A Radiomics-Based Classifier for the Progression of Oropharyngeal Cancer Treated with Definitive Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohorts
2.2. Feature Extraction
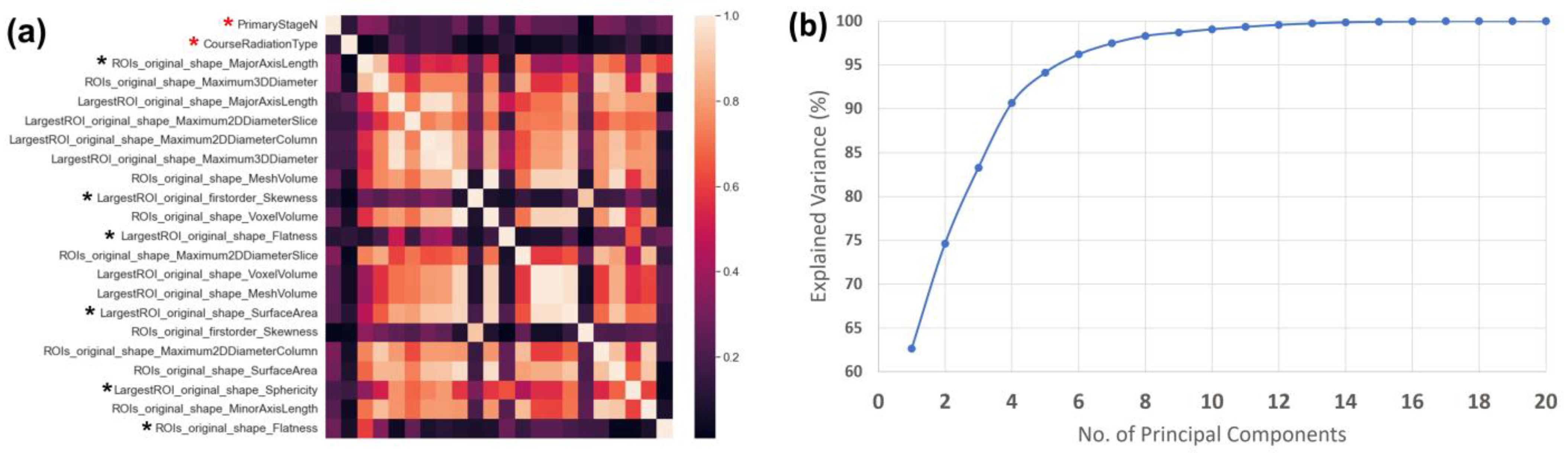
2.3. Dimensionality Reduction
2.4. Data Balancing
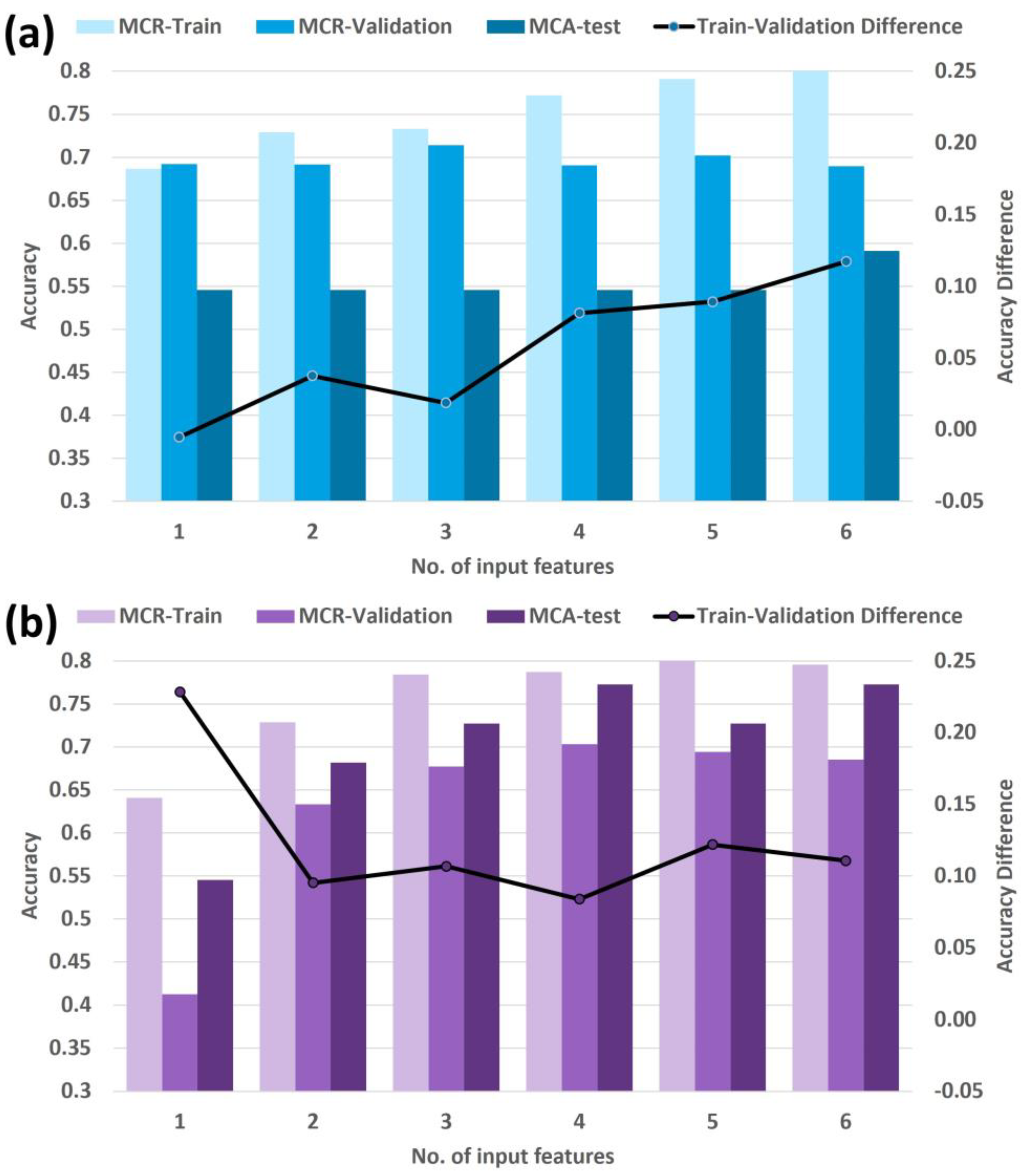
2.5. Feature Selection, Model Construction, and Testing
3. Results
3.1. Dimensionality Reduction
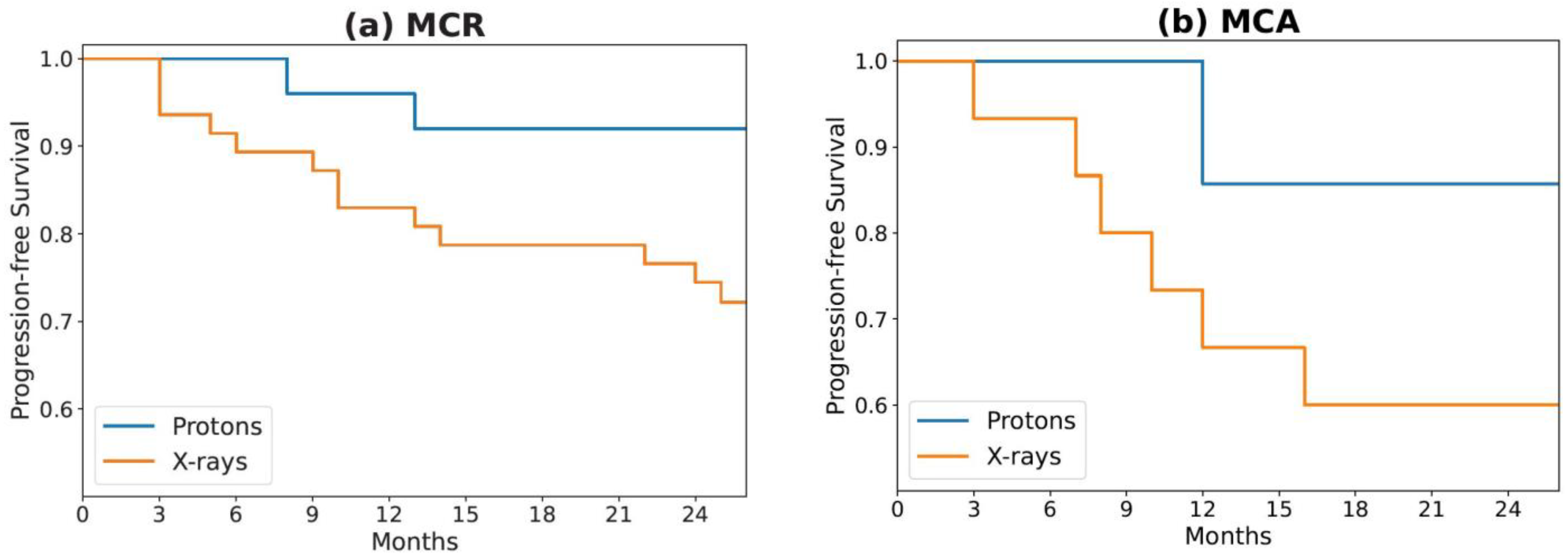
3.2. Feature Selection, Model Construction, and Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fakhry, C.; Krapcho, M.; Eisele, D.W.; D’Souza, G. Head and neck squamous cell cancers in the United States are rare and the risk now is higher among white individuals compared with black individuals. Cancer 2018, 124, 2125–2133. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- D’Souza, G.; Westra, W.H.; Wang, S.J.; van Zante, A.; Wentz, A.; Kluz, N.; Rettig, E.; Ryan, W.R.; Ha, P.K.; Kang, H.; et al. Differences in the Prevalence of Human Papillomavirus (HPV) in Head and Neck Squamous Cell Cancers by Sex, Race, Anatomic Tumor Site, and HPV Detection Method. JAMA Oncol. 2017, 3, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrom, K.R.; Calzada, G.; Hanby, J.D.; Garden, A.S.; Glisson, B.S.; Li, G.; Roberts, D.B.; Weber, R.S.; Sturgis, E.M. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: A staging system in need of repair. Cancer 2013, 119, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner, H., Jr.; Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. 2003, 21, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Denis, F.; Garaud, P.; Bardet, E.; Alfonsi, M.; Sire, C.; Germain, T.; Bergerot, P.; Rhein, B.; Tortochaux, J.; Calais, G. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J. Clin. Oncol. 2004, 22, 69–76. [Google Scholar] [CrossRef]
- Ma, D.J.; Price, K.A.; Moore, E.J.; Patel, S.H.; Hinni, M.L.; Garcia, J.J.; Graner, D.E.; Foster, N.R.; Ginos, B.; Neben-Wittich, M.; et al. Phase II Evaluation of Aggressive Dose De-Escalation for Adjuvant Chemoradiotherapy in Human Papillomavirus-Associated Oropharynx Squamous Cell Carcinoma. J. Clin. Oncol. 2019, 37, 1909–1918. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef]
