson, M.; Svanström, H.; Caspersen, S.; Munkholm, P.; Hviid, A.; Jess, T. Association between Tumor Necrosis Factor-α Antagonists and Risk of Cancer in Patients with Inflammatory Bowel Disease. JAMA 2014, 311, 2406–2413. [Google Scholar] [CrossRef] [Green Version]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Bonovas, S. Systematic Review with Meta-Analysis: Biologics and Risk of Infection or Cancer in Elderly Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2020, 51, 820–830. [Google Scholar] [CrossRef]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Biologic Therapies and Risk of Infection and Malignancy in Patients with Inflammatory Bowel Disease: A Systematic Review and Network Meta-Analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2016, 14, 1385–1397.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljung, T.; Karlén, P.; Schmidt, D.; Hellström, P.M.; Lapidus, A.; Janczewska, I.; Sjöqvist, U.; Löfberg, R. Infliximab in Inflammatory Bowel Disease: Clinical Outcome in a Population Based Cohort from Stockholm County. Gut 2004, 53, 849–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, H.S.; van Oijen, M.G.H.; de Jong, D.J. Serious Events with Infliximab in Patients with Inflammatory Bowel Disease: A 9-Year Cohort Study in the Netherlands. Drug Saf. 2008, 31, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Fidder, H.; Schnitzler, F.; Ferrante, M.; Noman, M.; Katsanos, K.; Segaert, S.; Henckaerts, L.; Assche, G.V.; Vermeire, S.; Rutgeerts, P. Long-Term Safety of Infliximab for the Treatment of Inflammatory Bowel Disease: A Single-Centre Cohort Study. Gut 2009, 58, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, G.T.; Mowat, A.; Potts, L.; Cahill, A.; Mowat, C.; Lees, C.W.; Hare, N.C.; Wilson, J.A.; Boulton-Jones, R.; Priest, M.; et al. Efficacy and Complications of Adalimumab Treatment for Medically-Refractory Crohn’s Disease: Analysis of Nationwide Experience in Scotland (2004–2008). Aliment. Pharmacol. Ther. 2009, 29, 527–534. [Google Scholar] [CrossRef]
- Lees, C.W.; Ali, A.I.; Thompson, A.I.; Ho, G.-T.; Forsythe, R.O.; Marquez, L.; Cochrane, C.J.; Aitken, S.; Fennell, J.; Rogers, P.; et al. The Safety Profile of Anti-Tumour Necrosis Factor Therapy in Inflammatory Bowel Disease in Clinical Practice: Analysis of 620 Patient-Years Follow-Up. Aliment. Pharmacol. Ther. 2009, 29, 286–297. [Google Scholar] [CrossRef]
- Karmiris, K.; Paintaud, G.; Noman, M.; Magdelaine-Beuzelin, C.; Ferrante, M.; Degenne, D.; Claes, K.; Coopman, T.; Van Schuerbeek, N.; Van Assche, G.; et al. Influence of Trough Serum Levels and Immunogenicity on Long-Term Outcome of Adalimumab Therapy in Crohn’s Disease. Gastroenterology 2009, 137, 1628–1640. [Google Scholar] [CrossRef]
- Biancone, L.; Petruzziello, C.; Orlando, A.; Kohn, A.; Ardizzone, S.; Daperno, M.; Angelucci, E.; Castiglione, F.; D’Incà, R.; Zorzi, F.; et al. Cancer in Crohn’s Disease Patients Treated with Infliximab: A Long-Term Multicenter Matched Pair Study. Inflamm. Bowel Dis. 2011, 17, 758–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, H.; Haneda, S.; Shibata, C.; Miura, K.; Nagao, M.; Ohnuma, S.; Kohyama, A.; Unno, M. Adenocarcinoma Associated with Perianal Fistulas in Crohn’s Disease. Anticancer Res. 2013, 33, 685–689. [Google Scholar] [PubMed]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Safdi, M.; Popp, J.W.; Langholff, W.; Sandborn, W.J. Infliximab for Crohn’s Disease: More Than 13 Years of Real-World Experience. Inflamm. Bowel Dis. 2018, 24, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Cottone, M.; Kohn, A.; Daperno, M.; Armuzzi, A.; Guidi, L.; D’Inca, R.; Bossa, F.; Angelucci, E.; Biancone, L.; Gionchetti, P.; et al. Advanced Age Is an Independent Risk Factor for Severe Infections and Mortality in Patients Given Anti–Tumor Necrosis Factor Therapy for Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2011, 9, 30–35. [Google Scholar] [CrossRef]
