Mucinous and Signet-Ring Cell Colonic Adenocarcinoma in Inflammatory Bowel Disease: A Case–Control Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Protocol
2.2. Study Population
2.3. Statistical Analysis
3. Results
3.1. IBD Patients with CRC
3.2. Colorectal Cancer in IBD
3.3. Standard versus Mucinous/Signet-Ring Cell Adenocarcinoma in IBD
3.4. Non-IBD Patients with CRC
3.5. Characteristics of CRC in Patients with versus without IBD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo—Anal pouch disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Gomollòn, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: Diagnosis and medical management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Ording, A.G.; Horvath-Puho, E.; Erichsen, R.; Long, M.D.; Baron, J.A.; Lash, T.L.; Sørensen, H.T. Five-year mortality in colorectal cancer patients with ulcerative colitis or Crohn’s disease: A nationwide population-based cohort study. Inflamm. Bowel Dis. 2013, 19, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.K.; Feuerstein, J.D.; Nguyen, G.C.; Velayos, F.S. AGA clinical practice update on endoscopic surveillance and management of colorectal dysplasia in Inflammatory Bowel Diseases: Expert Review. Gastroenterology 2021, 161, 1043–1051.e4. [Google Scholar] [CrossRef]
- Weitz, J.; Koch, M.; Debus, J.; Höhler, T.; Galle, P.R.; Büchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: A metanalysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef]
- Beaugerie, L.; Itzkowitz, S.H. Cancers complicating Inflammatory Bowel Disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef]
- Herrinton, L.J.; Liu, L.; Levin, T.R.; Allison, J.E.; Lewis, J.D.; Velayos, F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology 2012, 143, 382–389. [Google Scholar] [CrossRef]
- Rutter, M.; Saunders, B.; Wilkinson, K.; Rumbles, S.; Schofield, G.; Kamm, M.; Williams, C.; Price, A.; Talbot, I.; Forbes, A. Severity of inflammation is a risk factor for colorectal neoplasia in Ulcerative Colitis. Gastroenterology 2004, 126, 451–459. [Google Scholar] [CrossRef]
- Gupta, R.B.; Harpaz, N.; Itzkowitz, S.; Hossain, S.; Matula, S.; Kornbluth, A.; Bodian, C.; Ullman, T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in Ulcerative Colitis: A Cohort Study. Gastroenterology 2007, 133, 1099–1105. [Google Scholar] [CrossRef]
- Soetikno, R.M.; Lin, O.S.; Heidenreich, P.A.; Young, H.S.; Blackstone, M.O. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: A meta-analysis. Gastrointest. Endosc. 2002, 56, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, A.M.; Mahmoud, R.; Lutgens, M.W.M.D.; Oldenburg, B. Surveillance and management of colorectal dysplasia and cancer in inflammatory bowel disease: Current practice and future perspectives. Eur. J. Intern. Med. 2021, 93, 35–41. [Google Scholar] [CrossRef]
