Reassessing Breast Cancer-Associated Fibroblasts (CAFs) Interactions with Other Stromal Components and Clinico-Pathologic Parameters by Using Immunohistochemistry and Digital Image Analysis (DIA)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Selection of Patients and Ethical Considerations
2.2. Initial Processing, Histology, and Selection of Formalin-Fixed Paraffin-Embedded (FFPE) Specimens for Immunohistochemistry
2.3. Immunohistochemistry
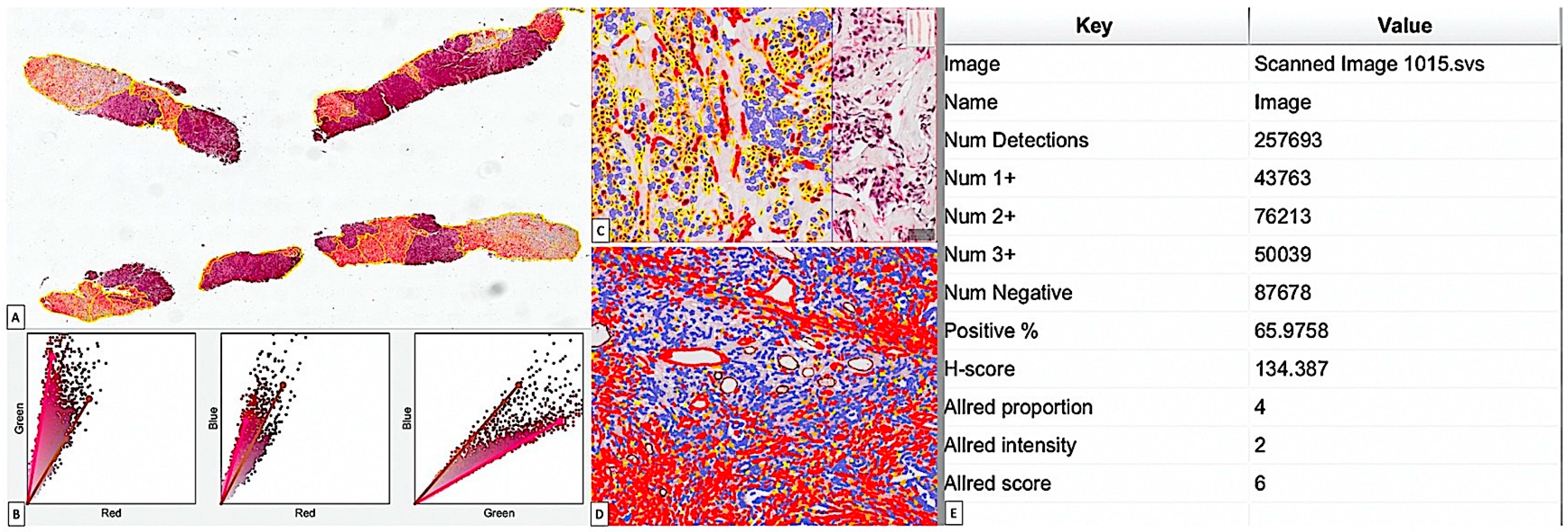
2.4. Image Acquisition and Digital Image Analysis (DIA)
2.5. Statistical Analysis
3. Results
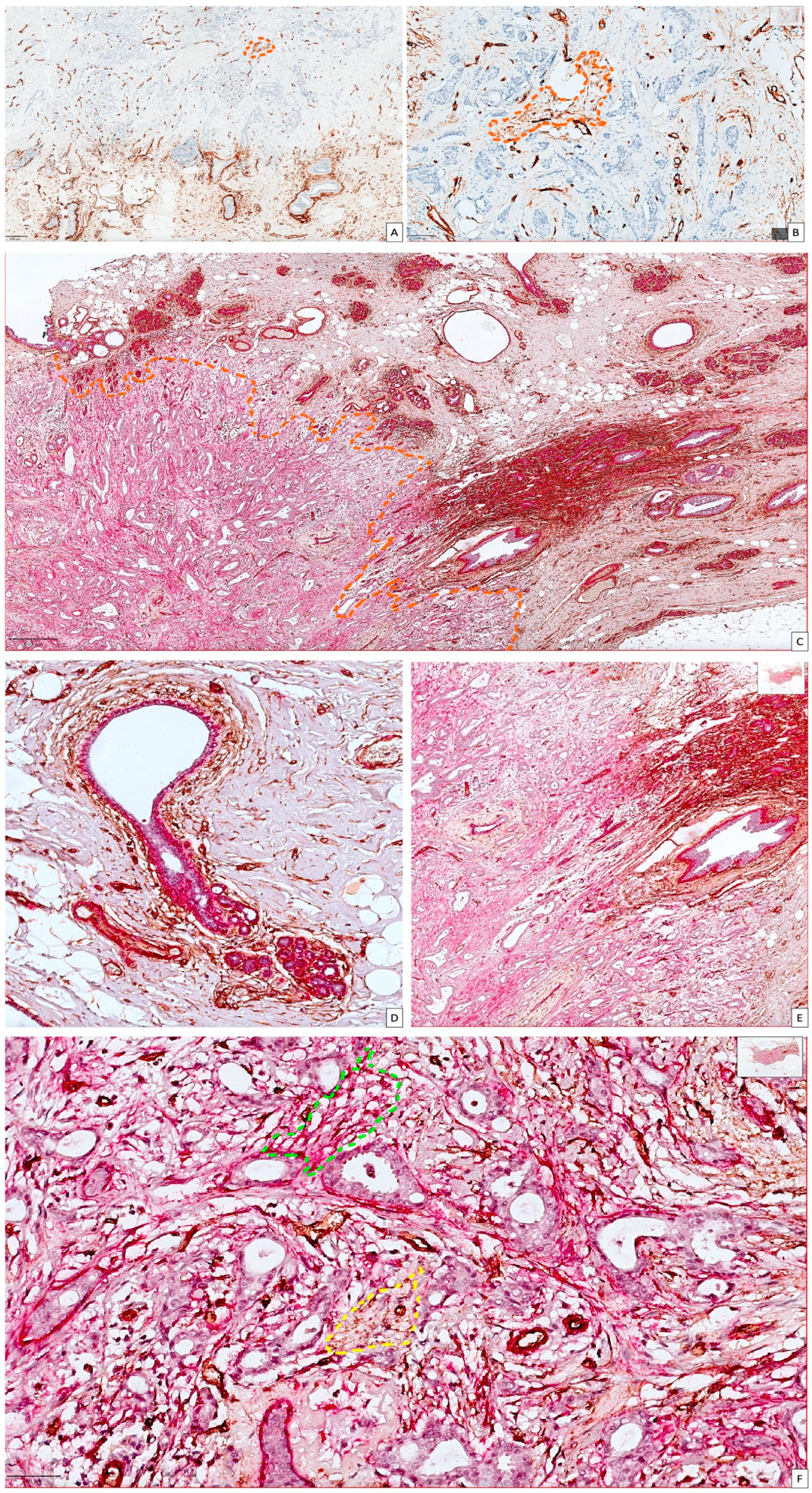
3.1. General Considerations on Conventional Microscopic Assessment of Stromal CD34 and αSMA in Normal Breast Tissue and BC
3.2. Critical Overview of Digital Image Analysis for BC Stromal CAF Assessment by Using Immunohistochemistry and QuPath Platform
3.2.1. αSMA_CAF Digital Image Analysis
3.2.2. CD34_CAF Image Analysis
3.3. DIA Impact on Stromal CD34/αSMA_CAF Assessment Related to BC Molecular Subtypes and Clinic-Pathologic Data
3.3.1. CD34/αSMA_CAF Interplay Is Highly Dependent on Age and Strongly Influences Survival and Stromal Components in Luminal A (LA_BC) Subtype
3.3.2. αSMA_SS Variability Is Age-Dependent but Also Influences Tumor Grade (G), TLS, and Immature Tumor Blood Vessels Dynamics for Luminal B (LB)_BC Subtype
3.3.3. HER2_BC Subtype Is CD34_CAF-Dependent but Not Influenced by αSMA_CAFs
3.3.4. Luminal B-HER2 (LB-HER2) BC Is Influenced by Both Types of CAFs, but Each of Them Had Significant Impact on Different Stromal Vascular Components and Clinic-Pathologic Parameters
3.3.5. CD34_CAFs Are Key Players of TNBC_BC Stroma Influencing G, NPI, Invasion, Recurrence, and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, S.; Zou, Y.; Tang, Y.; Yang, A.; Liang, J.Y.; Wu, L.; Tian, W.; Xiao, W.; Xie, X.; Yang, L.; et al. Landscape of cancer-associated fibroblasts identifies the secreted biglycan as a protumor and immunosuppressive factor in triple-negative breast cancer. Oncoimmunology 2022, 11, 2020984. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, S.; Zou, Y.; Tang, Y.; Tian, W.; Wong, C.W.; Wu, S.; Ou, X.; Zhao, W.; Cai, M.; et al. Turning up a new pattern: Identification of cancer-associated fibroblast-related clusters in TNBC. Front. Immunol. 2022, 13, 1022147. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Liang, J.Y.; Tang, Y.; Xie, J.; Zou, Y.; Yang, A.; Shao, N.; Kuang, X.; Ji, F.; Liu, X.; et al. Dissecting the role of cancer-associated fibroblast-derived biglycan as a potential therapeutic target in immunotherapy resistance: A tumor bulk and single-cell transcriptomic study. Clin. Transl. Med. 2023, 13, e1189. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ye, F.; Kong, Y.; Hu, X.; Deng, X.; Xie, J.; Song, C.; Ou, X.; Wu, S.; Wu, L.; et al. The Single-Cell Landscape of Intratumoral Heterogeneity and The Immunosuppressive Microenvironment in Liver and Brain Metastases of Breast Cancer. Adv. Sci. 2023, 10, e2203699. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Zou, X.; Li, M.; Zhang, H.; Du, Y.; Wang, J.; Peng, C.; Dong, C.; Hou, Z. CHST2-mediated sulfation of MECA79 antigens is critical for breast cancer cell migration and metastasis. Cell Death Dis. 2023, 14, 288. [Google Scholar] [CrossRef]
- Cîmpean, A.M.; Raica, M.; Nariţa, D. Diagnostic significance of the immunoexpression of CD34 and smooth muscle cell actin in benign and malignant tumors of the breast. Rom. J. Morphol. Embryol. 2005, 46, 123–129. [Google Scholar]
- Barth, P.J.; Ebrahimsade, S.; Ramaswamy, A.; Moll, R. CD34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Arch. 2002, 440, 298–303. [Google Scholar] [CrossRef]
- Yazhou, C.; Wenlv, S.; Weidong, Z.; Licun, W. Clinicopathological significance of stromal myofibroblasts in invasive ductal carcinoma of the breast. Tumor Biol. 2004, 25, 290–295. [Google Scholar] [CrossRef]