- Fakhry, C.; Zhang, Q.; Nguyen-Tan, P.F.; Rosenthal, D.; El-Naggar, A.; Garden, A.S.; Soulieres, D.; Trotti, A.; Avizonis, V.; Ridge, J.A.; et al. Human Papillomavirus and Overall Survival After Progression of Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 2014, 32, 3365–3373. [Google Scholar] [CrossRef]
- Anzai, Y.; Chang, C.P.; Rowe, K.; Snyder, J.; Deshmukh, V.; Newman, M.; Fraser, A.; Smith, K.; Date, A.; Galvao, C.; et al. Surveillance Imaging with PET/CT and CT and/or MRI for Head and Neck Cancer and Mortality: A Population-based Study. Radiology 2023, 307, e212915. [Google Scholar] [CrossRef]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef]
- Haider, S.P.; Burtness, B.; Yarbrough, W.G.; Payabvash, S. Applications of radiomics in precision diagnosis, prognostication and treatment planning of head and neck squamous cell carcinomas. Cancers Head Neck 2020, 5, 6. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Mowery, Y.M.; Vergalasova, I.; Rushing, C.N.; Choudhury, K.R.; Niedzwiecki, D.; Wu, Q.; Yoo, D.S.; Das, S.; Wong, T.Z.; Brizel, D.M. Early (18)F-FDG-PET Response During Radiation Therapy for HPV-Related Oropharyngeal Cancer May Predict Disease Recurrence. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 969–976. [Google Scholar] [CrossRef]
- Kim, J.W.; Oh, J.S.; Roh, J.L.; Kim, J.S.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Prognostic significance of standardized uptake value and metabolic tumour volume on (1)(8)F-FDG PET/CT in oropharyngeal squamous cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1353–1361. [Google Scholar] [CrossRef]
- Lim, R.; Eaton, A.; Lee, N.Y.; Setton, J.; Ohri, N.; Rao, S.; Wong, R.; Fury, M.; Schoder, H. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J. Nucl. Med. 2012, 53, 1506–1513. [Google Scholar] [CrossRef]
- Dibble, E.H.; Alvarez, A.C.; Truong, M.T.; Mercier, G.; Cook, E.F.; Subramaniam, R.M. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: Adding value to clinical staging. J. Nucl. Med. 2012, 53, 709–715. [Google Scholar] [CrossRef]
