Effects on the Hypothalamo-Pituitary Axis in Patients with CNS or Head and Neck Tumors following Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Tumor Types
3.2. Radiotherapy Modalities
- Pituitary radiotherapy modality in non-pituitary tumorsAccording to our literature search, non-PG tumor series (21 studies) were treated with 3DCRT in 12, IMRT in one, SBRT in one, and protons alone or combined with photons in four studies. Thus, normofractionated photon and proton techniques appeared as the most commonly used for non-PG tumours.
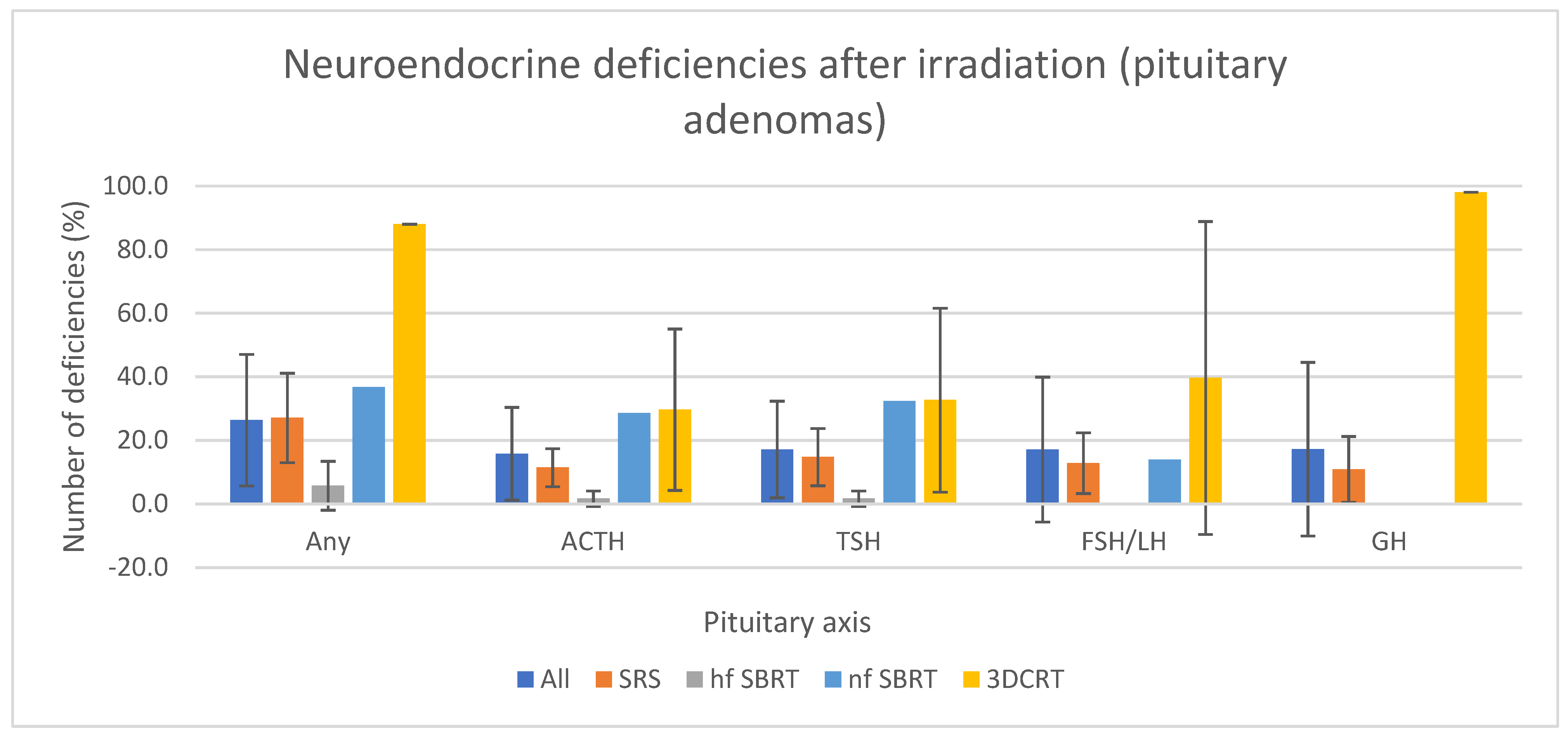
- Pituitary radiotherapy modality in pituitary tumorsIn contrast, 88% (21/24) of pituitary adenoma studies used SRS (single fraction) (16/24) or SBRT (6/24) in 3 to 5 fractions. The remaining three studies used 3DCRT. Thus, SRS/hypofractionated SBRT appeared as the most commonly used radiotherapy technique for PG tumours.
- Pituitary doses in non-pituitary tumorsIn non-PG, the mean dose to the pituitary gland was 47.6 (±12.9) Gy, ranging from 0 to 79 Gy (calculated from historical data of series indicated in Table 1). Most studies used photons (13/23), almost exclusively 3DCRT and the mean dose delivered to the pituitary gland was 47.2 (±9.4) Gy, ranging from 6 to 73 Gy. Four studies [10,11,15,27] used protons alone or associated with photons to treat gliomas, meningiomas, chordomas or chondrosarcomas. Among these studies, the mean pituitary dose was 57.9 (±9.2) Gy, ranging from 0.6 to 72.8 Gy. Six studies did not report the radiation technique, and five did not report the dose to the pituitary gland.Different total doses to the PG seemed to result in different reported pituitary deficiency rates, as illustrated by a 14% rate of patients developing new pituitary impairments after a median dose to the pituitary gland of 13 Gy and a median follow-up of 16 years [16] opposed to a 93% rate after 46 Gy and a median follow-up of 2 years [17]. However, such variations leave little confidence in interpreting deficits as they originate from retrospective studies. Prospective studies with systematic dosages are still needed, assuming that the excess cost of endocrine monitoring would probably be compensated by reducing the health expenses associated with managing complications.
- Pituitary doses in pituitary tumorsConsidering pituitary adenomas, the mean dose to the PG across studies was 19.7 (±4.2) Gy for SRS, 34.7 (±14.4) Gy for SBRT (52.2 for normofractionated SBRT and 26.0 for hypofractionated SBRT) and 49.1 (±3.7) Gy for 3DCRT. The mean tumor volume treated by SRS was 3.1 ± 1.6 cm3 and 4.4 (±0.7) cm3 by hypofractionated SBRT (calculated from historical series in Table 2). Only one article reported tumor volume treated by normofractionated SBRT and another by 3DCRT, respectively 8 cm3 and 1.9 cm3 [35,40]. As pituitary dose was not reported in SRS studies, Table 2 lists the margin dose, i.e., the isodose including the tumor, here the PG.
