True Prevalence of Unforeseen N2 Disease in NSCLC: A Systematic Review + Meta-Analysis
Abstract
Simple Summary
Abstract
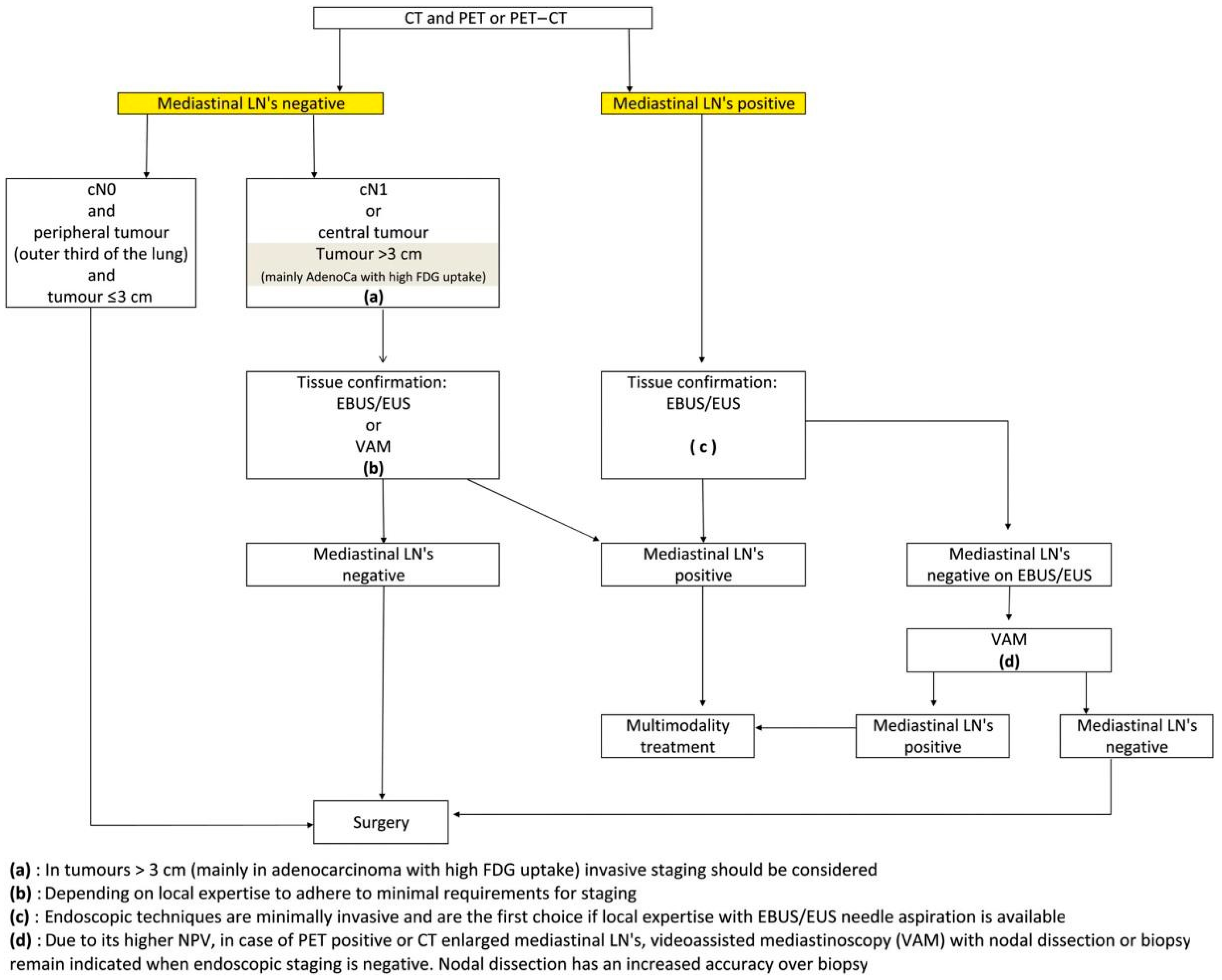
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Statistical Methods
2.3. Quality Assessment
3. Results
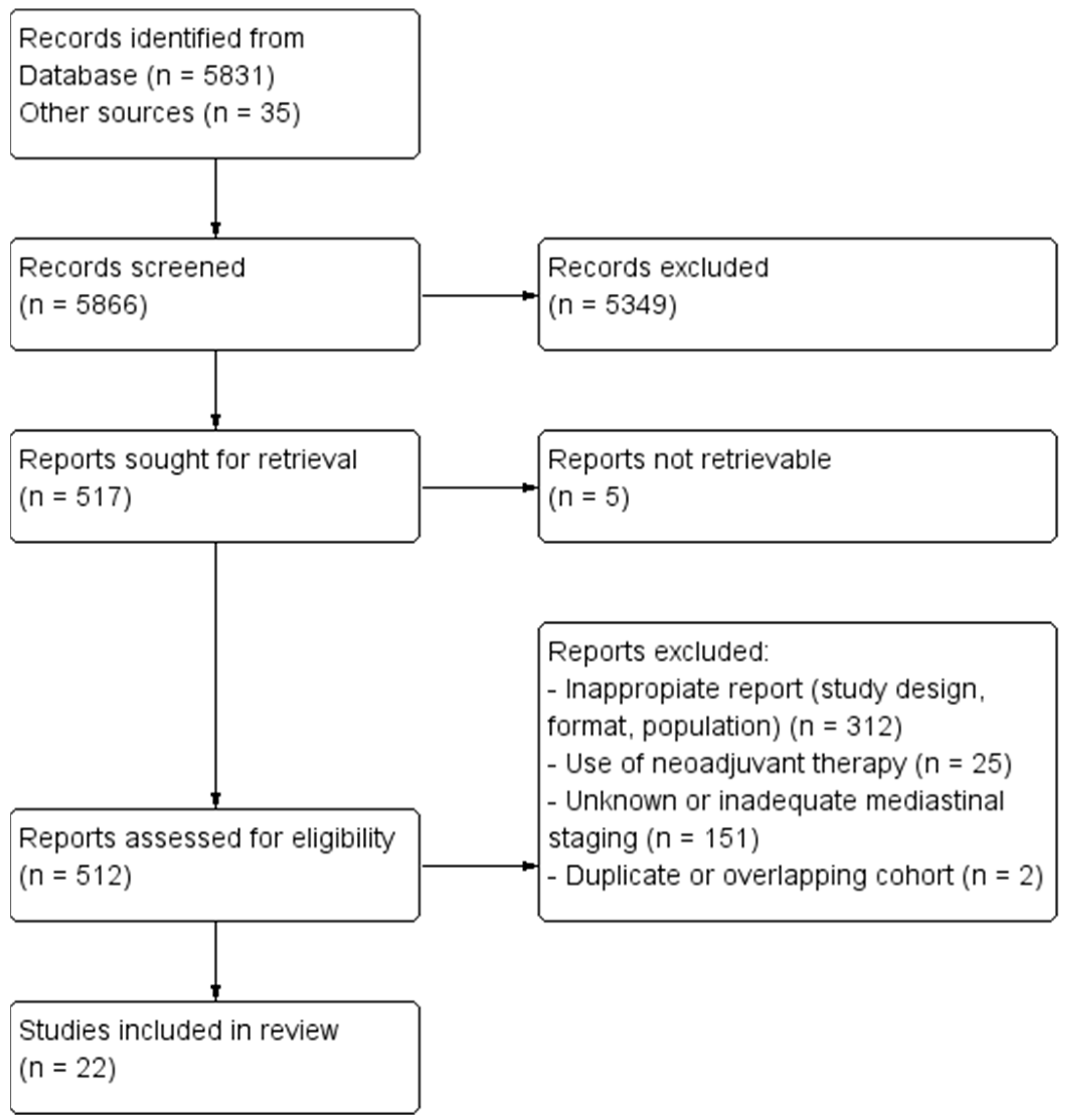
3.1. Search Results
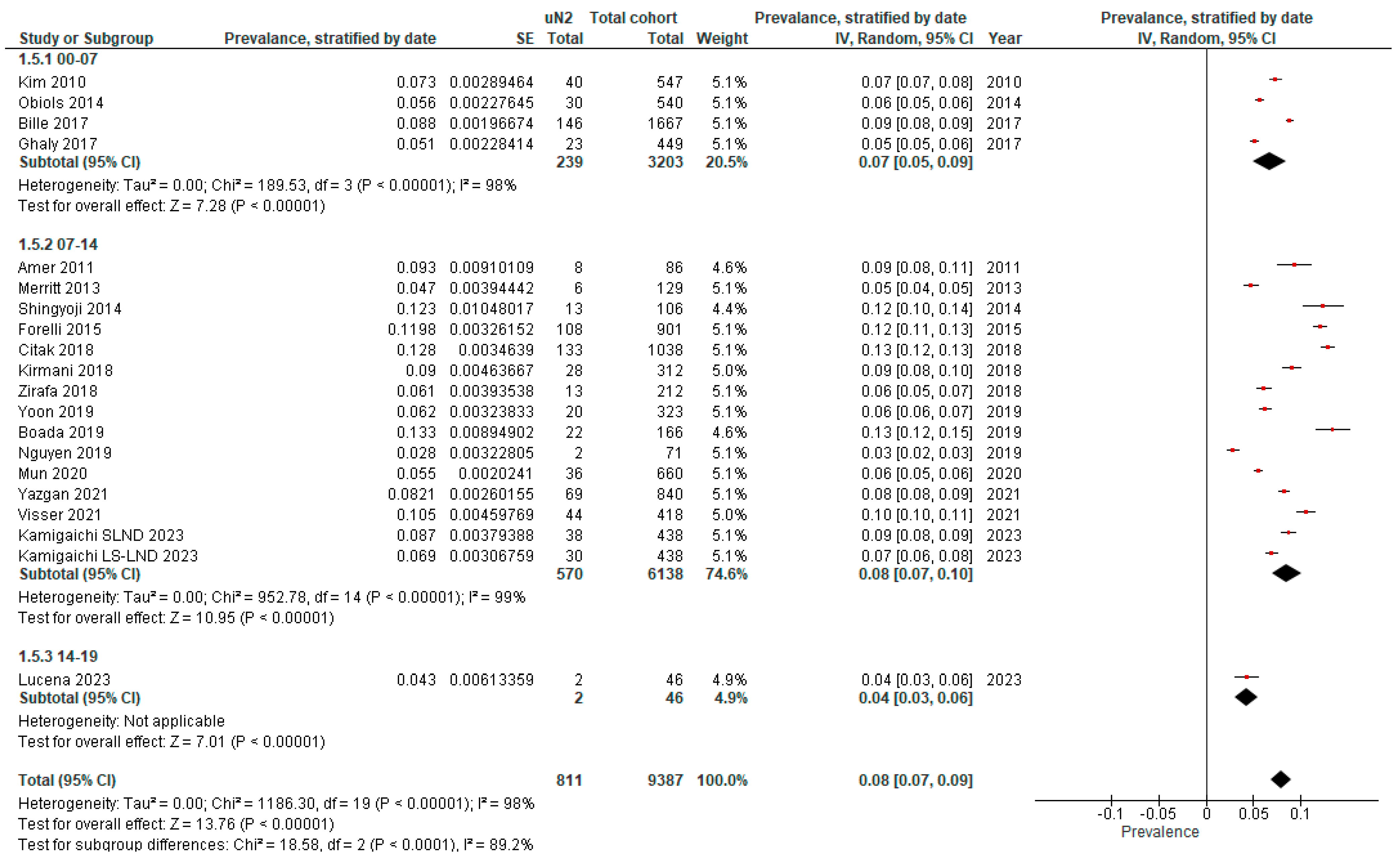
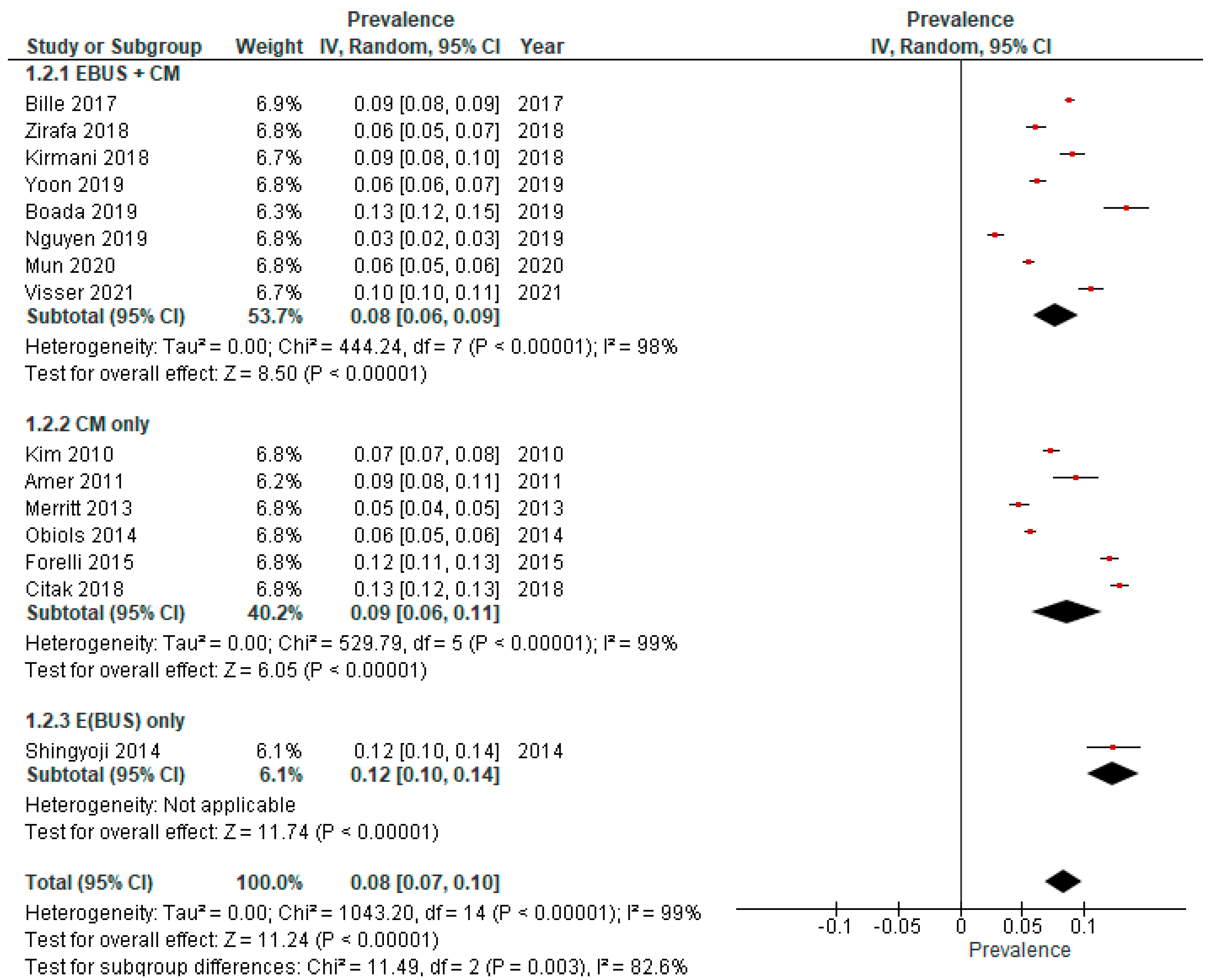
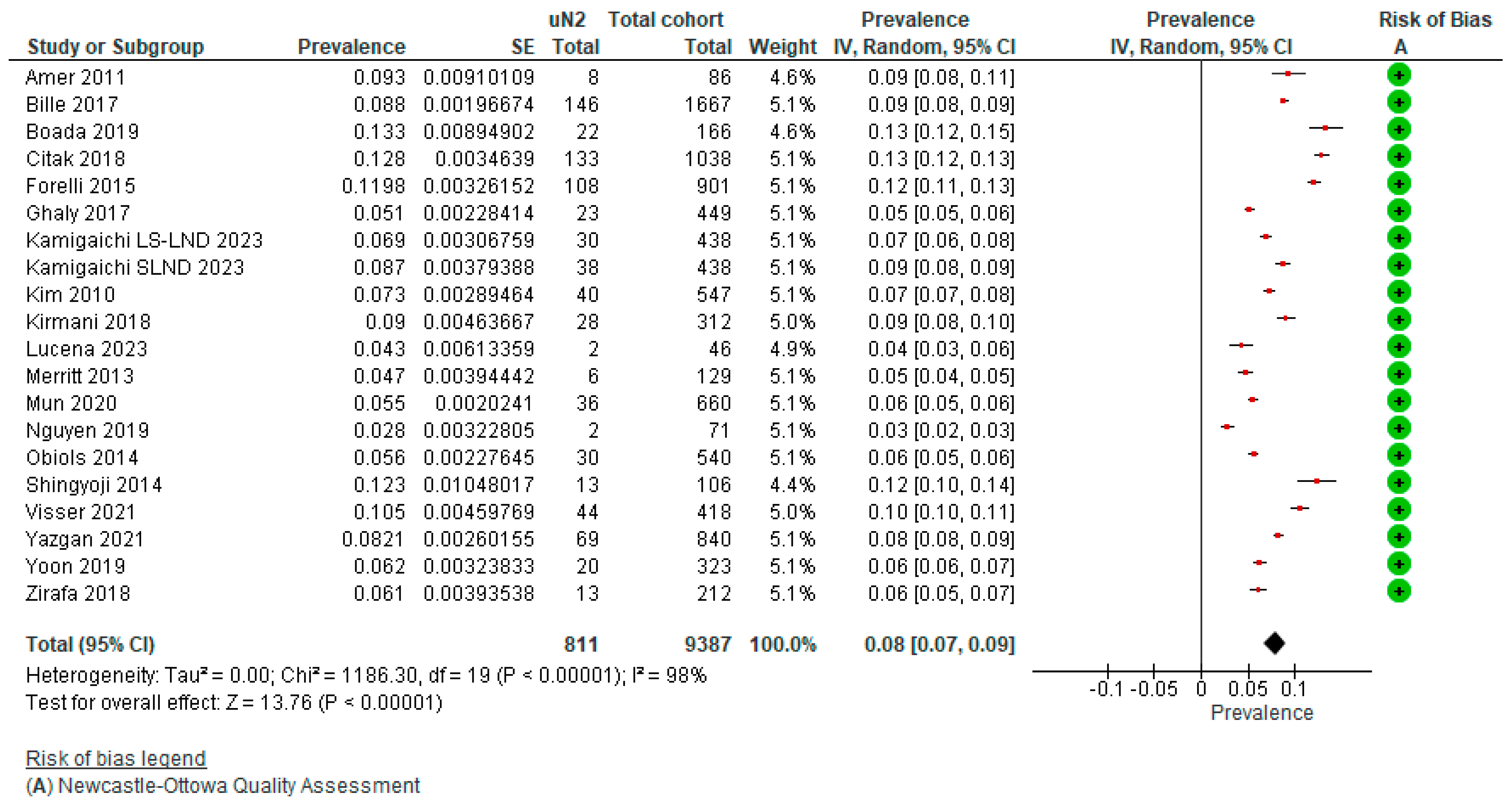
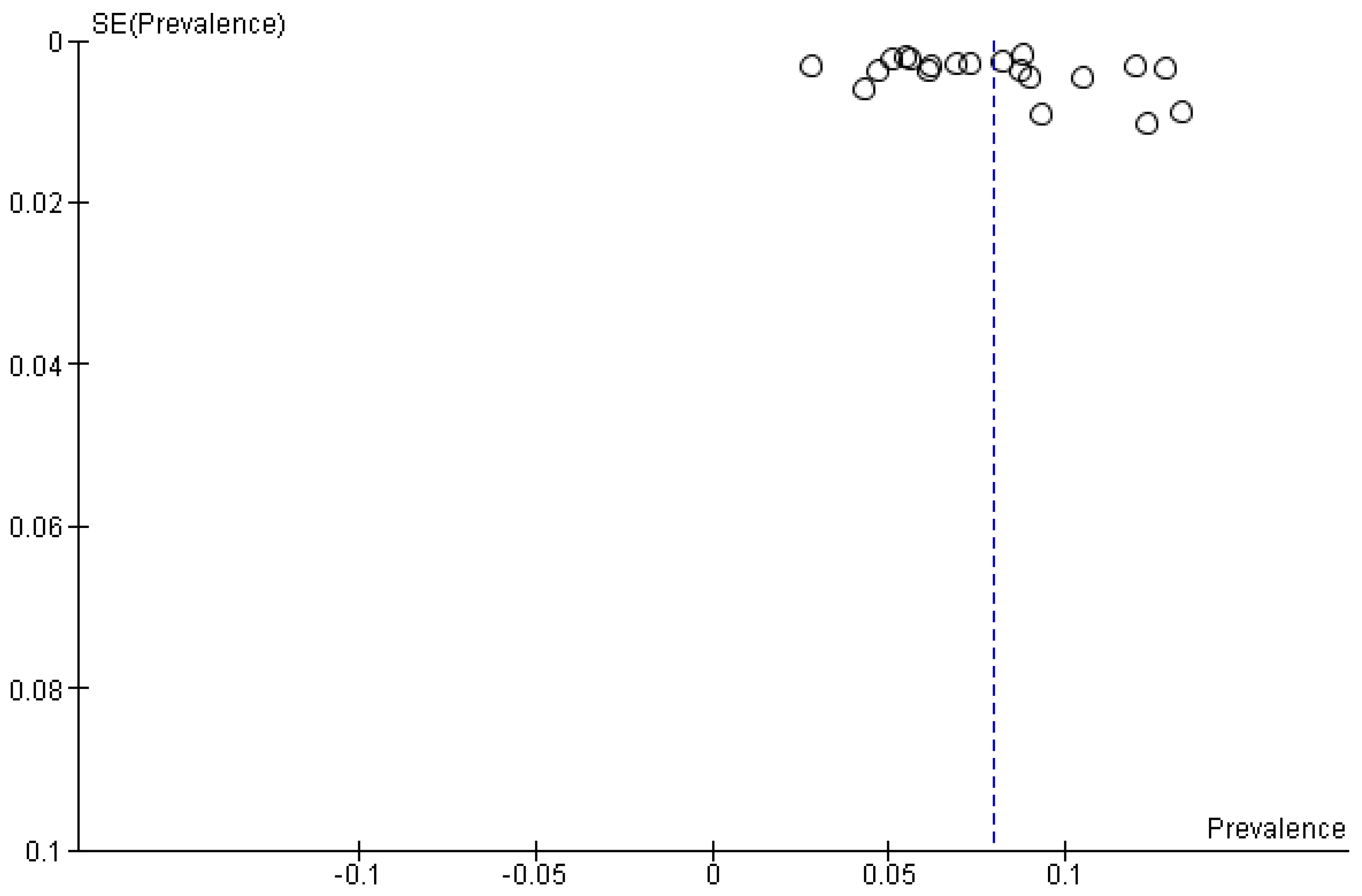
3.2. True Prevalence of Unforeseen N2 (uN2)
| Author, Year and Journal of Publication | Origin and Time Span | Preoperative Invasive Mediastinal Staging | Type of Surgery | Intraoperative Mediastinal Staging | Full Cohort | Unforeseen N2 | ||
|---|---|---|---|---|---|---|---|---|
| Indications | Method | N | N | % | ||||
| Amer et al., 2011. Eur J Cardiothorac [20] | UK, 07–11 | Single mediastinal LN > 15 mm (short axis) or low-to-moderate FDG-avidity | CM | Open + VATS | SLND | 86 | 8 | 9.3 |
| Bille et al., 2017. Eur J Cardiothorac Surg [15] | USA, 00–12 | LN ≥ 10 mm (short axis), SUVmax ≥ 2.5, exclusion if suspicious N2 | EBUS + CM | Open + VATS | SLND | 1667 | 146 | 8.8 |
| Boada et al., 2019. Lung Cancer [19] | Spain, 11–17 | FDG-avid or LN > 10 mm (short axis), central tumors, T > 3 cm | EBUS + CM | Open + VATS | SLND | 323 | 20 | 6.2 |
| Citak et al., 2018. Zentralbl Chir [13] | Turkey, 10–17 | LN ≥ 10 mm (short axis), SUVmax ≥ 3, central or tumor > 3 cm | CM (+extended CM if indicated) | Open | SLND | 1038 | 133 | 12.8 |
| Fiorelli et al., 2015. Thorac Cardiovasc Surg [16] | Italy, 06–12 | LN ≥ 10 mm (short axis) or SUVmax > 2.5, if present exclusion | CM | Open | SLND or MLNS | 901 | 108 | 12.0 |
| Ghaly et al., 2017. Ann Thorac Surg [18] | USA, 00–15 | Exclusion if LN ≥ 10 mm (short axis), SUVmax ≥ 2.5, centrally located or tumor > 2 cm | na | Open + RATS + VATS | SLND | 449 | 23 | 5.1 |
| Kamigaichi et al., 2023. Eur J Cardiothorac Surg [21] | Japan, 06–14 | Mediastinal LN > 10 mm (short axis) or FDG-avid LN | EBUS | ns | LS-LND | 438 | 30 | 6.9 |
| Kamigaichi et al., 2023. Eur J Cardiothorac Surg [21] | Japan, 06–14 | Mediastinal LN > 10 mm (short axis) or FDG-avid LN | EBUS | ns | SLND | 438 | 38 | 8.7 |
| Kim et al., 2010. J Thorac Cardiovasc Surg [10] | South Korea, 04–08 | Not specified | CM | VATS | SLND | 547 | 40 | 7.3 |
| Kirmani et al., 2018. J Thorac Dis [22] | UK, 06–10 | LN > 10 mm (short axis), SUVmax > 2 | E(BUS) + CM | ns | MLNS | 312 | 28 | 9.0 |
| Lucena et al., 2023. Respir Med [11] | Spain, 15–19 | LN ≥ 10 mm (short axis) or FDG-avid LN, centrally located or tumor > 3 cm | EBUS + CM | ns | SLND | 46 | 2 | 4.3 |
| Merritt et al., 2013. Ann Thorac Surg [23] | USA, 08–12 | LN > 10 mm (short axis) or SUVmax > 2.5, if present exclusion | CM | Open + VATS | SLND or MLNS | 129 | 6 | 4.7 |
| Mun et al., 2020. Eur J Cardiothorac Surg [24] | Japan, 08–16 | Suspicious N1–2 | EBUS (if technically not possible: CM) | VATS | LS-LND | 660 | 36 | 5.5 |
| Nguyen et al., 2019. Eur J Cardiothorac Surg [25] | USA, 04–13 | Not specified | CM (+EBUS a) | RATS | SLND b | 71 | 2 | 2.8 |
| Obiols et al., 2014. Ann Thorac Surg [14] | Spain, 04–10 | Central tumors, tumors with low FDG-uptake, LN > 16 mm or FDG-avid N1 | CM (+extended CM if indicated) | Open + VATS | SLND | 540 | 30 | 5.6 |
| Shingyoji et al., 2014. Ann Thorac Surg [26] | Japan, 08–13 | FDG-avid or LN > 10 mm (short axis), central tumors or T > 2 cm. | EBUS | Open + VATS | SLND | 106 | 13 | 12.3 |
| Visser et al., 2021. Lung Cancer [27] | Netherlands, 07–19 | Mediastinal LN ≥ 10 mm (short axis) or FDG-avid LN | CM and/or EBUS | ns | SLND | 418 | 44 | 10.5 |
| Yazgan et al., 2021. Acta Chir Belg [28] | Turkey, 07–20 | Not specified | EBUS and/or CM | Open + VATS | ns | 840 | 69 | 8.2 |
| Yoon et al., 2019. Korean J Thorac Cardiovasc Surg [17] | South Korea, 05–14 | Multiple N1 or N2 FDG-avid LN | E(BUS) + CM | ns | SLND | 166 | 22 | 13.3 |
| Zirafa et al., 2018. Surg Endosc [29] | Italy, 13–16 | LN ≥ 10 mm (short axis), SUVmax ≥ 3 | EBUS + CM | Open + RATS | SLND | 212 | 13 | 6.1 |
3.3. Subcategories of Mediastinal Nodal Metastasis
| Author, year and Journal of Publication | Origin and Time Span | Preoperative Invasive Mediastinal Staging | Type of Surgery | Intraoperative Mediastinal Staging | Full Cohort | cN-Description (uN2) | Unforeseen N2 | Single (N2a2) | Multiple (N2b) | Skip (N2a1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indications | Method | N | cN0 | cN1 | N | N | % | N | % | N | % | ||||