- Cheng, N.M.; Fang, Y.H.; Lee, L.Y.; Chang, J.T.; Tsan, D.L.; Ng, S.H.; Wang, H.M.; Liao, C.T.; Yang, L.Y.; Hsu, C.H.; et al. Zone-size nonuniformity of 18F-FDG PET regional textural features predicts survival in patients with oropharyngeal cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 419–428. [Google Scholar] [CrossRef]
- Folkert, M.R.; Setton, J.; Apte, A.P.; Grkovski, M.; Young, R.J.; Schoder, H.; Thorstad, W.L.; Lee, N.Y.; Deasy, J.O.; Oh, J.H. Predictive modeling of outcomes following definitive chemoradiotherapy for oropharyngeal cancer based on FDG-PET image characteristics. Phys. Med. Biol. 2017, 62, 5327–5343. [Google Scholar] [CrossRef]
- Vallieres, M.; Kay-Rivest, E.; Perrin, L.J.; Liem, X.; Furstoss, C.; Aerts, H.; Khaouam, N.; Nguyen-Tan, P.F.; Wang, C.S.; Sultanem, K.; et al. Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer. Sci. Rep. 2017, 7, 10117. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Qian, J.; Herman, M.G.; Brinkmann, D.H.; Laack, N.N.; Kemp, B.J.; Hunt, C.H.; Lowe, V.; Pafundi, D.H. Prediction of MGMT Status for Glioblastoma Patients Using Radiomics Feature Extraction From (18)F-DOPA-PET Imaging. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1339–1346. [Google Scholar] [CrossRef]
- Lucien, F.; Kim, Y.; Qian, J.; Orme, J.J.; Zhang, H.; Arafa, A.; Abraha, F.; Thapa, I.; Tryggestad, E.J.; Harmsen, W.S.; et al. Tumor-Derived Extracellular Vesicles Predict Clinical Outcomes in Oligometastatic Prostate Cancer and Suppress Antitumor Immunity. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 725–737. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Batista, G.E.; Prati, R.C.; Monard, M.C. A study of the behavior of several methods for balancing machine learning training data. ACM SIGKDD Explor. Newsl. 2004, 6, 20–29. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Parmar, C.; Grossmann, P.; Rietveld, D.; Rietbergen, M.M.; Lambin, P.; Aerts, H.J. Radiomic Machine-Learning Classifiers for Prognostic Biomarkers of Head and Neck Cancer. Front. Oncol. 2015, 5, 272. [Google Scholar] [CrossRef]