3.3. Hypothalamus Dose
3.4. Pituitary Hormone Deficiencies by Endocrine Axis
- 5
- Pituitary hormone deficiencies in non-pituitary tumors
- 6
- Pituitary hormone deficiencies in pituitary tumors
3.5. Posterior Pituitary Gland Deficiency
3.6. Radiotherapy Planning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosén, T.; Bengtsson, B.-Å. Premature Mortality Due to Cardiovascular Disease in Hypopituitarism. Lancet 1990, 336, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.; Holden, N.; Hills, R.; Wheatley, K.; Clayton, R.; Bates, A.; Sheppard, M.; Stewart, P. Association between Premature Mortality and Hypopituitarism. Lancet 2001, 357, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Mazziotti, G.; Frara, S.; Giustina, A. Pituitary Diseases and Bone. Endocr. Rev. 2018, 39, 440–488. [Google Scholar] [CrossRef] [PubMed]
- Noël, G.; Antoni, D. Organs at Risk Radiation Dose Constraints. Cancer/Radiothérapie 2022, 26, 59–75. [Google Scholar] [CrossRef]
- Littley, M.D.; Shalet, S.M.; Beardwell, C.G.; Robinson, E.L.; Sutton, M.L. Radiation-Induced Hypopituitarism Is Dose-Dependent. Clin. Endocrinol. 1989, 31, 363–373. [Google Scholar] [CrossRef]
- Darzy, K.H.; Shalet, S.M. Hypopituitarism Following Radiotherapy. Pituitary 2009, 12, 40–50. [Google Scholar] [CrossRef]
- Partoune, E.; Virzi, M.; Vander Veken, L.; Renard, L.; Maiter, D. Occurrence of Pituitary Hormone Deficits in Relation to Both Pituitary and Hypothalamic Doses after Radiotherapy for Skull Base Meningioma. Clin. Endocrinol. 2021, 95, 460–468. [Google Scholar] [CrossRef]
- Raymond, P.; Klein, M.; Cuny, T.; Klein, O.; Salleron, J.; Bernier-Chastagner, V. High Prevalence of Anterior Pituitary Deficiencies after Cranial Radiation Therapy for Skull Base Meningiomas. BMC Cancer 2021, 21, 1346. [Google Scholar] [CrossRef]
- Gebauer, J.; Mehta, P.; Fahlbusch, F.B.; Schmid, S.M.; Rades, D.; Janssen, S. Hypothalamic-Pituitary Axis Dysfunction after Whole Brain Radiotherapy—A Cohort Study. Anticancer. Res. 2020, 40, 5787–5792. [Google Scholar] [CrossRef]
- Tabrizi, S.; Yeap, B.Y.; Sherman, J.C.; Nachtigall, L.B.; Colvin, M.K.; Dworkin, M.; Fullerton, B.C.; Daartz, J.; Royce, T.J.; Oh, K.S.; et al. Long-Term Outcomes and Late Adverse Effects of a Prospective Study on Proton Radiotherapy for Patients with Low-Grade Glioma. Radiother. Oncol. 2019, 137, 95–101. [Google Scholar] [CrossRef]
- Lamba, N.; Bussiere, M.R.; Niemierko, A.; Abedi, P.; Fullerton, B.C.; Loeffler, J.S.; Oh, K.S.; Nachtigall, L.B.; Shih, H.A. Hypopituitarism After Cranial Irradiation for Meningiomas: A Single-Institution Experience. Pract. Radiat. Oncol. 2019, 9, e266–e273. [Google Scholar] [CrossRef] [PubMed]
- Kyriakakis, N.; Lynch, J.; Orme, S.M.; Gerrard, G.; Hatfield, P.; Short, S.C.; Loughrey, C.; Murray, R.D. Hypothalamic-pituitary Axis Irradiation Dose Thresholds for the Development of Hypopituitarism in Adult-onset Gliomas. Clin. Endocrinol. 2019, 91, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Handisurya, A.; Rumpold, T.; Caucig-Lütgendorf, C.; Flechl, B.; Preusser, M.; Ilhan-Mutlu, A.; Dieckmann, K.; Widhalm, G.; Grisold, A.; Wöhrer, A.; et al. Are Hypothyroidism and Hypogonadism Clinically Relevant in Patients with Malignant Gliomas? A Longitudinal Trial in Patients with Glioma. Radiother. Oncol. 2019, 130, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kyriakakis, N.; Lynch, J.; Orme, S.M.; Gerrard, G.; Hatfield, P.; Loughrey, C.; Short, S.C.; Murray, R.D. Pituitary Dysfunction Following Cranial Radiotherapy for Adult-Onset Nonpituitary Brain Tumours. Clin. Endocrinol. 2016, 84, 372–379. [Google Scholar] [CrossRef]
- De Marzi, L.; Feuvret, L.; Boulé, T.; Habrand, J.-L.; Martin, F.; Calugaru, V.; Fournier-Bidoz, N.; Ferrand, R.; Mazal, A. Use of GEUD for Predicting Ear and Pituitary Gland Damage Following Proton and Photon Radiation Therapy. BJR 2015, 88, 20140413. [Google Scholar] [CrossRef]
- Seland, M.; Bjøro, T.; Furre, T.; Schreiner, T.; Bollerslev, J.; Fosså, S.D.; Loge, J.H.; Holte, H.; Kiserud, C.E. Hormonal Dysfunction Is Frequent in Cancer Survivors Treated with Radiotherapy to the Head and Neck Region. J. Cancer Surviv. 2015, 9, 630–640. [Google Scholar] [CrossRef]
- Ipekci, S.; Cakir, M.; Kiyici, A.; Koc, O.; Artac, M. Radiotherapy-Induced Hypopituitarism in Nasopharyngeal Carcinoma: The Tip of an Iceberg. Exp. Clin. Endocrinol. Diabetes 2015, 123, 411–418. [Google Scholar] [CrossRef]
