Radiomics of Tumor Heterogeneity in 18F-FDG-PET-CT for Predicting Response to Immune Checkpoint Inhibition in Therapy-Naïve Patients with Advanced Non-Small-Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Baseline 18F-FDG-PET-CT Imaging
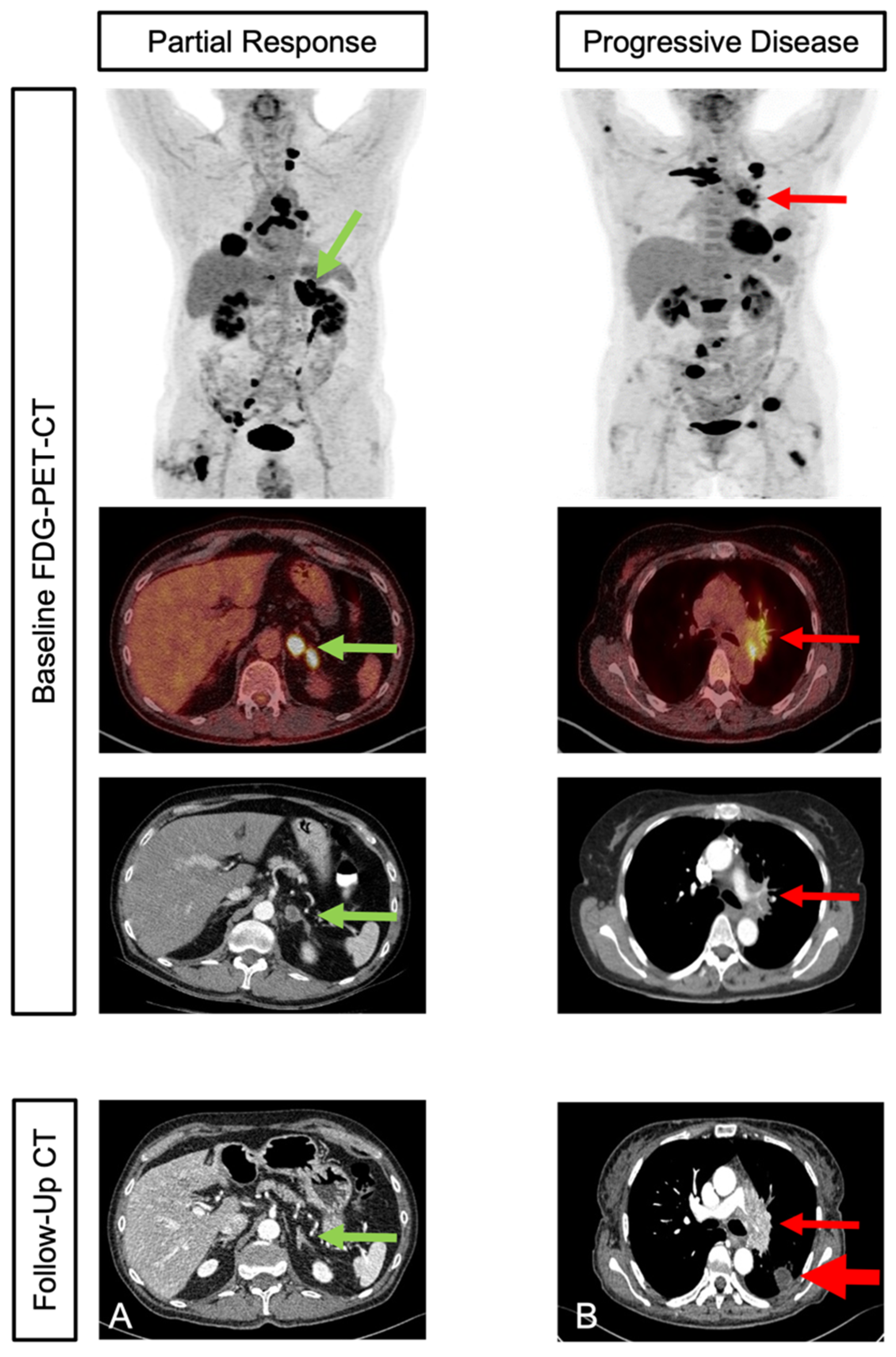
2.4. Response Assessment and Follow-Up
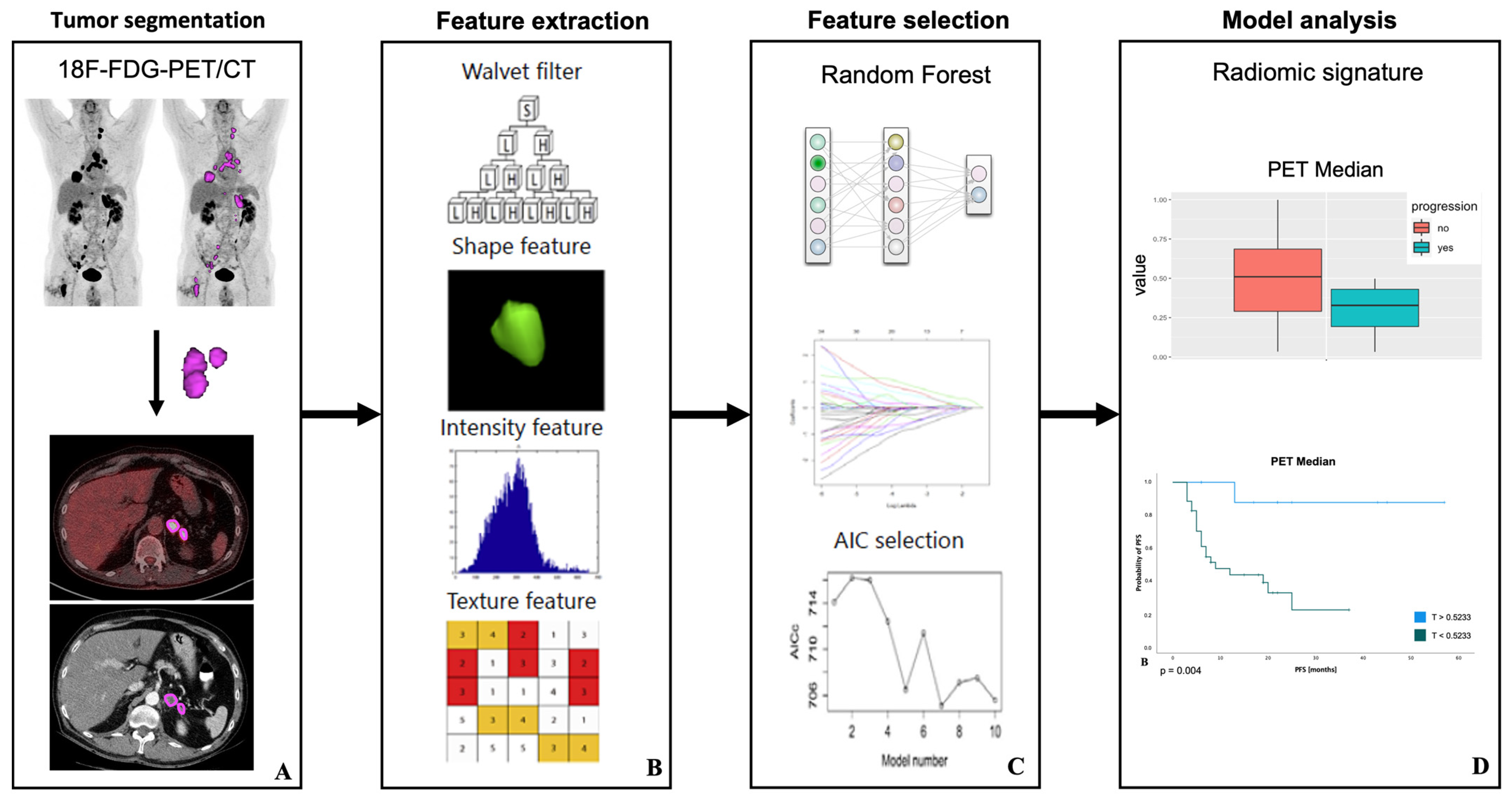
2.5. Image Segmentation and Feature Extraction
2.6. Feature Selection and Model Analysis
2.7. Survival Analysis
2.8. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Treatment
3.3. Response and Clinical Outcome
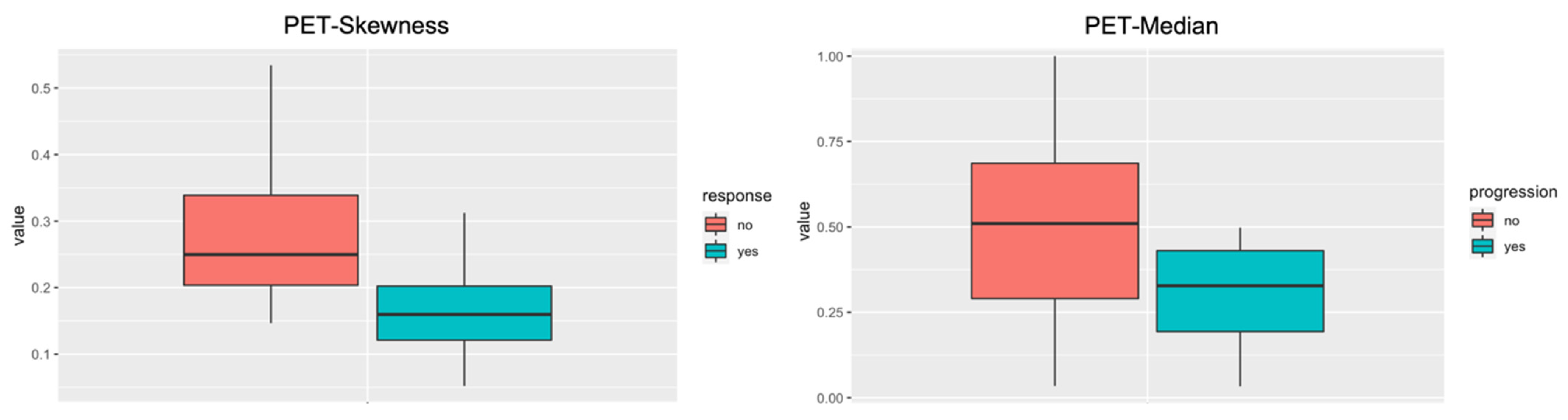
3.4. Outcome Prediction
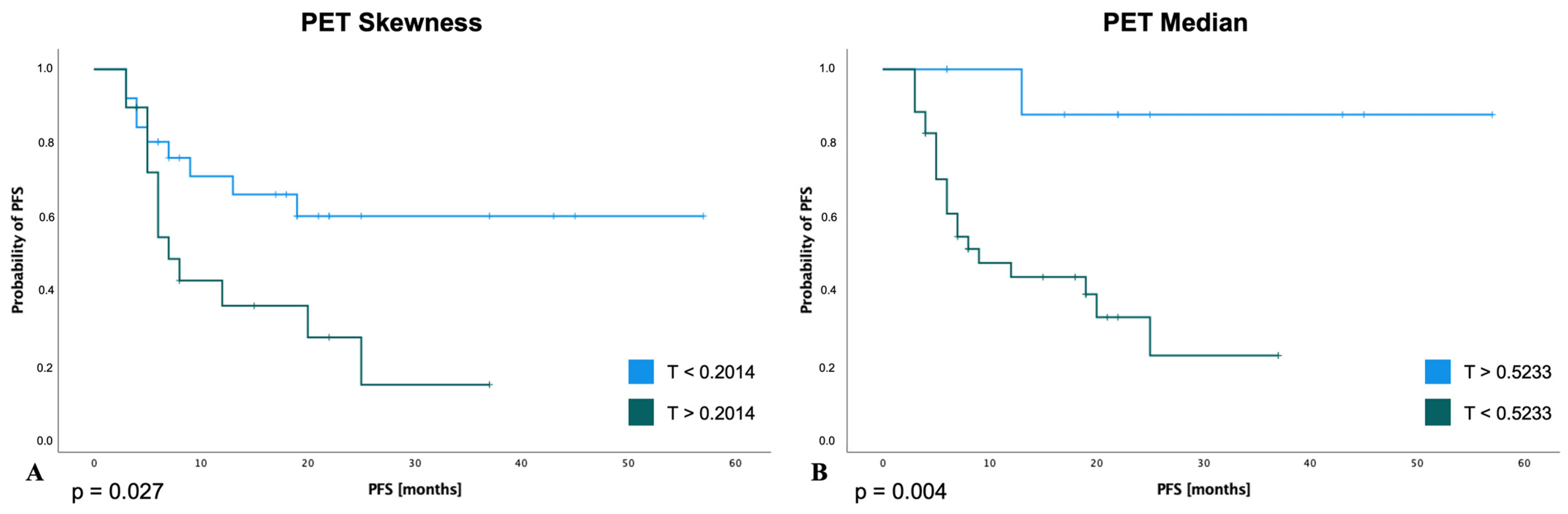
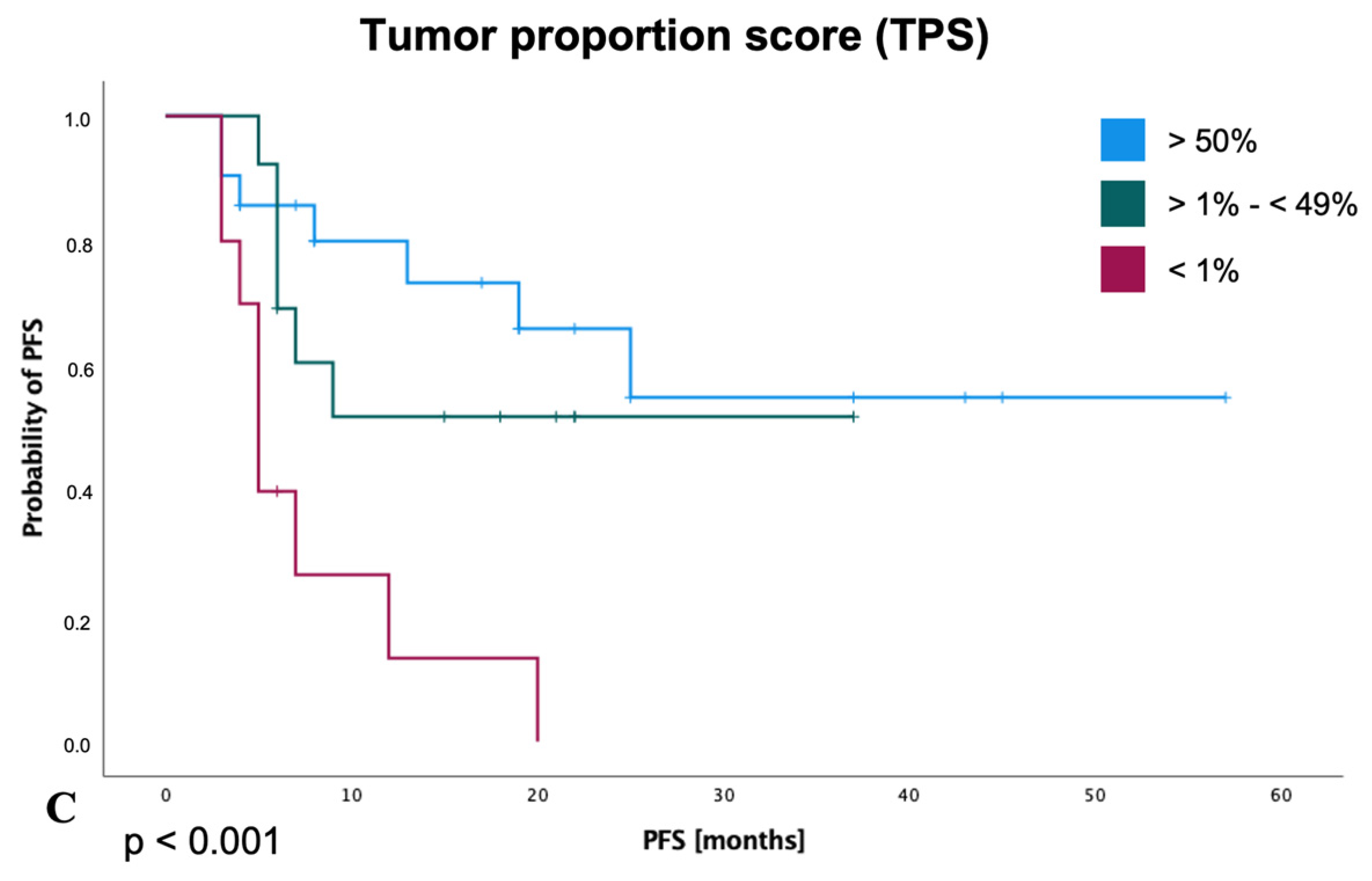
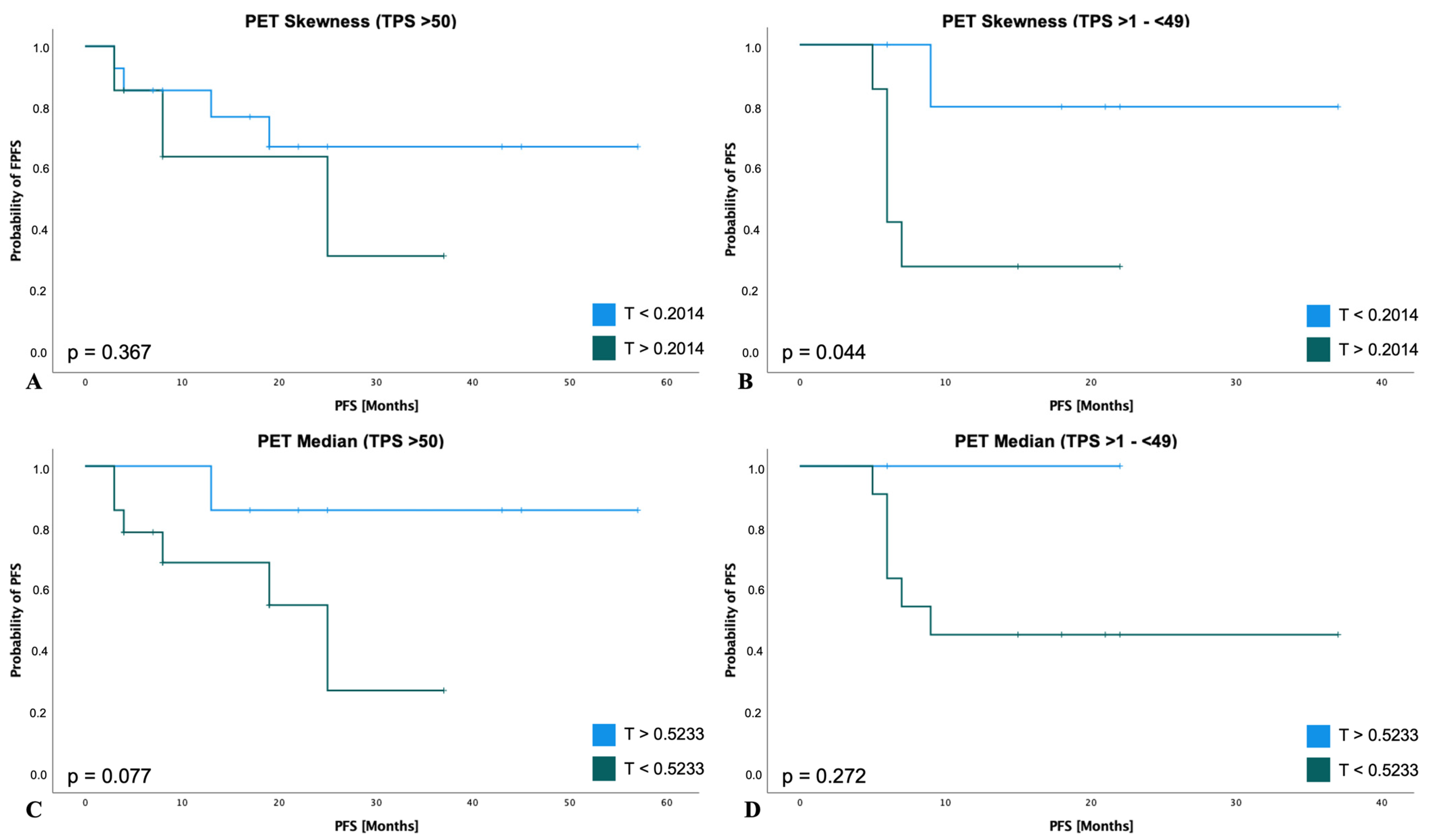
3.5. Survival Analysis for PFS
4. Discussion
4.1. Radiomic Features as Potential Markers for Predicting Treatment Response and Survival
4.2. PET-CT Derived Radiomic Features in Lung Cancer
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bade, B.C.; dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Reck, M.; Heigener, D.F.; Mok, T.; Soria, J.C.; Rabe, K.F. Management of Non-Small-Cell Lung Cancer: Recent Developments. Lancet 2013, 382, 709–719. [Google Scholar] [CrossRef]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Herbst, R.S.; Goldberg, S.B. Selecting the Optimal Immunotherapy Regimen in Driver-Negative Metastatic NSCLC. Nat. Rev. Clin. Oncol. 2021, 18, 625–644. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer with PD-L1 of at Least 50%: A Multicentre, Open-Label, Global, Phase 3, Randomised, Controlled Trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; de Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Doebele, R.C.; Kerr, K.M. Comparing and Contrasting Predictive Biomarkers for Immunotherapy and Targeted Therapy of NSCLC. Nat. Rev. Clin. Oncol. 2019, 16, 341–355. [Google Scholar] [CrossRef]
- Farsad, M. FDG PET/CT in the Staging of Lung Cancer. Curr. Radiopharm. 2020, 13, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Kandathil, A.; Kay, F.U.; Butt, Y.M.; Wachsmann, J.W.; Subramaniam, R.M. Role of FDG PET/CT in the Eighth Edition of TNM Staging of Non-Small Cell Lung Cancer. Radiographics 2018, 38, 2134–2149. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, M.; Gilardi, L.; Ferrari, M.E.; Piperno, G.; Travaini, L.L.; Timmerman, R.; Botta, F.; Baroni, G.; Grana, C.M.; Ronchi, S.; et al. Role of Interim 18 F-FDG-PET/CT for the Early Prediction of Clinical Outcomes of Non-Small Cell Lung Cancer (NSCLC) during Radiotherapy or Chemo-Radiotherapy. A Systematic Review. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Morland, D.; Triumbari, E.K.A.; Boldrini, L.; Gatta, R.; Pizzuto, D.; Annunziata, S. Radiomics in Oncological PET Imaging: A Systematic Review—Part 1, Supradiaphragmatic Cancers. Diagnostics 2022, 12, 1329. [Google Scholar] [CrossRef]
