Elafin as a Prognostic Marker in Esophageal Squamous Cell Carcinoma: A Pilot Study Using Three-Dimensional Imaging and Genomic Profiling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of ESCC Patients to Provide Sample for 3-D Imaging
2.2. Sample Preparation for 3-D Imaging and Confocal Imaging Acquisition
2.3. Processing of 3-D Tissue Images and 3-D Tissue Modeling
2.4. Elafin Expression in Tissue Arrays of Resected ESCC Specimens
2.5. Elafin mRNA Expression in TCGA Dataset
2.6. Cell Lines and Cell Culture
2.7. Plasmids, Short Hairpin RNAs, and Lentivirus Production
2.8. Quantitative Real-Time RT-PCR (TaqMan Assay)
2.9. Immunoblot Analysis
2.10. Cell Proliferation Assay
2.11. Cell Motility and Invasion Assays
2.12. Statistical Analysis
3. Results
3.1. Selection of Locoregional ESCC Patients for 3-D Tissue Imaging
3.2. Distinct 3-D Spatial Distribution of Elafin Expression in ESCC Patients with Different Prognoses
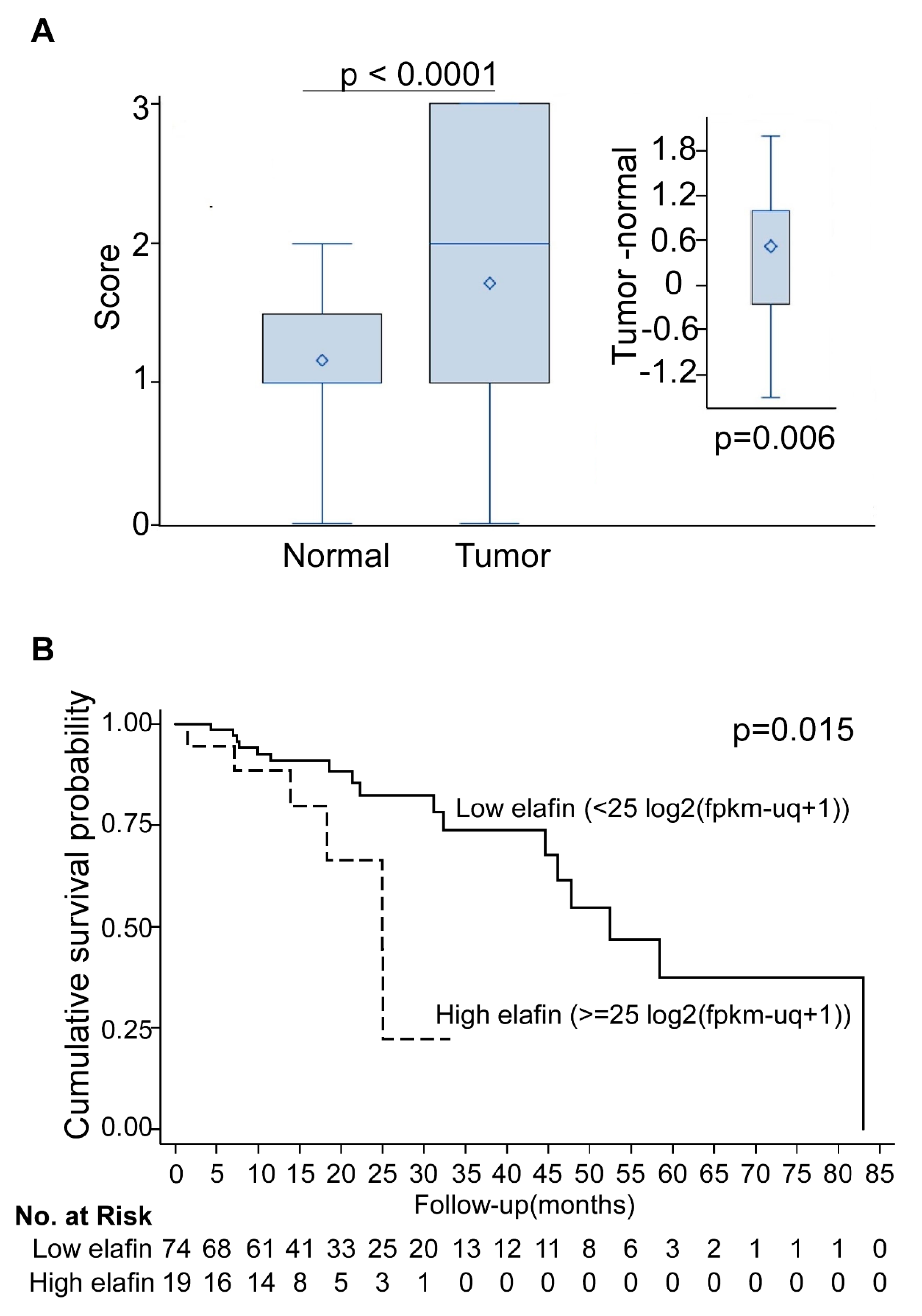
3.3. Overexpression of Elafin Protein in Tissue Arrays of Resected ESCC Specimens
3.4. Elafin Increased Proliferation, Migration, and Invasion of the ESCC Cell Lines
3.5. Elafin Induced Epithelial-to-Mesenchymal Transition (EMT)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, G.; McCormack, V.; Abedi-Ardekani, B.; Arnold, M.; Camargo, M.C.; Dar, N.A.; Dawsey, S.M.; Etemadi, A.; Fitzgerald, R.C.; Fleischer, D.E.; et al. International cancer seminars: A focus on esophageal squamous cell carcinoma. Ann. Oncol. 2017, 28, 2086–2093. [Google Scholar] [CrossRef]
- Abnet, C.C.; Arnold, M.; Wei, W.Q. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Njei, B.; McCarty, T.R.; Birk, J.W. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J. Gastroenterol. Hepatol. 2016, 31, 1141–1146. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xia, J.; Wang, K.; Zhang, J. Serum autoantibodies in the early detection of esophageal cancer: A systematic review. Tumour. Biol. 2015, 36, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.C.; Wang, Y.K.; Chen, Y.H.; Wu, C.C.; Wu, M.C.; Chen, W.C.; Wang, W.L.; Lin, H.S.; Chen, C.C.; Chou, S.H.; et al. High Serum Elafin Prediction of Poor Prognosis of Locoregional Esophageal Squamous Cell Carcinoma. Cancers 2021, 13, 3082. [Google Scholar] [CrossRef] [PubMed]
- Yazbeck, R.; Jaenisch, S.E.; Watson, D.I. From blood to breath: New horizons for esophageal cancer biomarkers. World J. Gastroenterol. 2016, 22, 10077–10083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Egami, H.; Kurizaki, T.; Ohmachi, H.; Hayashi, N.; Okino, T.; Shibata, Y.; Schalkwijk, J.; Ogawa, M. Immunohistochemical expression of SKALP/elafin in squamous cell carcinoma of the oesophagus. Br. J. Cancer 1997, 76, 1081–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westin, U.; Nystrom, M.; Ljungcrantz, I.; Eriksson, B.; Ohlsson, K. The presence of elafin, SLPI, IL1-RA and STNFalpha RI in head and neck squamous cell carcinomas and their relation to the degree of tumour differentiation. Mediat. Inflamm. 2002, 11, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.; Weldon, S.; Taggart, C.C. SLPI and elafin: Multifunctional antiproteases of the WFDC family. Biochem. Soc. Trans. 2011, 39, 1437–1440. [Google Scholar] [CrossRef] [Green Version]
- Shaw, L.; Wiedow, O. Therapeutic potential of human elafin. Biochem. Soc. Trans. 2011, 39, 1450–1454. [Google Scholar] [CrossRef]
- Alam, S.R.; Newby, D.E.; Henriksen, P.A. Role of the endogenous elastase inhibitor, elafin, in cardiovascular injury: From epithelium to endothelium. Biochem. Pharmacol. 2012, 83, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Egami, H.; Yamashita, J.; Takai, E.; Tamori, Y.; Fujino, N.; Kitaoka, M.; Schalkwijk, J.; Ogawa, M. Immunohistochemical expression of SKALP/elafin in squamous cell carcinoma of human lung. Oncol. Rep. 2002, 9, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.Y.; Lin, C.W.; Enikolopov, G.; Sibley, E.; Chiang, A.S.; Tang, S.C. Microtome-free 