- Abraham, R.; Simha, J.B.; Iyengar, S. Medical datamining with a new algorithm for feature selection and naive bayesian classifier. In Proceedings of the 10th International Conference on Information Technology (ICIT 2007), Rourkela, India, 17–20 December 2007; pp. 44–49. [Google Scholar]
- Cheng, N.M.; Fang, Y.H.; Chang, J.T.; Huang, C.G.; Tsan, D.L.; Ng, S.H.; Wang, H.M.; Lin, C.Y.; Liao, C.T.; Yen, T.C. Textural features of pretreatment 18F-FDG PET/CT images: Prognostic significance in patients with advanced T-stage oropharyngeal squamous cell carcinoma. J. Nucl. Med. 2013, 54, 1703–1709. [Google Scholar] [CrossRef]
- Orlhac, F.; Eertink, J.J.; Cottereau, A.S.; Zijlstra, J.M.; Thieblemont, C.; Meignan, M.; Boellaard, R.; Buvat, I. A Guide to ComBat Harmonization of Imaging Biomarkers in Multicenter Studies. J. Nucl. Med. 2022, 63, 172–179. [Google Scholar] [CrossRef]
- Promteangtrong, C.; Siripongsatian, D.; Jantarato, A.; Kunawudhi, A.; Kiatkittikul, P.; Yaset, S.; Boonkawin, N.; Chotipanich, C. Head-to-Head Comparison of (68)Ga-FAPI-46 and (18)F-FDG PET/CT for Evaluation of Head and Neck Squamous Cell Carcinoma: A Single-Center Exploratory Study. J. Nucl. Med. 2022, 63, 1155–1161. [Google Scholar] [CrossRef]
- Huang, R.; Pu, Y.; Huang, S.; Yang, C.; Yang, F.; Pu, Y.; Li, J.; Chen, L.; Huang, Y. FAPI-PET/CT in Cancer Imaging: A Potential Novel Molecule of the Century. Front. Oncol. 2022, 12, 854658. [Google Scholar] [CrossRef]

| MCR | MCA | ||||
|---|---|---|---|---|---|
| Radiation Type | X-rays | Protons | X-rays | Protons | |
| Total Patients | 47 | 25 | 15 | 7 | |
| Any Progression | |||||
| Yes | 15 | 2 | 7 | 1 | |
| No | 32 | 23 | 8 | 6 | |
| Age at Diagnosis (y) | |||||
| Mean | 62.5 | 64.9 | 63.2 | 65.8 | |
| Range | 48.1–77.7 | 42.3–81.0 | 46.4–86.9 | 55.1–76.3 | |
| T Category | |||||
| T0 | 0 | 0 | 0 | 0 | |
| T1 | 5 | 0 | 0 | 0 | |
| T2 | 15 | 3 | 4 | 5 | |
| T3 | 8 | 9 | 4 | 1 | |
| T4 | 19 | 13 | 7 | 1 | |
| N Category | |||||
| N0 | 6 | 1 | 0 | 1 | |
| N1 | 1 | 7 | 4 | 1 | |
| N2 | 35 | 17 | 9 | 5 | |
| N3 | 5 | 0 | 2 | 0 | |
| Concurrent Chemotherapy | |||||
| Yes | 45 | 24 | 15 | 7 | |
| No | 2 | 1 | 0 | 0 | |
| Dimensionality Reduction Method | ||
