Prostate Cancer and Its Mimics—A Pictorial Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Anterior Fibromuscular Stroma
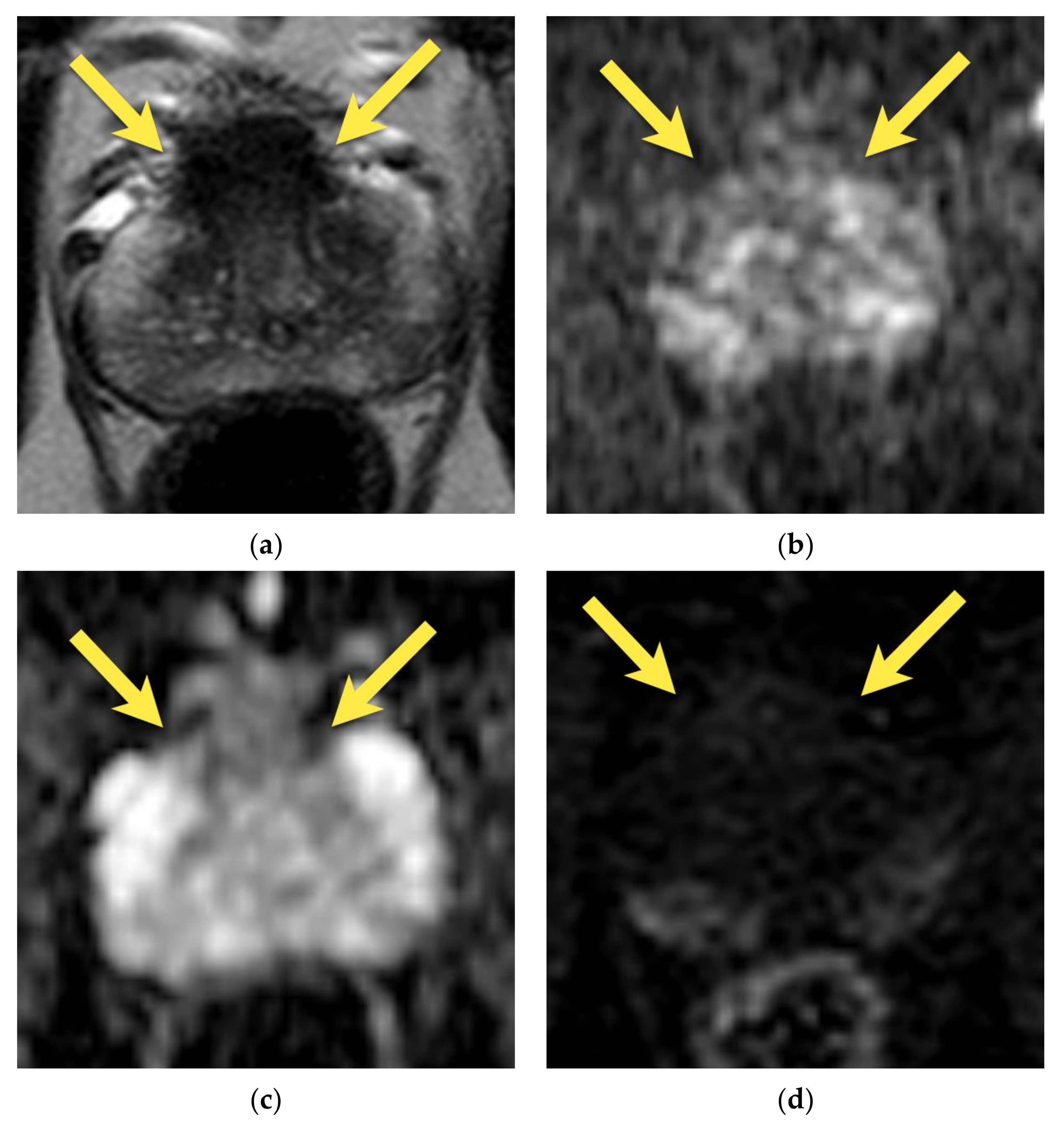
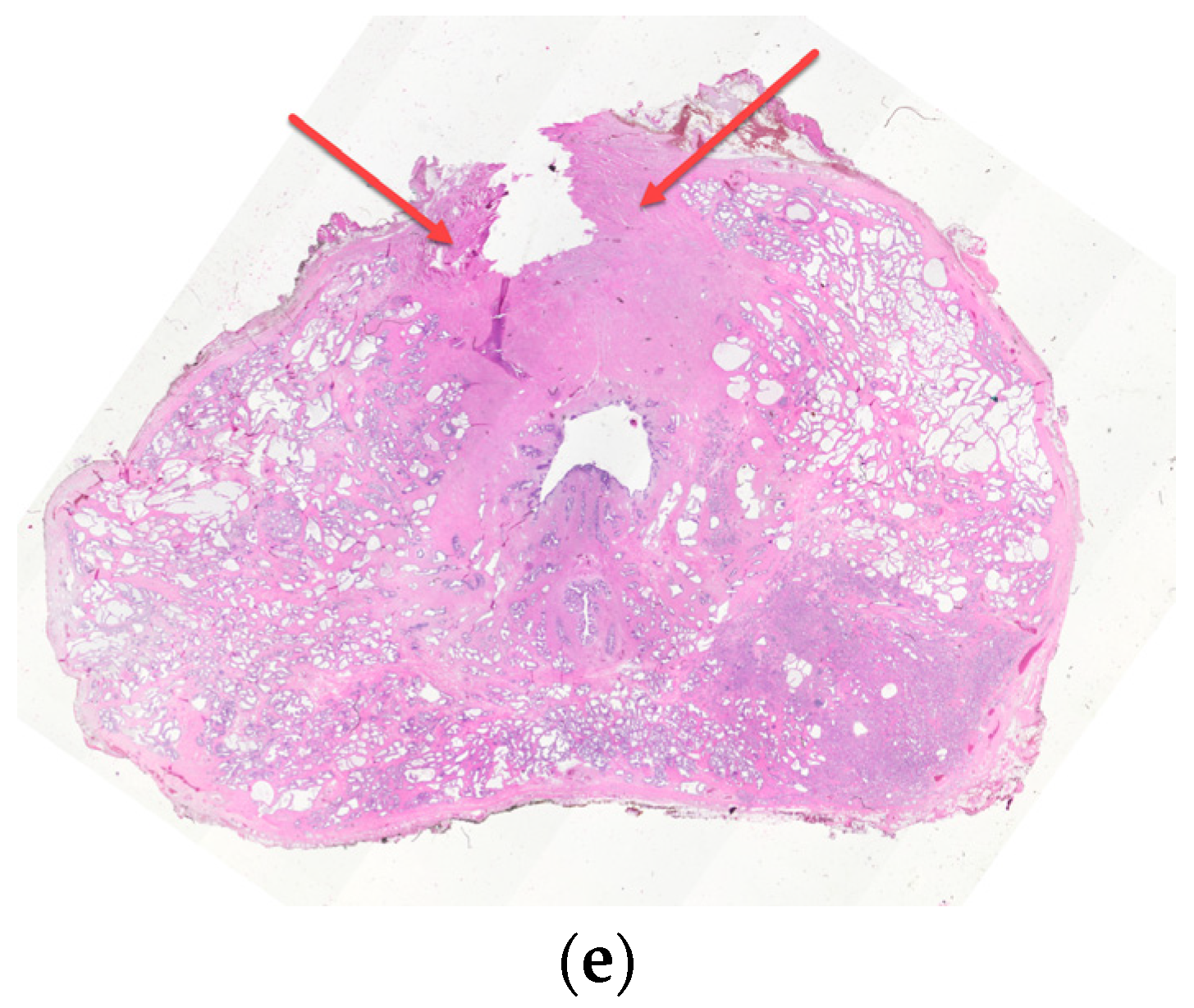
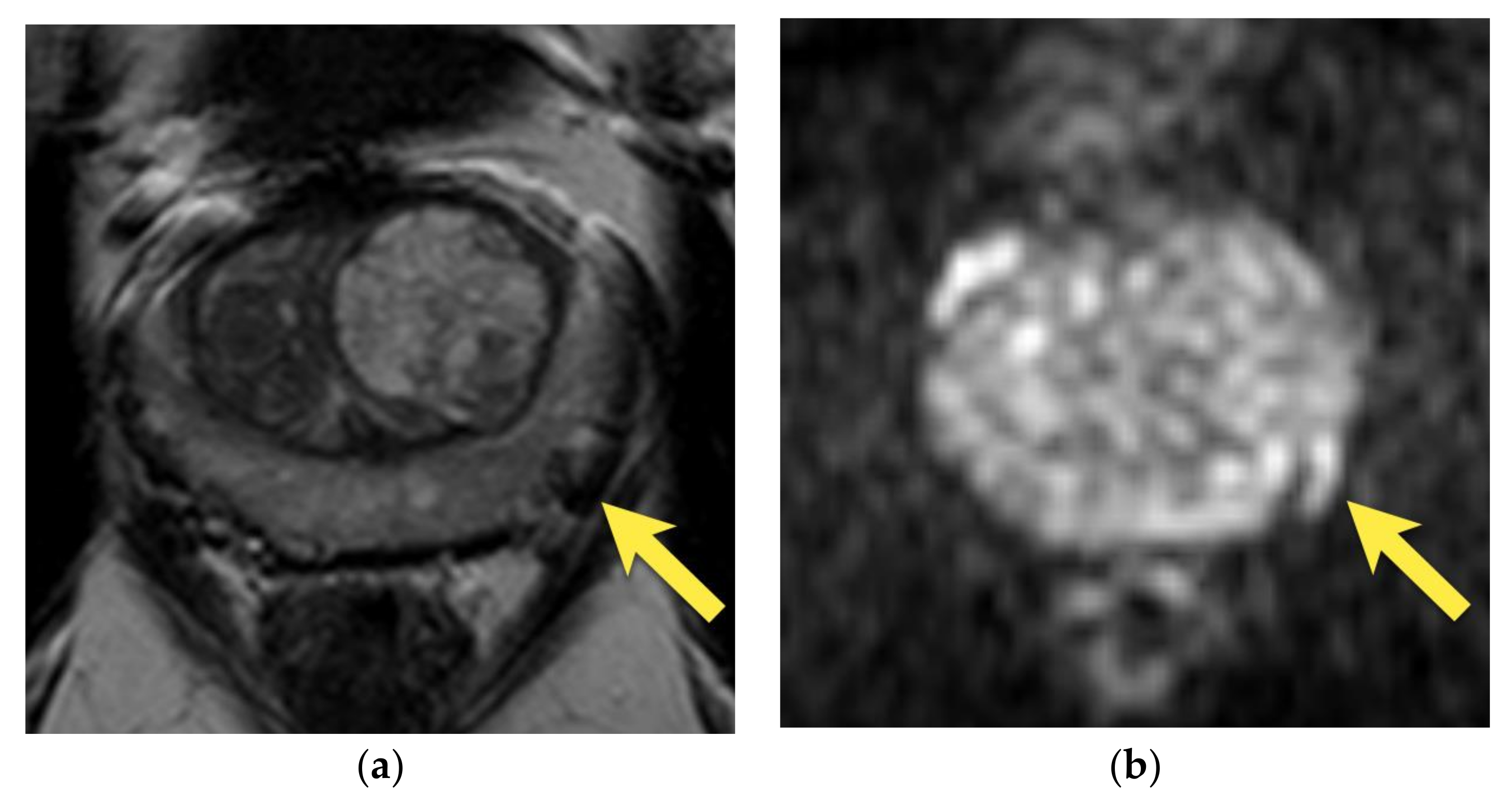