- Appelman-Dijkstra, N.M.; Malgo, F.; Neelis, K.J.; Coremans, I.; Biermasz, N.R.; Pereira, A.M. Pituitary Dysfunction in Adult Patients after Cranial Irradiation for Head and Nasopharyngeal Tumours. Radiother. Oncol. 2014, 113, 102–107. [Google Scholar] [CrossRef]
- Ratnasingam, J.; Karim, N.; Paramasivam, S.S.; Ibrahim, L.; Lim, L.L.; Tan, A.T.B.; Vethakkan, S.R.; Jalaludin, A.; Chan, S.P. Hypothalamic Pituitary Dysfunction amongst Nasopharyngeal Cancer Survivors. Pituitary 2015, 18, 448–455. [Google Scholar] [CrossRef]
- Sara, M.; Claudio, F.; Marco, L.; Roberto, L.; Elena, M.; Micaela, M.; Lucia, P.; Elena, B.; Marina, S.; Michele, R. Time Course of Hypothalamic-Pituitary Deficiency in Adults Receiving Cranial Radiotherapy for Primary Extrasellar Brain Tumors. Radiother. Oncol. 2011, 99, 23–28. [Google Scholar] [CrossRef]
- Minniti, G.; Clarke, E.; Cavallo, L.; Osti, M.F.; Esposito, V.; Cantore, G.; Cappabianca, P.; Enrici, R.M. Fractionated Stereotactic Conformal Radiotherapy for Large Benign Skull Base Meningiomas. Radiat. Oncol. 2011, 6, 36. [Google Scholar] [CrossRef]
- Snyers, A.; Janssens, G.O.R.J.; Twickler, M.B.; Hermus, A.R.; Takes, R.P.; Kappelle, A.C.; Merkx, M.A.W.; Dirix, P.; Kaanders, J.H.A.M. Malignant Tumors of the Nasal Cavity and Paranasal Sinuses: Long-Term Outcome and Morbidity with Emphasis on Hypothalamic-Pituitary Deficiency. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1343–1351. [Google Scholar] [CrossRef]
- Bhandare, N.; Kennedy, L.; Malyapa, R.S.; Morris, C.G.; Mendenhall, W.M. Hypopituitarism after Radiotherapy for Extracranial Head and Neck Cancers. Head Neck 2008, 30, 1182–1192. [Google Scholar] [CrossRef]
- Schneider, H.J.; Rovere, S.; Corneli, G.; Croce, C.G.; Gasco, V.; Rudà, R.; Grottoli, S.; Stalla, G.K.; Soffietti, R.; Ghigo, E.; et al. Endocrine Dysfunction in Patients Operated on for Non-Pituitary Intracranial Tumors. Eur. J. Endocrinol. 2006, 155, 559–566. [Google Scholar] [CrossRef]
- Agha, A.; Sherlock, M.; Brennan, S.; O’Connor, S.A.; O’Sullivan, E.; Rogers, B.; Faul, C.; Rawluk, D.; Tormey, W.; Thompson, C.J. Hypothalamic-Pituitary Dysfunction after Irradiation of Nonpituitary Brain Tumors in Adults. J. Clin. Endocrinol. Metab. 2005, 90, 6355–6360. [Google Scholar] [CrossRef]
- Johannesen, T.B.; Henrik Lien, H.; Håkon Hole, K.; Lote, K. Radiological and Clinical Assessment of Long-Term Brain Tumour Survivors after Radiotherapy. Radiother. Oncol. 2003, 69, 169–176. [Google Scholar] [CrossRef]
- Pai, H.H.; Thornton, A.; Katznelson, L.; Finkelstein, D.M.; Adams, J.A.; Fullerton, B.C.; Loeffler, J.S.; Leibsch, N.J.; Klibanski, A.; Munzenrider, J.E. Hypothalamic/Pituitary Function Following High-Dose Conformal Radiotherapy to the Base of Skull: Demonstration of a Dose–Effect Relationship Using Dose–Volume Histogram Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1079–1092. [Google Scholar] [CrossRef]
- Sumodhee, S.; Atallah, V.; Kinj, R.; Doyen, J.; L’Homel, B.; Gillon, P.; Paquis, P.; Almairac, F.; Hieronimus, S.; Schiappa, R.; et al. Fractionated Stereotactic Radiation Therapy for Pituitary Adenomas: An Alternative Escalating Protocol of Hypofractionated Stereotactic Radiotherapy Delivering 35 Gy in 5 Fractions. Cancer/Radiothérapie 2022, 26, 557–562. [Google Scholar] [CrossRef]
- Graffeo, C.S.; Perry, A.; Link, M.J.; Brown, P.D.; Young, W.F.; Pollock, B.E. Biological Effective Dose as a Predictor of Hypopituitarism After Single-Fraction Pituitary Adenoma Radiosurgery: Dosimetric Analysis and Cohort Study of Patients Treated Using Contemporary Techniques. Neurosurgery 2021, 89, S119. [Google Scholar] [CrossRef]
- Uygur, M.M.; Deyneli, O.; Yavuz, D.G. Long-Term Endocrinological Outcomes of Gamma Knife Radiosurgery in Acromegaly Patients. Growth Horm. IGF Res. 2020, 55, 101335. [Google Scholar] [CrossRef]
- Zibar Tomšić, K.; Dušek, T.; Kraljević, I.; Heinrich, Z.; Solak, M.; Vučinović, A.; Ozretić, D.; Mihailović Marasanov, S.; Hršak, H.; Kaštelan, D. Hypopituitarism after Gamma Knife Radiosurgery for Pituitary Adenoma. Endocr. Res. 2017, 42, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Ramesh, A.; Xu, Z.; Vance, M.L.; Schlesinger, D.; Sheehan, J.P. Gamma Knife Radiosurgery in Patients with Persistent Acromegaly or Cushing’s Disease: Long-Term Risk of Hypopituitarism. Clin. Endocrinol. 2016, 84, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Sato, K.; Nomura, R.; Tabei, Y.; Suzuki, I.; Yokota, N.; Inoue, M.; Ohta, S.; Yamada, S.; Shibamoto, Y. Long-Term Results of Hypofractionated Stereotactic Radiotherapy with CyberKnife for Growth Hormone-Secreting Pituitary Adenoma: Evaluation by the Cortina Consensus. J. Neurooncol. 2016, 128, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Puataweepong, P.; Dhanachai, M.; Hansasuta, A.; Dangprasert, S.; Swangsilpa, T.; Sitathanee, C.; Jiarpinitnun, C.; Vitoonpanich, P.; Yongvithisatid, P. The Clinical Outcome of Hypofractionated Stereotactic Radiotherapy with CyberKnife Robotic Radiosurgery for Perioptic Pituitary Adenoma. Technol. Cancer Res. Treat. 2016, 15, NP10–NP15. [Google Scholar] [CrossRef]