- Lai, L.A.; Risques, R.A.; Bronner, M.P.; Rabinovitch, P.S.; Crispin, D.; Chen, R.; Brentnall, T.A. Pan-colonic field defects are detected by CGH in the colons of UC patients with dysplasia/cancer. Cancer Lett. 2012, 320, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Liu, G.; Wu, X.; Lai, K.K.; Shen, B.; Liu, X. Overexpression of p53 predicts colorectal neoplasia risk in patients with inflammatory bowel disease and mucosa changes indefinite for dysplasia. Gastroenterol. Rep. 2015, 3, 344–349. [Google Scholar] [CrossRef]
- Kang, H.; O’Connell, J.B.; Maggard, M.A.; Sack, J.; Ko, C.Y. A 10-year outcomes evaluation of mucinous and signet ring cell carcinoma of the colon and rectum. Dis. Colon Rectum 2005, 48, 1161–1168. [Google Scholar] [CrossRef]
- Choi, P.M.; Zelig, M.P. Similarity of colorectal cancer in Crohn’s disease and ulcerative colitis: Implications for carcinogenesis and prevention. Gut 1994, 35, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Al Sulais, E.; Axelrad, J.E.; Balendran, K.; Burisch, J.; de Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohns Colitis 2022, 17, 827–854. [Google Scholar] [CrossRef]
- Wolf, T.; Lewis, A.; Beaugerie, L.; Svrcek, M.; Kirchgesner, J.; Saint-Antoine IBD Network. Risk of colorectal neoplasia according to histologic disease activity in patients with inflammatory bowel disease and colonic post-inflammatory polyps. Aliment. Pharmacol. Ther. 2023, 57, 1445–1452. [Google Scholar] [CrossRef]
- Mahmoud, R.; Shah, S.C.; Ten Hove, J.R.; Torres, J.; Mooiweer, E.; Castaneda, D.; Glass, J.; Elman, J.; Kumar, A.; Axelrad, J.; et al. Dutch Initiative on Crohn and Colitis. No association between pseudopolyps and colorectal neoplasia in patients with Inflammatory Bowel Diseases. Gastroenterology 2019, 156, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System, 4th ed.; World Health Organization Classification of Tumours: Geneva, Swizerland, 2010. [Google Scholar]
- Lu, C.; Schardey, J.; Zhang, T.; Crispin, A.; Wirth, U.; Karcz, K.W.; Bazhin, A.V.; Andrassy, J.; Werner, J.; Kühn, F. Survival outcomes and clinicopathological features in inflammatory bowel disease-associated colorectal cancer: A Systematic Review and Meta-analysis. Ann. Surg. 2022, 276, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Galata, C.; Hirsch, D.; Reindl, W.; Post, S.; Kienle, P.; Boutros, M.; Gaiser, T.; Horisberger, K. Clinical and histopathologic features of colorectal adenocarcinoma in Crohn’s Disease. J. Clin. Gastroenterol. 