- Carles, M.; Fechter, T.; Martí-Bonmatí, L.; Baltas, D.; Mix, M. Experimental Phantom Evaluation to Identify Robust Positron Emission Tomography (PET) Radiomic Features. EJNMMI Phys. 2021, 8, 46. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563. [Google Scholar] [CrossRef]
- Polverari, G.; Ceci, F.; Bertaglia, V.; Reale, M.L.; Rampado, O.; Gallio, E.; Passera, R.; Liberini, V.; Scapoli, P.; Arena, V.; et al. 18F-FDG Pet Parameters and Radiomics Features Analysis in Advanced Nsclc Treated with Immunotherapy as Predictors of Therapy Response and Survival. Cancers 2020, 12, 1163. [Google Scholar] [CrossRef]
- Valentinuzzi, D.; Vrankar, M.; Boc, N.; Ahac, V.; Zupancic, Z.; Unk, M.; Skalic, K.; Zagar, I.; Studen, A.; Simoncic, U.; et al. [18F]FDG PET Immunotherapy Radiomics Signature (IRADIOMICS) Predicts Response of Non-Small-Cell Lung Cancer Patients Treated with Pembrolizumab. Radiol. Oncol. 2020, 54, 285. [Google Scholar] [CrossRef]
- Mu, W.; Tunali, I.; Gray, J.E.; Qi, J.; Schabath, M.B.; Gillies, R.J. Radiomics of 18F-FDG PET/CT Images Predicts Clinical Benefit of Advanced NSCLC Patients to Checkpoint Blockade Immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, L.; Mo, X.; You, J.; Chen, L.; Fang, J.; Wang, F.; Jin, Z.; Zhang, B.; Zhang, S. Current Status and Quality of Radiomic Studies for Predicting Immunotherapy Response and Outcome in Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 345–360. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.H.; Schneider, B.J.; Temin, S.; Baker, S.; Brahmer, J.; Ellis, P.M.; Gaspar, L.E.; Haddad, R.Y.; Hesketh, P.J.; Jain, D.; et al. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2020, 38, 1608–1632. [Google Scholar] [CrossRef]