- D’Haens, G.; Reinisch, W.; Colombel, J.-F.; Panes, J.; Ghosh, S.; Prantera, C.; Lindgren, S.; Hommes, D.W.; Huang, Z.; Boice, J.; et al. Five-Year Safety Data From ENCORE, a European Observational Safety Registry for Adults with Crohn’s Disease Treated with Infliximab [Remicade®] or Conventional Therapy. J. Crohns Colitis 2017, 11, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Panés, J.; Lindsay, J.O.; Teich, N.; Lindgren, S.; Colombel, J.-F.; Cornillie, F.; Flynn, H.A.; Huyck, S.; Stryszak, P.; Yao, R.; et al. Five-Year Safety Data From OPUS, a European Observational Safety Registry for Adults with Ulcerative Colitis Treated with Originator Infliximab [Remicade®] or Conventional Therapy. J. Crohns Colitis 2019, 13, 1148–1157. [Google Scholar] [CrossRef]
- Yang, C.; Huang, J.; Huang, X.; Huang, S.; Cheng, J.; Liao, W.; Chen, X.; Wang, X.; Dai, S. Risk of Lymphoma in Patients With Inflammatory Bowel Disease Treated with Anti-Tumour Necrosis Factor Alpha Agents: A Systematic Review and Meta-Analysis. J. Crohns Colitis 2018, 12, 1042–1052. [Google Scholar] [CrossRef]
- Tassone, D.; Basnayake, C.; Wright, E.; Lust, M.; Kamm, M.A.; Niewiadomski, O.; Schulberg, J.; Flanagan, E.; Samyue, M.T.; Fry, M.S.; et al. Risk Factors for Malignancy and Serious Infection in Patients with Inflammatory Bowel Disease: A Retrospective Analysis. Intern. Med. J. 2023. [Google Scholar] [CrossRef]
- D’Haens, G.; Reinisch, W.; Panaccione, R.; Satsangi, J.; Petersson, J.; Bereswill, M.; Arikan, D.; Perotti, E.; Robinson, A.M.; Kalabic, J.; et al. Lymphoma Risk and Overall Safety Profile of Adalimumab in Patients with Crohn’s Disease with up to 6 Years of Follow-Up in the Pyramid Registry. Am. J. Gastroenterol. 2018, 113, 872–882. [Google Scholar] [CrossRef]
- Esse, S.; Mason, K.J.; Green, A.C.; Warren, R.B. Melanoma risk in patients treated with biologic therapy for common inflammatory diseases: A systematic review and meta-analysis. JAMA Dermatol. 2020, 156, 787–794. [Google Scholar] [CrossRef]
- McAuliffe, M.E.; Lanes, S.; Leach, T.; Parikh, A.; Faich, G.; Porter, J.; Holick, C.; Esposito, D.; Zhao, Y.; Fox, I. Occurrence of Adverse Events among Patients with Inflammatory Bowel Disease in the HealthCore Integrated Research Database. Curr. Med. Res. Opin. 2015, 31, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Scott, F.I.; Mamtani, R.; Brensinger, C.M.; Haynes, K.; Chiesa-Fuxench, Z.C.; Zhang, J.; Chen, L.; Xie, F.; Yun, H.; Osterman, M.T.; et al. Risk of Non-Melanoma Skin Cancer in Patients with a History of NMSC with the Use of Immunosuppressant and Biologic Agents in Autoimmune Disease. JAMA Dermatol. 2016, 152, 164–172. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef] [Green Version]
- Khanna, R.; Bressler, B.; Levesque, B.G.; Zou, G.; Stitt, L.W.; Greenberg, G.R.; Panaccione, R.; Bitton, A.; Paré, P.; Vermeire, S.; et al. Early Combined Immunosuppression for the Management of Crohn’s Disease (REACT): A Cluster Randomised Controlled Trial. Lancet Lond. Engl. 2015, 386, 1825–1834. [Google Scholar] [CrossRef]