| Amer et al., 2011. Eur J Cardiothorac [20] | UK, 07–11 | Single mediastinal LN > 15 mm (short axis) or low-to-moderate FDG-avidity | CM | Open + VATS | SLND | 86 | 7 | 1 | 8 | 2 | 2.3 | 4 | 4.7 | 2 | 2.3 |
| Obiols et al., 2014. Ann Thorac Surg [14] | Spain, 04–10 | Central tumors, tumors with low FDG-uptake, LN > 16 mm or FDG-avid N1 | CM (+extended CM if indicated) | Open + VATS | SLND | 540 | 30 | 9 | 1.7 | 5 | 0.9 | 16 | 3.0 | ||
| Park et al., 2019. J Thorac Oncol [30] | South Korea, 04–14 | ns | EBUS + CM | ns | Ns | nr | 495 | 257 | 107 | 131 | |||||
| Yazgan et al., 2021. Acta Chir Belg [28] | Turkey, 07–20 | ns | EBUS or/and CM | Open + VATS | Ns | 840 | 69 | 38 | 4.5 | 22 | 2.6 | 31 | 3.7 | ||
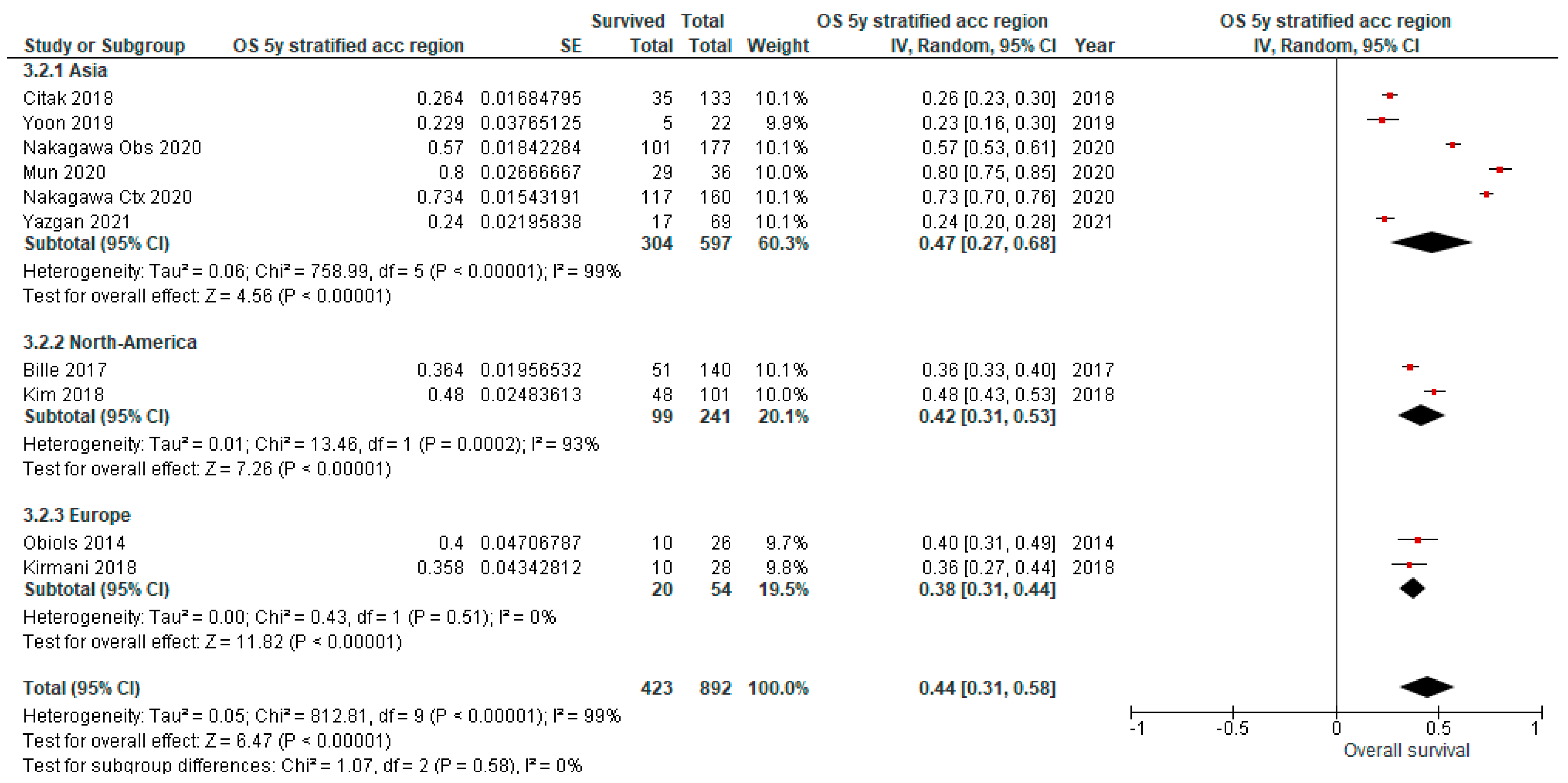
3.4. Overall Survival (OS) Outcomes of uN2
| Author, Year and Journal of Publication | Origin and Time Span | N | Mean Age (y ± SD) | Type of Surgery | Intraoperative Mediastinal Staging (%) | Adjuvant Chemotherapy (%) | Adjuvant Radiotherapy (%) | Adjuvant Chemo-Radiotherapy (%) | Overall Survival (%) | DFS (%) | Remarks | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 10 | 1 | 3 | 5 | ||||||||||
| Bille et al., 2017. Eur J Cardiothorac Surg [15] | USA, 00–12 | 140 | Open + VATS | SLND | 36.4 | Patients underwent lobectomy, segmentectomy or wedge resection. | ||||||||||
| Citak et al., 2018. Zentralbl Chir [13] | Turkey, 10–17 | 133 | 58.6 ± 8.1 | Open | SLND | 26.4 | ||||||||||
| Kim et al., 2018. J Thorac Dis [8] | USA, 00–11 | 101 | 65 ± 11 | ns | 84/98 (85.7) | 84/97 (86.6) | 48 | 24 | 10% underwent pneumonectomy. | |||||||
| Kirmani et al., 2018. J Thorac Dis [22] | UK, 06–10 | 28 | 66.5 | MLNS | 35.8 | Patients underwent lobectomy, segmentectomy or pneumonectomy. | ||||||||||
| Mun et al., 2020. Eur J Cardiothorac Surg [24] | Japan, 08–16 | 36 | VATS | LS-LND | 11 (30.6) | 80 | ||||||||||
| Nakagawa et al., 2020. Eur J Cardiothorac Surg [9] | Japan, 05–16 | 177 | 65.3 ± 8.9 | SLND 123 (69.5) MLNS 54 (30.5) | 0 | 57 | 34.9 | 27.5 | ||||||||
| 160 | 60.9 ± 9.3 | SLND 135 (84.4) MLNS 25 (15.6) | 160 (100) | 73.4 | 45.2 | 34.5 | ||||||||||
| Obiols et al., 2014. Ann Thorac Surg [14] | Spain, 04–10 | 26 | Open + VATS | SLND | 1 (3.9) | 79 | 40 | Excluded postoperative deaths | ||||||||
| Yazgan et al., 2021. Acta Chir Belg [28] | Turkey, 07–20 | 69 | Open + VATS | ns | 24.0 | |||||||||||
| Yoon et al., 2019. Korean J Thorac Cardiovasc Surg [17] | South Korea, 05–14 | 22 | 64.1 ± 9.9 | SLND | 22.9 | Patient underwent segmentectomy, (bi)lobectomy or pneumonectomy. | ||||||||||
3.5. Disease-Free Survival Outcomes of uN2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix B


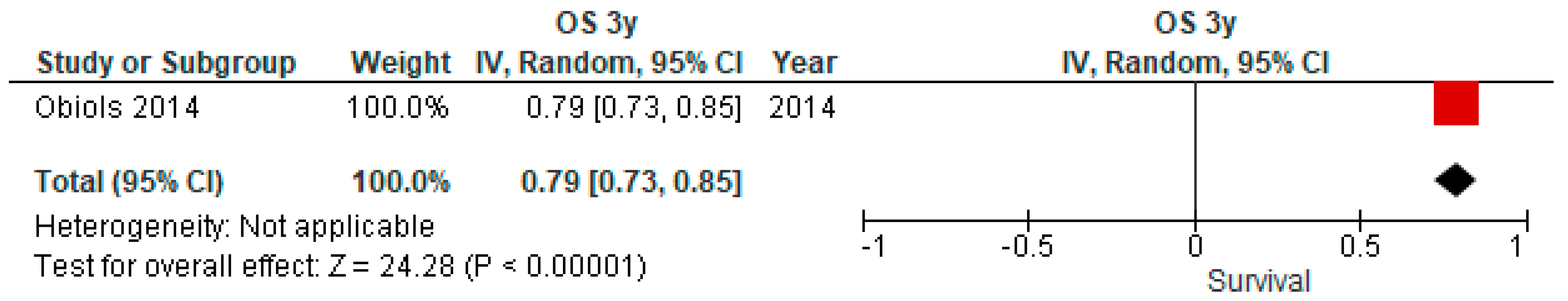

References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- De Leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Turna, A.; Van Schil, P.; Venuta, F.; Waller, D.; et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Mannam, G.C.; Kaplan, D.K.; Michail, P. Surgical management of non-small-cell lung cancer with ipsilateral mediastinal node metastasis (N2 disease). J. Thorac. Cardiovasc. Surg. 1994, 107, 19–27; discussion 27–28. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J.; Ojha, B.; Bryant, A.S.; Raghuveer, V.; Mountz, J.M.; Bartolucci, A.A. The accuracy of integrated PET-CT compared with dedicated pet alone for the staging of patients with nonsmall cell lung cancer. Ann. Thorac. Surg. 2004, 78, 1017–1023. [Google Scholar] [CrossRef]
- Rankin, S. PET/CT for staging and monitoring non small cell lung cancer. Cancer Imaging 2008, 8, S27–S31. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.; Ga, S.W.; Zello, G.; Petersen, J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality If Nonrandomized Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 24 June 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kim, M.P.; Correa, A.M.; Hofstetter, W.L.; Mehran, R.J.; Rice, D.C.; Roth, J.A.; Vaporciyan, A.A.; Walsh, G.L.; Erasmus, J.J.; Swisher, S.G. Occult stage IIIA-N2 patients have excellent overall survival with initial surgery. J. Thorac. Dis. 2018, 10, 6670–6676. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yoshida, Y.; Yotsukura, M.; Watanabe, S.-I. Pattern of recurrence of pN2 non-small-cell lung cancer: Should postoperative radiotherapy be reconsidered? Eur. J. Cardio-Thorac. Surg. 2021, 59, 109–115. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.S.; Kim, J.; Shim, Y.M.; Kim, K. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 140, 1288–1293. [Google Scholar] [CrossRef]