3-dimensional confocal imaging method for visualization of mouse intestine with subcellular-level resolution. Gastroenterology 2009, 137, 453–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.A.; Chung, Y.C.; Shen, M.Y.; Pan, S.T.; Kuo, C.W.; Peng, S.J.; Pasricha, P.J.; Tang, S.C. Perivascular Interstitial Cells of Cajal in Human Colon. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 102–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, A.S.; Lin, W.Y.; Liu, H.P.; Pszczolkowski, M.A.; Fu, T.F.; Chiu, S.L.; Holbrook, G.L. Insect NMDA receptors mediate juvenile hormone biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Chiang, A.S. High-resolution confocal imaging and three-dimensional rendering. Methods 2003, 30, 86–93. [Google Scholar] [CrossRef]
- Chiang, A.S.; Lin, C.Y.; Chuang, C.C.; Chang, H.M.; Hsieh, C.H.; Yeh, C.W.; Shih, C.T.; Wu, J.J.; Wang, G.T.; Chen, Y.C.; et al. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr. Biol. 2011, 21, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.K.; Chang, W.S.; Wu, I.C.; Li, L.H.; Yang, S.F.; Chen, J.Y.; Hsu, M.C.; Chen, S.H.; Wu, D.C.; Lee, J.M.; et al. Molecular characterization of invasive subpopulations from an esophageal squamous cell carcinoma cell line. Anticancer Res. 2010, 30, 727–736. [Google Scholar]
- Nara, K.; Ito, S.; Ito, T.; Suzuki, Y.; Ghoneim, M.A.; Tachibana, S.; Hirose, S. Elastase inhibitor elafin is a new type of proteinase inhibitor which has a transglutaminase-mediated anchoring sequence termed “cementoin”. J. Biochem. 1994, 115, 441–448. [Google Scholar] [CrossRef]
- Steinert, P.M.; Marekov, L.N. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J. Biol. Chem. 1995, 270, 17702–17711. [Google Scholar] [CrossRef] [Green Version]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef]
- Alkemade, H.A.; Molhuizen, H.O.; van Vlijmen-Willems, I.M.; van Haelst, U.J.; Schalkwijk, J. Differential expression of SKALP/Elafin in human epidermal tumors. Am. J. Pathol. 1993, 143, 1679–1687. [Google Scholar]