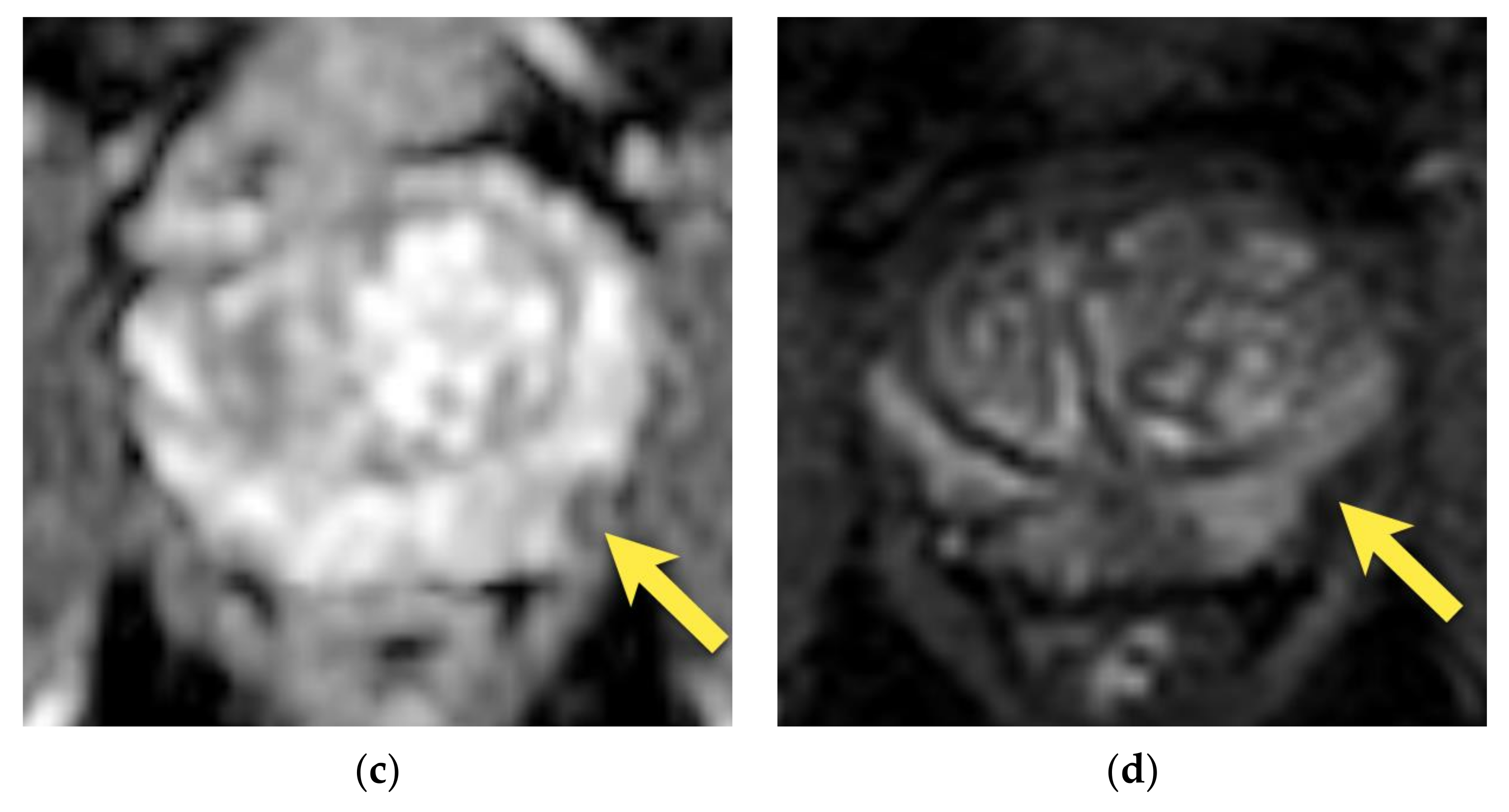
3. Periprostatic Venous Plexus
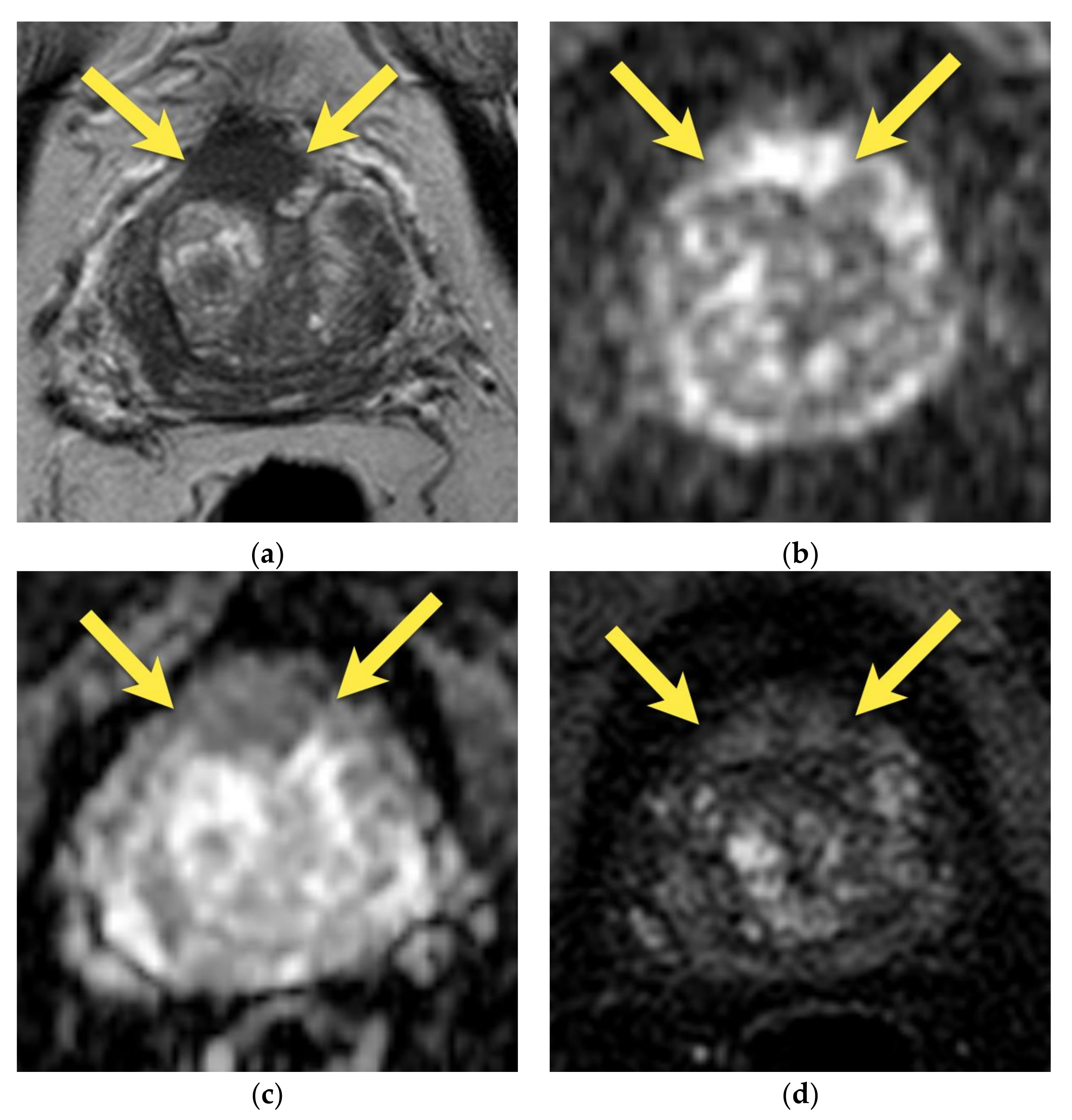