- Chauhan, H.; Abraham, A.; Phillips, J.R.; Pringle, J.H.; Walker, R.A.; Jones, J.L. There is more than one kind of myofibroblast: Analysis of CD34 expression in benign, in situ, and invasive breast lesions. J. Clin. Pathol. 2003, 56, 271–276. [Google Scholar] [CrossRef][Green Version]
- Catteau, X.; Simon, P.; Vanhaeverbeek, M.; Noël, J.C. Variable stromal periductular expression of CD34 and smooth muscle actin (SMA) in intraductal carcinoma of the breast. PLoS ONE 2013, 8, e57773. [Google Scholar] [CrossRef]
- Catteau, X.; Simon, P.; Jondet, M.; Vanhaeverbeek, M.; Noël, J.C. Quantification of stromal reaction in breast carcinoma and its correlation with tumor grade and free progression survival. PLoS ONE 2019, 14, e0210263. [Google Scholar] [CrossRef]
- Artacho-Cordón, A.; Artacho-Cordón, F.; Ríos-Arrabal, S.; Calvente, I.; Núñez, M.I. Tumor microenvironment and breast cancer progression: A complex scenario. Cancer Biol. Ther. 2012, 13, 14–24. [Google Scholar] [CrossRef]
- Martins, D.; Schmitt, F. Microenvironment in breast tumorigenesis: Friend or foe? Histol. Histopathol. 2019, 34, 13–24. [Google Scholar] [CrossRef]
- Onuchic, V.; Hartmaier, R.J.; Boone, D.N.; Samuels, M.L.; Patel, R.Y.; White, W.M.; Garovic, V.D.; Oesterreich, S.; Roth, M.E.; Lee, A.V.; et al. Epigenomic Deconvolution of Breast Tumors Reveals Metabolic Coupling between Constituent Cell Types. Cell Rep. 2016, 17, 2075–2086. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Lee, Y.K.; Koo, J.S. Expression of cancer-associated fibroblast-related proteins in adipose stroma of breast cancer. Tumor Biol. 2015, 36, 8685–8695. [Google Scholar] [CrossRef]
- Andrade de Oliveira, K.; Sengupta, S.; Yadav, A.K.; Clarke, R. The complex nature of heterogeneity and its roles in breast cancer biology and therapeutic responsiveness. Front. Endocrinol. 2023, 14, 1083048. [Google Scholar] [CrossRef]
- Westhoff, C.C.; Jank, P.; Jacke, C.O.; Albert, U.S.; Ebrahimsade, S.; Barth, P.J.; Moll, R. Prognostic relevance of the loss of stromal CD34 positive fibroblasts in invasive lobular carcinoma of the breast. Virchows Arch. 2020, 477, 717–724. [Google Scholar] [CrossRef]
- Westhoff, C.C.; Jacke, C.O.; Albert, U.S.; Moll, R. Stromal subtypes of invasive lobular carcinoma of the breast: Absence of CD34-positive stromal fibrocytes correlate with presence of α-smooth muscle actin-positive myofibroblasts. In Der Pathologe; Moch, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 39, (Suppl. S2). [Google Scholar]
- Dzobo, K.; Senthebane, D.A.; Dandara, C. The Tumor Microenvironment in Tumorigenesis and Therapy Resistance Revisited. Cancers 2023, 15, 376. [Google Scholar] [CrossRef]
- Forsare, C.; Vistrand, S.; Ehinger, A.; Lövgren, K.; Rydén, L.; Fernö, M.; Narbe, U. The Prognostic Role of Intratumoral Stromal Content in Lobular Breast Cancer. Cancers 2022, 14, 941. [Google Scholar] [CrossRef]
- Elwakeel, E.; Weigert, A. Breast Cancer CAFs: Spectrum of Phenotypes and Promising Targeting Avenues. Int. J. Mol. Sci. 2021, 22, 11636. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González, M.; Sáez, F.J.; Aparicio, F.; Díaz-Flores, L., Jr.; Madrid, J.F. Human resident CD34+ stromal cells/telocytes have progenitor capacity and are a source of αSMA+ cells during repair. Histol. Histopathol. 2015, 30, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.J.; Waise, S.; Ellis, M.J.; Lopez, M.A.; Pun, W.Y.; Taylor, J.; Parker, R.; Kimbley, L.M.; Chee, S.J.; Shaw, E.C.; et al. Single-cell analysis reveals prognostic fibroblast subpopulations linked to molecular and immunological subtypes of lung cancer. Nat. Commun. 2023, 14, 387. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; González-Gómez, M.; García, M.D.P.; Palmas, M.; Carrasco, J.L.; Madrid, J.F.; Díaz-Flores, L., Jr. Delimiting CD34+ Stromal Cells/Telocytes Are Resident Mesenchymal Cells That Participate in Neovessel Formation in Skin Kaposi Sarcoma. Int. J. Mol. Sci. 2023, 24, 3793. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; Sáez, F.J.; Díaz-Flores, L., Jr.; Valladares, F.; Madrid, J.F. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol. Histopathol. 2014, 29, 831–870. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González-Gómez, M.; Rodríguez-Rodriguez, R.; Hernández-León, N.; Díaz-Flores, L., Jr.; Carrasco, J.L. Cd34+ Stromal Cells/Telocytes in Normal and Pathological Skin. Int. J. Mol. Sci. 2021, 22, 7342. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; González-Gómez, M.; García, M.P.; Díaz-Flores, L., Jr.; Carrasco, J.L.; Martín-Vasallo, P. CD34+ Stromal Cells/Telocytes as a Source of Cancer-Associated Fibroblasts (CAFs) in Invasive Lobular Carcinoma of the Breast. Int. J. Mol. Sci. 2021, 22, 3686. [Google Scholar] [CrossRef]
- Bonacho, T.; Rodrigues, F.; Liberal, J. Immunohistochemistry for diagnosis and prognosis of breast cancer: A review. Biotech. Histochem. 2020, 95, 71–91. [Google Scholar] [CrossRef]
- Ray, U.; Pathoulas, C.L.; Thirusangu, P.; Purcell, J.W.; Kannan, N.; Shridhar, V. Exploiting LRRC15 as a Novel Therapeutic Target in Cancer. Cancer Res. 2022, 82, 1675–1681. [Google Scholar] [CrossRef]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef]
- Jiang, P.; Chen, Y.; Liu, B. Prognostic Efficacy of Tumor-Stroma Ratio in Women with Breast Cancer: A Meta-Analysis of Cohort Studies. Front. Oncol. 2021, 11, 731409. [Google Scholar] [CrossRef]
- Yan, D.; Ju, X.; Luo, B.; Guan, F.; He, H.; Yan, H.; Yuan, J. Tumour stroma ratio is a potential predictor for 5-year disease-free survival in breast cancer. BMC Cancer 2022, 22, 1082. [Google Scholar] [CrossRef]
- Le, M.K.; Odate, T.; Kawai, M.; Oishi, N.; Kondo, T. Investigating the role of core needle biopsy in evaluating tumor-stroma ratio (TSR) of invasive breast cancer: A retrospective study. Breast Cancer Res. Treat. 2023, 197, 113–121. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, Y.Y.; Luo, Y.H.; Zheng, J.S.; Lin, Z.H.; Xiong, B.; Wang, L.W. Proposal of an automated tumor-stromal ratio assessment algorithm and a nomogram for prognosis in early-stage invasive breast cancer. Cancer Med. 2023, 12, 131–145. [Google Scholar] [CrossRef]
- Qian, X.; Xiao, F.; Chen, Y.Y.; Yuan, J.P.; Liu, X.H.; Wang, L.W.; Xiong, B. Computerized Assessment of the Tumor-stromal Ratio and Proposal of a Novel Nomogram for Predicting Survival in Invasive Breast Cancer. J. Cancer 2021, 12, 3427–3438. [Google Scholar] [CrossRef]
- Hanna, M.G.; Pantanowitz, L.; Evans, A.J. Overview of contemporary guidelines in digital pathology: What is available in 2015 and what still needs to be addressed? J. Clin. Pathol. 2015, 68, 499–505. [Google Scholar] [CrossRef]
- Samuelson, M.I.; Chen, S.J.; Boukhar, S.A.; Schnieders, E.M.; Walhof, M.L.; Bellizzi, A.M.; Robinson, R.A.; Rajan, K.D.A. Rapid Validation of Whole-Slide Imaging for Primary Histopathology Diagnosis. Am. J. Clin. Pathol. 2021, 155, 638–648. [Google Scholar] [CrossRef]
- Millar, E.K.; Browne, L.H.; Beretov, J.; Lee, K.; Lynch, J.; Swarbrick, A.; Graham, P.H. Tumour Stroma Ratio Assessment Using Digital Image Analysis Predicts Survival in Triple Negative and Luminal Breast Cancer. Cancers 2020, 12, 3749. [Google Scholar] [CrossRef]
- Wu, D.; Hacking, S.M.; Chavarria, H.; Abdelwahed, M.; Nasim, M. Computational portraits of the tumoral microenvironment in human breast cancer. Virchows Arch. 2022, 481, 367–385. [Google Scholar] [CrossRef]
- Lumongga, F.; Dharmajaya, R.; Siregar, K.B.; Delyuzar; Handjari, D.R.; Jusuf, N.K.; Munir, D.; Asrul. Correlation between Intensity of Vimentin Immuno-expression in Young Women with Triple Negative Breast Cancer and Its Cliniocopathological Parameters. Med. Arch. 2022, 76, 454–457. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Available online: https://pubmed.ncbi.nlm.nih.gov/?term=QuPath+breast+stroma (accessed on 22 March 2023).