- Boström, J.P.; Kinfe, T.; Meyer, A.; Pintea, B.; Gerlach, R.; Surber, G.; Lammering, G.; Hamm, K. Treatment of Acromegaly Patients with Risk-Adapted Single or Fractionated Stereotactic High-Precision Radiotherapy: High Local Control and Low Toxicity in a Pooled Series. Strahlenther. Onkol. 2015, 191, 477–485. [Google Scholar] [CrossRef]
- Lee, C.-C.; Vance, M.L.; Xu, Z.; Yen, C.-P.; Schlesinger, D.; Dodson, B.; Sheehan, J. Stereotactic Radiosurgery for Acromegaly. J. Clin. Endocrinol. Metab. 2014, 99, 1273–1281. [Google Scholar] [CrossRef]
- Starke, R.M.; Williams, B.J.; Jane, J.A.; Sheehan, J.P. Gamma Knife Surgery for Patients with Nonfunctioning Pituitary Macroadenomas: Predictors of Tumor Control, Neurological Deficits, and Hypopituitarism: Clinical Article. J. Neurosurg. 2012, 117, 129–135. [Google Scholar] [CrossRef]
- Park, K.-J.; Kano, H.; Parry, P.V.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D.; Kondziolka, D. Long-Term Outcomes After Gamma Knife Stereotactic Radiosurgery for Nonfunctional Pituitary Adenomas. Neurosurgery 2011, 69, 1188–1199. [Google Scholar] [CrossRef]
- Iwata, H.; Sato, K.; Tatewaki, K.; Yokota, N.; Inoue, M.; Baba, Y.; Shibamoto, Y. Hypofractionated Stereotactic Radiotherapy with CyberKnife for Nonfunctioning Pituitary Adenoma: High Local Control with Low Toxicity. Neuro-Oncology 2011, 13, 916–922. [Google Scholar] [CrossRef]
- González, B.; Vargas, G.; Espinosa-de-los-Monteros, A.L.; Sosa, E.; Mercado, M. Efficacy and Safety of Radiotherapy in Acromegaly. Arch. Med. Res. 2011, 42, 48–52. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Pouratian, N.; Steiner, L.; Laws, E.R.; Vance, M.L. Gamma Knife Surgery for Pituitary Adenomas: Factors Related to Radiological and Endocrine Outcomes: Clinical Article. JNS 2011, 114, 303–309. [Google Scholar] [CrossRef]
- Feigl, G.C.; Pistracher, K.; Berghold, A.; Mokry, M. Pituitary Insufficiency as a Side Effect after Radiosurgery for Pituitary Adenomas: The Role of the Hypothalamus: Clinical Article. JNS 2010, 113, 153–159. [Google Scholar] [CrossRef]
- Leenstra, J.L.; Tanaka, S.; Kline, R.W.; Brown, P.D.; Link, M.J.; Nippoldt, T.B.; Young, W.F.; Pollock, B.E. Factors Associated with Endocrine Deficits after Stereotactic Radiosurgery of Pituitary Adenomas. Neurosurgery 2010, 67, 27–33. [Google Scholar] [CrossRef]
- Castinetti, F.; Nagai, M.; Morange, I.; Dufour, H.; Caron, P.; Chanson, P.; Cortet-Rudelli, C.; Kuhn, J.-M.; Conte-Devolx, B.; Regis, J.; et al. Long-Term Results of Stereotactic Radiosurgery in Secretory Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2009, 94, 3400–3407. [Google Scholar] [CrossRef]
- Langsenlehner, T.; Stiegler, C.; Quehenberger, F.; Feigl, G.C.; Jakse, G.; Mokry, M.; Langsenlehner, U.; Kapp, K.S.; Mayer, R. Long-Term Follow-up of Patients with Pituitary Macroadenomas after Postoperative Radiation Therapy: Analysis of Tumor Control and Functional Outcome. Strahlenther. Onkol. 2007, 183, 241–247. [Google Scholar] [CrossRef]
- Liščák, R.; Vladyka, V.; Marek, J.; Šimonová, G.; Vymazal, J. Gamma Knife Radiosurgery for Endocrine-Inactive Pituitary Adenomas. Acta Neurochir. 2007, 149, 999–1006. [Google Scholar] [CrossRef]
- Mingione, V.; Steiner, M.; Steiner, L. Gamma Surgery in the Treatment of Nonsecretory Pituitary Macroadenoma. J. Neurosurg. 2006, 104, 8. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Bates, P.; Carson, M.N.; Stewart, P.M.; Wass, J.A.H. Conventional Pituitary Irradiation Is Effective in Lowering Serum Growth Hormone and Insulin-Like Growth Factor-I in Patients with Acromegaly. J. Clin. Endocrinol. Metab. 2006, 91, 1239–1245. [Google Scholar] [CrossRef]
- Pouratian, N.; Sheehan, J.; Jagannathan, J.; Laws, E.R.; Steiner, L.; Vance, M.L. Gamma Knife Radiosurgery for Medically and Surgically Refractory Prolactinomas. Neurosurgery 2006, 59, 255–266. [Google Scholar] [CrossRef]
- Colin, P.; Jovenin, N.; Delemer, B.; Caron, J.; Grulet, H.; Hecart, A.-C.; Lukas, C.; Bazin, A.; Bernard, M.-H.; Scherpereel, B.; et al. Treatment of Pituitary Adenomas by Fractionated Stereotactic Radiotherapy: A Prospective Study of 110 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 333–341. [Google Scholar] [CrossRef]