2018, 52, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Akarca, F.G.; Yozu, M.; Alpert, L.; Kővári, B.P.; Zhao, L.; Salomao, M.; Liao, X.; Westerhoff, M.; Lauwers, G.Y.; Choi, W.T. Non-conventional dysplasia is frequently associated with low-grade tubuloglandular and mucinous adenocarcinomas in inflammatory bowel disease. Histopathology 2023, 83, 276–285. [Google Scholar] [CrossRef]
- Hartman, D.J.; Binion, D.G.; Regueiro, M.D.; Miller, C.; Herbst, C.; Pai, R.K. Distinct histopathologic and molecular alterations in Inflammatory Bowel Disease-Associated Intestinal Adenocarcinoma: c-MYC amplification is common and associated with mucinous/signet ring cell differentiation. Inflamm. Bowel Dis. 2018, 24, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Cen, S.; Ding, G.; Wu, W. Mucinous colorectal adenocarcinoma: Clinical pathology and treatment options. Cancer Commun. 2019, 39, 13. [Google Scholar] [CrossRef]
- Festa, S.; Zerboni, G.; Derikx, L.A.A.P.; Ribaldone, D.G.; Dragoni, G.; Buskens, C.J.; van Dijkum, E.N.; Pugliese, D.; Panzuto, F.; Krela-Kaźmierczak, I.; et al. Gastroenteropancreatic Neuroendocrine Neoplasms in patients with Inflammatory Bowel Disease: An ECCO CONFER Multicentre Case Series. J. Crohns Colitis 2022, 16, 940–945. [Google Scholar] [CrossRef]
- Neri, B.; Mossa, M.; Scucchi, L.; Sena, G.; Palmieri, G.; Biancone, L. Histological scores in inflammatory bowel disease. J. Dig. Dis. 2021, 22, 9–22. [Google Scholar] [CrossRef]
- Leopoldo, S.; Lorena, B.; Cinzia, A.; Gabriella, D.C.; Angela Luciana, B.; Renato, C.; Antonio, M.; Carlo, S.; Cristina, P.; Stefano, C.; et al. Two subtypes of mucinous adenocarcinoma of the colorectum: Clinicopathological and genetic features. Ann. Surg. Oncol. 2008, 15, 1429–1439. [Google Scholar] [CrossRef]
- Svrcek, M.; Nunes, P.B.; Villanacci, V.; Beaugerie, L.; Rogler, G.; De Hertogh, G.; Tripathi, M.; Feakins, R.; on behalf the H-ECCO Group. Clinicopathological and Molecular Specificities of Inflammatory Bowel Disease-Related Colorectal Neoplastic Lesions: The Role of Inflammation. J. Crohns Colitis. 2018, 12, 1486–1498. [Google Scholar] [CrossRef]
- Beaugerie, L.; Carrat, F.; Nahon, S.; Zeitoun, J.D.; Sabaté, J.M.; Peyrin-Biroulet, L.; Colombel, J.F.; Allez, M.; Fléjou, J.F.; Kirchgesner, J.; et al. High risk of anal and rectal cancer in patients with anal and/or perianal Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 892–899. [Google Scholar] [CrossRef]