- Lucena, C.M.; Martin-Deleon, R.; Boada, M.; Marrades, R.M.; Sánchez, D.; Sánchez, M.; Vollmer, I.; Martínez, D.; Fontana, A.; Reguart, N.; et al. Integral mediastinal staging in patients with NON-SMALL cell lung cancer and risk factors for occult N2 disease. Respir. Med. 2023, 208, 107132. [Google Scholar] [CrossRef]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; A Waller, D.; Passlick, B.; Zielinski, M.; Junker, K.; Rendina, E.A.; Ris, H.-B. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- Citak, N.; Aksoy, Y.; Isgörücü, Ö.; Obuz, C.; Acikmese, B.; Buyukkale, S.; Metin, M.; Sayar, A. A Comparison of the Currently Used Nodal Stage Classification with the Number of Metastatic Lymph Nodes and the Number of Metastatic Lymph Node Stations for Non-Small Cell Lung Cancer; Which of These Is the Best Prognostic Factor? Zentralbl. Chir. 2019, 145, 565–573. (In English) [Google Scholar] [CrossRef]
- Obiols, C.; Call, S.; Rami-Porta, R.; Trujillo-Reyes, J.C.; Saumench, R.; Iglesias, M.; Serra-Mitjans, M.; Gonzalez-Pont, G.; Belda-Sanchís, J. Survival of Patients With Unsuspected pN2 Non-Small Cell Lung Cancer After an Accurate Preoperative Mediastinal Staging. Ann. Thorac. Surg. 2014, 97, 957–964. [Google Scholar] [CrossRef]
- Bille, A.; Woo, K.M.; Ahmad, U.; Rizk, N.P.; Jones, D.R. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur. J. Cardio-Thorac. Surg. 2017, 51, 674–679. [Google Scholar] [CrossRef]
- Fiorelli, A.; Sagan, D.; Mackiewicz, L.; Cagini, L.; Scarnecchia, E.; Chiodini, P.; Caronia, F.P.; Puma, F.; Ragusa, M.; Santini, M. Incidence, Risk Factors, and Analysis of Survival of Unexpected N2 Disease in Stage I Non–Small Cell Lung Cancer. Thorac. Cardiovasc. Surg. 2015, 63, 558–567. [Google Scholar] [CrossRef]
- Yoon, T.-H.; Lee, C.-H.; Park, K.-S.; Bae, C.-H.; Cho, J.-W.; Jang, J.-S. Preoperative Risk Factors for Pathologic N2 Metastasis in Positron Emission Tomography-Computed Tomography–Diagnosed N0–1 Non-Small Cell Lung Cancer. Korean J. Thorac. Cardiovasc. Surg. 2019, 52, 221–226. [Google Scholar] [CrossRef]
- Ghaly, G.; Rahouma, M.; Kamel, M.K.; Nasar, A.; Harrison, S.; Nguyen, A.B.; Port, J.; Stiles, B.M.; Altorki, N.K.; Lee, P.C. Clinical Predictors of Nodal Metastases in Peripherally Clinical T1a N0 Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2017, 104, 1153–1158. [Google Scholar] [CrossRef]
- Boada, M.; Guzmán, R.; Montesinos, M.; Libreros, A.; Guirao, A.; Sánchez-Lorente, D.; Gimferrer, J.; Agustí, A.; Molins, L. Upstaging, Centrality and Survival in Early Stage Non-Small Cell Lung Cancer Video-Assisted Surgery. Lung Cancer 2019, 134, 254–258. [Google Scholar] [CrossRef]
- Amer, K.; Khan, A.-Z.; Singh, N.; Addis, B.; Jogai, S.; Harden, S.; Peebles, C.; Brown, I. Video-assisted thoracic surgery systematic mediastinal nodal dissection and stage migration: Impact on clinical pathway. Eur. J. Cardio-Thorac. Surg. 2011, 40, 1474–1482. [Google Scholar] [CrossRef]
- Kamigaichi, A.; Aokage, K.; Ikeno, T.; Wakabayashi, M.; Miyoshi, T.; Tane, K.; Samejima, J.; Tsuboi, M. Long-term survival outcomes after lobe-specific nodal dissection in patients with early non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2023, 63, ezad016. [Google Scholar] [CrossRef]
- Kirmani, B.H.; Volpi, S.; Aresu, G.; Peryt, A.; Win, T.; Coonar, A.S. Long term and disease-free survival following surgical resection of occult N2 lung cancer. J. Thorac. Dis. 2018, 10, 4806–4811. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.E.; Hoang, C.D.; Shrager, J.B. Lymph Node Evaluation Achieved by Open Lobectomy Compared with Thoracoscopic Lobectomy for N0 Lung Cancer. Ann. Thorac. Surg. 2013, 96, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Mun, M.; Nakao, M.; Matsuura, Y.; Ichinose, J.; Okumura, S. Oncological outcomes after lobe-specific mediastinal lymph node dissection via multiport video-assisted thoracoscopic surgery. Eur. J. Cardio-Thorac. Surg. 2020, 58 (Suppl. S1), i92–i99. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Gharagozloo, F.; Tempesta, B.; Meyer, M.; Gruessner, A. Long-term results of robotic anatomical segmentectomy for early-stage non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2018, 55, 427–433. [Google Scholar] [CrossRef]
