Health Outcomes with Curative and Palliative Therapies in Real World: Role of the Quality of Life Summary Score in Thoracic Oncology Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Selection
2.2. Acquisition of Data
2.3. Patient-Reported Outcomes (PROs)
- Global QOL is estimated by global health score (GHS) and is based on two questions (score range 1–7). A higher GHS (range 0–100) means a better global QOL;
- The FS is composed of five domains, each with a 4-point score range: (a) physical, (b) role, (c) emotional, (d) cognitive, and (e) social functioning. These FSs are estimated by two questions per status, except physical functioning, which is assessed by five questions. A higher calculated FS score means a better status (range 0–100);
- The SS measures cancer-specific symptoms by 1 or 2 questions on a 4-point score scale. The higher the calculated symptom score (range 0–100), the more complaints the patient has.
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Health Status as Reported by the Patient per Group
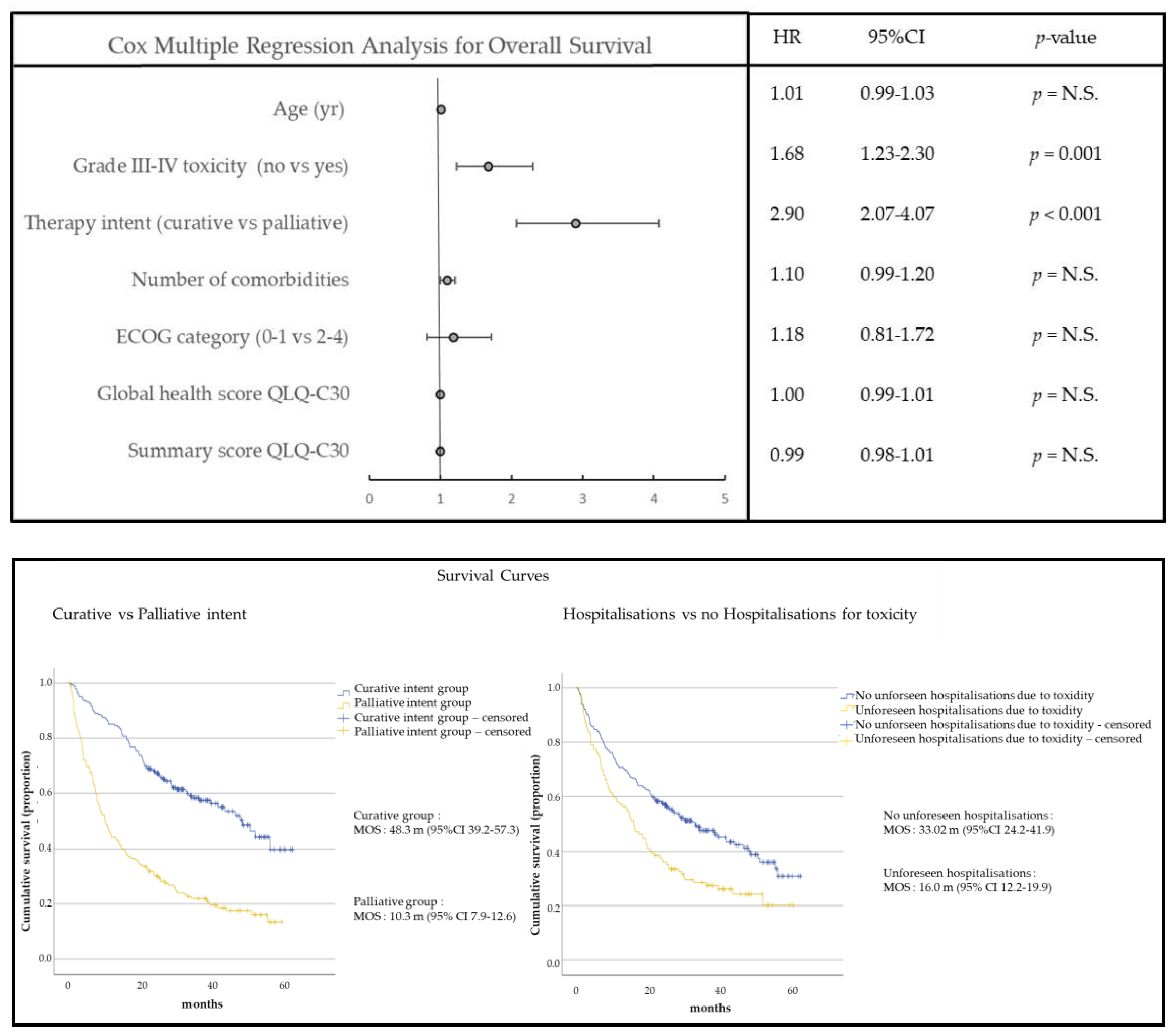
3.3. Univariable and Multivariable Cox Regression Model to Predict Survival
3.4. Multilinear Regression Model for GHS and QSS One Year after Diagnosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Soria, J.C.; Peters, S. Early and locally advanced non-small-cell lung cancer: An update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann. Oncol. 2021, 32, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Garinet, S.; Wang, P.; Mansuet-Lupo, A.; Fournel, L.; Wislez, M.; Blons, H. Updated Prognostic Factors in Localized NSCLC. Cancers 2022, 14, 1400. [Google Scholar] [CrossRef] [PubMed]
- Metzenmacher, M.; Griesinger, F.; Hummel, H.D.; Elender, C.; Schäfer, H.; de Wit, M.; Kaiser, U.; Kern, J.; Jänicke, M.; Spring, L.; et al. Prognostic factors in nonsmall cell lung cancer: Insights from the German CRISP registry. Eur. Respir. J. 2023, 61, 2201336. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, K.T.; Kaur, H.; Muthu, V.; Aggarwal, A.N.; Behera, D.; Singh, N. Interconversion of two commonly used performance tools: An analysis of 5844 paired assessments in 1501 lung cancer patients. World J. Clin. Oncol. 2018, 9, 140–147. [Google Scholar] [CrossRef]
- Maltoni, M.; Amadori, D. Prognosis in advanced cancer. Hematol. Oncol. Clin. N. Am. 2002, 16, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Ando, Y.; Hasegawa, Y.; Shimokata, K.; Minami, H.; Wakai, K.; Ohno, Y.; Sakai, S. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non-small cell lung cancer. Br. J. Cancer 2001, 85, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Shahrokni, A. Moving beyond Karnofsky and ECOG Performance Status Assessments with New Technologies. J. Oncol. 2016, 2016, 6186543. [Google Scholar] [CrossRef] [Green Version]
- Blagden, S.P.; Charman, S.C.; Sharples, L.D.; Magee, L.R.; Gilligan, D. Performance status score: Do patients and their oncologists agree? Br. J. Cancer 2003, 89, 1022–1027. [Google Scholar] [CrossRef]