- Pollock, B.E.; Nippoldt, T.B.; Stafford, S.L.; Foote, R.L.; Abboud, C.F. Results of Stereotactic Radiosurgery in Patients with Hormone-Producing Pituitary Adenomas: Factors Associated with Endocrine Normalization. J. Neurosurg. 2002, 97, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.; Pineda, R.; Sabatier, N.; Ludwig, M. 60 Years of Neuroendocrinology: The Posterior Pituitary, from Geoffrey Harris to Our Present Understanding. J. Endocrinol. 2015, 226, T173–T185. [Google Scholar] [CrossRef]
- Wu, Q.; Mohan, R.; Niemierko, A.; Schmidt-Ullrich, R. Optimization of Intensity-Modulated Radiotherapy Plans Based on the Equivalent Uniform Dose. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, M.; Eekers, D.B.P.; Alapetite, C.; Burnet, N.G.; Calugaru, V.; Coremans, I.E.M.; Fossati, P.; Høyer, M.; Langendijk, J.A.; Méndez Romero, A.; et al. Radiation Dose Constraints for Organs at Risk in Neuro-Oncology; the European Particle Therapy Network Consensus. Radiother. Oncol. 2018, 128, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Baumert, B.G.; Bendszus, M.; Bozzao, A.; Brada, M.; Fariselli, L.; Fiorentino, A.; Ganswindt, U.; Grosu, A.L.; Lagerwaard, F.L.; et al. ESTRO ACROP Guideline for Target Volume Delineation of Skull Base Tumors. Radiother. Oncol. 2021, 156, 80–94. [Google Scholar] [CrossRef]
- Verhelst, J.; Mattsson, A.F.; Camacho-Hübner, C.; Luger, A.; Abs, R. The Prevalence of the Metabolic Syndrome and Associated Cardiovascular Complications in Adult-Onset GHD during GH Replacement: A KIMS Analysis. Endocr. Connect. 2018, 7, 653–662. [Google Scholar] [CrossRef]
- Gazzaruso, C.; Gola, M.; Karamouzis, I.; Giubbini, R.; Giustina, A. Cardiovascular Risk in Adult Patients with Growth Hormone (GH) Deficiency and Following Substitution With GH—An Update. J. Clin. Endocrinol. Metab. 2014, 99, 18–29. [Google Scholar] [CrossRef]
- Ranke, M.B.; Wit, J.M. Growth Hormone—Past, Present and Future. Nat. Rev. Endocrinol. 2018, 14, 285–300. [Google Scholar] [CrossRef]
- van Bunderen, C.C.; van Nieuwpoort, I.C.; Arwert, L.I.; Heymans, M.W.; Franken, A.A.M.; Koppeschaar, H.P.F.; van der Lely, A.J.; Drent, M.L. Does Growth Hormone Replacement Therapy Reduce Mortality in Adults with Growth Hormone Deficiency? Data from the Dutch National Registry of Growth Hormone Treatment in Adults. J. Clin. Endocrinol. Metab. 2011, 96, 3151–3159. [Google Scholar] [CrossRef]
- Murray, P.G.; Higham, C.E.; Clayton, P.E. 60 Years of Neuroendocrinology: The Hypothalamo-GH Axis: The Past 60 Years. J. Endocrinol. 2015, 226, T123–T140. [Google Scholar] [CrossRef]
- Toledano, Y.; Lubetsky, A.; Shimon, I. Acquired Prolactin Deficiency in Patients with Disorders of the Hypothalamic-Pituitary Axis. J. Endocrinol. Investig. 2007, 30, 268–273. [Google Scholar] [CrossRef]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men with Hypogonadism: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef]
- Ilovayskaya, I.; Zektser, V.; Lazebnik, L. Similarity of Female Central (Hypogonadotropic) Hypogonadism and Postmenopause. Climacteric 2017, 20, 356–361. [Google Scholar] [CrossRef]
- Greenman, Y. Prolactinomas and Menopause: Any Changes in Management? Pituitary 2020, 23, 58–64. [Google Scholar] [CrossRef]
- Boehm, U.; Bouloux, P.-M.; Dattani, M.T.; de Roux, N.; Dodé, C.; Dunkel, L.; Dwyer, A.A.; Giacobini, P.; Hardelin, J.-P.; Juul, A.; et al. European Consensus Statement on Congenital Hypogonadotropic Hypogonadism—Pathogenesis, Diagnosis and Treatment. Nat. Rev. Endocrinol. 2015, 11, 547–564. [Google Scholar] [CrossRef]
- Young, J.; Xu, C.; Papadakis, G.E.; Acierno, J.S.; Maione, L.; Hietamäki, J.; Raivio, T.; Pitteloud, N. Clinical Management of Congenital Hypogonadotropic Hypogonadism. Endocr. Rev. 2019, 40, 669–710. [Google Scholar] [CrossRef]
- Yoo, T.-K.; Han, K.D.; Kim, D.; Ahn, J.; Park, W.-C.; Chae, B.J. Hormone Replacement Therapy, Breast Cancer Risk Factors, and Breast Cancer Risk: A Nationwide Population-Based Cohort. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1341–1347. [Google Scholar] [CrossRef]
- Olivius, C.; Landin-Wilhelmsen, K.; Olsson, D.S.; Johannsson, G.; Tivesten, Å. Prevalence and Treatment of Central Hypogonadism and Hypoandrogenism in Women with Hypopituitarism. Pituitary 2018, 21, 445–453. [Google Scholar] [CrossRef]