- Shingyoji, M.; Nakajima, T.; Yoshino, M.; Yoshida, Y.; Ashinuma, H.; Itakura, M.; Tatsumi, K.; Iizasa, T. Endobronchial Ultrasonography for Positron Emission Tomography and Computed Tomography–Negative Lymph Node Staging in Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2014, 98, 1762–1767. [Google Scholar] [CrossRef]
- Visser, M.P.J.; van Grimbergen, I.; Hölters, J.; Barendregt, W.B.; Vermeer, L.C.; Vreuls, W.; Janssen, J. Performance insights of endobronchial ultrasonography (EBUS) and mediastinoscopy for mediastinal lymph node staging in lung cancer. Lung Cancer 2021, 156, 122–128. [Google Scholar] [CrossRef]
- Yazgan, S.; Üçvet, A.; Türk, Y.; Gürsoy, S. The impact of dissection of station 9 on survival and the necessity of pulmonary ligament division during upper lobectomy for lung cancer. Acta Chir. Belg. 2023, 123, 148–155. [Google Scholar] [CrossRef]
- Zirafa, C.; Aprile, V.; Ricciardi, S.; Romano, G.; Davini, F.; Cavaliere, I.; Alì, G.; Fontanini, G.; Melfi, F. Nodal upstaging evaluation in NSCLC patients treated by robotic lobectomy. Surg. Endosc. 2018, 33, 153–158. [Google Scholar] [CrossRef]
- Park, B.J.; Kim, T.H.; Shin, S.; Kim, H.K.; Choi, Y.S.; Kim, J.; Zo, J.I.; Shim, Y.M.; Cho, J.H. Recommended Change in the N Descriptor Proposed by the International Association for the Study of Lung Cancer: A Validation Study. J. Thorac. Oncol. 2019, 14, 1962–1969. [Google Scholar] [CrossRef]
- Asamura, H.; Chansky, K.; Crowley, J.; Goldstraw, P.; Rusch, V.W.; Vansteenkiste, J.F.; Watanabe, H.; Wu, Y.-L.; Zielinski, M.; Ball, D.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 1675–1684. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv1–iv21. [Google Scholar] [CrossRef]
- Van Schil, P.E.; Berzenji, L.; Yogeswaran, S.K.; Hendriks, J.M.; Lauwers, P. Surgical Management of Stage IIIA Non-Small Cell Lung Cancer. Front. Oncol. 2017, 7, 249. [Google Scholar] [CrossRef]
- Yun, J.K.; Bok, J.S.; Lee, G.D.; Kim, H.R.; Kim, Y.-H.; Kim, D.K.; Park, S.-I.; Choi, S. Long-term outcomes of upfront surgery in patients with resectable pathological N2 non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2020, 58, 59–69. [Google Scholar] [CrossRef]
- van Klaveren, R.J.; Festen, J.; Otten, H.J.; Cox, A.L.; de Graaf, R.; Lacquet, L.K. Prognosis of unsuspected but completely resectable N2 non-small cell lung cancer. Ann. Thorac. Surg. 1993, 56, 300–304. [Google Scholar] [CrossRef]
- Albain, K.S.; Swann, R.S.; Rusch, V.W.; Turrisi, A.T., III; A Shepherd, F.A.; Smith, C.; Chen, Y.; Livingston, R.B.; Feins, R.H.; Gandara, D.R.; et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet 2009, 374, 379–386. [Google Scholar] [CrossRef]
- Farrow, N.E.; An, S.J.; Speicher, P.J.; Harpole, D.H., Jr.; D’Amico, T.A.; Klapper, J.A.; Hartwig, M.G.; Tong, B.C. Disparities in guideline-concordant treatment for node-positive, non–small cell lung cancer following surgery. J. Thorac. Cardiovasc. Surg. 2019, 160, 261–271.e1. [Google Scholar] [CrossRef]
- Detterbeck, F. What to do with “Surprise” N2?: Intraoperative Management of Patients with Non-small Cell Lung Cancer. J. Thorac. Oncol. 2008, 3, 289–302. [Google Scholar] [CrossRef]
- Osarogiagbon, R.U. Management of screening-detected stage I lung cancer. J. Thorac. Dis. 2016, 8, E1416–E1419. [Google Scholar] [CrossRef]
- Rusch, V.W.; Asamura, H.; Watanabe, H.; Giroux, D.J.; Rami-Porta, R.; Goldstraw, P. The IASLC Lung Cancer Staging Project: A Proposal for a New International Lymph Node Map in the Forthcoming Seventh Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2009, 4, 568–577. [Google Scholar] [CrossRef]
- Lee, B.E.; Redwine, J.; Foster, C.; Abella, E.; Lown, T.; Lau, D.; Follette, D. Mediastinoscopy might not be necessary in patients with non–small cell lung cancer with mediastinal lymph nodes having a maximum standardized uptake value of less than 5.3. J. Thorac. Cardiovasc. Surg. 2008, 135, 615–619. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Bryant, A.S. Maximum Standardized Uptake Values on Positron Emission Tomography of Esophageal Cancer Predicts Stage, Tumor Biology, and Survival. Ann. Thorac. Surg. 2006, 82, 391–394; discussion 394–395. [Google Scholar] [CrossRef] [PubMed]