- Roll, W.; Evers, G.; Strotmann, R.; Albring, J.; Reicherts, C.; Noto, B.; Weckesser, M.; Lenz, G.; Schäfers, M.; Stelljes, M. Fluorodeoxyglucose F 18 for the Assessment of Acute Intestinal Graft-versus-Host Disease and Prediction of Response to Immunosuppressive Therapy. Transpl. Cell. Ther. 2021, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and Clarification: From the RECIST Committee. Eur. J. Cancer 2016, 62, 132. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50, 122S. [Google Scholar] [CrossRef]
- Kuruva, M.; Mittal, B.; Abrar, M.; Kashyap, R.; Bhattacharya, A. Multivariate Analysis of Various Factors Affecting Background Liver and Mediastinal Standardized Uptake Values. Indian J. Nucl. Med. 2012, 27, 20. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gu, D.; Wei, J.; Yang, C.; Rao, S.; Wang, W.; Chen, C.; Ding, Y.; Tian, J.; Zeng, M. A Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer 2019, 8, 373–386. [Google Scholar] [CrossRef]
- Chang, R.; Qi, S.; Yue, Y.; Zhang, X.; Song, J.; Qian, W. Predictive Radiomic Models for the Chemotherapy Response in Non-Small-Cell Lung Cancer Based on Computerized-Tomography Images. Front. Oncol. 2021, 11, 646190. [Google Scholar] [CrossRef]