- Giesinger, J.M.; Kieffer, J.M.; Fayers, P.M.; Groenvold, M.; Petersen, M.A.; Scott, N.W.; Sprangers, M.A.G.; Velikova, G.; Aaronson, N.K. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J. Clin. Epidemiol. 2016, 69, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangha, O.; Stucki, G.; Liang, M.H.; Fossel, A.H.; Katz, J.N. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003, 49, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.S.; van Bommel, A.C.; Stowell, C.; Abrahm, J.L.; Baker, M.; Baldotto, C.S.; Baldwin, D.R.; Borthwick, D.; Carbone, D.P.; Chen, A.B.; et al. Defining a standard set of patient-centred outcomes for lung cancer. Eur. Respir. J. 2016, 48, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Maringwa, J.; Quinten, C.; King, M.; Ringash, J.; Osoba, D.; Coens, C.; Martinelli, F.; Reeve, B.B.; Gotay, C.; Greimel, E.; et al. Minimal clinically meaningful differences for the EORTC QLQ-C30 and EORTC QLQ-BN20 scales in brain cancer patients. Ann. Oncol. 2011, 22, 2107–2112. [Google Scholar] [CrossRef]
- Di Maio, M. Quality of life: An important element of treatment value. Lancet Oncol. 2017, 18, 1557–1558. [Google Scholar] [CrossRef]
- Porter, M.E. A strategy for health care reform--toward a value-based system. N. Engl. J. Med. 2009, 361, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Lehto, R.H. Symptom burden in lung cancer: Management updates. Lung Cancer Manag. 2016, 5, 61–78. [Google Scholar] [CrossRef]
- Kenny, P.M.; King, M.T.; Viney, R.C.; Boyer, M.J.; Pollicino, C.A.; McLean, J.M.; Pollicino, C.A.; McLean, J.M.; Fulham, M.J.; McCaughan, B.C. Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. J. Clin. Oncol. 2008, 26, 233–241. [Google Scholar] [CrossRef]
- Hirpara, D.H.; Gupta, V.; Davis, L.E.; Zhao, H.; Hallet, J.; Mahar, A.L.; Sutradhar, R.; Doherty, M.; Louie, A.V.; Kidane, B.; et al. Severe symptoms persist for Up to one year after diagnosis of stage I-III lung cancer: An analysis of province-wide patient reported outcomes. Lung Cancer 2020, 142, 80–89. [Google Scholar] [CrossRef]
- Li, W.W.; Lee, T.W.; Yim, A.P. Quality of life after lung cancer resection. Thorac. Surg. Clin. 2004, 14, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, C.; Mazzocco, K.; Monzani, D.; Pavan, F.; Casiraghi, M.; Spaggiari, L.; Monturano, M. One-Year Quality of Life Trends in Early-Stage Lung Cancer Patients After Lobectomy. Front. Psychol. 2020, 11, 534428. [Google Scholar] [CrossRef] [PubMed]
- Pompili, C. Quality of life after lung resection for lung cancer. J. Thorac. Dis. 2015, 7 (Suppl. 2), S138–S144. [Google Scholar] [PubMed]
- Yucel, B.; Akkaş, E.A.; Okur, Y.; Eren, A.A.; Eren, M.F.; Karapinar, H.; Babacan, N.A.; Kılıçkap, S. The impact of radiotherapy on quality of life for cancer patients: A longitudinal study. Support Care Cancer 2014, 22, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.A.H.; Vercauter, P.; Verbeke, L.; Beelen, R.; Dooms, C.; Tournoy, K.G. Health Outcomes for Definite Concurrent Chemoradiation in Locally Advanced Non-Small Cell Lung Cancer: A Prospective Study. Respiration 2019, 97, 310–318. [Google Scholar] [CrossRef]