- Downey, R.J.; Akhurst, T.; Gonen, M.; Vincent, A.; Bains, M.S.; Larson, S.; Rusch, V. Preoperative F-18 Fluorodeoxyglucose-Positron Emission Tomography Maximal Standardized Uptake Value Predicts Survival After Lung Cancer Resection. J. Clin. Oncol. 2004, 22, 3255–3260. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Nakayama, H.; Kondo, H.; Tsuchiya, R.; Shimosato, Y.; Naruke, T. Lymph node involvement, recurrence, and prognosis in resected small, peripheral, non-small-cell lung carcinomas: Are these carcinomas candidates for video-assisted lobectomy? J. Thorac. Cardiovasc. Surg. 1996, 111, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nagai, K.; Yoshida, J.; Nishimura, M.; Takahashi, K.; Nishiwaki, Y. Clinical predictors of N2 disease in the setting of a negative computed tomographic scan in patients with lung cancer. J. Thorac. Cardiovasc. Surg. 1999, 117, 593–598. [Google Scholar] [CrossRef]
- Wang, C.L.; Li, Y.; Yue, D.S.; Zhang, L.M.; Zhang, Z.F.; Sun, B.S. Value of the metastatic lymph node ratio for predicting the prognosis of non-small-cell lung cancer patients. World J. Surg. 2012, 36, 455–462. [Google Scholar] [CrossRef]
- Defranchi, S.A.; Cassivi, S.D.; Nichols, F.C.; Allen, M.S.; Shen, K.R.; Deschamps, C.; Wigle, D.A. N2 Disease in T1 Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2009, 88, 924–928. [Google Scholar] [CrossRef]
- Ketchedjian, A.; Daly, B.D.; Fernando, H.C.; Florin, L.; Hunter, C.J.; Morelli, D.M.; Shemin, R.J. Location as an important predictor of lymph node involvement for pulmonary adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2006, 132, 544–548. [Google Scholar] [CrossRef]
- Hishida, T.; Yoshida, J.; Nishimura, M.; Nishiwaki, Y.; Nagai, K. Problems in the current diagnostic standards of clinical N1 non-small cell lung cancer. Thorax 2007, 63, 526–531. [Google Scholar] [CrossRef]
- De Leyn, P.; Lardinois, D.; Van Schil, P.E.; Rami-Porta, R.; Passlick, B.; Zielinski, M.; Waller, D.A.; Lerut, T.; Weder, W. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2007, 32, 1–8. [Google Scholar] [CrossRef]
- Gunluoglu, M.Z.; Melek, H.; Medetoglu, B.; Demir, A.; Kara, H.V.; Dincer, S.I. The validity of preoperative lymph node staging guidelines of European Society of Thoracic Surgeons in non-small-cell lung cancer patients. Eur. J. Cardio-Thorac. Surg. 2010, 40, 287–290. [Google Scholar] [CrossRef]
- Birim, O.; Kappetein, A.P.; Stijnen, T.; Bogers, A.J. Meta-Analysis of Positron Emission Tomographic and Computed Tomographic Imaging in Detecting Mediastinal Lymph Node Metastases in Nonsmall Cell Lung Cancer. Ann. Thorac. Surg. 2005, 79, 375–382. [Google Scholar] [CrossRef]
- Martínez, A.H.; Jiménez, M.G.; Vicente, A.G.; Hidalgo, J.L.-T.; Colon, M.; van Gómez López, O.; Soriano Castrejón, Á.M.; Atance, P.L. Ratio between maximum standardized uptake value of N1 lymph nodes and tumor predicts N2 disease in patients with non-small cell lung cancer in 18F-FDG PET-CT scan. Rev. Española Med. Nucl. Imagen Mol. 2016, 35, 159–164. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Gould, M.K.; Margolis, M.L.; Tanoue, L.T.; McCrory, D.; Toloza, E.; Detterbeck, F. Noninvasive Staging of Non-small Cell Lung Cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007, 132 (Suppl. S3), 178S–201S. [Google Scholar] [CrossRef]
- van Tinteren, H.; Hoekstra, O.S.; Smit, E.F.; Bergh, J.H.v.D.; Schreurs, A.J.; Stallaert, R.A.; van Velthoven, P.C.; Comans, E.F.; Diepenhorst, F.W.; Verboom, P.; et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: The PLUS multicentre randomised trial. Lancet 2002, 359, 1388–1393. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.S.; Kim, K.; Shim, Y.M.; Park, K.; Ahn, Y.C.; Lee, K.S.; Choi, J.Y.; Kim, J. Outcomes of Mediastinoscopy and Surgery with or without Neoadjuvant Therapy in Patients with Non-small Cell Lung Cancer Who are N2 Negative on Positron Emission Tomography and Computed Tomography. J. Thorac. Oncol. 2011, 6, 336–342. [Google Scholar] [CrossRef]
- Annema, J.T.; van Meerbeeck, J.P.; Rintoul, R.C.; Dooms, C.; Deschepper, E.; Dekkers, O.M.; De Leyn, P.; Braun, J.; Carroll, N.R.; Praet, M.; et al. Mediastinoscopy vs Endosonography for Mediastinal Nodal Staging of Lung Cancer: A randomized trial. JAMA 2010, 304, 2245–2252. [Google Scholar] [CrossRef]
- Bousema, J.E.; van Dorp, M.; Noyez, V.J.; Dijkgraaf, M.G.; Annema, J.T.; van den Broek, F.J. Unforeseen N2 Disease after Negative Endosonography Findings with or without Confirmatory Mediastinoscopy in Resectable Non–Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2019, 14, 979–992. [Google Scholar] [CrossRef]