- Du, W.; Mah, J.T.; Lee, J.; Sankila, R.; Sankaranarayanan, R.; Chia, K.S. Incidence and survival of mucinous adenocarcinoma of the colorectum: A population-based study from an Asian country. Dis. Colon Rectum 2004, 47, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Connelly, J.H.; Robey-Cafferty, S.S.; Cleary, K.R. Mucinous carcinomas of the colon and rectum. An analysis of 62 stage B and C lesions. Arch. Pathol. Lab. Med. 1991, 115, 1022–1025. [Google Scholar] [PubMed]
- Neri, B.; Scribano, M.L.; Armuzzi, A.; Castiglione, F.; D’Incà, R.; Orlando, A.; Festa, S.; Riegler, G.; Fries, W.; Meucci, G.; et al. Incident Colorectal Cancer in Inflammatory Bowel Disease. Cancers 2022, 14, 721. [Google Scholar] [CrossRef] [PubMed]
- Symonds, D.A.; Vickery, A.L. Mucinous carcinoma of the colon and rectum. Cancer 1976, 37, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, T.B.; Seim, E. Influence of mucinous components on survival in colorectal adenocarcinomas: A multivariate analysis. J. Clin. Pathol. 1988, 41, 1068–1072. [Google Scholar] [CrossRef] [PubMed]



| IBD Patients (n = 40) | Standard Adenocarcinoma (n = 23) | Mucinous/Signet-Ring Cell Adenocarcinoma (n = 17) | p | |
|---|---|---|---|---|
| Age at diagnosis of CRC, median (range) | 54 (29–80) | 61 (30–80) | 53 (29–80) | 0.61 |
| Age at diagnosis of IBD, median (range) | 35.5 (29–80) | 38 (12–80) | 29 (17–73) | 0.56 |
| Gender (F), n (%) | 13 (32.5%) | 8 (34.8%) | 5 (29.4%) | 0.98 |
| IBD duration, median (range) | 21 (1–48) | 22 (3–48) | 21 (1–38) | 0.77 |
| Time interval between diagnosis of IBD and CRC, median (range) | 16 (1–45) | 14 (1–45) | 17 (1–36) | 0.74 |
| Ulcerative colitis, n (%) | 24 (60%) | 14 (60.9%) | 10 (58.8%) | 0.84 |
| E1 | 7 (29.2%) | 4 (28.6%) | 3 (30%) | 0.70 |
| E2 | 5 (20.8%) | 3 (21.4%) | 2 (20%) | 0.67 |
| E3 | 12 (50%) | 7 (50%) | 5 (50%) | 0.67 |
| Crohn’s disease, n (%) | 16 (40%) | 9 (39.1%) | 7 (41.2%) | 0.84 |
| L1 | 5 (31.3%) | 2 (22.2%) | 3 (42.8%) | 0.73 |
| L2 | 3 (18.7%) | 1 (11.1%) | 2 (28.6%) | 0.8 |
| L3 | 8 (50%) | 6 (66.7%) | 2 (28.6%) | 0.31 |
| L4 | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| B1 | 4 (25%) | 1 (11.1%) | 3 (42.8%) | 0.38 |
| B2 | 8 (50%) | 7 (77.8%) | 1 (14.4%) | 0.04 |
| B3 | 4 (25%) | 1 (11.1%) | 3 (42.8%) | 0.38 |
| Perianal disease, n (%) | 8 (50%) | 3 (33.3%) | 5 (29.4%) | 0.37 |
| IBD-related surgery, n (%) | 12 (30%) | 7 (77.8%) | 5 (29.4%) | 0.78 |
| Smoking habits, n (%) | ||||
| Yes | 3 (7.5%) | 1 (4.3%) | 2 (11.7%) | 0.78 |
| No/Ex | 37 (92.5%) | 22 (95.7%) | 15 (88.3%) | |
| EIMs, n (%) | 7 (17.5%) | 4 (44.4%) | 3 (17.6%) | 0.68 |
| Thiopurines, n (%) | 11 (27.5%) | 5 (21.7%) | 6 (35.3%) | 0.55 |