- Udovcic, M.; Pena, R.H.; Patham, B.; Tabatabai, L.; Kansara, A. Hypothyroidism and the Heart. Methodist DeBakey Cardiovasc. J. 2017, 3, 55–59. [Google Scholar] [CrossRef]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Thyroid Hormones and Female Reproduction†. Biol. Reprod. 2018, 99, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Feldt-Rasmussen, U.; Effraimidis, G.; Klose, M. The Hypothalamus-Pituitary-Thyroid (HPT)-Axis and Its Role in Physiology and Pathophysiology of Other Hypothalamus-Pituitary Functions. Mol. Cell. Endocrinol. 2021, 525, 111173. [Google Scholar] [CrossRef] [PubMed]
- Charmandari, E.; Nicolaides, N.C.; Chrousos, G.P. Adrenal Insufficiency. Lancet 2014, 383, 2152–2167. [Google Scholar] [CrossRef] [PubMed]
- Martin-Grace, J.; Dineen, R.; Sherlock, M.; Thompson, C.J. Adrenal Insufficiency: Physiology, Clinical Presentation and Diagnostic Challenges. Clin. Chim. Acta 2020, 505, 78–91. [Google Scholar] [CrossRef]
- Chabre, O.; Goichot, B.; Zenaty, D.; Bertherat, J. Group 1. Epidemiology of Primary and Secondary Adrenal Insufficiency: Prevalence and Incidence, Acute Adrenal Insufficiency, Long-Term Morbidity and Mortality. Ann. D’endocrinologie 2017, 78, 490–494. [Google Scholar] [CrossRef]
- Quinkler, M.; Ekman, B.; Zhang, P.; Isidori, A.M.; Murray, R.D.; on behalf of the EU-AIR Investigators. Mortality Data from the European Adrenal Insufficiency Registry-Patient Characterization and Associations. Clin. Endocrinol. 2018, 89, 30–35. [Google Scholar] [CrossRef]
- Crouzeix, G.; Morello, R.; Thariat, J.; Morera, J.; Joubert, M.; Reznik, Y. Quality of Life but Not Cognition Is Impacted by Radiotherapy in Patients with Non-Functioning Pituitary Adenoma. Horm. Metab. Res. 2019, 51, 178–185. [Google Scholar] [CrossRef]
- Webb, S.M.; Crespo, I.; Santos, A.; Resmini, E.; Aulinas, A.; Valassi, E. Management of Endocrine Disease: Quality of Life Tools for the Management of Pituitary Disease. Eur. J. Endocrinol. 2017, 177, R13–R26. [Google Scholar] [CrossRef]
- Higham, C.E.; Johannsson, G.; Shalet, S.M. Hypopituitarism. Lancet 2016, 388, 2403–2415. [Google Scholar] [CrossRef]


| Author | Patients | Age at RT (Years) (Range) | Technique | Diagnosis | Median Follow-Up (Range) | Mean Dose to PG (Gy) | New Pituitary Impairment (%) | Median Time Since RT before Diagnosis of New Deficit (Years) (Range) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Prolactin | ACTH | TSH | FSH/LH | GH | Any | Prolactin | ACTH | TSH | FSH/LH | GH | |||||||
| Partoune 2021 [7] | 48 | 49.2 ± 12.0 | 3DCRT (15); IMRT (33) | Meningioma | 7.5 (1.4–18.1) | 48.9 (6.0–55.1) | 38 | 11 | 13 | 32 | 28 | 35 | NR | NR | 5.0 (1.0–9.2) | 1.9 (0.3–4.8) | 2.2 (0.9–6.0) | 1.9 (0.3–6.5) |
| Raymond 2021 [8] | 52 | 56.2 ± 14.1 | NR | Meningioma | 7 (5.0–10.0) | 47 ± 9.4 | 60.2 | 18.5 | 15.4 | 28 | 36.9 | 13.4 | NR | NR | NR | NR | NR | NR |
| Gebauer 2020 [9] | 26 | 58 (36–81) | WBRT (moderate hypofractionation) | Prophylactic for SCLC or therapeutic for brain metastases (NSCLC. SCLC. breast or urothelial) | 1.7 (0.5–12.6) | 34.4 (30–41.25) | 50 | 26.9 | 4.8 | 5 | 37.5 | 8 | NR | NR | NR | NR | NR | NR |
| Tabrizi 2019 [10] * | 20 | 37.5 (22–56) | Proton | Glioma | 6.8 | Dmax > 20 Gy RBE (60% half of which > 30 Gy), Dmax < 5 Gy RBE (40%) | 30 | NR | 20 | 15 | 10 | 0 | 0.9 (0.4–3.15) | NR | NR | NR | NR | NR |
| Lamba 2019 [11] | 74 | 53 (13–83) | Proton or photon (NR, including 2 SBRT) | Meningioma | 3.6 (0.25–20.8) | 51.4 (0.6–61.5) | 20 | 15 | 24 | 24 | 10 | 19 | NR | 1.0 (0.75–1.08) | 2.67 (0.17–8.67) | 1.5 (0.75–3.25) | 1.0 (0.75–2.5) | 0.92 (0.58–2.5) |
| Kyriakakis 2019 [12] | 58 | 41.2 ± 10.9 | 3DCRT | Glioma | 8.2 ± 5.2 | 36.7 ± 15.9 | 84.5 | 10.3 | 19 | 6.9 | 20.7 | 82.8 | NR | NR | NR | NR | NR | NR |
| Handisurya 2019 [13] * | 436 | 50 (19–83) | 3DCRT | Glioma | NR | NR | NR | 38.3 | NR | 9.2 | NR | NR | NR | NR | NR | NR | NR | NR |
| Kyriakakis 2016 [14] | 107 | 40.0 (26.9–53.1) | 3DCRT | Various brain tumors | 8 | NR | 88.8 | 15 | 23.4 | 11.2 | 34.6 | 86.9 | NR | NR | 3.9 (2.5–5.7) | 5.3 (1.8–14.2) | 4.6 (2.3–7.9) | 3.3 (2.1–5.0) |
| De Marzi 2015 [15] | 103 | NR | Photon 3DCRT and proton | Chordoma or chondrosarcoma | NR | 54.0 (1.8–72.8) | 44 | 29 | NR | 11 | NR | NR | NR | NR | NR | NR | NR | NR |
| Seland 2015 [16] | 140 | 42.5 (15–76) | NR | Haematological (93%) | 16.1 (15–29) | 13.0 (0–68.5) | 14 | NR | NR | 1.4 | 7.9 | 5 | NR | NR | NR | NR | NR | NR |
| Ipekci 2015 [17] | 30 | 45.2 ± 9.8 | 3DCRT or Co60 | Nasopharyngeal | 2.0 (0.8–11.1) | 46.23 | 93.3 | 43.3 | 73.3 | 26.7 | 6.7 | 76.7 | NR | NR | NR | NR | NR | NR |
| Appelman-Dijkstra 2014 [18] | 80 | 47.5 (18.6–89.7) | NR | Various brain and nasopharyngeal tumors | 6.0 (0.5–35.0) | 56.27 (40.0–70.0) | 62 | 21 | 31 | 14 | 25 | 33 | NR | 2.5 (0.5–21.0) | 6.0 (0.5–24.0) | 5.1 (1.5–10.3) | 7 (0.5–16.0) | 4.5 (0.5–35.0) |