- Yamaguchi, K.; Hara, Y.; Kitano, I.; Hamamoto, T.; Kiyomatsu, K.; Yamasaki, F.; Egashira, R.; Nakazono, T.; Irie, H. Tumor-stromal ratio (TSR) of invasive breast cancer: Correlation with multi-parametric breast MRI findings. Br. J. Radiol. 2019, 92, 20181032. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Bae, S.J.; Eun, N.; Ahn, S.G.; Jeong, J.; Cha, Y.J. Correlation of Yes-Associated Protein 1 with Stroma Type and Tumor Stiffness in Hormone-Receptor Positive Breast Cancer. Cancers 2022, 14, 4971. [Google Scholar] [CrossRef]
- Vangangelt, K.M.H.; Tollenaar, L.S.A.; van Pelt, G.W.; de Kruijf, E.M.; Dekker, T.J.A.; Kuppen, P.J.K.; Tollenaar, R.A.E.M.; Mesker, W.E. The prognostic value of tumor-stroma ratio in tumor-positive axillary lymph nodes of breast cancer patients. Int. J. Cancer 2018, 143, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yuan, J.P.; Chen, Y.Y.; Zhang, H.Y.; Wang, L.W.; Xiong, B. Prognostic Significance of the Tumor-Stromal Ratio in Invasive Breast Cancer and a Proposal of a New Ts-TNM Staging System. J. Oncol. 2020, 2020, 9050631. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Jiang, Z.; Ni, X.; Yang, S.; Jiao, P.; Wu, J.; Xiong, L.; Yuan, J.; Wang, J.; Jian, J.; et al. Machine Learning Quantified Tumor-Stroma Ratio Is an Independent Prognosticator in Muscle-Invasive Bladder Cancer. Int. J. Mol. Sci. 2023, 24, 2746. [Google Scholar] [CrossRef]
- Frisbie, L.; Buckanovich, R.J.; Coffman, L. Carcinoma-Associated Mesenchymal Stem/Stromal Cells: Architects of the Pro-tumorigenic Tumor Microenvironment. Stem Cells 2022, 40, 705–715. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, T.; Xia, R.; Wei, Y.; Wei, X. Targeting the tumor stroma for cancer therapy. Mol. Cancer 2022, 21, 208. [Google Scholar] [CrossRef]
- Mishra, P.J.; Banerjee, D. Visualizing Activated Myofibroblasts Resulting from Differentiation of Mesenchymal Stem Cells. Methods Mol. Biol. 2023, 2593, 83–92. [Google Scholar] [CrossRef]
- Bhatia, J.K.; Chaudhary, T.; Boruah, D.; Bharadwaj, R. Study of angiogenesis in invasive breast carcinoma by morphometry and immunohistochemistry. Med. J. Armed Forces India 2022, 78, 345–354. [Google Scholar] [CrossRef]
- Bujor, I.S.; Cioca, A.; Ceaușu, R.A.; Veaceslav, F.; Nica, C.; Cîmpean, A.M.; Raica, M. Evaluation of Vascular Proliferation in Molecular Subtypes of Breast Cancer. In Vivo 2018, 32, 79–83. [Google Scholar] [CrossRef]
- D’Alfonso, T.M.; Subramaniyam, S.; Ginter, P.S.; Mosquera, J.M.; Croyle, J.; Liu, Y.F.; Rubin, M.A.; Shin, S.J. Characterization of CD34-deficient myofibroblastomas of the breast. Breast J. 2018, 24, 55–61. [Google Scholar] [CrossRef]
- Schulze, A.B.; Schmidt, L.H.; Heitkötter, B.; Huss, S.; Mohr, M.; Marra, A.; Hillejan, L.; Görlich, D.; Barth, P.J.; Rehkämper, J.; et al. Prognostic impact of CD34 and SMA in cancer-associated fibroblasts in stage I-III NSCLC. Thorac. Cancer 2020, 11, 120–129. [Google Scholar] [CrossRef]
- Barth, P.J.; Ebrahimsade, S.; Hellinger, A.; Moll, R.; Ramaswamy, A. CD34+ fibrocytes in neoplastic and inflammatory pancreatic lesions. Virchows Arch. 2002, 440, 128–133. [Google Scholar] [CrossRef]
- Horn, L.C.; Schreiter, C.; Canzler, A.; Leonhardt, K.; Einenkel, J.; Hentschel, B. CD34(low) and SMA(high) represent stromal signature in uterine cervical cancer and are markers for peritumoral stromal remodeling. Ann. Diagn. Pathol. 2013, 17, 531–535. [Google Scholar] [CrossRef]
- Vathiotis, I.A.; Moutafi, M.K.; Divakar, P.; Aung, T.N.; Qing, T.; Fernandez, A.; Yaghoobi, V.; El-Abed, S.; Wang, Y.; Guillaume, S.; et al. Alpha-smooth Muscle Actin Expression in the Stroma Predicts Resistance to Trastuzumab in Patients with Early-stage HER2-positive Breast Cancer. Clin. Cancer Res. 2021, 27, 6156–6163. [Google Scholar] [CrossRef]
- Nguyen, M.; De Ninno, A.; Mencattini, A.; Mermet-Meillon, F.; Fornabaio, G.; Evans, S.S.; Cossutta, M.; Khira, Y.; Han, W.; Sirven, P.; et al. Dissecting Effects of Anti-cancer Drugs and Cancer-Associated Fibroblasts by On-Chip Reconstitution of Immunocompetent Tumor Microenvironments. Cell Rep. 2018, 25, 3884–3893.e3. [Google Scholar] [CrossRef]
- Ishii, G.; Ishii, T. Review of cancer-associated fibroblasts and their microenvironment in post-chemotherapy recurrence. Hum. Cell 2020, 33, 938–945. [Google Scholar] [CrossRef]
- San Martin, R.; Barron, D.A.; Tuxhorn, J.A.; Ressler, S.J.; Hayward, S.W.; Shen, X.; Laucirica, R.; Wheeler, T.M.; Gutierrez, C.; Ayala, G.E.; et al. Recruitment of CD34+ fibroblasts in tumor-associated reactive stroma: The reactive microvasculature hypothesis. Am. J. Pathol. 2014, 184, 1860–1870. [Google Scholar] [CrossRef]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020, 39, e104063. [Google Scholar] [CrossRef]
- Yamashita, M.; Ogawa, T.; Zhang, X.; Hanamura, N.; Kashikura, Y.; Takamura, M.; Yoneda, M.; Shiraishi, T. Role of stromal myofibroblasts in invasive breast cancer: Stromal expression of alpha-smooth muscle actin correlates with worse clinical outcome. Breast Cancer 2012, 19, 170–176. [Google Scholar] [CrossRef]
- Catteau, X.; Simon, P.; Noël, J.C. Myofibroblastic stromal reaction and lymph node status in invasive breast carcinoma: Possible role of the TGF-β1/TGF-βR1 pathway. BMC Cancer 2014, 14, 499. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.R.; Ahn, E.J.; Kim, Y.J.; Salam, S.M.A.; Noh, M.G.; Kim, S.S.; Jung, T.Y.; Kim, I.Y.; Kim, C.H.; Lee, K.H.; et al. Different Expression and Clinical Implications of Cancer-Associated Fibroblast (CAF) Markers in Brain Metastases. J. Cancer 2023, 14, 464–479. [Google Scholar] [CrossRef]
- Kennel, K.B.; Bozlar, M.; De Valk, A.F.; Greten, F.R. Cancer-Associated Fibroblasts in Inflammation and Antitumor Immunity. Clin. Cancer Res. 2023, 29, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Toullec, A.; Gerald, D.; Despouy, G.; Bourachot, B.; Cardon, M.; Lefort, S.; Richardson, M.; Rigaill, G.; Parrini, M.C.; Lucchesi, C.; et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol. Med. 2010, 2, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Benyahia, Z.; Dussault, N.; Cayol, M.; Sigaud, R.; Berenguer-Daizé, C.; Delfino, C.; Tounsi, A.; Garcia, S.; Martin, P.M.; Mabrouk, K.; et al. Stromal fibroblasts present in breast carcinomas promote tumor growth and angiogenesis through adrenomedullin secretion. Oncotarget 2017, 8, 15744–15762. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.e10. [Google Scholar] [CrossRef]
- Germain, C.; Devi-Marulkar, P.; Knockaert, S.; Biton, J.; Kaplon, H.; Letaïef, L.; Goc, J.; Seguin-Givelet, A.; Gossot, D.; Girard, N.; et al. Tertiary Lymphoid Structure-B Cells Narrow Regulatory T Cells Impact in Lung Cancer Patients. Front. Immunol. 2021, 12, 626776. [Google Scholar] [CrossRef]
- Bonneau, C.; Eliès, A.; Kieffer, Y.; Bourachot, B.; Ladoire, S.; Pelon, F.; Hequet, D.; Guinebretière, J.M.; Blanchet, C.; Vincent-Salomon, A.; et al. A subset of activated fibroblasts is associated with distant relapse in early luminal breast cancer. Breast Cancer Res. 2020, 22, 76. [Google Scholar] [CrossRef]
- Kieffer, Y.; Hocine, H.R.; Gentric, G.; Pelon, F.; Bernard, C.; Bourachot, B.; Lameiras, S.; Albergante, L.; Bonneau, C.; Guyard, A.; et al. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020, 10, 1330–1351. [Google Scholar] [CrossRef]
- Barb, A.C.; Pasca Fenesan, M.; Pirtea, M.; Margan, M.M.; Tomescu, L.; Melnic, E.; Cimpean, A.M. Tertiary Lymphoid Structures (TLSs) and Stromal Blood Vessels Have Significant and Heterogeneous Impact on Recurrence, Lymphovascular and Perineural Invasion amongst Breast Cancer Molecular Subtypes. Cells 2023, 12, 1176. [Google Scholar] [CrossRef]
- Available online: https://pubmed.ncbi.nlm.nih.gov/?term=CD34%20%2C%20smooth%20muscle%20actin%20breast%20cancer (accessed on 6 May 2023).