- Caruso, J.A.; Akli, S.; Pageon, L.; Hunt, K.K.; Keyomarsi, K. The serine protease inhibitor elafin maintains normal growth control by opposing the mitogenic effects of neutrophil elastase. Oncogene 2015, 34, 3556–3567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labidi-Galy, S.I.; Clauss, A.; Ng, V.; Duraisamy, S.; Elias, K.M.; Piao, H.Y.; Bilal, E.; Davidowitz, R.A.; Lu, Y.; Badalian-Very, G.; et al. Elafin drives poor outcome in high-grade serous ovarian cancers and basal-like breast tumors. Oncogene 2015, 34, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Hellstrom, K.E.; Hellstrom, I. Elafin selectively regulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol. Oncol. 2012, 125, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, M.C.; Gonzalez, S.F.; Welin, J.; Fuxe, J. Epithelial-mesenchymal transition in cancer metastasis through the lymphatic system. Mol. Oncol. 2017, 11, 781–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, K.T.; Yang, J. Epithelial-mesenchymal transition in tumor metastasis. Mol. Oncol. 2017, 11, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef]
- Kulasingam, V.; Diamandis, E.P. Proteomics analysis of conditioned media from three breast cancer cell lines: A mine for biomarkers and therapeutic targets. Mol. Cell Proteomics 2007, 6, 1997–2011. [Google Scholar] [CrossRef] [Green Version]
- Yokota, T.; Bui, T.; Liu, Y.; Yi, M.; Hunt, K.K.; Keyomarsi, K. Differential regulation of elafin in normal and tumor-derived mammary epithelial cells is mediated by CCAAT/enhancer binding protein beta. Cancer Res. 2007, 67, 11272–11283. [Google Scholar] [CrossRef] [Green Version]
- Caruso, J.A.; Hunt, K.K.; Keyomarsi, K. The neutrophil elastase inhibitor elafin triggers rb-mediated growth arrest and caspase-dependent apoptosis in breast cancer. Cancer Res. 2010, 70, 7125–7136. [Google Scholar] [CrossRef] [Green Version]