- Yang, P.; Cheville, A.L.; Wampfler, J.A.; Garces, Y.I.; Jatoi, A.; Clark, M.M.; Cassivi, S.D.; Midthun, D.E.; Marks, R.S.; Aubry, M.-C.; et al. Quality of life and symptom burden among long-term lung cancer survivors. J. Thorac. Oncol. 2012, 7, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haenen, V.; Evenepoel, M.; De Baerdemaecker, T.; Meeus, M.; Devoogdt, N.; Morlion, B.; Dams, L.; Van Dijck, S.; Van der Gucht, E.; De Vrieze, T.; et al. Pain prevalence and characteristics in survivors of solid cancers: A systematic review and meta-analysis. Support Care Cancer 2022, 31, 85. [Google Scholar] [CrossRef] [PubMed]
- Snijders, R.A.H.; Brom, L.; Theunissen, M.; van den Beuken-van Everdingen, M.H.J. Update on Prevalence of Pain in Patients with Cancer 2022: A Systematic Literature Review and Meta-Analysis. Cancers 2023, 15, 591. [Google Scholar] [CrossRef]
- Chabowski, M.; Jankowska-Polańska, B.; Lomper, K.; Janczak, D. The effect of coping strategy on quality of life in patients with NSCLC. Cancer Manag. Res. 2018, 10, 4085–4093. [Google Scholar] [CrossRef] [Green Version]
- Polański, J.; Dudek, K.; Mazur, G.; Chabowski, M. Effect of nutritional status on psychological functioning and coping in patients with lung cancer. Nutrition 2023, 109, 111970. [Google Scholar] [CrossRef]
- Tjong, M.C.; Doherty, M.; Tan, H.; Chan, W.C.; Zhao, H.; Hallet, J.; Darling, G.; Kidane, B.; Wright, F.C.; Mahar, A.; et al. Province-Wide Analysis of Patient-Reported Outcomes for Stage IV Non-Small Cell Lung Cancer. Oncologist 2021, 26, e1800–e1811. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weijst, L.; Lievens, Y.; Schrauwen, W.; Surmont, V. Health-Related Quality of Life in Advanced Non-small Cell Lung Cancer: A Methodological Appraisal Based on a Systematic Literature Review. Front. Oncol. 2019, 9, 715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahmer, J.R.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, A.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): A multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017, 18, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Thongprasert, S.; Duffield, E.; Saijo, N.; Wu, Y.L.; Yang, J.C.; Chu, D.T.; Liao, M.; Chen, Y.M.; Kuo, H.P.; Negoro, S.; et al. Health-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). J. Thorac. Oncol. 2011, 6, 1872–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.P.; William, F.; et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, T.; Takada, M.; Kubo, A.; Matsumura, A.; Fukai, S.; Tamura, A.; Saito, R.; Maruyama, Y.; Kawahara, M.; Ou, S.H.L.; et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: A comprehensive analysis of 26,957 patients with NSCLC. J. Thorac. Oncol. 2010, 5, 620–630. [Google Scholar] [CrossRef] [Green Version]