- Agrawal, S.; Fritchie, K.J.; Fernandez, A.P.; Ko, J.S.; Bergfeld, W.; Rubin, B.P.; Billings, S.D. The capillary lobule variant of radiation-associated angiosarcoma in the setting of breast cancer: A diagnostic pitfall. J. Cutan. Pathol. 2023, 50, 140–146. [Google Scholar] [CrossRef]
- Strait, A.M.; Bridge, J.A.; Iafrate, A.J.; Li, M.M.; Xu, F.; Tsongalis, G.J.; Linos, K. Mammary-type Myofibroblastoma with Leiomyomatous Differentiation: A Rare Variant with Potential Pitfalls. Int. J. Surg. Pathol. 2022, 30, 200–206. [Google Scholar] [CrossRef]
- Zhao, L.; Komforti, M.K.; Dawson, A.; Rowe, J.J. Periductal Stromal Tumor of the Breast: One Institution’s Review of 6 Tumors Over a 22 Year Period with Immunohistochemical Analysis. Int. J. Surg. Pathol. 2022, 30, 370–377. [Google Scholar] [CrossRef]
- Neuzillet, C.; Nicolle, R.; Raffenne, J.; Tijeras-Raballand, A.; Brunel, A.; Astorgues-Xerri, L.; Vacher, S.; Arbateraz, F.; Fanjul, M.; Hilmi, M.; et al. Periostin- and podoplanin-positive cancer-associated fibroblast subtypes cooperate to shape the inflamed tumor microenvironment in aggressive pancreatic adenocarcinoma. J. Pathol. 2022, 258, 408–425. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, T.; Yuan, Y.; Zhu, Y. What is new in cancer-associated fibroblast biomarkers? Cell Commun. Signal. 2023, 21, 96. [Google Scholar] [CrossRef]
- Saw, P.E.; Chen, J.; Song, E. Targeting CAFs to overcome anticancer therapeutic resistance. Trends Cancer 2022, 8, 527–555. [Google Scholar] [CrossRef]
- Du, R.; Zhang, X.; Lu, X.; Ma, X.; Guo, X.; Shi, C.; Ren, X.; Ma, X.; He, Y.; Gao, Y.; et al. PDPN positive CAFs contribute to HER2 positive breast cancer resistance to trastuzumab by inhibiting antibody-dependent NK cell-mediated cytotoxicity. Drug Resist. Updates 2023, 68, 100947. [Google Scholar] [CrossRef]
- Kato, T.; Furusawa, A.; Okada, R.; Inagaki, F.; Wakiyama, H.; Furumoto, H.; Fukushima, H.; Okuyama, S.; Choyke, P.L.; Kobayashi, H. Near-Infrared Photoimmunotherapy Targeting Podoplanin-Expressing Cancer Cells and Cancer-Associated Fibroblasts. Mol. Cancer Ther. 2023, 22, 75–88. [Google Scholar] [CrossRef]



| S_SMA_I | S_SMA_D | SMA_STROMAL SCORE | SMA_H-SCORE | S_CD34_I | S_CD34_D | ||
|---|---|---|---|---|---|---|---|
| SMA_STROMAL SCORE | Pearson’s r | 0.693 * | 0.189 | — | |||
| p-value | 0.038 | 0.626 | — | ||||
| 95% CI Upper | 0.929 | 0.758 | — | ||||
| 95% CI Lower | 0.054 | −0.543 | — | ||||
| Spearman’s rho | 0.709 * | 0.189 | — | ||||
| p-value | 0.032 | 0.626 | — | ||||
| Kendall’s Tau B | 0.685 * | 0.189 | — | ||||
| p-value | 0.045 | 0.593 | — | ||||
| SMA_H-SCORE | Pearson’s r | −0.079 | 0.000 | −0.095 | — | ||
| p-value | 0.839 | 1.000 | 0.808 | — | |||
| 95% CI Upper | 0.617 | 0.664 | 0.607 | — | |||
| 95% CI Lower | −0.706 | −0.664 | −0.714 | — | |||
| Spearman’s rho | 0.040 | 0.000 | 0 | — | |||
| p-value | 0.919 | 1.000 | 1 | — | |||
| Kendall’s Tau B | 0.075 | 0.000 | 0 | — | |||
| p-value | 0.801 | 1.000 | 1.000 | — | |||
| S_CD34_I | Pearson’s r | −0.105 | −0.286 | −0.378 | −0.113 | — | |
| p-value | 0.788 | 0.456 | 0.316 | 0.772 | — | ||
| 95% CI Upper | 0.601 | 0.467 | 0.382 | 0.596 | — | ||
| 95% CI Lower | −0.719 | −0.798 | −0.833 | −0.723 | — | ||
| Spearman’s rho | −0.124 | −0.286 | −0.378 | 0.000 | — | ||
| p-value | 0.751 | 0.456 | 0.316 | 1.000 | — | ||
| Kendall’s Tau B | −0.120 | −0.286 | −0.378 | 0.000 | — | ||
| p-value | 0.726 | 0.419 | 0.285 | 1.000 | — | ||
| S_CD34_D | Pearson’s r | 0.490 | −0.668 * | 0.000 | 0.363 | 0.134 | — |
| p-value | 0.180 | 0.049 | 1.000 | 0.337 | 0.732 | — | |
| 95% CI Upper | 0.871 | −0.007 | 0.664 | 0.828 | 0.733 | — | |
| 95% CI Lower | −0.258 | −0.923 | −0.664 | −0.397 | −0.582 | — | |
| Spearman’s rho | 0.551 | −0.624 | 0.100 | 0.329 | 0.113 | — | |
| p-value | 0.124 | 0.073 | 0.798 | 0.388 | 0.771 | — | |
| Kendall’s Tau B | 0.502 | −0.600 | 0.096 | 0.272 | 0.109 | — | |
| p-value | 0.127 | 0.078 | 0.777 | 0.355 | 0.748 | — | |
| CD34_STROMAL SCORE | Pearson’s r | 0.341 | −0.679 * | −0.189 | 0.234 | 0.607 | 0.869 ** |
| p-value | 0.370 | 0.044 | 0.626 | 0.544 | 0.083 | 0.002 | |
| 95% CI Upper | 0.819 | −0.026 | 0.543 | 0.778 | 0.906 | 0.972 | |