- Hunt, K.K.; Wingate, H.; Yokota, T.; Liu, Y.; Mills, G.B.; Zhang, F.; Fang, B.; Su, C.H.; Zhang, M.; Yi, M.; et al. Elafin, an inhibitor of elastase, is a prognostic indicator in breast cancer. Breast Cancer Res. 2013, 15, 3. [Google Scholar] [CrossRef]
- Caruso, J.A.; Karakas, C.; Zhang, J.; Yi, M.; Albarracin, C.; Sahin, A.; Bondy, M.; Liu, J.; Hunt, K.K.; Keyomarsi, K. Elafin is downregulated during breast and ovarian tumorigenesis but its residual expression predicts recurrence. Breast Cancer Res. 2014, 16, 3417. [Google Scholar] [CrossRef]
- Clauss, A.; Ng, V.; Liu, J.; Piao, H.; Russo, M.; Vena, N.; Sheng, Q.; Hirsch, M.S.; Bonome, T.; Matulonis, U.; et al. Overexpression of elafin in ovarian carcinoma is driven by genomic gains and activation of the nuclear factor kappaB pathway and is associated with poor overall survival. Neoplasia 2010, 12, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tian, Y.; Wu, T.; Dai, Y.; Wang, W.; Teng, G. High Expression and Clinical Significance of Elafin in Colorectal Cancer. Gastroenterol. Res. Pract. 2019, 2019, 4946824. [Google Scholar] [CrossRef]
- Saidi, A.; Javerzat, S.; Bellahcène, A.; De Vos, J.; Bello, L.; Castronovo, V.; Deprez, M.; Loiseau, H.; Bikfalvi, A.; Hagedorn, M. Experimental anti-angiogenesis causes upregulation of genes associated with poor survival in glioblastoma. Int. J. Cancer 2008, 122, 2187–2198. [Google Scholar] [CrossRef]
- Verbovšek, U.; Motaln, H.; Rotter, A.; Atai, N.A.; Gruden, K.; Van Noorden, C.J.; Lah, T.T. Expression analysis of all protease genes reveals cathepsin K to be overexpressed in glioblastoma. PLoS ONE 2014, 9, e111819. [Google Scholar] [CrossRef]
- Yu, K.S.; Lee, Y.; Kim, C.M.; Park, E.C.; Choi, J.; Lim, D.S.; Chung, Y.H.; Koh, S.S. The protease inhibitor, elafin, induces p53-dependent apoptosis in human melanoma cells. Int. J. Cancer 2010, 127, 1308–1320. [Google Scholar] [CrossRef]
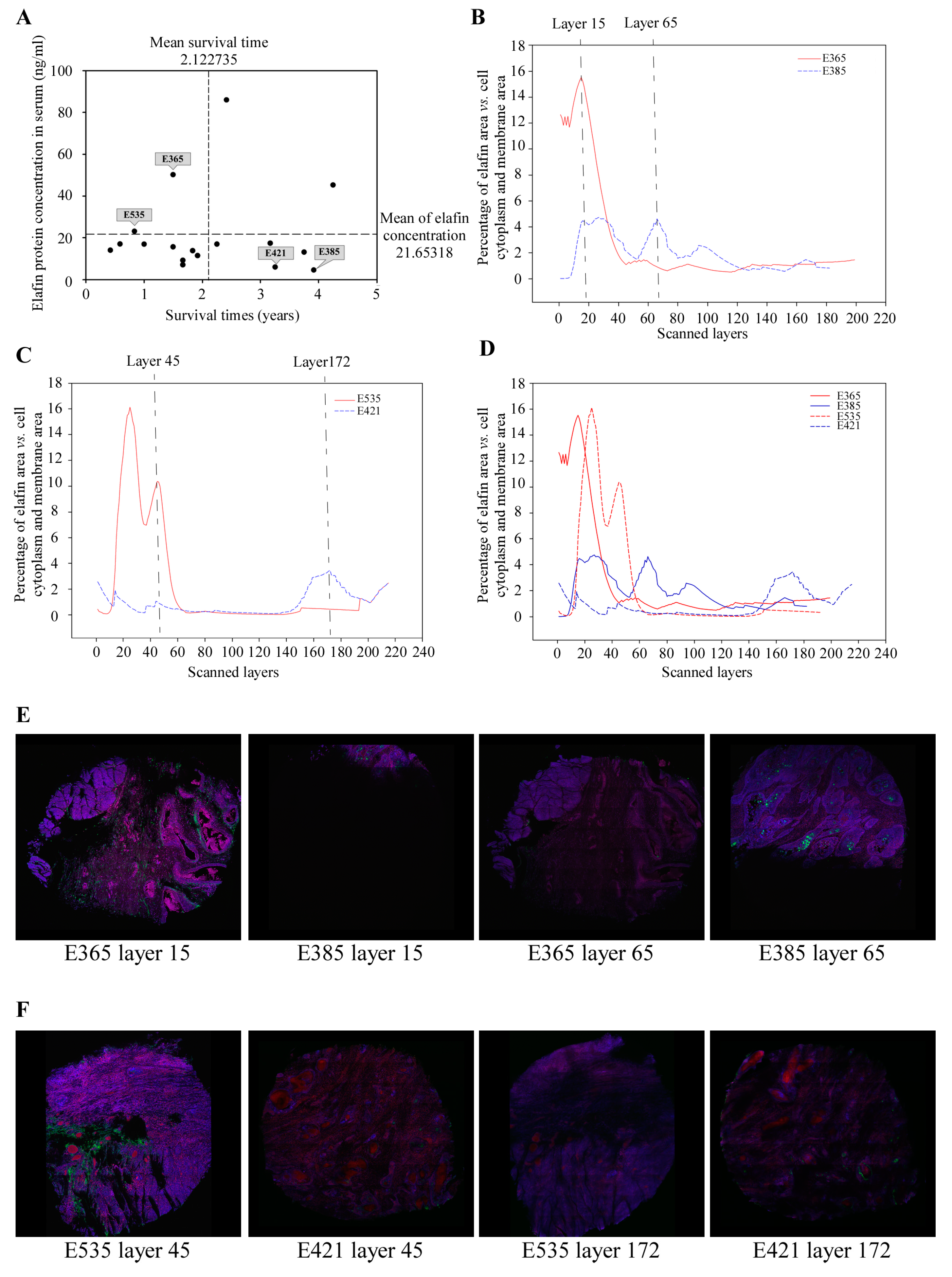