- Khan, J.N.; Singh, A.; Nazir, S.A.; Kanagala, P.; Gershlick, A.H.; McCann, G.P. Comparison of Cardiovascular Magnetic Resonance Feature Tracking and Tagging for the Assessment of Left Ventricular Systolic Strain in Acute Myocardial Infarction. Eur. J. Radiol. 2015, 84, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Gyorffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A Comprehensive and Straightforward Web Application Enabling Rapid Biomarker Cutoff Optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Shields, M.D.; Marin-Acevedo, J.A.; Pellini, B. Immunotherapy for Advanced Non-Small Cell Lung Cancer: A Decade of Progress. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e105–e127. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csöszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Garassino, M.C.; Gadgeel, S.M.; Speranza, G.; Felip, E.; Esteban Gonzalez, E.; Domine Gomez, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.; Peled, N.; et al. 973MO KEYNOTE-189 5-Year Update: First-Line Pembrolizumab (Pembro) + Pemetrexed (Pem) and Platinum vs Placebo (Pbo) + Pem and Platinum for Metastatic Nonsquamous NSCLC. Ann. Oncol. 2022, 33, S992–S993. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; van Schil, P.E.; Hellmann, M.D.; et al. Metastatic Non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Seban, R.D.; Mezquita, L.; Berenbaum, A.; Dercle, L.; Botticella, A.; le Pechoux, C.; Caramella, C.; Deutsch, E.; Grimaldi, S.; Adam, J.; et al. Baseline Metabolic Tumor Burden on FDG PET/CT Scans Predicts Outcome in Advanced NSCLC Patients Treated with Immune Checkpoint Inhibitors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wroblewski, K.; Liao, S.; Kampalath, R.; Penney, B.C.; Zhang, Y.; Pu, Y. Prognostic Value of Metabolic Tumor Burden from (18)F-FDG PET in Surgical Patients with Non-Small-Cell Lung Cancer. Acad. Radiol. 2013, 20, 32–40. [Google Scholar] [CrossRef]