| 95% CI Lower | −0.418 | −0.926 | −0.758 | −0.509 | −0.095 | 0.483 | |
| Spearman’s rho | 0.403 | −0.661 | −0.097 | 0.301 | 0.606 | 0.855 ** | |
| p-value | 0.283 | 0.053 | 0.804 | 0.430 | 0.084 | 0.003 | |
| Kendall’s Tau B | 0.344 | −0.617 | −0.091 | 0.225 | 0.566 | 0.825 ** | |
| p-value | 0.281 | 0.062 | 0.784 | 0.433 | 0.087 | 0.01 | |
| CD34_H-SCORE | Pearson’s r | 0.328 | −0.711 * | −0.233 | 0.449 | 0.252 | 0.948 *** |
| p-value | 0.389 | 0.032 | 0.546 | 0.225 | 0.514 | <0.001 | |
| 95% CI Upper | 0.815 | −0.089 | 0.510 | 0.858 | 0.785 | 0.989 | |
| 95% CI Lower | −0.430 | −0.934 | −0.777 | −0.306 | −0.495 | 0.768 | |
| Spearman’s rho | 0.347 | −0.574 | −0.092 | 0.294 | 0.313 | 0.921 *** | |
| p-value | 0.361 | 0.106 | 0.814 | 0.442 | 0.412 | <0.001 | |
| Kendall’s Tau B | 0.268 | −0.504 | −0.081 | 0.171 | 0.275 | 0.840 ** | |
| p-value | 0.372 | 0.104 | 0.795 | 0.527 | 0.376 | 0.005 | |
| TLS | Pearson’s r | 0.367 | 0.357 | 0.756 * | −0.132 | −0.286 | −0.267 |
| p-value | 0.331 | 0.345 | 0.018 | 0.735 | 0.456 | 0.487 | |
| 95% CI Upper | 0.829 | 0.825 | 0.945 | 0.583 | 0.467 | 0.483 | |
| 95% CI Lower | −0.393 | −0.402 | 0.184 | −0.732 | −0.798 | −0.791 | |
| Spearman’s rho | 0.371 | 0.357 | 0.756 * | −0.104 | −0.286 | −0.170 | |
| p-value | 0.325 | 0.345 | 0.018 | 0.791 | 0.456 | 0.662 | |
| Kendall’s Tau B | 0.359 | 0.357 | 0.756 * | −0.089 | −0.286 | −0.164 | |
| p-value | 0.294 | 0.312 | 0.033 | 0.770 | 0.419 | 0.630 |
| H-SCORE | S_SMA_I | S_SMA_D | S-SCORE | IMBV_CD34+/SMA- | ||
|---|---|---|---|---|---|---|
| S_SMA_I | Pearson’s r | 0.506 * | — | |||
| p-value | 0.027 | — | ||||
| 95% CI Upper | 0.781 | — | ||||
| 95% CI Lower | 0.068 | — | ||||
| Spearman’s rho | 0.388 | — | ||||
| p-value | 0.101 | — | ||||
| Kendall’s Tau B | 0.338 | — | ||||
| p-value | 0.073 | — | ||||
| S_SMA_D | Pearson’s r | 0.765 *** | 0.056 | — | ||
| p-value | <0.001 | 0.821 | — | |||
| 95% CI Upper | 0.905 | 0.497 | — | |||
| 95% CI Lower | 0.476 | −0.409 | — | |||
| Spearman’s rho | 0.871 *** | 0.092 | — | |||
| p-value | <0.001 | 0.707 | — | |||
| Kendall’s Tau B | 0.745 *** | 0.099 | — | |||
| p-value | <0.001 | 0.654 | — | |||
| S-SCORE | Pearson’s r | 0.870 *** | 0.685 ** | 0.731 *** | — | |
| p-value | <0.001 | 0.001 | <0.001 | — | ||
| 95% CI Upper | 0.949 | 0.869 | 0.890 | — | ||
| 95% CI Lower | 0.687 | 0.335 | 0.414 | — | ||
| Spearman’s rho | 0.895 *** | 0.603 ** | 0.813 *** | — | ||
| p-value | <0.001 | 0.006 | <0.001 | — | ||
| Kendall’s Tau B | 0.779 *** | 0.555 ** | 0.722 *** | — | ||
| p-value | <0.001 | 0.008 | <0.001 | — | ||
| IMBV_CD34+/SMA- | Pearson’s r | −0.378 | −0.434 | −0.370 | −0.532 * | — |
| p-value | 0.111 | 0.064 | 0.119 | 0.019 | — | |
| 95% CI Upper | 0.092 | 0.026 | 0.101 | −0.103 | — | |
| 95% CI Lower | −0.71 | −0.742 | −0.706 | −0.794 | — | |
| Spearman’s rho | −0.354 | −0.346 | −0.352 | −0.446 | — | |
| p-value | 0.137 | 0.147 | 0.139 | 0.056 | — | |
| Kendall’s Tau B | −0.187 | −0.267 | −0.293 | −0.339 | — | |
| p-value | 0.285 | 0.179 | 0.149 | 0.079 | — | |
| TLS | Pearson’s r | −0.617 ** | −0.519 * | −0.396 | −0.579 ** | 0.540 * |
| p-value | 0.005 | 0.023 | 0.094 | 0.009 | 0.017 | |
| 95% CI Upper | −0.227 | −0.085 | 0.071 | −0.170 | 0.798 | |
| 95% CI Lower | −0.837 | −0.788 | −0.720 | −0.818 | 0.113 | |
| Spearman’s rho | −0.603 ** | −0.505 * | −0.380 | −0.561 * | 0.544 * | |
| p-value | 0.006 | 0.028 | 0.108 | 0.013 | 0.016 | |
| Kendall’s Tau B | −0.505 * | −0.482 * | −0.372 | −0.519 * | 0.479 * | |
| p-value | 0.010 | 0.032 | 0.107 | 0.017 | 0.021 | |
| G | Pearson’s r | 0.280 | 0.367 | 0.331 | 0.484 * | −0.271 |
| p-value | 0.245 | 0.122 | 0.167 | 0.036 | 0.261 | |
| 95% CI Upper | 0.652 | 0.704 | 0.682 | 0.769 | 0.208 | |
| 95% CI Lower | −0.199 | −0.104 | −0.145 | 0.038 | −0.646 | |
| Spearman’s rho | 0.289 | 0.345 | 0.364 | 0.466 * | −0.421 | |
| p-value | 0.230 | 0.148 | 0.126 | 0.044 | 0.073 | |
| Kendall’s Tau B | 0.238 | 0.327 | 0.348 | 0.434 * | −0.370 | |
| p-value | 0.221 | 0.140 | 0.125 | 0.043 | 0.070 | |
| AGE | Pearson’s r | 0.354 | 0.358 | 0.358 | 0.527 * | −0.459 * |
| p-value | 0.137 | 0.133 | 0.132 | 0.020 | 0.048 | |
| 95% CI Upper | 0.696 | 0.699 | 0.699 | 0.792 | −0.006 | |
| 95% CI Lower | −0.119 | −0.115 | −0.115 | 0.096 | −0.756 | |