- Li, T.C.; Li, C.I.; Tseng, C.H.; Lin, K.S.; Yang, S.Y.; Chen, C.Y.; Hsia, T.C.; Lee, Y.D.; Lin, C.C. Quality of life predicts survival in patients with non-small cell lung cancer. BMC Public Health 2012, 12, 790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, Y.H.; Kim, Y.A.; Sim, J.A.; Shin, A.S.; Chang, Y.J.; Lee, J.; Kim, M.S.; Shim, Y.M.; Zo, J., III. Prognostic value of quality of life score in disease-free survivors of surgically-treated lung cancer. BMC Cancer 2016, 16, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinten, C.; Coens, C.; Mauer, M.; Comte, S.; Sprangers, M.A.; Cleeland, C.; Osoba, D.; Bjordal, K.; Bottomley, A. Baseline quality of life as a prognostic indicator of survival: A meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009, 10, 865–871. [Google Scholar] [CrossRef]
- Jacot, W.; Colinet, B.; Bertrand, D.; Lacombe, S.; Bozonnat, M.C.; Daurès, J.P.; Pujol, J.L. Quality of life and comorbidity score as prognostic determinants in non-small-cell lung cancer patients. Ann. Oncol. 2008, 19, 1458–1464. [Google Scholar] [CrossRef]
- Fiteni, F.; Vernerey, D.; Bonnetain, F.; Vaylet, F.; Sennélart, H.; Trédaniel, J.; Moro-Sibilot, D.; Herman, D.; Laizé, H.; Masson, P.; et al. Prognostic value of health-related quality of life for overall survival in elderly non-small-cell lung cancer patients. Eur. J. Cancer 2016, 52, 120–128. [Google Scholar] [CrossRef]
- Efficace, F.; Bottomley, A.; Smit, E.F.; Lianes, P.; Legrand, C.; Debruyne, C.; Schramel, F.; Smit, H.J.; Gaafar, R.; Biesma, B.; et al. Is a patient’s self-reported health-related quality of life a prognostic factor for survival in non-small-cell lung cancer patients? A multivariate analysis of prognostic factors of EORTC study 08975. Ann. Oncol. 2006, 17, 1698–1704. [Google Scholar] [CrossRef]
- Hong, Y.J.; Han, S.; Lim, J.U.; Kang, H.S.; Kim, S.K.; Kim, J.W.; Lee, S.H.; Kim, S.J.; Yeo, C.D. Association Between Quality of Life Questionnaire at Diagnosis and Survival in Patients With Lung Cancer. Clin. Lung Cancer 2023, 24, 459–466. [Google Scholar] [CrossRef]
- Husson, O.; de Rooij, B.H.; Kieffer, J.; Oerlemans, S.; Mols, F.; Aaronson, N.K.; van der Graaf, W.T.A.; van de Poll-Franse, L.V. The EORTC QLQ-C30 Summary Score as Prognostic Factor for Survival of Patients with Cancer in the “Real-World”: Results from the Population-Based PROFILES Registry. Oncologist 2020, 25, e722–e732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarewicz, M.A.; Wlodarczyk, D.; Johansen Reidunsdatter, R. Decision Tree Analyses for Prediction of QoL over a One-Year Period in Breast Cancer Patients: An Added Value of Patient-Reported Outcomes. Cancers 2023, 15, 2474. [Google Scholar] [CrossRef]
- Lehto, U.S.; Ojanen, M.; Kellokumpu-Lehtinen, P. Predictors of quality of life in newly diagnosed melanoma and breast cancer patients. Ann. Oncol. 2005, 16, 805–816. [Google Scholar] [CrossRef]