| Biologics, n (%) * | 11 (27.5%) | 6 (26.1%) | 5 (29.4%) | 0.9 |
| Infliximab | 9 | 5 | 4 | |
| Adalimumab | 5 | 1 | 4 | |
| Golimumab | 1 | 1 | 0 | |
| Vedolizumab | 2 | 2 | 0 | |
| Ustekinumab | 1 | 1 | 0 |
| IBD Patients (n = 40) | Standard Adenocarcinoma (n = 23) | Mucinous/Signet-Ring Cell Adenocarcinoma (n = 17) | p | |
|---|---|---|---|---|
| CRC symptoms, n (%) | 16 (40%) | 8 (34.8%) | 8 (47.1%) | 0.64 |
| CRC diagnostic modality | ||||
| Colonoscopy | 32 (80%) | 20 (86.9%) | 12 (70.6%) | 0.37 |
| Imaging | 3 (7.5%) | 0 (0%) | 3 (17.6%) | 0.13 |
| Intraoperative | 5 (12.5%) | 3 (13.1%) | 2 (11.8%) | 0.71 |
| Concomitant adenoma, n (%) | 5 (12.5%) | 4 (17.4%) | 1 (5.9%) | 0.54 |
| Previous history of adenoma, n (%) | 10 (25%) | 7 (30.4%) | 3 (17.6%) | 0.57 |
| Family history of CRC, n (%) | 7 (12.5%) | 5 (21.7%) | 2 (11.8%) | 0.68 |
| CRC stage at diagnosis | ||||
| I | 7 (17.5%) | 6 (26.1%) | 1 (5.9%) | 0.21 |
| II | 13 (32.5%) | 9 (39.1%) | 4 (23.5%) | 0.48 |
| III | 12 (30%) | 5 (21.7%) | 7 (41.2%) | 0.32 |
| IV | 8 (20%) | 3 (13.1%) | 5 (29.4%) | 0.37 |
| Metastasis at CRC diagnosis, n (%) | 14 (35%) | 6 (26.1%) | 8 (47.1%) | 0.29 |
| CRC site, n (%) | ||||
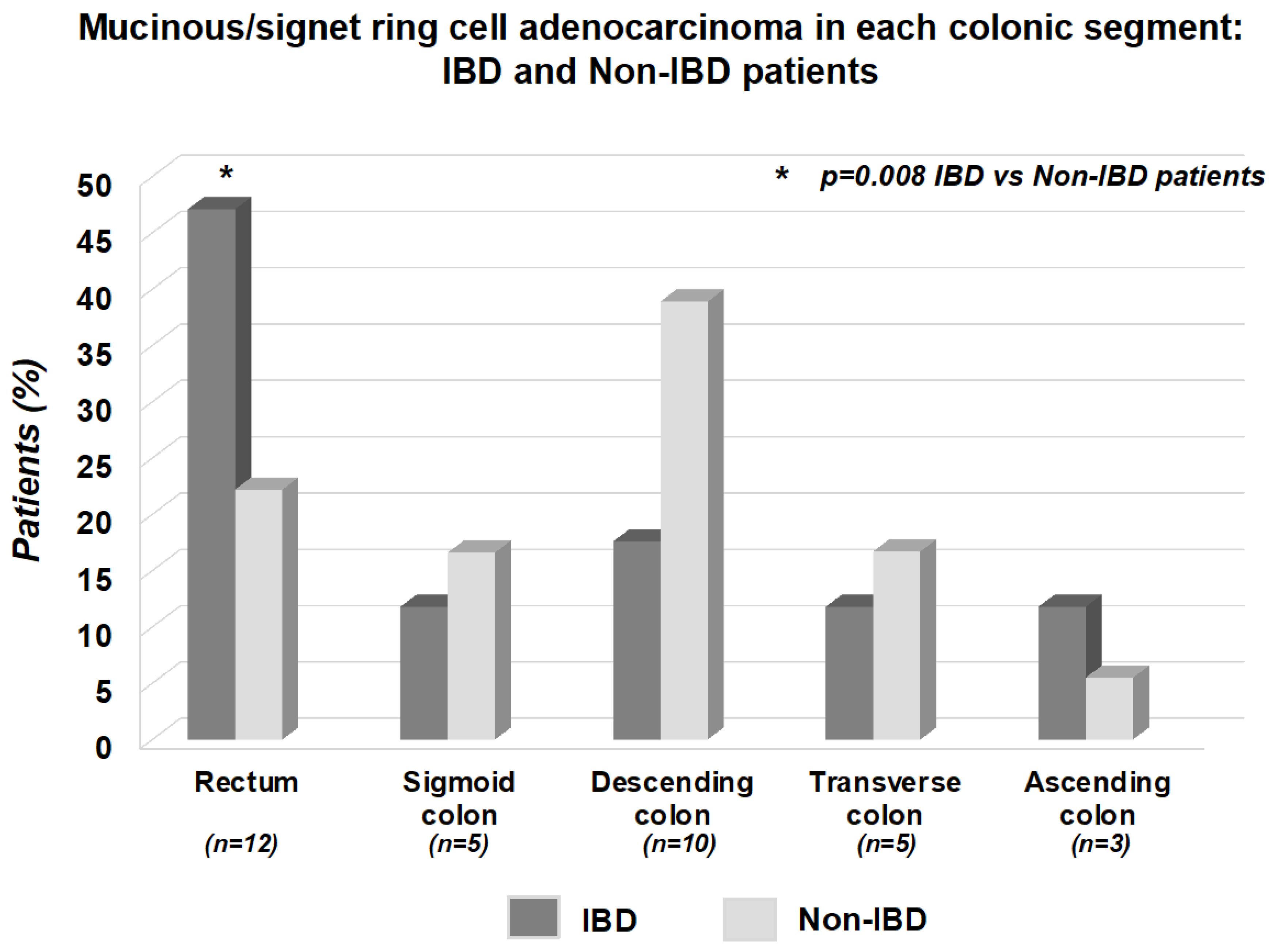
| Rectum | 12 (30%) | 4 (17.4%) | 8 (47.1%) | 0.04 |
| Sigmoid colon | 9 (22.5%) | 7 (30.4%) | 2 (11.8%) | 0.31 |
| Descending colon | 8 (20%) | 5 (21.7%) | 3 (17.6%) | 0.93 |
| Transverse colon | 2 (5%) | 0 (0%) | 2 (11.8%) | 0.34 |
| Coecum/Ascending colon | 9 (22.5%) | 7 (30.4%) | 2 (11.8%) | 0.31 |
| Left colon | 29 (72.5%) | 16 (69.6%) | 13 (76.5%) | 0.09 |
| Right colon | 11 (27.5%) | 7 (30.4%) | 4 (23.5%) | |
| Surgery for CRC, n (%) | 37 (92.5%) | 20 (86.9%) | 17 (100%) | 0.34 |
| Type of surgery, n (%) | ||||
| EMR | 3 (7.5%) | 3 (13.1%) | 0 (0%) | 0.34 |
| Hemicolectomy | 8 (20%) | 5 (21.7%) | 3 (17.6%) | 0.93 |
| Subtotal colectomy with IRA | 9 (22.5%) | 5 (21.7%) | 4 (23.5%) | 0.8 |
| Proctocolectomy and ileostomy | 10 (25%) | 5 (21.7%) | 4 (23.5%) | 0.8 |
| Proctocolectomy and IPAA | 5 (12.5%) | 2 (8.7%) | 3 (17.6%) | 0.71 |
| Palliative stoma | 2 (5%) | 0 (0%) | 2 (11.8%) | 0.34 |
| Anterior rectal resection | 2 (5%) | 1 (4.3%) | 1 (5.9%) | 0.6 |
| Neoadjuvant RT, n (%) | 1 (2.5%) | 0 (0%) | 1 (5.9%) | 0.87 |
| Neoadjuvant CHT, n (%) | 1 (2.5%) | 1 (4.3%) | 0 (0%) | 0.87 |
| Adjuvant RT, n (%) | 1 (2.5%) | 0 (0%) | 1 (5.9%) | 0.87 |
| Adjuvant CHT, n (%) | 15 (37.5%) | 9 (39.1%) | 6 (35.3%) | 0.93 |
| CRC-related death, n (%) | 8 (20%) | 3 (13.1%) | 5 (29.4%) | 0.37 |
| Survival time, median (range) | 61.5 (1–269) | 37.5 (1–269) | 18 (4–111) | 0.09 |