- Panaccione, R.; Ghosh, S.; Middleton, S.; Márquez, J.R.; Scott, B.B.; Flint, L.; van Hoogstraten, H.J.F.; Chen, A.C.; Zheng, H.; Danese, S.; et al. Combination Therapy with Infliximab and Azathioprine Is Superior to Monotherapy with Either Agent in Ulcerative Colitis. Gastroenterology 2014, 146, 392–400.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchgesner, J.; Lemaitre, M.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Risk of Serious and Opportunistic Infections Associated with Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018, 155, 337–346.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Diamond, R.H.; Langholff, W.; Londhe, A.; Sandborn, W.J. Drug Therapies and the Risk of Malignancy in Crohn’s Disease: Results from the TREATTM Registry. Am. J. Gastroenterol. 2014, 109, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, D.S.; Osterman, M.T.; Diamond, R.H.; Porter, D.; Blonski, W.C.; Wasik, M.; Sampat, S.; Mendizabal, M.; Lin, M.V.; Lichtenstein, G.R. A Systematic Review of Factors That Contribute to Hepatosplenic T-Cell Lymphoma in Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2011, 9, 36–41.e1. [Google Scholar] [CrossRef]
- Osterman, M.T.; Sandborn, W.J.; Colombel, J.-F.; Robinson, A.M.; Lau, W.; Huang, B.; Pollack, P.F.; Thakkar, R.B.; Lewis, J.D. Increased Risk of Malignancy with Adalimumab Combination Therapy, Compared with Monotherapy, for Crohn’s Disease. Gastroenterology 2014, 146, 941–949. [Google Scholar] [CrossRef]
- Cohen, R.D.; Bhayat, F.; Blake, A.; Travis, S. The Safety Profile of Vedolizumab in Ulcerative Colitis and Crohn’s Disease: 4 Years of Global Post-Marketing Data. J. Crohns Colitis 2020, 14, 192–204. [Google Scholar] [CrossRef]
- Card, T.; Ungaro, R.; Bhayat, F.; Blake, A.; Hantsbarger, G.; Travis, S. Vedolizumab Use Is Not Associated with Increased Malignancy Incidence: GEMINI LTS Study Results and Post-Marketing Data. Aliment. Pharmacol. Ther. 2020, 51, 149–157. [Google Scholar] [CrossRef]
- Dahiya, D.S.; Chandan, S.; Bapaye, J.; Mohan, B.P.; Ramai, D.; Kassab, L.L.; Chandan, O.C.; Dulai, P.S.; Kochhar, G.S. Safety and Effectiveness of Vedolizumab in Elderly Patients with Inflammatory Bowel Disease: A Systematic Review & Meta-Analysis. J. Clin. Gastroenterol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Huang, K.; Liu, J.; Xia, W.; Tian, C.; Yao, L.; Cao, Q.; Chen, H. Effectiveness and Safety of Vedolizumab for Ulcerative Colitis: A Single-Center Retrospective Real-World Study in China. Front. Pharmacol. 2023, 14, 1188751. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.S.; Ventimiglia, M.; Orlando, A. Effectiveness and Safety of Vedolizumab in Inflammatory Bowel Disease: A Comprehensive Meta-Analysis of Observational Studies. J. Crohns Colitis 2023. online ahead of print. [Google Scholar] [CrossRef]
- Lin, W.-C.; Tai, W.-C.; Chang, C.-H.; Tu, C.-H.; Feng, I.-C.; Shieh, M.-J.; Chung, C.-S.; Yen, H.-H.; Chou, J.-W.; Wong, J.-M.; et al. Real-World Evidence of Effectiveness and Safety of Vedolizumab for Inflammatory Bowel Disease in Taiwan: A Prospective Nationwide Registry (VIOLET) Study. Inflamm. Bowel Dis. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Burgevin, A.; Caron, B.; Sasson, A.; Luc, A.; Netter, P.; Baumann, C.; Ananthakrishnan, A.N.; Peyrin-Biroulet, L. Comparative Safety of Ustekinumab and Vedolizumab in Older Patients with Inflammatory Bowel Disease: A Bicentric Cohort Study. J. Clin. Med. 2022, 11, 6967. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Iversen, A.T.; Allin, K.H.; Jess, T. Comparative Outcomes and Safety of Vedolizumab vs Tumor Necrosis Factor Antagonists for Older Adults with Inflammatory Bowel Diseases. JAMA Netw. Open 2022, 5, e2234200. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Danese, S.; O’Brien, C.D.; Ott, E.; Marano, C.; Baker, T.; Zhou, Y.; Volger, S.; Tikhonov, I.; et al. Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis of Results from Phase 2/3 Studies. Inflamm. Bowel Dis. 2021, 27, 994–1007. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 578–590.e4. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Rowbotham, D.S.; Danese, S.; Sandborn, W.J.; Miao, Y.; Zhang, H.; Tikhonov, I.; Panaccione, R.; Hisamatsu, T.; Scherl, E.J.; et al. Efficacy and Safety of Maintenance Ustekinumab for Ulcerative Colitis Through 3 Years: UNIFI Long-Term Extension. J. Crohns Colitis 2022, 16, 1222–1234. [Google Scholar] [CrossRef]