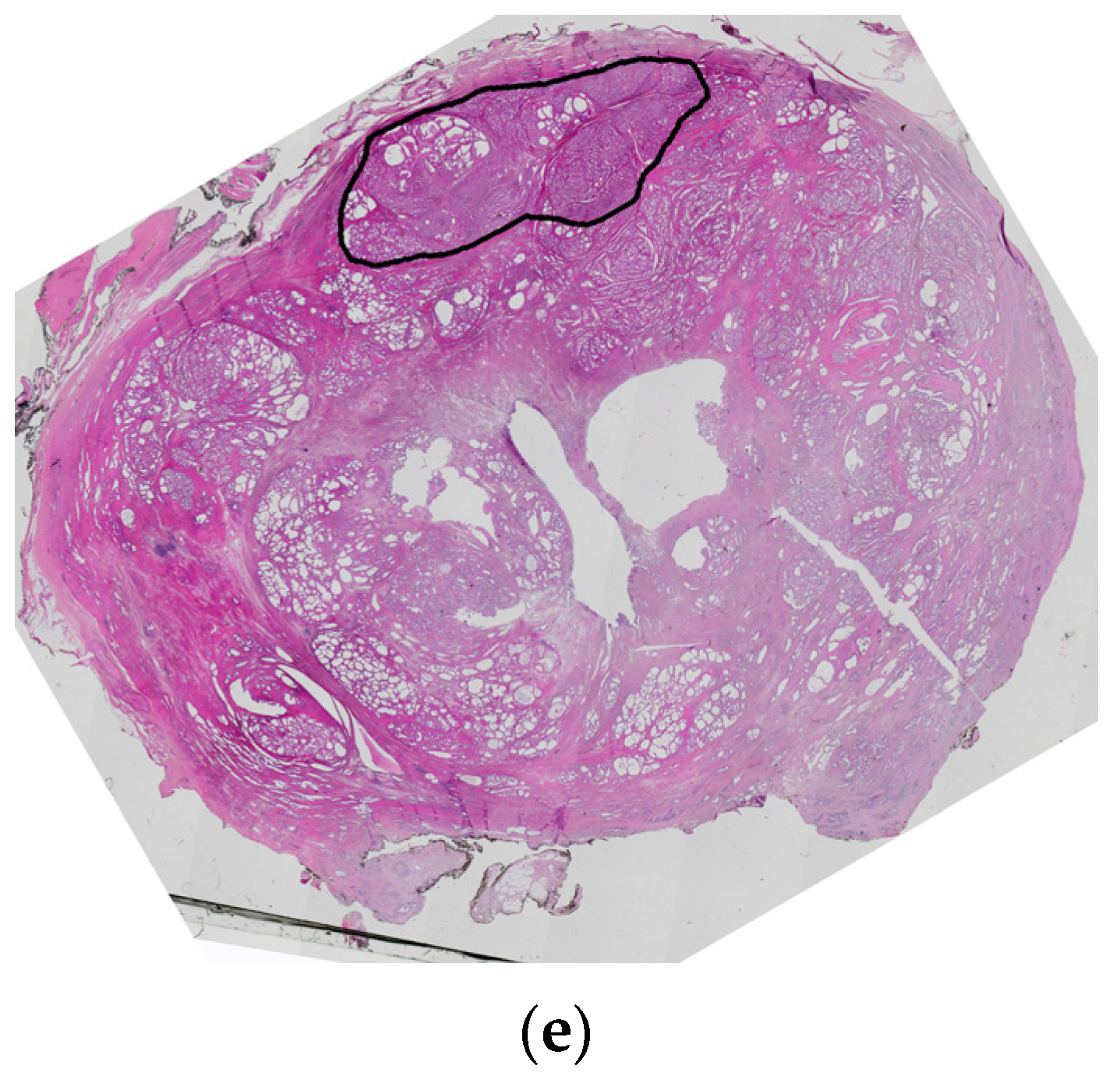
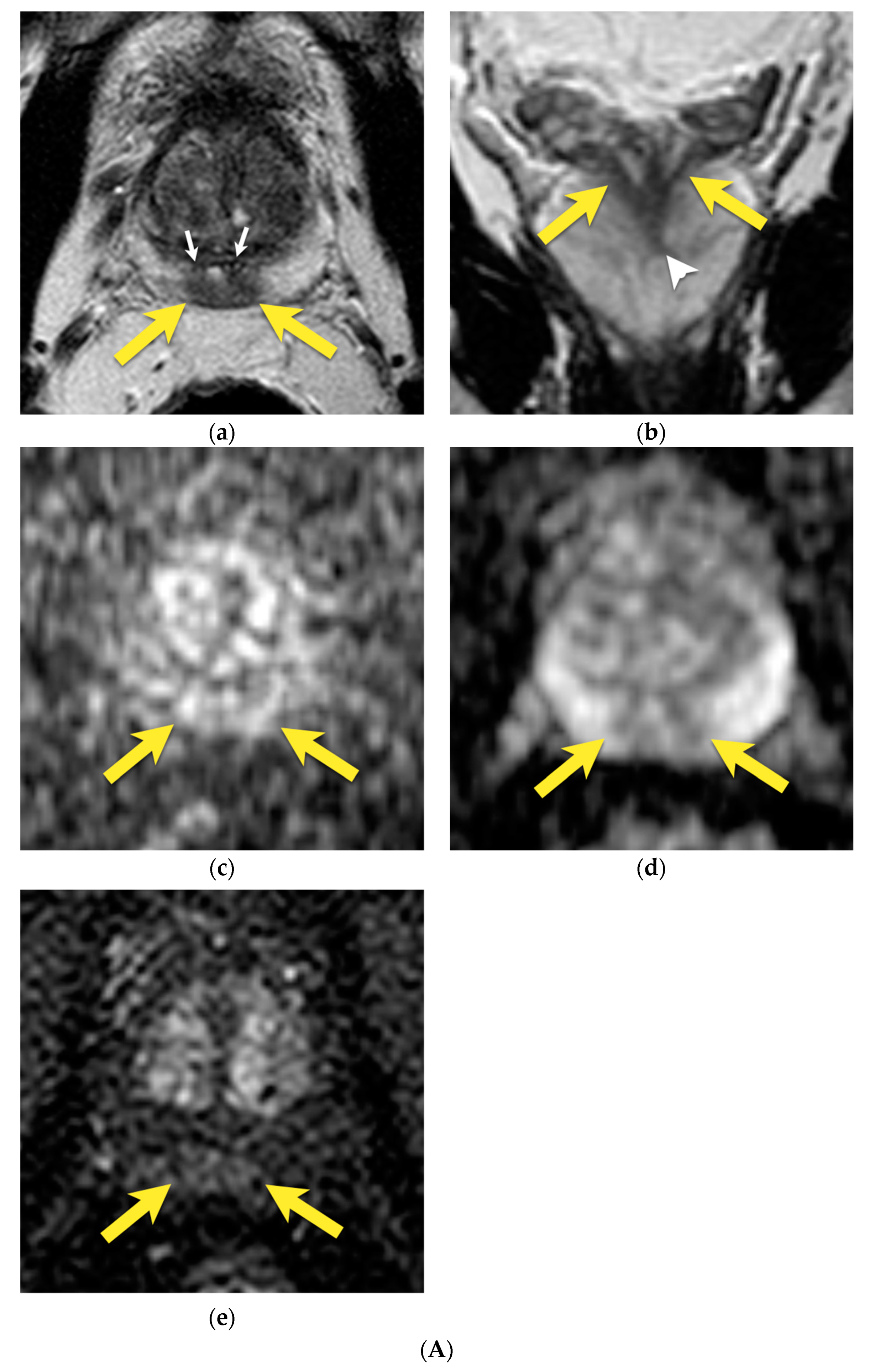
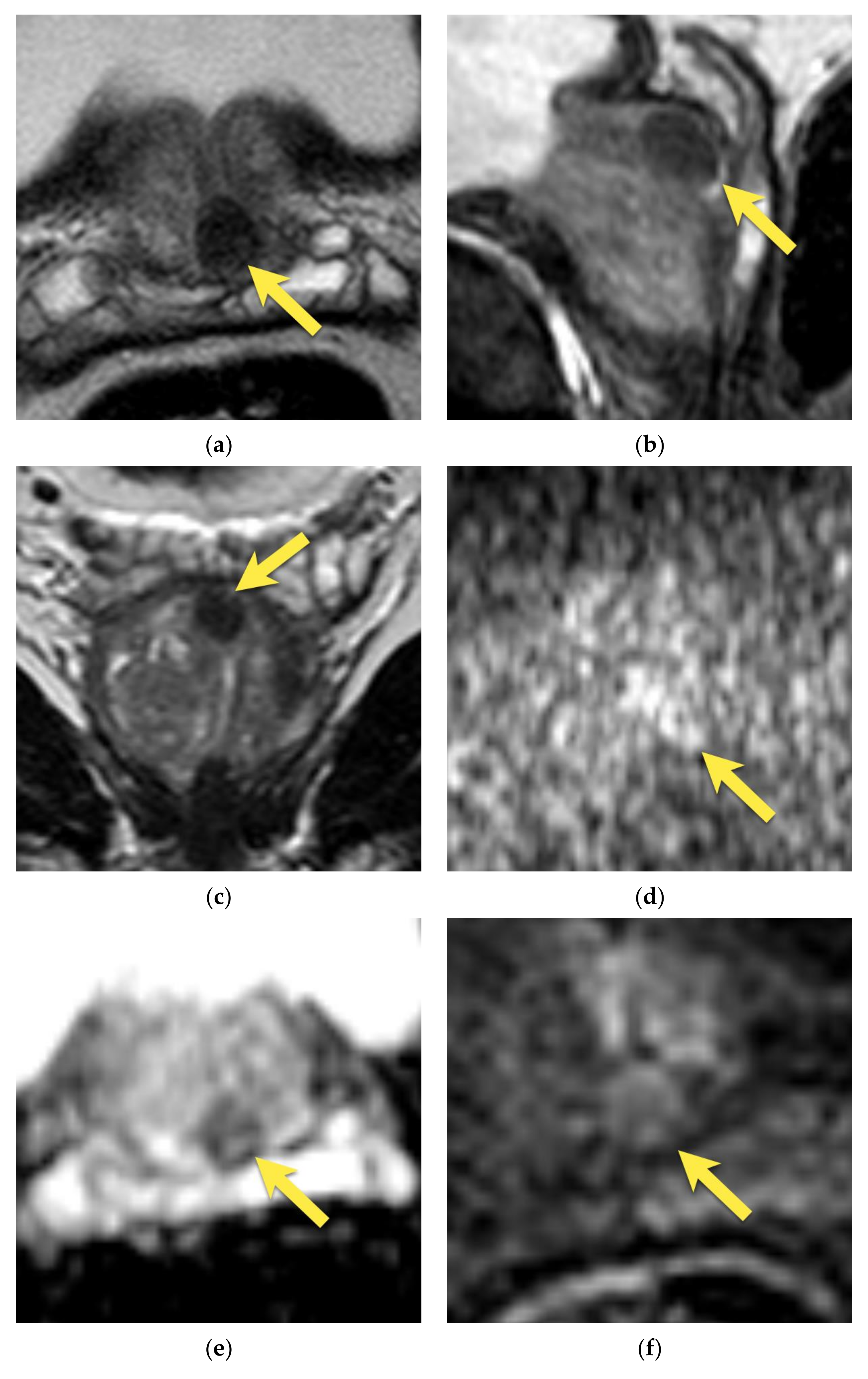