- Bousema, J.E.; Dijkgraaf, M.G.; van der Heijden, E.H.; Verhagen, A.F.; Annema, J.T.; Broek, F.J.V.D.; Papen-Botterhuis, N.E.; Soud, M.Y.-E.; van Boven, W.J.; Daniels, J.M.; et al. Endosonography with or Without Confirmatory Mediastinoscopy for Resectable Lung Cancer: A Randomized Clinical Trial. J. Clin. Oncol. 2023, 5, JCO2201728. [Google Scholar] [CrossRef]
- Darling, G.E.; Allen, M.S.; Decker, P.A.; Ballman, K.; Malthaner, R.A.; Inculet, R.I.; Jones, D.R.; McKenna, R.J.; Landreneau, R.J.; Rusch, V.W.; et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non–small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J. Thorac. Cardiovasc. Surg. 2011, 141, 662–670. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Bryant, A.S.; Minnich, D.J. Complete Thoracic Mediastinal Lymphadenectomy Leads to a Higher Rate of Pathologically Proven N2 Disease in Patients with Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2012, 94, 902–906. [Google Scholar] [CrossRef]
- Jeon, H.W.; Moon, M.H.; Kim, K.S.; Du Kim, Y.; Wang, Y.P.; Park, H.J.; Kil Park, J. Extent of Removal for Mediastinal Nodal Stations for Patients with Clinical Stage I Non-Small Cell Lung Cancer: Effect on Outcome. Thorac. Cardiovasc. Surg. 2014, 62, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, S.; Phan, K.; Yan, T.D.; Zhang, L.; Yang, Y.; Wu, N. Mediastinal lymphadenectomy fulfilling NCCN criteria may improve the outcome of clinical N0–1 and pathological N2 non-small cell lung cancer. J. Thorac. Dis. 2016, 8, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.M.; Vangel, M.G.; Wagner, H.; Schiller, J.H.; Herskovic, A.; Komaki, R.; Marks, R.S.; Perry, M.C.; Livingston, R.B.; Johnson, D.H. Prolonged survival in patients with resected non–small cell lung cancer and single-level N2 disease. J. Thorac. Cardiovasc. Surg. 2004, 128, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Yang, X.; Bai, J.; Yang, J.; Manegold, C.; Wu, Y. Complete mediastinal lymphadenectomy: The core component of the multidisciplinary therapy in resectable non-small cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2008, 34, 187–195. [Google Scholar] [CrossRef]
- Asamura, H.; Nakayama, H.; Kondo, H.; Tsuchiya, R.; Naruke, T. Lobe-specific extent of systematic lymph node dissection for non–small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J. Thorac. Cardiovasc. Surg. 1999, 117, 1102–1111. [Google Scholar] [CrossRef]
- Kim, A.W. Lymph Node Drainage Patterns and Micrometastasis in Lung Cancer. Semin. Thorac. Cardiovasc. Surg. 2009, 21, 298–308. [Google Scholar] [CrossRef]
- Deng, H.-Y.; Zhou, J.; Wang, R.-L.; Jiang, R.; Zhu, D.-X.; Tang, X.-J.; Zhou, Q. Lobe-Specific Lymph Node Dissection for Clinical Early-Stage (cIA) Peripheral Non-small Cell Lung Cancer Patients: What and How? Ann. Surg. Oncol. 2019, 27, 472–480. [Google Scholar] [CrossRef]
- Abughararah, T.Z.; Jeong, Y.H.; Alabbood, F.; Chong, Y.; Yun, J.K.; Lee, G.D.; Choi, S.; Kim, H.R.; Kim, Y.-H.; Kim, D.K.; et al. Lobe-specific lymph node dissection in stage IA non-small-cell lung cancer: A retrospective cohort study. Eur. J. Cardio-Thorac. Surg. 2020, 59, 783–790. [Google Scholar] [CrossRef]
- Riquet, M. Anatomic basis of lymphatic spread from carcinoma of the lung to the mediastinum: Surgical and prognostic implications. Surg. Radiol. Anat. 1993, 15, 271–277. [Google Scholar] [CrossRef]
- Garelli, E.; Olland, A.; Santelmo, N.; Renaud, S.; Falcoz, P.-E.; Weingertner, N.; Massard, G. Microscopic N2 disease exhibits a better prognosis in resected non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2016, 50, 322–328. [Google Scholar] [CrossRef]
- Renaud, S.; Falcoz, P.-E.; Olland, A.; Reeb, J.; Santelmo, N.; Massard, G. Mediastinal downstaging after induction treatment is not a significant prognostic factor to select patients who would benefit from surgery: The clinical value of the lymph node ratio. Interact. Cardiovasc. Thorac. Surg. 2014, 20, 222–227. [Google Scholar] [CrossRef]
- Wei, S.; Asamura, H.; Kawachi, R.; Sakurai, H.; Watanabe, S. Which is the Better Prognostic Factor for Resected Non-small Cell Lung Cancer: The Number of Metastatic Lymph Nodes or the Currently Used Nodal Stage Classification? J. Thorac. Oncol. 2011, 6, 310–318. [Google Scholar] [CrossRef]