- Chaparro, M.; Garre, A.; Iborra, M.; Sierra-Ausín, M.; Barreiro-de Acosta, M.; Fernández-Clotet, A.; de Castro, L.; Boscá-Watts, M.; Casanova, M.J.; López-García, A.; et al. Effectiveness and Safety of Ustekinumab in Ulcerative Colitis: Real-World Evidence from the ENEIDA Registry. J. Crohns Colitis 2021, 15, 1846–1851. [Google Scholar] [CrossRef]
- Casas-Deza, D.; Lamuela-Calvo, L.J.; Gomollón, F.; Arbonés-Mainar, J.M.; Caballol, B.; Gisbert, J.P.; Rivero, M.; Sánchez-Rodríguez, E.; Arias García, L.; Gutiérrez Casbas, A.; et al. Effectiveness and Safety of Ustekinumab in Elderly Patients with Crohn’s Disease: Real World Evidence From the ENEIDA Registry. J. Crohns Colitis 2023, 17, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Regueiro, M.; Yun, H.; Su, C.; DiBonaventura, M.; Lawendy, N.; Nduaka, C.I.; Koram, N.; Cappelleri, J.C.; Chan, G.; et al. Tofacitinib Treatment Safety in Moderate to Severe Ulcerative Colitis: Comparison of Observational Population Cohort Data From the IBM MarketScan® Administrative Claims Database with Tofacitinib Trial Data. Inflamm. Bowel Dis. 2020, 27, 1394–1408. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Vranic, I.; Su, C.; Rousell, S.; Niezychowski, W.; Study A3921063 Investigators. Tofacitinib, an Oral Janus Kinase Inhibitor, in Active Ulcerative Colitis. N. Engl. J. Med. 2012, 367, 616–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Lawendy, N.; Danese, S.; Su, C.; Loftus, E.V.; Hart, A.; Dotan, I.; Damião, A.O.M.C.; Judd, D.T.; Guo, X.; et al. Safety and Efficacy of Tofacitinib for Treatment of Ulcerative Colitis: Final Analysis of OCTAVE Open, an Open-label, Long-term Extension Study with up to 7.0 Years of Treatment. Aliment. Pharmacol. Ther. 2022, 55, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Panés, J.; D’Haens, G.R.; Sands, B.E.; Su, C.; Moscariello, M.; Jones, T.; Pedersen, R.; Friedman, G.S.; Lawendy, N.; et al. Safety of Tofacitinib for Treatment of Ulcerative Colitis, Based on 4.4 Years of Data From Global Clinical Trials. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 1541–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeire, S.; Su, C.; Lawendy, N.; Kobayashi, T.; Sandborn, W.J.; Rubin, D.T.; Modesto, I.; Gardiner, S.; Kulisek, N.; Zhang, H.; et al. Outcomes of Tofacitinib Dose Reduction in Patients with Ulcerative Colitis in Stable Remission from the Randomised RIVETING Trial. J. Crohns Colitis 2021, 15, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Bressler, B.; Francisconi, C.; Vermeire, S.; Lawendy, N.; Salese, L.; Sawyerr, G.; Shi, H.; Su, C.; Judd, D.T.; et al. Assessment of Safety and Efficacy of Tofacitinib, Stratified by Age, in Patients from the Ulcerative Colitis Clinical Program. Inflamm. Bowel Dis. 2022, 29, 27–41. [Google Scholar] [CrossRef]
- Olivera, P.A.; Lasa, J.S.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Safety of Janus Kinase Inhibitors in Patients with Inflammatory Bowel Diseases or Other Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1554–1573.e12. [Google Scholar] [CrossRef]
- Admin, S.; European Crohn’s and Colitis Organisation—ECCO—P260. An Analysis of Non-Melanoma Skin Cancer Rates in the Tofacitinib Ulcerative Colitis Clinical Programme. Available online: https://www.ecco-ibd.eu/publications/congress-abstracts/item/p260-an-analysis-of-non-melanoma-skin-cancer-rates-in-the-tofacitinib-ulcerative-colitis-clinical-programme.html (accessed on 30 May 2023).