- Ramirez, R.A.; Lu, J.; Thomas, K.E.H. Quality of life for non-small cell lung cancer patients in the age of immunotherapy. Transl. Lung Cancer Res. 2018, 7 (Suppl. 2), S149–S152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zhang, Q.; Zhang, T.; Li, L.; Xu, C. Quality of life in patients with non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: A systematic review and meta-analysis. World J. Surg. Oncol. 2022, 20, 333. [Google Scholar] [CrossRef] [PubMed]
- Iovoli, A.J.; Yu, B.; Ma, S.J.; Farrugia, M.K.; Dexter, E.U.; Yendamuri, S.; Bouchard, E.G.; Singh, A.K. Quality of Life after Stereotactic Body Radiation Therapy or Surgery for Early-Stage NSCLC: A Systematic Review. JTO Clin. Res. Rep. 2022, 3, 100417. [Google Scholar] [CrossRef]




| All (n = 375) | Curative Group (n = 203) | Palliative Group (n = 172) | Significance | |
|---|---|---|---|---|
| Age, median (range) | 68.0 (38.0–96.0) | 69.0 (41.0–93.0) | 67.5 (38.0–96.0) | p = N.S. |
| Sex, male n (%) | 245 (65.3) | 140 (69.0) | 105 (61.0) | p = N.S. |
| BMI, mean (SEM) | 25.6 (0.3) | 26.4 (0.4) | 24.7 (0.3) | p = 0.002 |
| WHO, n (%) | p = 0.02 | |||
| 297 (79.2) | 170 (83.7) | 127 (73.8) | |
| 78 (20.8) | 33 (16.3) | 45 (26.2) | |
| Smoking, n (%) | p = N.S. | |||
| 324 (86.4) | 180 (88.7) | 144 (83.7) | |
| 51 (13.6) | 23 (11.3) | 28 (16.3) | |
| Cancer type, n (%) | p < 0.001 | |||
| 298 (79.5) | 150 (73.9) | 148 (86.0) | |
| 35 (9.3) | 17 (8.4) | 18 (10.5) | |
| 42 (11.2) | 36 (17.7) | 6 (3.5) | |
| Comorbidities, n (%) | p = N.S. | |||
| 138 (36.8) | 64 (31.5) | 74 (43.0) | |
| 147 (39.2) | 84 (41.4) | 63 (36.6) | |
| 90 (24.0) | 55 (27.1) | 35 (20.3) | |
| Stage (clinical), n (%) | p < 0.001 | |||
| 97 (25.9) | 97 (47.8) | - | |
| 25 (6.7) | 25 (12.3) | - | |
| 88 (23.5) | 81 (39.9) | 7 (4.1) | |
| 165 (44.0) | - | 165 (95.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tournoy, K.G.; Adam, V.; Muylle, I.; De Rijck, H.; Everaert, E.; Eqlimi, E.; van Meerbeeck, J.P.; Vercauter, P. Health Outcomes with Curative and Palliative Therapies in Real World: Role of the Quality of Life Summary Score in Thoracic Oncology Patients. Cancers 2023, 15, 3821. https://doi.org/10.3390/cancers15153821
Tournoy KG, Adam V, Muylle I, De Rijck H, Everaert E, Eqlimi E, van Meerbeeck JP, Vercauter P. Health Outcomes with Curative and Palliative Therapies in Real World: Role of the Quality of Life Summary Score in Thoracic Oncology Patients. Cancers. 2023; 15(15):3821. https://doi.org/10.3390/cancers15153821
Chicago/Turabian StyleTournoy, Kurt G., Valerie Adam, Inge Muylle, Helene De Rijck, Ellen Everaert, Ehsan Eqlimi, Jan P. van Meerbeeck, and Piet Vercauter. 2023. "Health Outcomes with Curative and Palliative Therapies in Real World: Role of the Quality of Life Summary Score in Thoracic Oncology Patients" Cancers 15, no. 15: 3821. https://doi.org/10.3390/cancers15153821
APA StyleTournoy, K. G., Adam, V., Muylle, I., De Rijck, H., Everaert, E., Eqlimi, E., van Meerbeeck, J. P., & Vercauter, P. (2023). Health Outcomes with Curative and Palliative Therapies in Real World: Role of the Quality of Life Summary Score in Thoracic Oncology Patients. Cancers, 15(15), 3821. https://doi.org/10.3390/cancers15153821