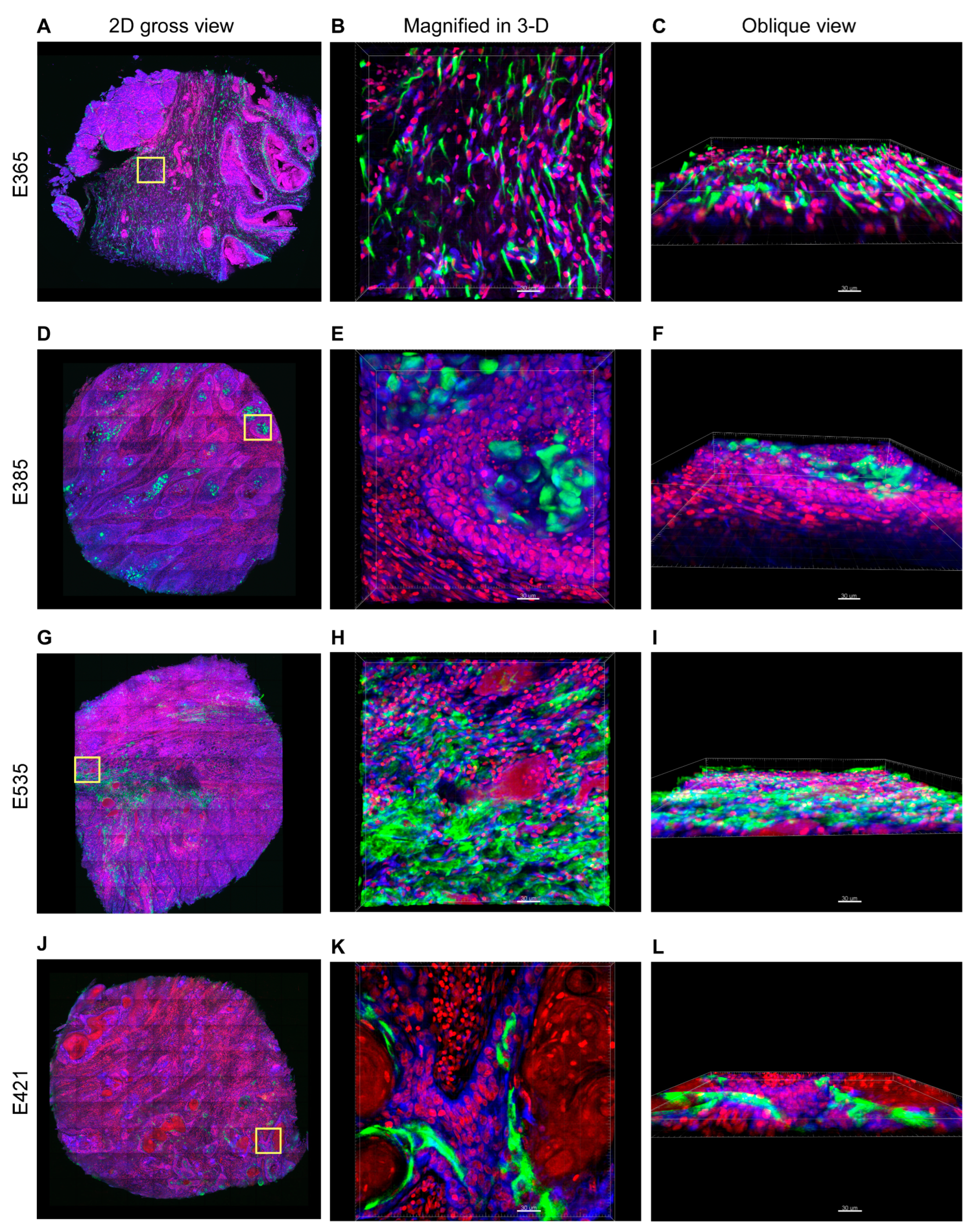


| Pair 1 | Pair 2 | |||
|---|---|---|---|---|
| E365 | E385 | E535 | E421 | |
| Gender | Male | Male | Male | Male |
| Age | 53 | 53 | 49 | 60 |
| Stage | IIb | IIIa | IIIa | IIIa |
| Treatment | OP then CCRT | OP then CCRT | OP | OP |
| Serum elafin (ng/mL) | 50.28 | 4.67 | 23.06 | 6.09 |
| Survival time (Months) | 18 | 47 | 10 | 39 |
| Scanned layers a (Thickness, μm) | 199 (139.3) | 182 (127.4) | 190 (133.0) | 215 (150.5) |
| Layers of selection b (Thickness, μm) | 1st–64th (45.5) | 10th–118th (76.3) | 13th–60th (33.6) | 1st–20th (14.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-C.; Wu, C.-C.; Liu, Y.-P.; Zhuo, G.-Y.; Wang, Y.-K.; Chen, Y.-H.; Chen, C.-C.; Wang, Y.-H.; Wu, M.-T.; Wu, I.-C. Elafin as a Prognostic Marker in Esophageal Squamous Cell Carcinoma: A Pilot Study Using Three-Dimensional Imaging and Genomic Profiling. Cancers 2023, 15, 3825. https://doi.org/10.3390/cancers15153825
Chen W-C, Wu C-C, Liu Y-P, Zhuo G-Y, Wang Y-K, Chen Y-H, Chen C-C, Wang Y-H, Wu M-T, Wu I-C. Elafin as a Prognostic Marker in Esophageal Squamous Cell Carcinoma: A Pilot Study Using Three-Dimensional Imaging and Genomic Profiling. Cancers. 2023; 15(15):3825. https://doi.org/10.3390/cancers15153825
Chicago/Turabian StyleChen, Wei-Chung, Chun-Chieh Wu, Yu-Peng Liu, Guan-Yu Zhuo, Yao-Kuang Wang, Yi-Hsun Chen, Chu-Chih Chen, Yin-Han Wang, Ming-Tsang Wu, and I-Chen Wu. 2023. "Elafin as a Prognostic Marker in Esophageal Squamous Cell Carcinoma: A Pilot Study Using Three-Dimensional Imaging and Genomic Profiling" Cancers 15, no. 15: 3825. https://doi.org/10.3390/cancers15153825
APA StyleChen, W.-C., Wu, C.-C., Liu, Y.-P., Zhuo, G.-Y., Wang, Y.-K., Chen, Y.-H., Chen, C.-C., Wang, Y.-H., Wu, M.-T., & Wu, I.-C. (2023). Elafin as a Prognostic Marker in Esophageal Squamous Cell Carcinoma: A Pilot Study Using Three-Dimensional Imaging and Genomic Profiling. Cancers, 15(15), 3825. https://doi.org/10.3390/cancers15153825