- Saji, H.; Tsuboi, M.; Shimada, Y.; Kato, Y.; Yoshida, K.; Nomura, M.; Matsubayashi, J.; Nagao, T.; Kakihana, M.; Usuda, J.; et al. A Proposal for Combination of Total Number and Anatomical Location of Involved Lymph Nodes for Nodal Classification in Non-small Cell Lung Cancer. Chest 2013, 143, 1618–1625. [Google Scholar] [CrossRef]
- Samejima, J.; Ito, H.; Nagashima, T.; Nemoto, D.; Eriguchi, D.; Nakayama, H.; Ikeda, N.; Okada, M. Anatomical location and number of metastatic lymph nodes for prognosis of non-small cell lung cancer. J. Thorac. Dis. 2021, 13, 4083–4093. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Su, H.; She, Y.; Dai, C.; Zhao, M.; Gao, J.; Xie, H.; Ren, Y.; Xie, D.; Chen, C. Which N Descriptor Is More Predictive of Prognosis in Resected Non-small Cell Lung Cancer: The Number of Involved Nodal Stations or the Location- Based Patho-logical N Stage? Chest 2020, 159, 2458–2469. [Google Scholar] [CrossRef] [PubMed]
- Rusch, V.W.; Crowley, J.; Giroux, D.J.; Goldstraw, P.; Im, J.-G.; Tsuboi, M.; Tsuchiya, R.; Vansteenkiste, J. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming Seventh Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2007, 2, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Sonobe, M.; Date, H.; Wada, H.; Okubo, K.; Hamakawa, H.; Teramukai, S.; Matsumura, A.; Nakagawa, T.; Sumitomo, S.-I.; Miyamoto, Y.; et al. Prognostic factors after complete resection of pN2 non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2013, 146, 788–795. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Maniscalco, L.; Bryant, A.S. The Treatment of Patients with Stage IIIA Non-Small Cell Lung Cancer from N2 Disease: Who Returns to the Surgical Arena and Who Survives. Ann. Thorac. Surg. 2008, 86, 912–920; discussion 912–920. [Google Scholar] [CrossRef]
- Yoshino, I.; Yoshida, S.; Miyaoka, E.; Asamura, H.; Nomori, H.; Fujii, Y.; Nakanishi, Y.; Eguchi, K.; Mori, M.; Sawabata, N.; et al. Surgical Outcome of Stage IIIA- cN2/pN2 Non–Small-Cell Lung Cancer Patients in Japanese Lung Cancer Registry Study in 2004. J. Thorac. Oncol. 2012, 7, 850–855. [Google Scholar] [CrossRef]
- Misthos, P.; Sepsas, E.; Kokotsakis, J.; Skottis, I.; Lioulias, A. The Significance of One-Station N2 Disease in the Prognosis of Patients with Nonsmall-Cell Lung Cancer. Ann. Thorac. Surg. 2008, 86, 1626–1630. [Google Scholar] [CrossRef]
- Andersson, S.; Ilonen, I.; Järvinen, T.; Rauma, V.; Räsänen, J.; Salo, J. Surgically Treated Unsuspected N2-Positive NSCLC: Role of Extent and Location of Lymph Node Metastasis. Clin. Lung Cancer 2018, 19, 418–425. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, S.R.; Kim, H.R.; Han, J.-O.; Kim, Y.-H.; Kim, D.K.; Park, S.-I. Modern Outcome and Risk Analysis of Surgically Resected Occult N2 Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2014, 97, 1920–1925. [Google Scholar] [CrossRef]
- Zhong, C.; Yao, F.; Zhao, H. Clinical Outcomes of Thoracoscopic Lobectomy for Patients with Clinical N0 and Pathologic N2 Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2013, 95, 987–992. [Google Scholar] [CrossRef]
- Legras, A.; Mordant, P.; Arame, A.; Foucault, C.; Dujon, A.; Barthes, F.L.P.; Riquet, M. Long-Term Survival of Patients With pN2 Lung Cancer According to the Pattern of Lymphatic Spread. Ann. Thorac. Surg. 2014, 97, 1156–1162. [Google Scholar] [CrossRef]
- Sezen, C.B.; Aksoy, Y.; Sonmezoglu, Y.; Citak, N.; Saydam, O.; Metin, M. Prognostic factors for survival in patients with completely resected pN2 non-small-cell lung cancer. Acta Chir. Belg. 2021, 121, 23–29. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, J.B.; Keum, D.Y.; Hwang, I.; Park, C.K. Long Term Survival of Patients with Unsuspected N2 Disease in Non-Small Cell Lung Cancer. Korean J. Thorac. Cardiovasc. Surg. 2013, 46, 49–55. [Google Scholar] [CrossRef]
- Ohta, Y.; Shimizu, Y.; Minato, H.; Matsumoto, I.; Oda, M.; Watanabe, G. Results of Initial Operations in Non–Small Cell Lung Cancer Patients with Single-Level N2 Disease. Ann. Thorac. Surg. 2006, 81, 427–433. [Google Scholar] [CrossRef]
- Sayar, A.; Metin, M.; Büyükkale, S.; Kök, A.; Solak, O.; Yurt, S.; Gürses, A.; Citak, N. The Prognostic Significance of Metastasis to Lymph Nodes in Aortopulmonary Zone (Stations 5 and 6) in Completely Resected Left Upper Lobe Tumors. Thorac. Cardiovasc. Surg. 2015, 63, 568–576. [Google Scholar] [CrossRef]