| Ratnasingam 2015 [19] | 50 | 50 ± 6.7 | NR | Nasopharyngeal | 8 (3–21) | >40 | 82 | 30 | 40 | 4 | 22 | 78 | NR | NR | NR | NR | NR | NR |
| Sara 2011 [20] * | 26 | 38.5 (33–47) | 3DCRT | Various brain tumors | 2.67 (1.0–9.25) | 41.8 (30.7–49.8) | 38 | 0 | 22 | 14 | 4 | 29 | 1.92 (1.75–2.5) | NR | NR | NR | NR | NR |
| Minniti 2011 [21] * | 52 | 56 (34–74) | SBRT | Meningioma | 3.5 (0.75–6.0) | NR | 19 | NR | 7.7 | 7.7 | 13.5 | 17.3 | 3 | NR | NR | NR | NR | NR |
| Snyers 2009 [22] | 21 | 61(27–74) | 3DCRT | Sinonasal | 8.9 (0.9–21.1) | 51–56 | 62 | 9.5 | 19 | 14.3 | 19 | 23.8 | NR | NR | NR | NR | NR | NR |
| Bhandare 2008 [23] | 312 | NR | 3DCRT | Sinonasal | 5.6 | 62.4 (39–73) | 14.1 | 3.2 | 4.5 | 5.4 | 3.8 | 5.1 | 5.6 (4.5–8.1) | NR | NR | NR | NR | NR |
| Schneider 2006 [24] | 44 | NR (20–79) | NR | Various brain tumors | NR | NR | 38.6 | 6.8 | 18.2 | 15.9 | 29.5 | 27.3 | NR | NR | NR | NR | NR | NR |
| Agha 2005 [25] | 56 | 33.3 (21.3–45.3) | NR | Various brain tumors | 3.2 (1.0–12.5) | BED 54 (4–97) | 41 | 32 | 21 | 9 | 27 | 32 | NR | NR | NR | NR | NR | NR |
| Johannesen 2003 [26] | 25 | 38 (14–68) | 3DCRT | Glioma | 13.1 (6.0–25.6) | 54.0 (45.0–59.4) | 64 | NR | 4 | 56 | 28.6 | NR | NR | NR | 2 | 5.0 (0.3–15.0) | 0.1 (0.1–3.0) | NR |
| Pai 2001 [27] | 107 | 41.2 (17–75) | Proton and photon | Chordoma or chondrosarcoma | 5.5 | <68.4 (55.8–79) | 87 | 72 | 19 | 30 | 29 | NR | NR | 2.5 (0.6–14.3) | 3.6 (1.0–14.3) | 3.6 (0.5–14.3) | 4.0 (2.2–11.7) | NR |
| Author | Patients | Age at RT (Years) (Range) | Technique | Median Follow-Up (Range) | Tumor Volume (cm3) | Mean Dose to PG (Gy) | New Pituitary Impairment (%) | Median time Since RT before Diagnosis of New Deficit (Years) (Range) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | ACTH | TSH | FSH/LH | GH | Any | ACTH | TSH | FSH/LH | GH | |||||||
| Sumodhee 2022 [28] | 29 | 54 (23–86) | SBRT | 3.9 (1.0–10.1) | 4.8 (0.4–14.7) | 35 | 17 | 3.4 | 3.4 | NR | NR | NR | NR | NR | NR | NR |
| Graffeo 2021 [29] | 97 | 50 (38–57) | SRS | 4 (2.8–5.7) | 2.8 (1.4–4.4) | 15 (non-secreting)/25 (secreting) | 28 | 16 | 14 | 14 | 4 | 1.8 (1–3) | NR | NR | NR | NR |
| Uygur 2020 [30] | 110 | 49 ± 12 | SRS | 6.5 ± 4.7 | NR | 23.3 (16–30) | 5.4 | 0.9 | 0.9 | 2.72 | NR | NR | NR | NR | NR | NR |
| Zibar Tomšić 2017 [31] | 27 | 49 (23–74) | SRS | 6 (0.5–12) | 3.4 (0.1–16.8) | 14.4 (5.9–22.8) | 30 | 19 | 8 | 14 | NR | 3.5 (0.25–8.0) | NR | NR | NR | NR |
| Cohen-Inbar 2016 [32] | 60 | 41.5 (18–69) | SRS | 13.3 (5.0–23.2) | 1.3 (0.3–13.4) | 25 (6–30) | 58.3 | 18.3 | 26.7 | 28.3 | 33.3 | 5.1 (1.0–13.3) | >10 | 5 < x < 10 | 5 < x < 10 | 5 < x < 10 |
| Iwata 2016 [33] | 52 | 35 (14–67) | SBRT | 5 (2.3–11.4) | 4.4 (0.2–19.8) | 21 | 1.9 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Puataweepong 2016 [34] | 40 | 49.5 (26–68) | SBRT | 3.2 (1.2–5.9) | 3.4 (0.8–25.9) | 25 (20–28) | 0 | 0 | 0 | 0 | 0 | NR | NR | NR | NR | NR |
| Boström 2015 [35] * | 35 | 54 (30–75) | SRS/normofractionated SBRT | 8 (2–13) | 1.2 (SRS)/8.0(SBRT) | 20 (SRS)/54 (SBRT) | NR | 46.4 | NR | NR | NR | NR | NR | NR | NR | NR |
| Lee 2014 [36] | 136 | 44 (14–93) | SRS | 5.1 | 2.3 (0.3–16) | 25 (8.8–30) | 31.6 | NR | NR | NR | NR | 4.2 (0.7–10.6) | NR | NR | NR | NR |
| Starke 2012 [37] | 140 | 51 (21–82) | SRS | 4.2 (0.5–17) | 5.6 ± 5.6 | 18 (6–25) | 30.3 | 13.8 | 28.1 | 7.6 | 11.8 | NR | NR | NR | NR | NR |
| Park 2011 [38] | 88 | 53.7 (15.5–88.1) | SRS | 5.3 (0.5–15.1) | 3.5(0.4–28.1) | 13 | 24 | 10.7 | 9.7 | 6.9 | 5.7 | 2 (0.25–9.5) | NR | NR | NR | NR |
| Iwata 2011 [39] | 74 | 59 (16–82) | SBRT | 2.8 (1.8–9.9) | 5.1 (0.7–64.3) | 21/25 | 4.1 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| González 2011 [40] | 40 | 52.9 ± 12.1 | 3DCRT | 8 (7–12) | 1.9 | 52 | NR | 15 | 7 | 5 | NR | NR | NR | NR | NR | NR |
| Sheehan 2011 [41] | 418 | 44 (12–91) | SRS | 2.6 (0.5–10.3) | 1.9 (0.1–27) | 24 (9–30) | 24.4 | NR | NR | NR | NR | NR | NR | NR | NRN | NR |
| Feigl 2010 [42] | 108 | 51.9 (9.1–81.2) | SRS | 6.7 | 6 (0.2–80) | 11.0 (2.3–31.1) | 43.5 | 17.2 | 25.3 | 27.3 | 24.2 | NR | 3.0 (0.25–9.6) | 2.33 (0.25–9.3) | 2.25 (0.25–9.3) | 2.6 (0.25–4.7) |
| Leenstra 2010 [43] | 82 | 48 | SRS | 5.3 (1.1–11.2) | 2.7 ± 2.7 | 20 (11–30) | 41 | 14 | 22 | 21 | 13 | 2.67 (0.2–9.8) | 2.25 (1.0–9.2) | 2.25 (0.2–4.7) | 2.3 (0.4–6.7) | 2.7 (1.1–9.8) |
| Castinetti 2009 [44] | 76 | 42.7 (7–66) | SRS | 8 (5–13) | 0.5 (0.2–0.9) | 25 (12–40) | 22.4 | 11.8 | 9.2 | 14.5 | 6.6 | 4 (1–6) | NR | NR | NR | NR |