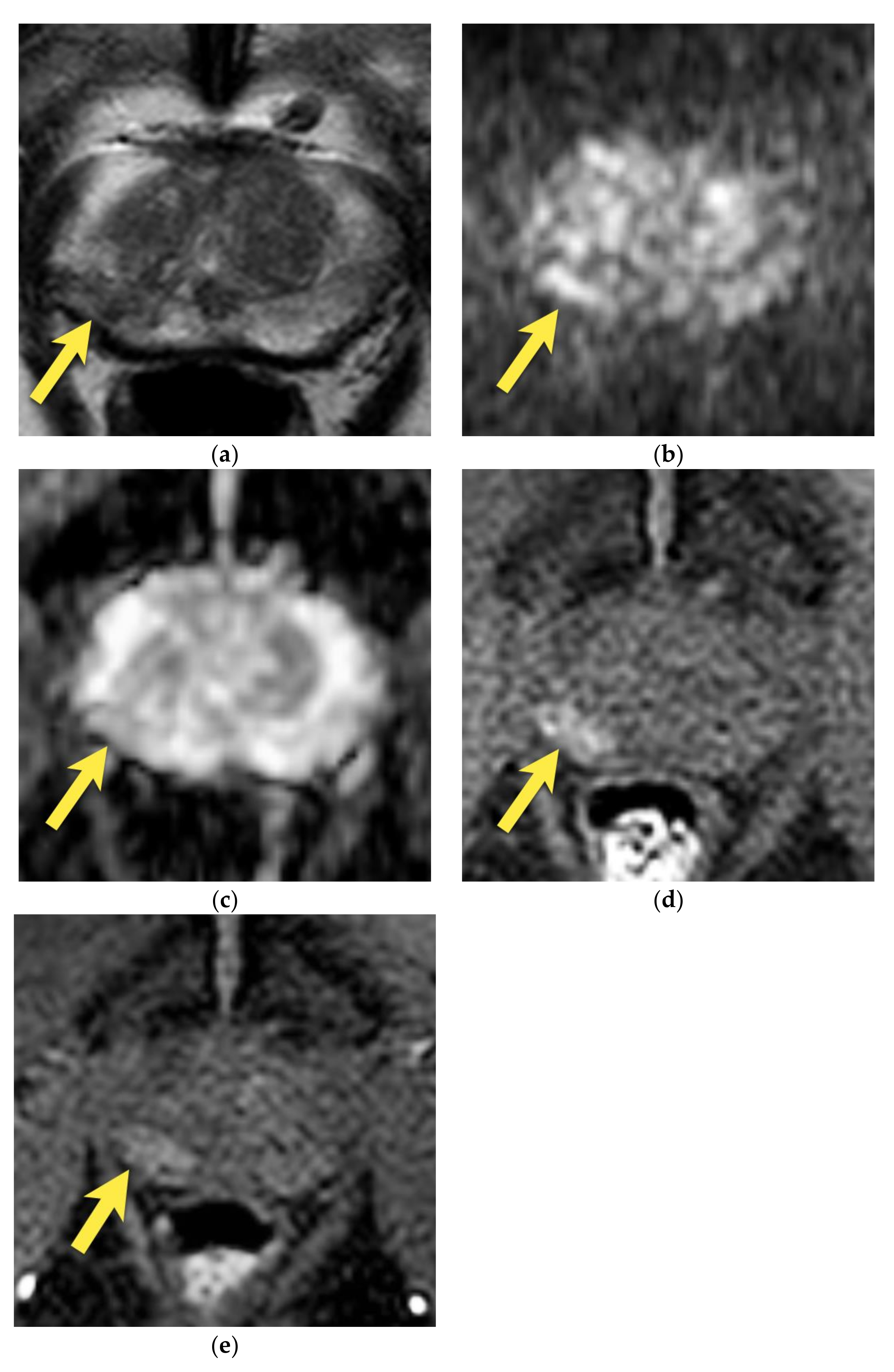
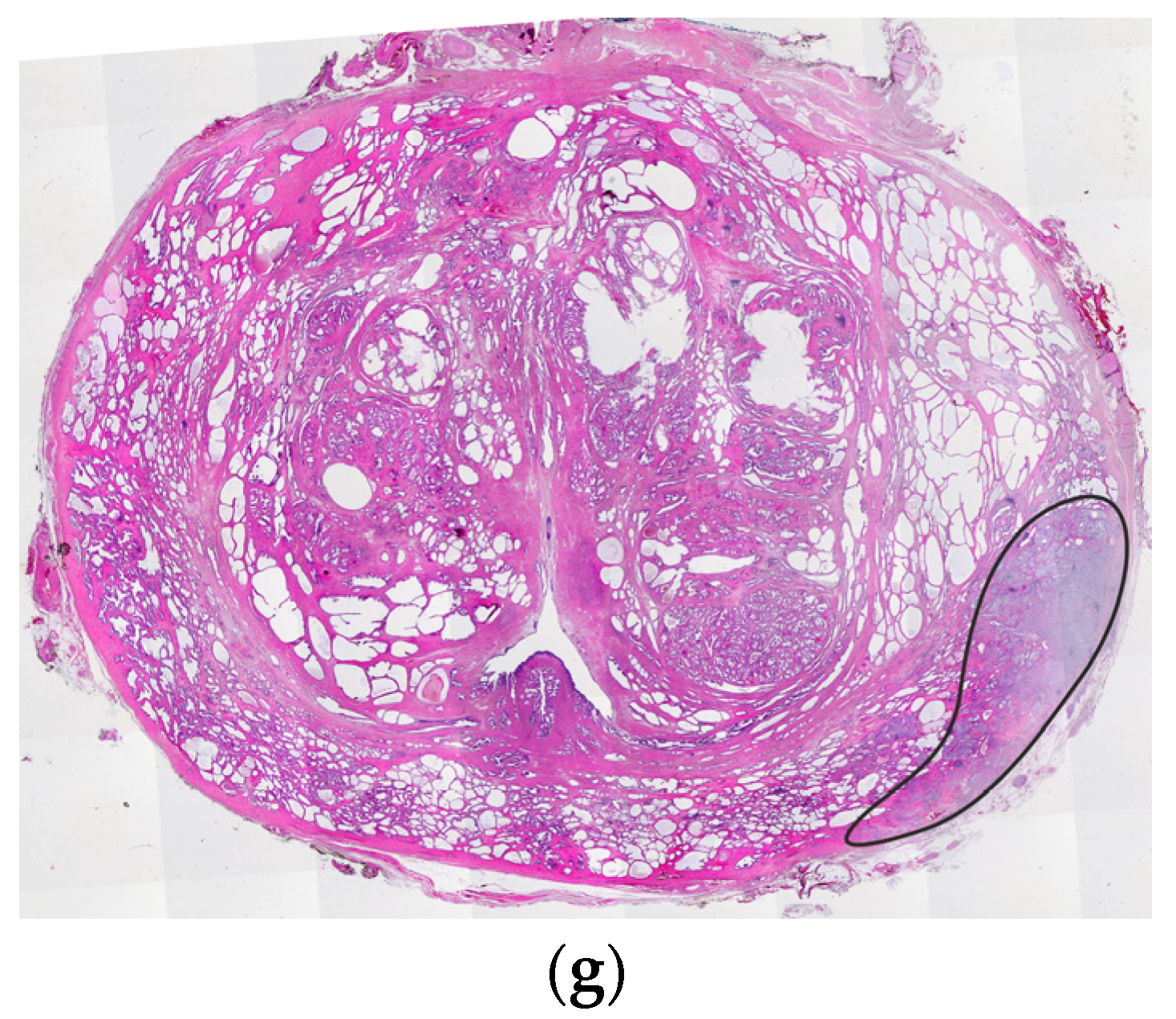
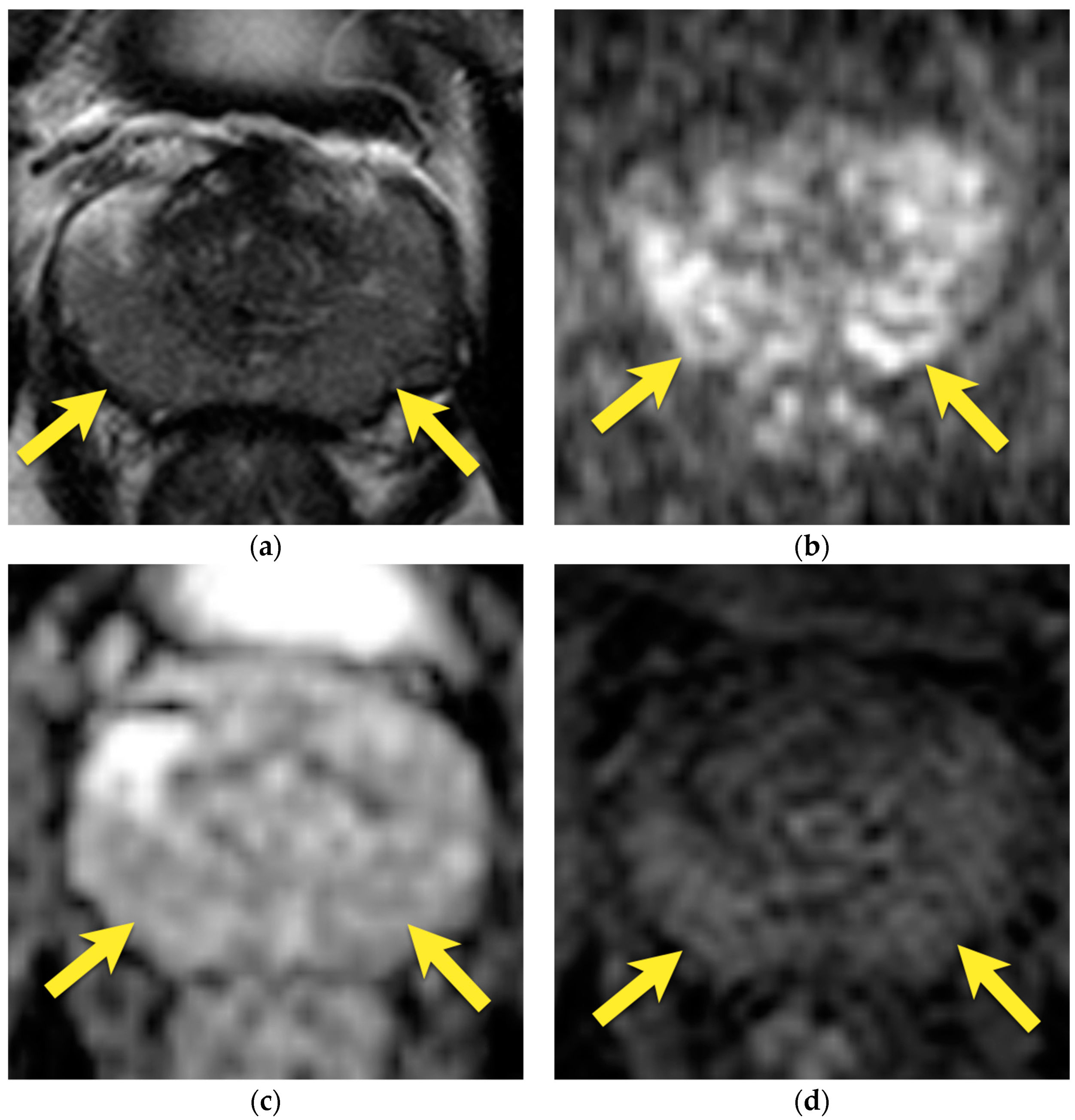