- Shapiro, M.; Kadakia, S.; Lim, J.; Breglio, A.; Wisnivesky, J.P.; Kaufman, A.; Lee, D.-S.; Flores, R.M. Lobe-Specific Mediastinal Nodal Dissection Is Sufficient During Lobectomy by Video-Assisted Thoracic Surgery or Thoracotomy for Early-Stage Lung Cancer. Chest 2013, 144, 1615–1621. [Google Scholar] [CrossRef]
- Patterson, G.; Piazza, D.; Pearson, F.; Todd, T.; Ginsberg, R.; Goldberg, M.; Waters, P.; Jones, D.; Ilves, R.; Cooper, J. Significance of Metastatic Disease in Subaortic Lymph Nodes. Ann. Thorac. Surg. 1987, 43, 155–159. [Google Scholar] [CrossRef]
- Wang, X.; Guo, H.; Hu, Q.; Ying, Y.; Chen, B. The Impact of Skip vs. Non-Skip N2 Lymph Node Metastasis on the Prognosis of Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Surg. 2021, 8, 749156. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, M.; Lococo, F.; Leuzzi, G.; Sperduti, I.; Petracca-Ciavarella, L.; Bria, E.; Mucilli, F.; Filosso, P.L.; Ratto, G.B.; Spaggiari, L.; et al. External validation of the N descriptor in the proposed tumour–node–metastasis subclassification for lung cancer: The crucial role of histological type, number of resected nodes and adjuvant therapy. Eur. J. Cardio-Thorac. Surg. 2020, 58, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Deleyn, P.; Schoonooghe, P.; Deneffe, G.; Vanraemdonck, D.; Coosemans, W.; Lerut, T.; Vansteenkiste, J. Surgery for non-small cell lung cancer with unsuspected metastasis to ipsilateral mediastinal or subcarinal nodes (N2 disease). Eur. J. Cardio-Thorac. Surg. 1996, 10, 649–654; discussion 654–655. [Google Scholar] [CrossRef]
- Liou, D.Z.; Chan, M.; Bhandari, P.; Lui, N.S.; Backhus, L.M.; Shrager, J.B.; Berry, M.F. Lobar versus sublobar resection in clinical stage IA primary lung cancer with occult N2 disease. Eur. J. Cardio-Thorac. Surg. 2022, 62, ezac440. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Bryant, A.S. Survival of Patients with Unsuspected N2 (Stage IIIA) Nonsmall-Cell Lung Cancer. Ann. Thorac. Surg. 2008, 86, 362–366; discussion 366–367. [Google Scholar] [CrossRef] [PubMed]
- Macia, I.; Ramos, R.; Moya, J.; Rivas, F.; Ureña, A.; Banque, M.; Escobar, I.; Rosado, G.; Rodriguez-Taboada, P. Survival of Patients with Non-Small Cell Lung Cancer According to Lymph Node Disease: Single pN1 vs Multiple pN1 vs Single Unsuspected pN2. Ann. Surg. Oncol. 2013, 20, 2413–2418. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Rosell, R.; De Lena, M.; Carpagnano, F.; Ramlau, R.; Gonzáles-Larriba, J.L.; Grodzki, T.; Pereira, J.R.; Le Groumellec, A.; Lorusso, V.; et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006, 7, 719–727, Erratum in Lancet Oncol. 2006, 7, 797. [Google Scholar] [CrossRef]
- Le Pechoux, C.; Pourel, N.; Barlesi, F.; Lerouge, D.; Antoni, D.; Lamezec, B.; Nestle, U.; Boisselier, P.; Dansin, E.; Paumier, A.; et al. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART, IFCT 0503): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 23, 104–114. [Google Scholar] [CrossRef]
- Nagasaka, M.; Gadgeel, S.M. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev. Anticancer. Ther. 2017, 18, 63–70. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes from the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef]
- Hao, X.; Li, W.; Li, W.; Gu, M.; Wang, Z.; Nakahashi, K.; Antonoff, M.B.; Adachi, H.; Zhou, S.; Xu, S. Re-evaluating the need for mediastinal lymph node dissection and exploring lncRNAs as biomarkers of N2 metastasis in T1 lung adenocarcinoma. Transl. Lung Cancer Res. 2022, 11, 1079–1088. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, W.K.; Charaf, Z.; Hendriks, J.M.H.; Van Schil, P.E. True Prevalence of Unforeseen N2 Disease in NSCLC: A Systematic Review + Meta-Analysis. Cancers 2023, 15, 3475. https://doi.org/10.3390/cancers15133475
Hui WK, Charaf Z, Hendriks JMH, Van Schil PE. True Prevalence of Unforeseen N2 Disease in NSCLC: A Systematic Review + Meta-Analysis. Cancers. 2023; 15(13):3475. https://doi.org/10.3390/cancers15133475
Chicago/Turabian StyleHui, Wing Kea, Zohra Charaf, Jeroen M. H. Hendriks, and Paul E. Van Schil. 2023. "True Prevalence of Unforeseen N2 Disease in NSCLC: A Systematic Review + Meta-Analysis" Cancers 15, no. 13: 3475. https://doi.org/10.3390/cancers15133475
APA StyleHui, W. K., Charaf, Z., Hendriks, J. M. H., & Van Schil, P. E. (2023). True Prevalence of Unforeseen N2 Disease in NSCLC: A Systematic Review + Meta-Analysis. Cancers, 15(13), 3475. https://doi.org/10.3390/cancers15133475