| Langsenlehner 2007 [45] | 87 | 51.3 (22.7–79.8) | 3DCRT | 10.5 (1.4–20.8) | NR | 50.4 (46–54) | 88 | 59 | 64 | 96 | 98 | NR | 7.08 (5.61–11.9) | 4.67 (3.1–6.91) | 1.96 (1.5–3.53) | NR |
| Liščák 2007 [46] | 79 | 54 (24–73) | SRS | 5 (3–10) | 3.5 (0.1–31.3) | 20 | 2.5 | 1.3 | 1.3 | 0 | 0 | NR | 4 | 7 | NR | NR |
| Mingione 2006 [47] | 61 | 51.1 (21–82) | SRS | 4 (0.5–10.6) | 4.8 (0.6–27) | 18.5 (5–25) | 19.7 | 6.6 | 14.8 | NR | 3.3 | 2.2 (0.7–8.9) | 1.4 (0.9–2.1) | 2.3 (0.7–8.9) | NR | 2.2 (1.1–3) |
| Jenkins 2006 [48] | 656 | 48 (14–80) | 3DCRT | 7 (3–13) | NR | 45 (10–55) | NR | 15 | 27 | 18 | NR | NR | NR | NR | NR | NR |
| Pouratian 2006 [49] | 28 | 43.1 (17–71) | SRS | 4.3 (1.25–10.2) | 3.0 (0.2–10.6) | 18.6 (0.3–25) | 29 | 7 | 18 | NR | 7 | 3.7 (2.75–4.25) | NR | NR | NR | NR |
| Colin 2005 [50] * | 110 | 50 (6–83) | Normofractionated SBRT | 6.7 (4.0–13.1) | NR | 50.4 | 36.7 | 28.6 | 32.3 | 13.9 | NR | NR | NR | NR | NR | NR |
| Pollock 2002 [51] | 43 | 42 | SRS | 3 (1–9) | 4.3 (0.4–17.3) | 20 (14.4–30) | 16 | 12 | 14 | 5 | NR | 5 | NR | NR | NR | NR |
| Pituitary Tumors | Non-Pituitary Head and Neck, Sinonasal and CNS Tumors | |
|---|---|---|
| Total dose on PG > 45 Gy (dose per fraction); No advantage on PG, any axis, between techniques | Wide range of total doses (examples: (sinonasal carcinoma = 70 Gy, meningioma 50–54 Gy) on PG may be <20 to 60 or over | |
| Conformal radiotherapy (head and neck and CNS irradiations before the year 2000, WBRT, TBI) | (2–4 Gy/f) No optimization on PG nor a healthy brain around tumor | (2–4 Gy/f), if using two opposed beams leaves little potential for dose optimization on PG (and might not be considered a priority versus other structures) In WBRT, dose to the brain = dose to the PG |
| Intensity-modulated radiotherapy | (≈1.8–2.2 Gy/f) Steep dose gradients, low dose bath ≈2–5 Gy to whole brain | Standard in most brain and head and neck primary tumors (≈<<2 Gy/f for tumors > 2 cm from PG) For tumors distant > 2 cm from PG, PG dose optimization might be reduced further. If PG is in the low-risk target volume (50 Gy in head and neck tumors), one might need to evaluate the trade-off, i.e., low-risk target volume coverage versus lower dose to PG, if decreasing the PG dose using extreme dose modulation improves the quality of life without increasing tumor failure risks |
| Stereotactic body radiotherapy | Frequently used for PG adenomas (≈4–5 Gy/f, sometimes ≈2 Gy/f) Very steep gradients ≈2 Gy to the whole brain Yet, significant dose to the carotids and nerves in the cavernous sinuses: Long-term vascular and neurotropic effects? | Frequently used for brain metastases (≈4–5 Gy/f) For tumors within 1 cm from PG, the PG dose may be optimized For tumors > 1 cm from PG, the PG dose could be lower than 20 Gy in most situations Dose to brain and radionecrosis might remain clinical issues in the mid-long term |
| Proton therapy | (≈2 Gy/f, in 2022 but arctherapy, hypofractionation and flash proton therapy may change this regimen), steep dose gradients ≈5–10 Gy in the 2–3 proton beams, no dose in the brain elsewhere | (≈2 Gy/f) For tumors within 1 cm from PG, PG dose may be optimized using technical sophistication (such as better range shifters, multi-field optimization, proton overshoot of in-front beams, DECT and reduced robustness constraints etc.) but not necessarily better than photon techniques. However, the proton beam allows significant sparing of distant brain tissue. For tumors > 1 cm, PT might be superior to IMRT and SBRT by avoiding low doses to the brain and vessels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouter, J.; Reznik, Y.; Thariat, J. Effects on the Hypothalamo-Pituitary Axis in Patients with CNS or Head and Neck Tumors following Radiotherapy. Cancers 2023, 15, 3820. https://doi.org/10.3390/cancers15153820
Bouter J, Reznik Y, Thariat J. Effects on the Hypothalamo-Pituitary Axis in Patients with CNS or Head and Neck Tumors following Radiotherapy. Cancers. 2023; 15(15):3820. https://doi.org/10.3390/cancers15153820
Chicago/Turabian StyleBouter, Jordan, Yves Reznik, and Juliette Thariat. 2023. "Effects on the Hypothalamo-Pituitary Axis in Patients with CNS or Head and Neck Tumors following Radiotherapy" Cancers 15, no. 15: 3820. https://doi.org/10.3390/cancers15153820
APA StyleBouter, J., Reznik, Y., & Thariat, J. (2023). Effects on the Hypothalamo-Pituitary Axis in Patients with CNS or Head and Neck Tumors following Radiotherapy. Cancers, 15(15), 3820. https://doi.org/10.3390/cancers15153820