- Vermeire, S.; Schreiber, S.; Petryka, R.; Kuehbacher, T.; Hebuterne, X.; Roblin, X.; Klopocka, M.; Goldis, A.; Wisniewska-Jarosinska, M.; Baranovsky, A.; et al. Clinical Remission in Patients with Moderate-to-Severe Crohn’s Disease Treated with Filgotinib (the FITZROY Study): Results from a Phase 2, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet 2017, 389, 266–275. [Google Scholar] [CrossRef]
- Schreiber, S.; Loftus, E.V.; Maaser, C.; Danese, S.; Rudolph, C.; Jongen, R.; Haas, A.D.; Oortwijn, A.; Vermeire, S. Su1565: Efficacy and Safety of Filgotinib in Patients with Ulcerative Colitis Stratified by Age: Post Hoc Analysis of the Phase 2b/3 Selection and Selectionlte Studies. Gastroenterology 2022, 162, S-632–S-633. [Google Scholar] [CrossRef]
- Van der Heijde, D.; Baraliakos, X.; Gensler, L.S.; Maksymowych, W.P.; Tseluyko, V.; Nadashkevich, O.; Abi-Saab, W.; Tasset, C.; Meuleners, L.; Besuyen, R.; et al. Efficacy and Safety of Filgotinib, a Selective Janus Kinase 1 Inhibitor, in Patients with Active Ankylosing Spondylitis (TORTUGA): Results from a Randomised, Placebo-Controlled, Phase 2 Trial. Lancet 2018, 392, 2378–2387. [Google Scholar] [CrossRef] [Green Version]
- Mease, P.; Coates, L.C.; Helliwell, P.S.; Stanislavchuk, M.; Rychlewska-Hanczewska, A.; Dudek, A.; Abi-Saab, W.; Tasset, C.; Meuleners, L.; Harrison, P.; et al. Efficacy and Safety of Filgotinib, a Selective Janus Kinase 1 Inhibitor, in Patients with Active Psoriatic Arthritis (EQUATOR): Results from a Randomised, Placebo-Controlled, Phase 2 Trial. Lancet 2018, 392, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Hibi, T.; Motoya, S.; Hisamatsu, T.; Hirai, F.; Watanabe, K.; Matsuoka, K.; Saruta, M.; Kobayashi, T.; Feagan, B.G.; Tasset, C.; et al. Efficacy and Safety of Filgotinib as Induction and Maintenance Therapy for Japanese Patients with Moderately to Severely Active Ulcerative Colitis: A Post-Hoc Analysis of the Phase 2b/3 SELECTION Trial. Intest. Res. 2023, 21, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Galapagos, N.V. A Long-Term Extension Study to Evaluate the Safety of Filgotinib in Subjects with Crohn’s Disease; U.S. National Library of Medicine: Washington, DC, USA, 2023.
- Galapagos, N.V. Combined Phase 3, Double-Blind, Randomized, Placebo-Controlled Studies Evaluating the Efficacy and Safety of Filgotinib in the Induction and Maintenance of Remission in Subjects with Moderately to Severely Active Crohn’s Disease; U.S. National Library of Medicine: Washington, DC, USA, 2022.
- Galapagos, N.V. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study to Evaluate the Testicular Safety of Filgotinib in Adult Males with Moderately to Severely Active Inflammatory Bowel Disease; U.S. National Library of Medicine: Washington, DC, USA, 2022.
- Mariette, X.; Aspeslagh, S.; Moriggl, R.; Rajendran, V.; Rudolph, C.; Van Hoek, P.; Verbruggen, N.; Watson, C.; Borchmann, S.; Stallmach, A. Malignancy Events in the Filgotinib Rheumatoid Arthritis and Ulcerative Colitis Clinical Development Programs. Arthritis Rheumatol. 2022, 74, 528–531. [Google Scholar]
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hébuterne, X.; D’Haens, G.; Nakase, H.; Panés, J.; et al. Upadacitinib as Induction and Maintenance Therapy for Moderately to Severely Active Ulcerative Colitis: Results from Three Phase 3, Multicentre, Double-Blind, Randomised Trials. Lancet 2022, 399, 2113–2128. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V.; Panés, J.; Lacerda, A.P.; Peyrin-Biroulet, L.; D’Haens, G.; Panaccione, R.; Reinisch, W.; Louis, E.; Chen, M.; Nakase, H.; et al. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2023, 388, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, R.; Collins, E.B.; Melmed, G.; Vermeire, S.; Danese, S.; Higgins, P.D.; Zhou, W.; Ilo, D.; Sharma, D.; Gonzalez, Y.S.; et al. Tu1449: Efficacy and Safety of Advanced Induction and Maintenance Therapies in Patients with Moderately to Severely Active Ulcerative Colitis: An Indirect Treatment Comparison Using Bayesian Network Meta-Analysis. Gastroenterology 2022, 162, S-965. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Wolf, D.C.; D’Haens, G.; Vermeire, S.; Hanauer, S.B.; Ghosh, S.; Smith, H.; Cravets, M.; Frohna, P.A.; et al. Ozanimod Induction and Maintenance Treatment for Ulcerative Colitis. N. Engl. J. Med. 2016, 374, 1754–1762. [Google Scholar] [CrossRef]