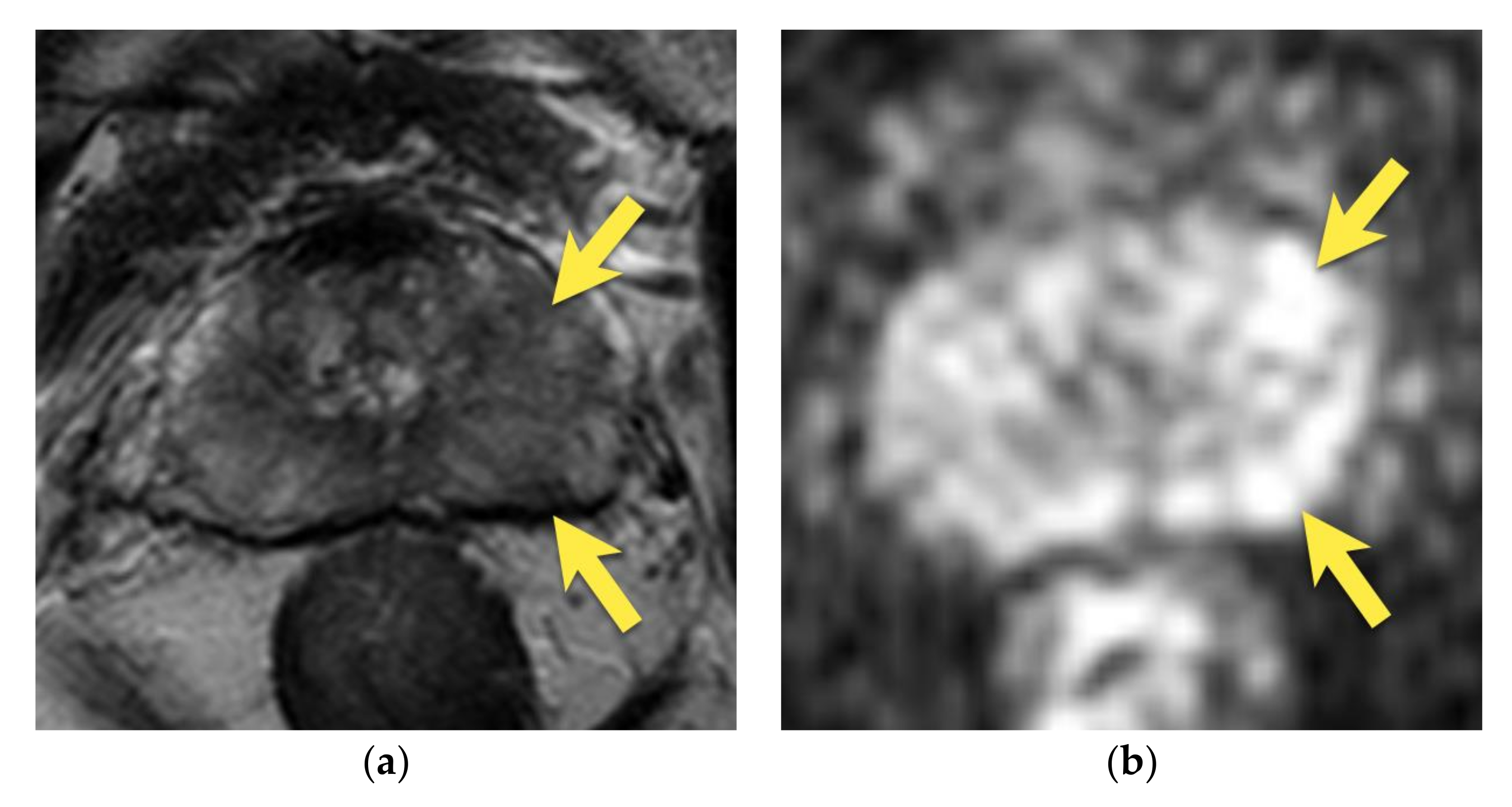
4. Central Zone
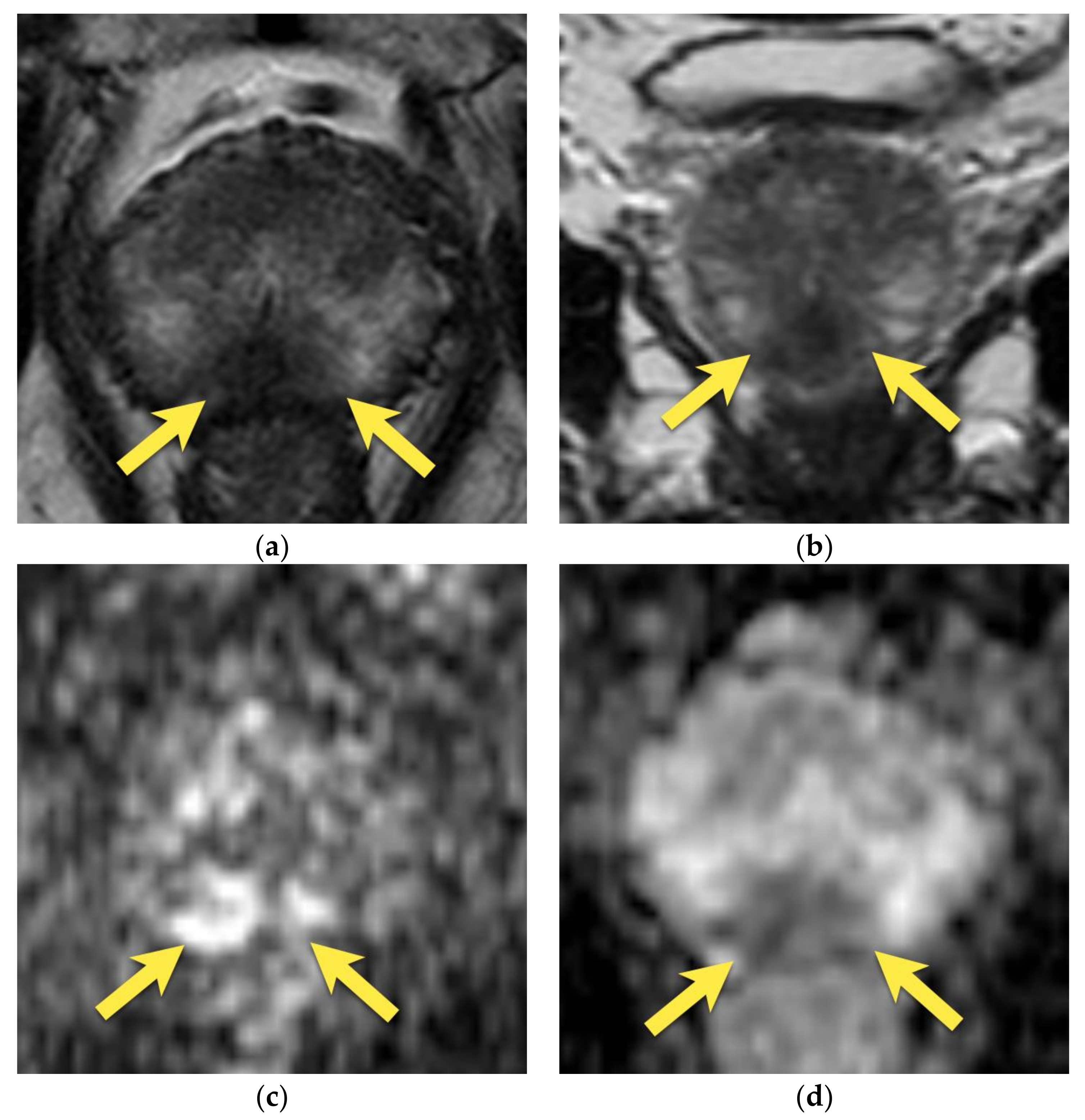