| Lost to follow-up, n (%) | 11 (27.5%) | 6 (26.1%) | 5 (29.4%) | 0.9 |
| Univariate Analysis | ||
|---|---|---|
| Risk Factors | OR [95% CI] | p |
| Female gender | 0.78 [0.2–3.01] | 0.72 |
| Crohn’s disease | 1.08 [0.3–3.91] | 0.89 |
| Ulcerative colitis | 0.91 [0.25–3.29] | 0.89 |
| IBD duration >10 years at diagnosis of CRC | 2.5 [0.6–10.04] | 0.19 |
| Age at diagnosis of IBD (<40 years) | 1.6 [0.46–6.09] | 0.43 |
| Smoking (yes/no) | 2.93 [0.24–35.32] | 0.39 |
| Perianal disease | 2.77 [0.56–13.76] | 0.21 |
| Family history of CRC | 0.48 [0.08–2.83] | 0.41 |
| History of thiopurines use | 1.9 [0.48–7.99] | 0.88 |
| History of biologics use | 1.18 [0.29–4.77] | 0.81 |
| History of mesalazine use | 0.7 [0.12–3.98] | 0.68 |
| Proctitis | 1.01 [0.19–5.29] | 0.98 |
| Left-sided colitis | 0.88 [0.13–6] | 0.9 |
| Pancolitis | 0.95 [0.24–3.78] | 0.94 |
| Non-fibrostricturing non-penetrating CD | 4.71 [0.44–49.94] | 0.19 |
| Fibrostricturing CD | 0.14 [0.01–1.29] | 0.08 |
| Penetrating CD | 4.71 [0.44–49.94] | 0.19 |
| Ileal CD | 2.25 [0.33–15.23] | 0.4 |
| Colonic CD | 2.93 [0.24–35.32] | 0.39 |
| Ileo-colonic CD | 0.37 [0.06–2.16] | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, B.; Mancone, R.; Savino, L.; Schiavone, S.; Formica, V.; Pizzi, F.; Salvatori, S.; Mossa, M.; Migliozzi, S.; Fiorillo, M.; et al. Mucinous and Signet-Ring Cell Colonic Adenocarcinoma in Inflammatory Bowel Disease: A Case–Control Study. Cancers 2023, 15, 3803. https://doi.org/10.3390/cancers15153803
Neri B, Mancone R, Savino L, Schiavone S, Formica V, Pizzi F, Salvatori S, Mossa M, Migliozzi S, Fiorillo M, et al. Mucinous and Signet-Ring Cell Colonic Adenocarcinoma in Inflammatory Bowel Disease: A Case–Control Study. Cancers. 2023; 15(15):3803. https://doi.org/10.3390/cancers15153803
Chicago/Turabian StyleNeri, Benedetto, Roberto Mancone, Luca Savino, Sara Schiavone, Vincenzo Formica, Francesca Pizzi, Silvia Salvatori, Michelangela Mossa, Stefano Migliozzi, Mariasofia Fiorillo, and et al. 2023. "Mucinous and Signet-Ring Cell Colonic Adenocarcinoma in Inflammatory Bowel Disease: A Case–Control Study" Cancers 15, no. 15: 3803. https://doi.org/10.3390/cancers15153803
APA StyleNeri, B., Mancone, R., Savino, L., Schiavone, S., Formica, V., Pizzi, F., Salvatori, S., Mossa, M., Migliozzi, S., Fiorillo, M., Morelli, C., Moscardelli, A., Lolli, E., Calabrese, E., Sica, G. S., Monteleone, G., & Biancone, L. (2023). Mucinous and Signet-Ring Cell Colonic Adenocarcinoma in Inflammatory Bowel Disease: A Case–Control Study. Cancers, 15(15), 3803. https://doi.org/10.3390/cancers15153803