- Panaccione, R.; Danese, S.; Wolf, D.C.; Canavan, J.B.; Jain, A.; Wu, H.; Petersen, A.; Charles, L.; Afzali, A.; Abreu, M.T. P405 Long-Term Safety of 3 Years of Ozanimod in Moderately to Severely Active Ulcerative Colitis: An Interim Analysis of the True North Open-Label Extension. J. Crohns Colitis 2023, 17, i534–i535. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Pellicano, R.; Vernero, M.; Caviglia, G.P.; Saracco, G.M.; Morino, M.; Astegiano, M. Dual Biological Therapy with Anti-TNF, Vedolizumab or Ustekinumab in Inflammatory Bowel Disease: A Systematic Review with Pool Analysis. Scand. J. Gastroenterol. 2019, 54, 407–413. [Google Scholar] [CrossRef]
- Sands, B.E.; Kozarek, R.; Spainhour, J.; Barish, C.F.; Becker, S.; Goldberg, L.; Katz, S.; Goldblum, R.; Harrigan, R.; Hilton, D.; et al. Safety and Tolerability of Concurrent Natalizumab Treatment for Patients with Crohn’s Disease Not in Remission While Receiving Infliximab. Inflamm. Bowel Dis. 2007, 13, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feagan, B.G.; Sands, B.E.; Sandborn, W.J.; Germinaro, M.; Vetter, M.; Shao, J.; Sheng, S.; Johanns, J.; Panés, J.; Tkachev, A.; et al. Guselkumab plus Golimumab Combination Therapy versus Guselkumab or Golimumab Monotherapy in Patients with Ulcerative Colitis (VEGA): A Randomised, Double-Blind, Controlled, Phase 2, Proof-of-Concept Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 307–320. [Google Scholar] [CrossRef]
- Alayo, Q.A.; Fenster, M.; Altayar, O.; Glassner, K.L.; Llano, E.; Clark-Snustad, K.; Patel, A.; Kwapisz, L.; Yarur, A.J.; Cohen, B.L.; et al. Systematic Review with Meta-Analysis: Safety and Effectiveness of Combining Biologics and Small Molecules in Inflammatory Bowel Disease. Crohns Colitis 360 2022, 4, otac002. [Google Scholar] [CrossRef] [PubMed]
- Berinstein, E.M.; Sheehan, J.L.; Jacob, J.; Steiner, C.A.; Stidham, R.W.; Shannon, C.; Bishu, S.; Levine, J.; Cohen-Mekelburg, S.A.; Waljee, A.K.; et al. Efficacy and Safety of Dual Targeted Therapy for Partially or Non-Responsive Inflammatory Bowel Disease: A Systematic Review of the Literature. Dig. Dis. Sci. 2023, 68, 2604–2623. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L. Management of Inflammatory Bowel Disease Patients with a Cancer History. Curr. Drug Targets 2014, 15, 1042–1048. [Google Scholar] [CrossRef]
- Holmer, A.K.; Luo, J.; Russ, K.B.; Park, S.; Yang, J.Y.; Ertem, F.; Dueker, J.; Nguyen, V.; Hong, S.; Zenger, C.; et al. Comparative Safety of Biologic Agents in Patients with Inflammatory Bowel Disease with Active or Recent Malignancy: A Multi-Center Cohort Study. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2023, 21, 1598–1606.e5. [Google Scholar] [CrossRef] [PubMed]
- Poullenot, F.; Amiot, A.; Nachury, M.; Viennot, S.; Altwegg, R.; Bouhnik, Y.; Abitbol, V.; Nancey, S.; Vuitton, L.; Peyrin-Biroulet, L.; et al. Comparative Risk of Incident Cancer in Patients with Inflammatory Bowel Disease with Prior Non-Digestive Malignancy According to Immunomodulator: A Multicentre Cohort Study. J. Crohns Colitis 2022, 16, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Vedamurthy, A.; Gangasani, N.; Ananthakrishnan, A.N. Vedolizumab or Tumor Necrosis Factor Antagonist Use and Risk of New or Recurrent Cancer in Patients with Inflammatory Bowel Disease with Prior Malignancy: A Retrospective Cohort Study. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 88–95. [Google Scholar] [CrossRef]