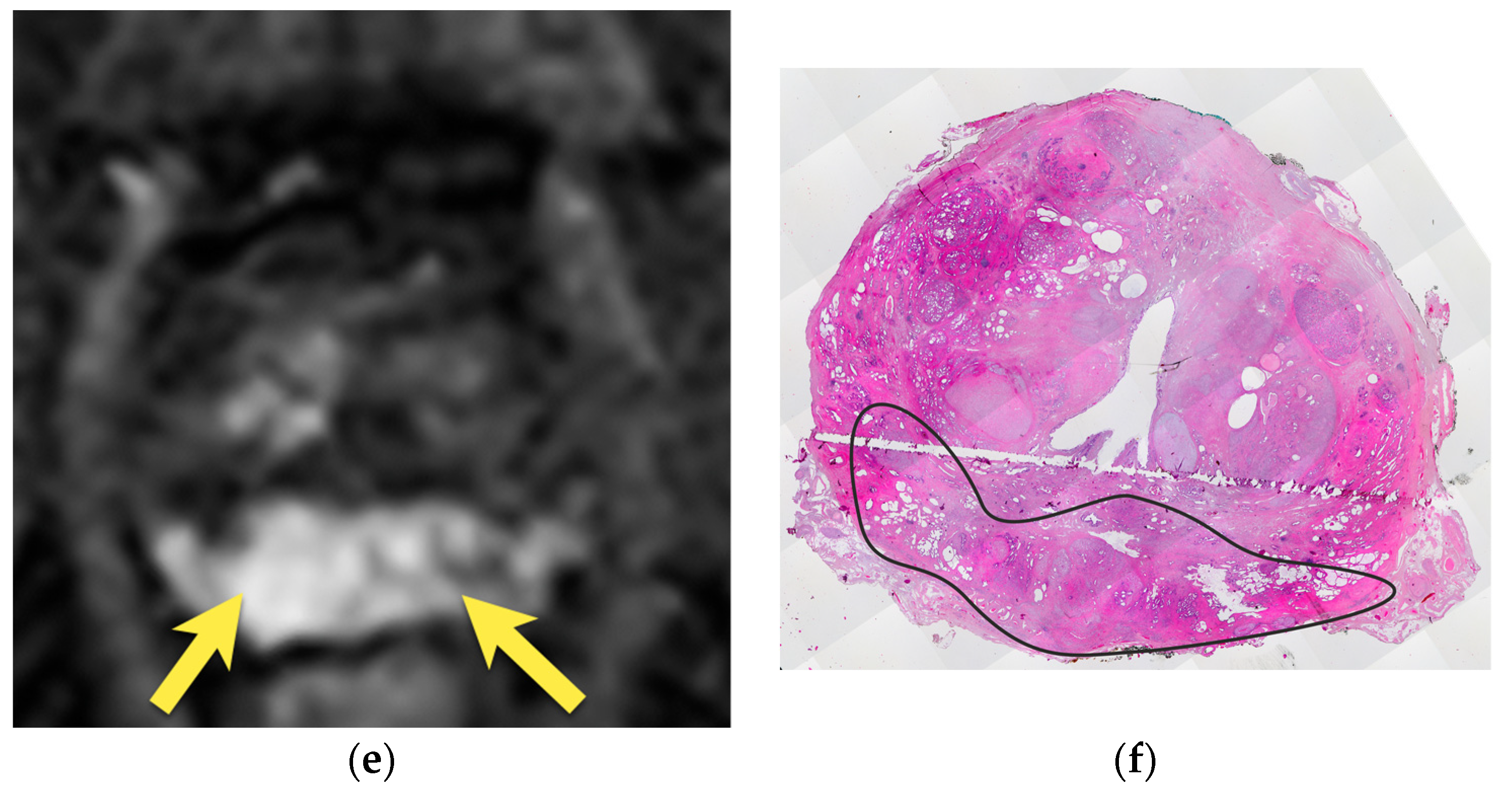
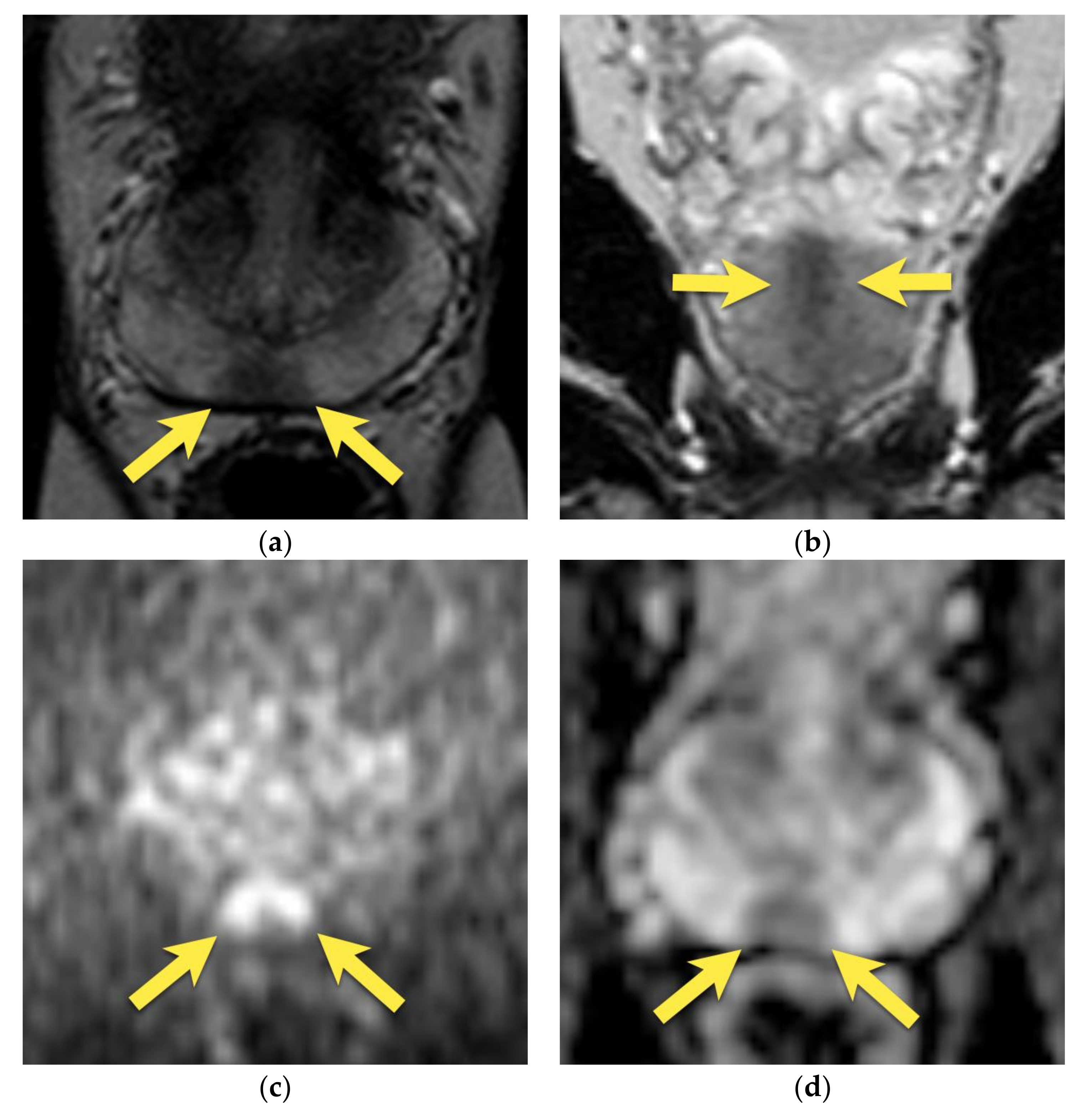
5. Hypointense Area in the Median