| Spearman’s rho | 0.339 | 0.308 | 0.345 | 0.474 * | −0.287 | |
| p-value | 0.156 | 0.199 | 0.148 | 0.040 | 0.234 | |
| Kendall’s Tau B | 0.226 | 0.238 | 0.287 | 0.360 | −0.235 | |
| p-value | 0.190 | 0.222 | 0.151 | 0.057 | 0.194 |
| S_SMA_I | SMA_STROMAL SCORE | SMA_H-SCORE | CD34_H-SCORE | S_CD34_D | AGE | IMBV_CD34+/SMA- | ||
|---|---|---|---|---|---|---|---|---|
| SMA_STROMAL SCORE | Pearson’s r | 1.000 *** | — | |||||
| p-value | <0.001 | — | ||||||
| Spearman’s rho | 1.000 *** | — | ||||||
| p-value | <0.001 | — | ||||||
| Kendall’s Tau B | 1.000 | — | ||||||
| p-value | 0.157 | — | ||||||
| SMA_H-SCORE | Pearson’s r | 0.814 | 0.814 | — | ||||
| p-value | 0.395 | 0.395 | — | |||||
| Spearman’s rho | 0.866 | 0.866 | — | |||||
| p-value | 0.333 | 0.333 | — | |||||
| Kendall’s Tau B | 0.816 | 0.816 | — | |||||
| p-value | 0.221 | 0.221 | — | |||||
| CD34_H-SCORE | Pearson’s r | −0.500 | −0.500 | −0.910 | — | |||
| p-value | 0.667 | 0.667 | 0.272 | — | ||||
| Spearman’s rho | −0.500 | −0.500 | −0.866 | — | ||||
| p-value | 0.667 | 0.667 | 0.333 | — | ||||
| Kendall’s Tau B | −0.500 | −0.500 | −0.816 | — | ||||
| p-value | 0.480 | 0.480 | 0.221 | — | ||||
| S_CD34_D | Pearson’s r | −0.500 | −0.500 | −0.910 | 1.000 *** | — | ||
| p-value | 0.667 | 0.667 | 0.272 | <0.001 | — | |||
| Spearman’s rho | −0.500 | −0.500 | −0.866 | 1.000 *** | — | |||
| p-value | 0.667 | 0.667 | 0.333 | <0.001 | — | |||
| Kendall’s Tau B | −0.500 | −0.500 | −0.816 | 1.000 | — | |||
| p-value | 0.480 | 0.480 | 0.221 | 0.157 | — | |||
| AGE | Pearson’s r | 0.500 | 0.500 | 0.910 | −1.000 *** | −1.000 *** | — | |
| p-value | 0.667 | 0.667 | 0.272 | <0.001 | <0.001 | — | ||
| Spearman’s rho | 0.500 | 0.500 | 0.866 | −1.000 *** | −1.000 *** | — | ||
| p-value | 0.667 | 0.667 | 0.333 | <0.001 | <0.001 | — | ||
| Kendall’s Tau B | 0.500 | 0.500 | 0.816 | −1.000 | −1.000 | — | ||
| p-value | 0.480 | 0.480 | 0.221 | 0.157 | 0.157 | — | ||
| MBV_CD34+/SMA+ | Pearson’s r | 0.000 | 0.000 | −0.581 | 0.866 | 0.866 | −0.866 | |
| p-value | 1.000 | 1.000 | 0.605 | 0.333 | 0.333 | 0.333 | ||
| Spearman’s rho | 0.000 | 0.000 | −0.500 | 0.866 | 0.866 | −0.866 | ||
| p-value | 1.000 | 1.000 | 1.000 | 0.333 | 0.333 | 0.333 | ||
| Kendall’s Tau B | 0.000 | 0.000 | −0.333 | 0.816 | 0.816 | −0.816 | ||
| p-value | 1.000 | 1.000 | 1.000 | 0.221 | 0.221 | 0.221 | ||
| IMBV_CD34+/SMA- | Pearson’s r | −0.500 | −0.500 | −0.910 | 1.000 *** | 1.000 *** | −1.000 *** | — |
| p-value | 0.667 | 0.667 | 0.272 | <0.001 | <0.001 | <0.001 | — | |
| Spearman’s rho | −0.500 | −0.500 | −0.866 | 1.000 *** | 1.000 *** | −1.000 *** | — | |
| p-value | 0.667 | 0.667 | 0.333 | <0.001 | <0.001 | <0.001 | — | |
| Kendall’s Tau B | −0.500 | −0.500 | −0.816 | 1.000 | 1.000 | −1.000 | — | |
| p-value | 0.480 | 0.480 | 0.221 | 0.157 | 0.157 | 0.157 | — | |
| LVI | Pearson’s r | −0.500 | −0.500 | −0.910 | 1.000 *** | 1.000 *** | −1.000 *** | 1.000 *** |
| p-value | 0.667 | 0.667 | 0.272 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Spearman’s rho | −0.500 | −0.500 | −0.866 | 1.000 *** | 1.000 *** | −1.000 *** | 1.000 *** | |
| p-value | 0.667 | 0.667 | 0.333 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Kendall’s Tau B | −0.500 | −0.500 | −0.816 | 1.000 | 1.000 | −1.000 | 1.000 | |
| p-value | 0.480 | 0.480 | 0.221 | 0.157 | 0.157 | 0.157 | 0.157 | |
| PnI | Pearson’s r | −0.500 | −0.500 | −0.910 | 1.000 *** | 1.000 *** | −1.000 *** | 1.000 *** |
| p-value | 0.667 | 0.667 | 0.272 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Spearman’s rho | −0.500 | −0.500 | −0.866 | 1.000 *** | 1.000 *** | −1.000 *** | 1.000 *** | |
| p-value | 0.667 | 0.667 | 0.333 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Kendall’s Tau B | −0.500 | −0.500 | −0.816 | 1.000 | 1.000 | −1.000 | 1.000 | |
| p-value | 0.480 | 0.480 | 0.221 | 0.157 | 0.157 | 0.157 | 0.157 | |
| R | Pearson’s r | −0.500 | −0.500 | −0.910 | 1.000 *** | 1.000 *** | −1.000 *** | 1.000 *** |
| p-value | 0.667 | 0.667 | 0.272 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Spearman’s rho | −0.500 | −0.500 | −0.866 | 1.000 *** | 1.000 *** | −1.000 *** | 1.000 *** | |