- Hong, S.J.; Zenger, C.; Pecoriello, J.; Pang, A.; Vallely, M.; Hudesman, D.P.; Chang, S.; Axelrad, J.E. Ustekinumab and Vedolizumab Are Not Associated with Subsequent Cancer in IBD Patients with Prior Malignancy. Inflamm. Bowel Dis. 2022, 28, 1826–1832. [Google Scholar] [CrossRef]
- Hasan, B.; Tandon, K.S.; Miret, R.; Khan, S.; Riaz, A.; Gonzalez, A.; Rahman, A.U.; Charles, R.; Narula, N.; Castro, F.J. Ustekinumab Does Not Increase Risk of New or Recurrent Cancer in Inflammatory Bowel Disease Patients with Prior Malignancy. J. Gastroenterol. Hepatol. 2022, 37, 1016–1021. [Google Scholar] [CrossRef]
- Khan, N.; Patel, D.; Trivedi, C.; Kavani, H.; Medvedeva, E.; Pernes, T.; Xie, D.; Lewis, J.; Yang, Y.-X. Repeated Occurrences of Basal Cell Cancer in Patients with Inflammatory Bowel Disease Treated with Immunosuppressive Medications. Am. J. Gastroenterol. 2020, 115, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Shelton, E.; Laharie, D.; Scott, F.I.; Mamtani, R.; Lewis, J.D.; Colombel, J.-F.; Ananthakrishnan, A.N. Cancer Recurrence Following Immune-Suppressive Therapies in Patients with Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2016, 151, 97–109.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ressing, M.; Blettner, M.; Klug, S.J. Data Analysis of Epidemiological Studies. Dtsch. Arztebl. Int. 2010, 107, 187–192. [Google Scholar] [CrossRef] [PubMed]
| Study | Year | Country | Study Design | Main Findings |
|---|---|---|---|---|
| Ekbom et al. [17] | 1991 | Sweden | Population-based study | CD: increased risk of squamous skin cancer (SIR 5.5). UC: increased risk of connective tissue (SIR 4) and brain (SIR 2.4) cancers. |
| Persson et al. [18] | 1994 | Sweden | Population-based study | Increased risk of bladder, lung, brain, and skin cancers. |
| Mellemkjaer et al. [19] | 1995 | Denmark | Population-based study | Increased risk of NMSC (RR 1.4) and hepatobiliary cancers (RR 2.3). |
| Bernstein et al. [20] | 2001 | Canada | Population-based study | Increased risk of liver and biliary tract cancers (IRR 3.96–5.22). |
| Jess et al. [21] | 2004 | Denmark | Population-based study | No increased risk of EICs. |
| Bhatia et al. [22] | 2006 | USA | Retrospective study | Correlation between IBD and cervical abnormalities. |
| Hemminki et al. [23] | 2008 | Sweden | Population-based study | UC: increased risk of liver (SIR 4.30), breast (SIR 1.25), and prostate (SIR 1.14) cancers. |
| Hemminki et al. [24] | 2009 | Sweden | Population-based study | CD: increased risk of liver, testis, and kidney cancers (SIR > 2). |
| Singh et al. [25] | 2009 | Canada | Population-based study | No association between IBD and cervical abnormalities. |
| Erichsen et al. [26] | 2009 | Denmark | Cohort study | Increased risk of cholangiocarcinoma (four-fold). |
| Lees et al. [27] | 2009 | UK | Case-control study | No increased risk of cervical abnormalities. |
| Long et al. [28] | 2012 | USA | Retrospective study | Increased risk of melanoma (IRR 1.29) and NMSC (IR 1.46). |
| Jussila et al. [29] | 2013 | Finland | Population-based study | CD: increased risk of biliary tract cancers (SIR 4.93). UC: increased risk of thyroid (SIR 1.93) and biliary tract (SIR 7.23) cancers. IBD: increased risk of basal cell skin cancers (SIR 1.29). |
| Jess et al. [30] | 2013 | Denmark | Population-based study | CD: increased risk of lung cancer (SIR 2.13) and cervical dysplasia (SIR 1.65). UC: increased risk of prostate cancer (SIR 1.82). |
| Kappelman et al. [31] | 2014 | Denmark | Population-based study | IBD: increased risk of smoking-related cancers (SIR 1.5), melanoma (SIR 1.4), and NMSC (SIR 1.8). CD: increased risk of gallbladder and biliary tract (SIR 2.4) cancers. UC: increased risk of liver (SIR 1.6) and gallbladder (SIR 2.5) cancers. |
| Rungoe et al. [32] | 2015 | Denmark | Population-based study | Increased risk of SILs and cervical cancers (IRR 1.15–1.55). |