- Lapa, P.; Oliveiros, B.; Marques, M.; Isidoro, J.; Alves, F.C.; Nascimento Costa, J.M.; Costa, G.; de Lima, J.P. Metabolic Tumor Burden Quantified on [18F]FDG PET/CT Improves TNM Staging of Lung Cancer Patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2169–2178. [Google Scholar] [CrossRef]
- Zhang, H.; Wroblewski, K.; Appelbaum, D.; Pu, Y. Independent Prognostic Value of Whole-Body Metabolic Tumor Burden from FDG-PET in Non-Small Cell Lung Cancer. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 181–191. [Google Scholar] [CrossRef]
- Ding, C.; Mao, X.; Li, N.; Huang, M.; Huang, Z.; Bao, W.; Li, H.; Fan, J. Metabolic Tumor Volume Derived from 18 F-FDG PET/CT as a Prognostic Parameter for Non-Small Cell Lung Cancer (NSCLC) Patients. Hell. J. Nucl. Med. 2022, 25, 63–70. [Google Scholar]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in Medical Imaging—“How-to” Guide and Critical Reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 13, 1. [Google Scholar] [CrossRef]
- Zerunian, M.; Caruso, D.; Zucchelli, A.; Polici, M.; Capalbo, C.; Filetti, M.; Mazzuca, F.; Marchetti, P.; Laghi, A. CT Based Radiomic Approach on First Line Pembrolizumab in Lung Cancer. Sci. Rep. 2021, 11, 6633. [Google Scholar] [CrossRef]