|---|---|---|
| No. of Input Features | Manually Filtered Set | Machine-Driven Set |
| 1 | Radiation Type | Radiation Type |
| 2 | Radiation Type, ROIs_MajorAxisLength | Radiation Type, PCA 3 |
| 3 | Radiation Type, ROIs_MajorAxisLength, LargestROI_Flatness | Radiation Type, PCA 3, PCA 6 |
| 4 | Radiation Type, ROIs_MajorAxisLength, LargestROI_Flatness, ROIs_Flatness | Radiation Type, PCA 3, PCA 6, PCA 4 |
| 5 | Radiation Type, ROIs_MajorAxisLength, LargestROI_Flatness, ROIs_Flatness, LargestROI_Skewness | Radiation Type, PCA 3, PCA 6, PCA 4, Primary Stage N |
| 6 | Radiation Type, ROIs_MajorAxisLength, LargestROI_Flatness, ROIs_Flatness, LargestROI_Skewness, LargestROI_SurfaceArea | Radiation Type, PCA 3, PCA 6, PCA 4, Primary Stage N, PCA 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, D.A.; Jeans, E.B.; Morris, L.K.; Shiraishi, S.; Laughlin, B.S.; Rong, Y.; Rwigema, J.-C.M.; Foote, R.L.; Herman, M.G.; Qian, J. A Radiomics-Based Classifier for the Progression of Oropharyngeal Cancer Treated with Definitive Radiotherapy. Cancers 2023, 15, 3715. https://doi.org/10.3390/cancers15143715
Garcia DA, Jeans EB, Morris LK, Shiraishi S, Laughlin BS, Rong Y, Rwigema J-CM, Foote RL, Herman MG, Qian J. A Radiomics-Based Classifier for the Progression of Oropharyngeal Cancer Treated with Definitive Radiotherapy. Cancers. 2023; 15(14):3715. https://doi.org/10.3390/cancers15143715
Chicago/Turabian StyleGarcia, Darwin A., Elizabeth B. Jeans, Lindsay K. Morris, Satomi Shiraishi, Brady S. Laughlin, Yi Rong, Jean-Claude M. Rwigema, Robert L. Foote, Michael G. Herman, and Jing Qian. 2023. "A Radiomics-Based Classifier for the Progression of Oropharyngeal Cancer Treated with Definitive Radiotherapy" Cancers 15, no. 14: 3715. https://doi.org/10.3390/cancers15143715
APA StyleGarcia, D. A., Jeans, E. B., Morris, L. K., Shiraishi, S., Laughlin, B. S., Rong, Y., Rwigema, J.-C. M., Foote, R. L., Herman, M. G., & Qian, J. (2023). A Radiomics-Based Classifier for the Progression of Oropharyngeal Cancer Treated with Definitive Radiotherapy. Cancers, 15(14), 3715. https://doi.org/10.3390/cancers15143715