| Kim et al. [33] | 2015 | USA | Population-based study | No association between IBD and cervical abnormalities. |
| van den Heuvel et al. [34] | 2016 | The Netherlands | Population-based study | CD: increased risk of skin cancer (SIR 1.55); decreased risk of breast cancer (SIR 0.11). UC: no increased risk of EICs. |
| Wilson et al. [35] | 2016 | UK | Population-based study | No increased risk of EICs. |
| Madanchi et al. [36] | 2016 | Switzerland | Retrospective study | Increased risk of urothelial cancer and cholangiocarcinoma. |
| Wadhwa et al. [37] | 2016 | USA | Case-control study | Increased risk of thyroid cancer (OR 1.97). |
| Hovde et al. [38] | 2017 | Norway | Population-based study | CD: increased risk of trachea/lungs cancer (SIR 2.91). UC: increased risk of breast (SIR 2) and liver/biliary (SIR 2.85) cancer; reduced risk of lung cancer (SIR = 0.79). |
| So et al. [39] | 2017 | China | Population-based study | CD: increased risk of renal-cell carcinoma (SIR 6.89), head and neck and CNS cancer (SIR 5.08), and NMSC (SIR 13.88). UC: increased risk of prostate cancer (SIR 2.47) and NMSC (SIR 9.05). |
| Jung et al. [40] | 2017 | Republic of Korea | Population-based study | CD: increased risk of liver (SIR 15.3) and pancreatic (SIR 8.6) cancers for women. UC: increased risk of prostate (SIR 3.5), CNS (SIR 6.1), and thyroid (SIR 2.2) for men; liver (SIR 4.4) and cervix uteri (SIR 5.7) for women. |
| Mosher et al. [41] | 2018 | USA | Case-control study | Increased risk of NMSC (RR 2.38), melanoma skin (RR 2.85), renal (RR 2.9), prostate (RR 1.7), and pancreatic (RR 4.23) cancers. |
| Loo et al. [42] | 2019 | Canada | Population-based study | Increased risk of breast (SIR 1.13), respiratory (SIR 1.16) cancers, and NMSC (SIR 22.62). |
| Burns et al. [43] | 2019 | USA | Retrospective study | Increased risk of prostate cancers (HR 4.84). |
| Taborelli et al. [44] | 2020 | Italy | Population-based study | UC: increased risk of corpus uteri (SIR 2.67) and kidney (SIR 2.06) cancers. CD: increased risk of thyroid cancer (SIR 5.58) and NMSC (SIR 1.86). |
| Everhov et al. [45] | 2020 | Denmark, Sweden | Population-based study | Increased risk of pancreatic cancer (SIR 9.04). |
| Wang et al. [46] | 2021 | China | Cohort study | Thyroid, cervical, hepatobiliary, and urinary tract cancers were the most common EICs. Patients with elderly-onset IBD are at higher risk of EICs (RR 2.83). |
| Goetgebuer et al. [47] | 2021 | The Netherlands | Case-control study | Increased risk of CIN2+ (SDR 1.27). |
| Wang et al. [48] | 2022 | United States | Population-based study | IBD: increased risk of lung cancer (OR 1.14) and melanoma (OR 1.19). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massano, A.; Bertin, L.; Zingone, F.; Buda, A.; Visaggi, P.; Bertani, L.; de Bortoli, N.; Fassan, M.; Scarpa, M.; Ruffolo, C.; et al. Extraintestinal Cancers in Inflammatory Bowel Disease: A Literature Review. Cancers 2023, 15, 3824. https://doi.org/10.3390/cancers15153824
Massano A, Bertin L, Zingone F, Buda A, Visaggi P, Bertani L, de Bortoli N, Fassan M, Scarpa M, Ruffolo C, et al. Extraintestinal Cancers in Inflammatory Bowel Disease: A Literature Review. Cancers. 2023; 15(15):3824. https://doi.org/10.3390/cancers15153824
Chicago/Turabian StyleMassano, Alessandro, Luisa Bertin, Fabiana Zingone, Andrea Buda, Pierfrancesco Visaggi, Lorenzo Bertani, Nicola de Bortoli, Matteo Fassan, Marco Scarpa, Cesare Ruffolo, and et al. 2023. "Extraintestinal Cancers in Inflammatory Bowel Disease: A Literature Review" Cancers 15, no. 15: 3824. https://doi.org/10.3390/cancers15153824
APA StyleMassano, A., Bertin, L., Zingone, F., Buda, A., Visaggi, P., Bertani, L., de Bortoli, N., Fassan, M., Scarpa, M., Ruffolo, C., Angriman, I., Bezzio, C., Casini, V., Ribaldone, D. G., Savarino, E. V., & Barberio, B. (2023). Extraintestinal Cancers in Inflammatory Bowel Disease: A Literature Review. Cancers, 15(15), 3824. https://doi.org/10.3390/cancers15153824