| p-value | 0.667 | 0.667 | 0.333 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Kendall’s Tau B | −0.500 | −0.500 | −0.816 | 1.000 | 1.000 | −1.000 | 1.000 | |
| p-value | 0.480 | 0.480 | 0.221 | 0.157 | 0.157 | 0.157 | 0.157 |
| S_SMA_D | SMA_H-SCORE | MBV_CD34+/SMA+ | ||
|---|---|---|---|---|
| SMA_H-SCORE | Pearson’s r | 0.824 * | — | |
| p-value | 0.023 | — | ||
| 95% CI Upper | 0.973 | — | ||
| 95% CI Lower | 0.186 | — | ||
| Spearman’s rho | 0.694 | — | ||
| p-value | 0.083 | — | ||
| Kendall’s Tau B | 0.620 | — | ||
| p-value | 0.071 | — | ||
| MBV_CD34+/SMA+ | Pearson’s r | 0.771 * | 0.508 | — |
| p-value | 0.043 | 0.244 | — | |
| 95% CI Upper | 0.964 | 0.912 | — | |
| 95% CI Lower | 0.042 | −0.397 | — | |
| Spearman’s rho | 0.701 | 0.342 | — | |
| p-value | 0.080 | 0.452 | — | |
| Kendall’s Tau B | 0.635 | 0.293 | — | |
| p-value | 0.068 | 0.362 | — | |
| MENOPAUSAL STATUS | Pearson’s r | 0.710 | 0.860 * | 0.517 |
| p-value | 0.074 | 0.013 | 0.235 | |
| 95% CI Upper | 0.953 | 0.979 | 0.914 | |
| 95% CI Lower | −0.092 | 0.303 | −0.387 | |
| Spearman’s rho | 0.683 | 0.791 * | 0.399 | |
| p-value | 0.091 | 0.034 | 0.375 | |
| Kendall’s Tau B | 0.653 | 0.690 | 0.354 | |
| p-value | 0.094 | 0.053 | 0.329 | |
| SURVIVAL (MONTHS) | Pearson’s r | 0.866 * | 0.508 | 0.785 * |
| p-value | 0.012 | 0.245 | 0.036 | |
| 95% CI Upper | 0.980 | 0.912 | 0.967 | |
| 95% CI Lower | 0.326 | −0.397 | 0.079 | |
| Spearman’s rho | 0.856 * | 0.378 | 0.836 * | |
| p-value | 0.014 | 0.403 | 0.019 | |
| Kendall’s Tau B | 0.751 * | 0.293 | 0.650 * | |
| p-value | 0.031 | 0.362 | 0.046 |
| S_CD34_D | CD34_STROMAL SCORE | CD34_H-SCORE | ||
|---|---|---|---|---|
| CD34_STROMAL SCORE | Pearson’s r | 1.000 *** | — | |
| p-value | <0.001 | — | ||
| 95% CI Upper | 1.000 | — | ||
| 95% CI Lower | 1.000 | — | ||
| Spearman’s rho | 1.000 *** | — | ||
| p-value | <0.001 | — | ||
| Kendall’s Tau B | 1.000 * | — | ||
| p-value | 0.014 | — | ||
| CD34_H-SCORE | Pearson’s r | 0.984 *** | 0.984 *** | — |
| p-value | <0.001 | <0.001 | — | |
| 95% CI Upper | 0.998 | 0.998 | — | |
| 95% CI Lower | 0.893 | 0.893 | — | |
| Spearman’s rho | 0.618 | 0.618 | — | |
| p-value | 0.139 | 0.139 | — | |
| Kendall’s Tau B | 0.548 | 0.548 | — | |
| p-value | 0.130 | 0.130 | — | |
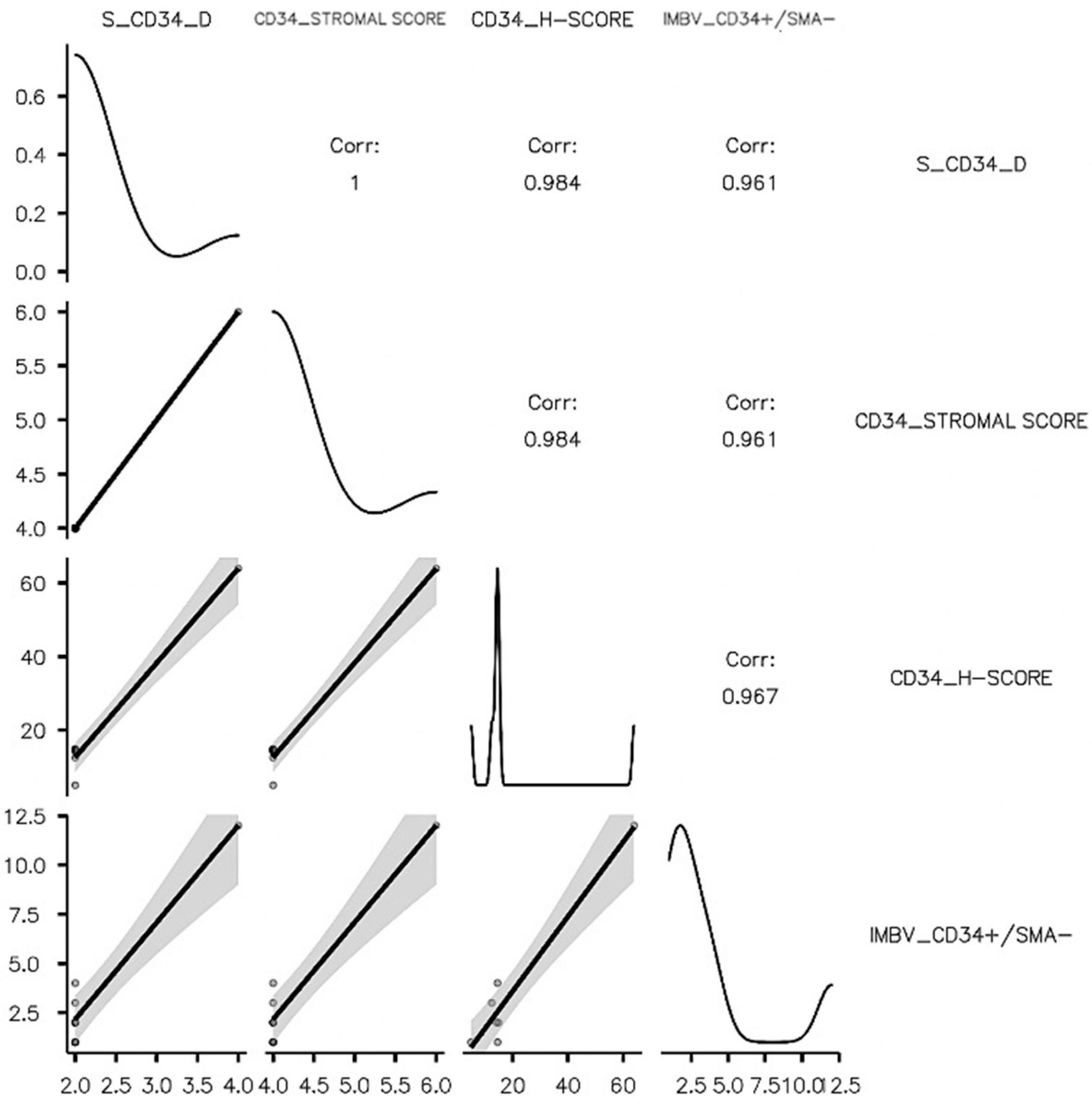
| IMBV_CD34+/SMA- | Pearson’s r | 0.961 *** | 0.961 *** | 0.967 *** |
| p-value | <0.001 | <0.001 | <0.001 | |
| 95% CI Upper | 0.994 | 0.994 | 0.995 | |
| 95% CI Lower | 0.753 | 0.753 | 0.785 | |
| Spearman’s rho | 0.624 | 0.624 | 0.505 | |
| p-value | 0.135 | 0.135 | 0.248 | |
| Kendall’s Tau B | 0.562 | 0.562 | 0.410 | |
| p-value | 0.127 | 0.127 | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barb, A.C.; Fenesan, M.P.; Pirtea, M.; Margan, M.-M.; Tomescu, L.; Ceban, E.; Cimpean, A.M.; Melnic, E. Reassessing Breast Cancer-Associated Fibroblasts (CAFs) Interactions with Other Stromal Components and Clinico-Pathologic Parameters by Using Immunohistochemistry and Digital Image Analysis (DIA). Cancers 2023, 15, 3823. https://doi.org/10.3390/cancers15153823
Barb AC, Fenesan MP, Pirtea M, Margan M-M, Tomescu L, Ceban E, Cimpean AM, Melnic E. Reassessing Breast Cancer-Associated Fibroblasts (CAFs) Interactions with Other Stromal Components and Clinico-Pathologic Parameters by Using Immunohistochemistry and Digital Image Analysis (DIA). Cancers. 2023; 15(15):3823. https://doi.org/10.3390/cancers15153823
Chicago/Turabian StyleBarb, Alina Cristina, Mihaela Pasca Fenesan, Marilena Pirtea, Mădălin-Marius Margan, Larisa Tomescu, Emil Ceban, Anca Maria Cimpean, and Eugen Melnic. 2023. "Reassessing Breast Cancer-Associated Fibroblasts (CAFs) Interactions with Other Stromal Components and Clinico-Pathologic Parameters by Using Immunohistochemistry and Digital Image Analysis (DIA)" Cancers 15, no. 15: 3823. https://doi.org/10.3390/cancers15153823
APA StyleBarb, A. C., Fenesan, M. P., Pirtea, M., Margan, M.-M., Tomescu, L., Ceban, E., Cimpean, A. M., & Melnic, E. (2023). Reassessing Breast Cancer-Associated Fibroblasts (CAFs) Interactions with Other Stromal Components and Clinico-Pathologic Parameters by Using Immunohistochemistry and Digital Image Analysis (DIA). Cancers, 15(15), 3823. https://doi.org/10.3390/cancers15153823