- Singh, A.; Horng, H.; Roshkovan, L.; Weeks, J.K.; Hershman, M.; Noël, P.; Luna, J.M.; Cohen, E.A.; Pantalone, L.; Shinohara, R.T.; et al. Development of a Robust Radiomic Biomarker of Progression-Free Survival in Advanced Non-Small Cell Lung Cancer Patients Treated with First-Line Immunotherapy. Sci. Rep. 2022, 12, 9993. [Google Scholar] [CrossRef] [PubMed]
- Sibille, L.; Seifert, R.; Avramovic, N.; Vehren, T.; Spottiswoode, B.; Zuehlsdorff, S.; Schäfers, M. 18F-FDG PET/CT Uptake Classification in Lymphoma and Lung Cancer by Using Deep Convolutional Neural Networks. Radiology 2020, 294, 445–452. [Google Scholar] [CrossRef] [PubMed]
| Subjects | 44 | |
|---|---|---|
| Male | 31 | 70.5% |
| Female | 13 | 29.5% |
| Age [Years] | 65 | (33–82) |
| Smoking status | ||
| Current | 10 | 22.7% |
| Former | 20 | 45.5% |
| Never | 2 | 4.5% |
| Unknown | 12 | 27.3% |
| Eastern Cooperative Oncology Group (ECOG) | ||
| ECOG 0 | 2 | 4.5% |
| ECOG 1 | 32 | 72.7% |
| ECOG 2 | 10 | 22.7% |
| Histology | ||
| Non-squamous carcinoma | 35 | 79.5% |
| Squamous carcinoma | 9 | 20.5% |
| Tumor Proportion Score (TPS)-PD-L1 | ||
| TPS > 50% | 21 | 47.7% |
| TPS > 1%–(<49%) | 13 | 29.5% |
| TPS < 1% | 10 | 22.7% |
| Response after Second Follow-Up (RECIST) | ||
| Complete Response (CR) | 1 | 2.3% |
| Partial Response (PR) | 17 | 38.6% |
| Stable Disease (SD) | 15 | 34.1% |
| Progressive Disease (PD) | 11 | 25.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura, D.; Schindler, P.; Masthoff, M.; Görlich, D.; Dittmann, M.; Heindel, W.; Schäfers, M.; Lenz, G.; Wardelmann, E.; Mohr, M.; et al. Radiomics of Tumor Heterogeneity in 18F-FDG-PET-CT for Predicting Response to Immune Checkpoint Inhibition in Therapy-Naïve Patients with Advanced Non-Small-Cell Lung Cancer. Cancers 2023, 15, 2297. https://doi.org/10.3390/cancers15082297
Ventura D, Schindler P, Masthoff M, Görlich D, Dittmann M, Heindel W, Schäfers M, Lenz G, Wardelmann E, Mohr M, et al. Radiomics of Tumor Heterogeneity in 18F-FDG-PET-CT for Predicting Response to Immune Checkpoint Inhibition in Therapy-Naïve Patients with Advanced Non-Small-Cell Lung Cancer. Cancers. 2023; 15(8):2297. https://doi.org/10.3390/cancers15082297
Chicago/Turabian StyleVentura, David, Philipp Schindler, Max Masthoff, Dennis Görlich, Matthias Dittmann, Walter Heindel, Michael Schäfers, Georg Lenz, Eva Wardelmann, Michael Mohr, and et al. 2023. "Radiomics of Tumor Heterogeneity in 18F-FDG-PET-CT for Predicting Response to Immune Checkpoint Inhibition in Therapy-Naïve Patients with Advanced Non-Small-Cell Lung Cancer" Cancers 15, no. 8: 2297. https://doi.org/10.3390/cancers15082297
APA StyleVentura, D., Schindler, P., Masthoff, M., Görlich, D., Dittmann, M., Heindel, W., Schäfers, M., Lenz, G., Wardelmann, E., Mohr, M., Kies, P., Bleckmann, A., Roll, W., & Evers, G. (2023). Radiomics of Tumor Heterogeneity in 18F-FDG-PET-CT for Predicting Response to Immune Checkpoint Inhibition in Therapy-Naïve Patients with Advanced Non-Small-Cell Lung Cancer. Cancers, 15(8), 2297. https://doi.org/10.3390/cancers15082297