Posterior Middle Gland or at the Base
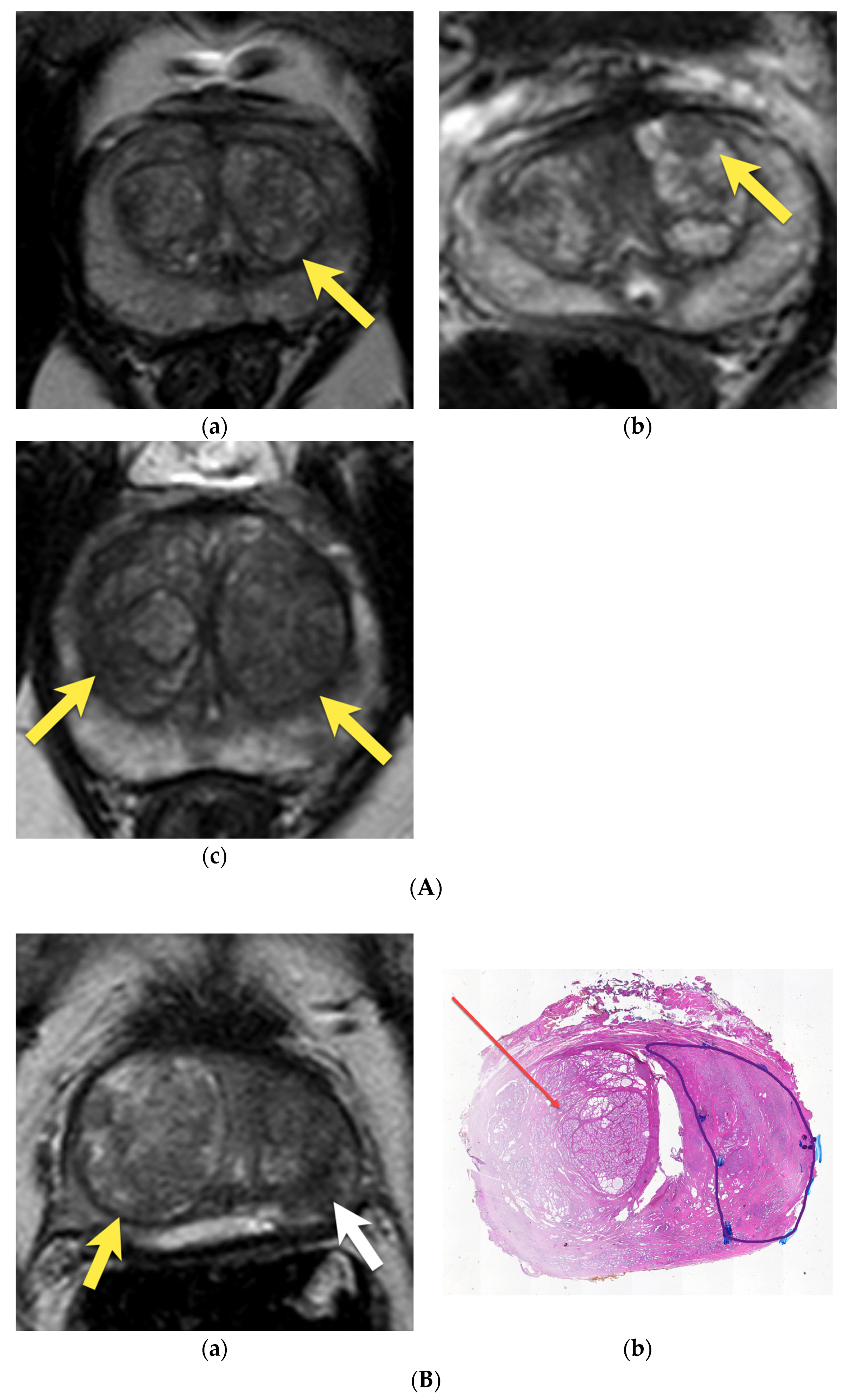
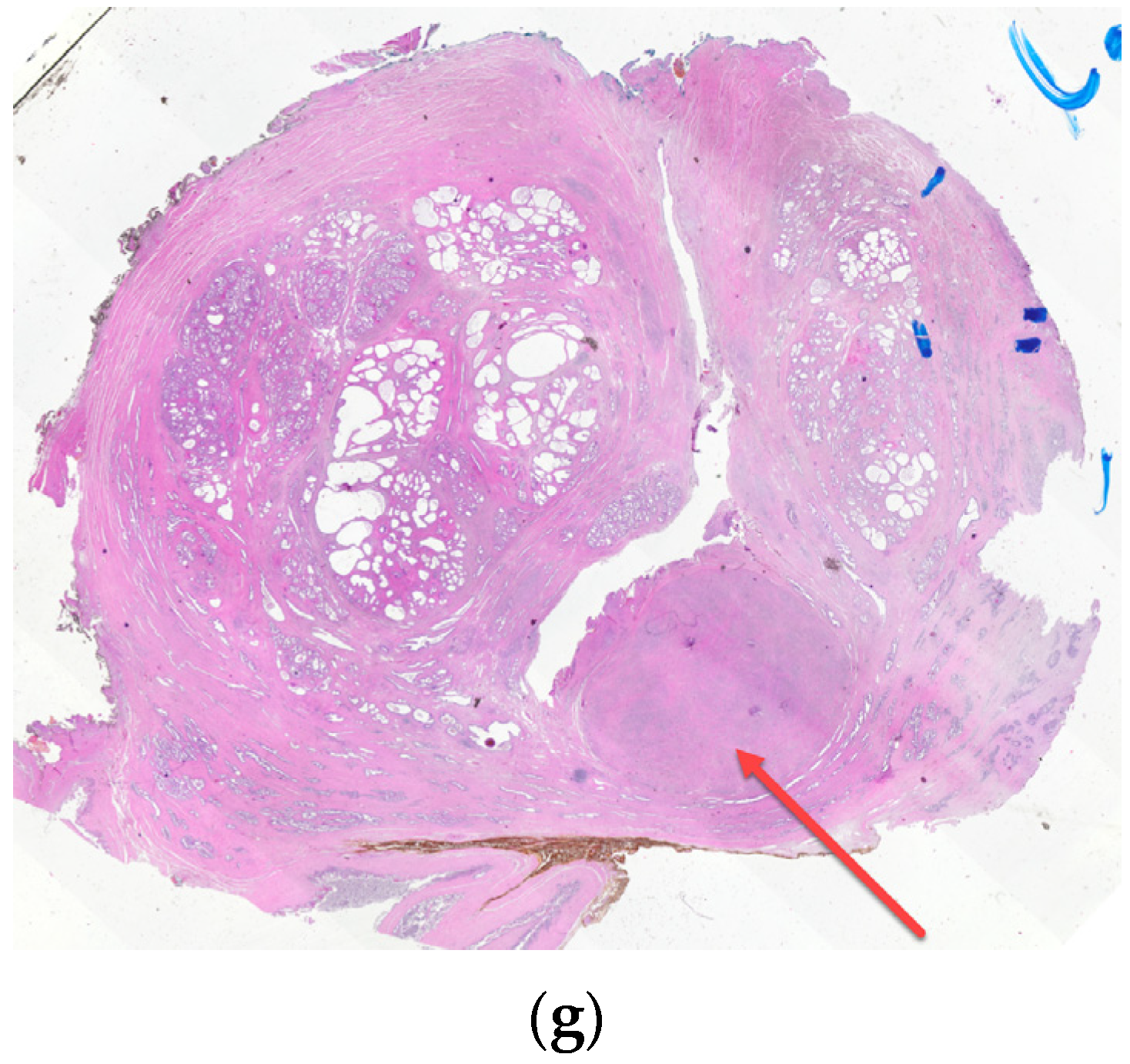
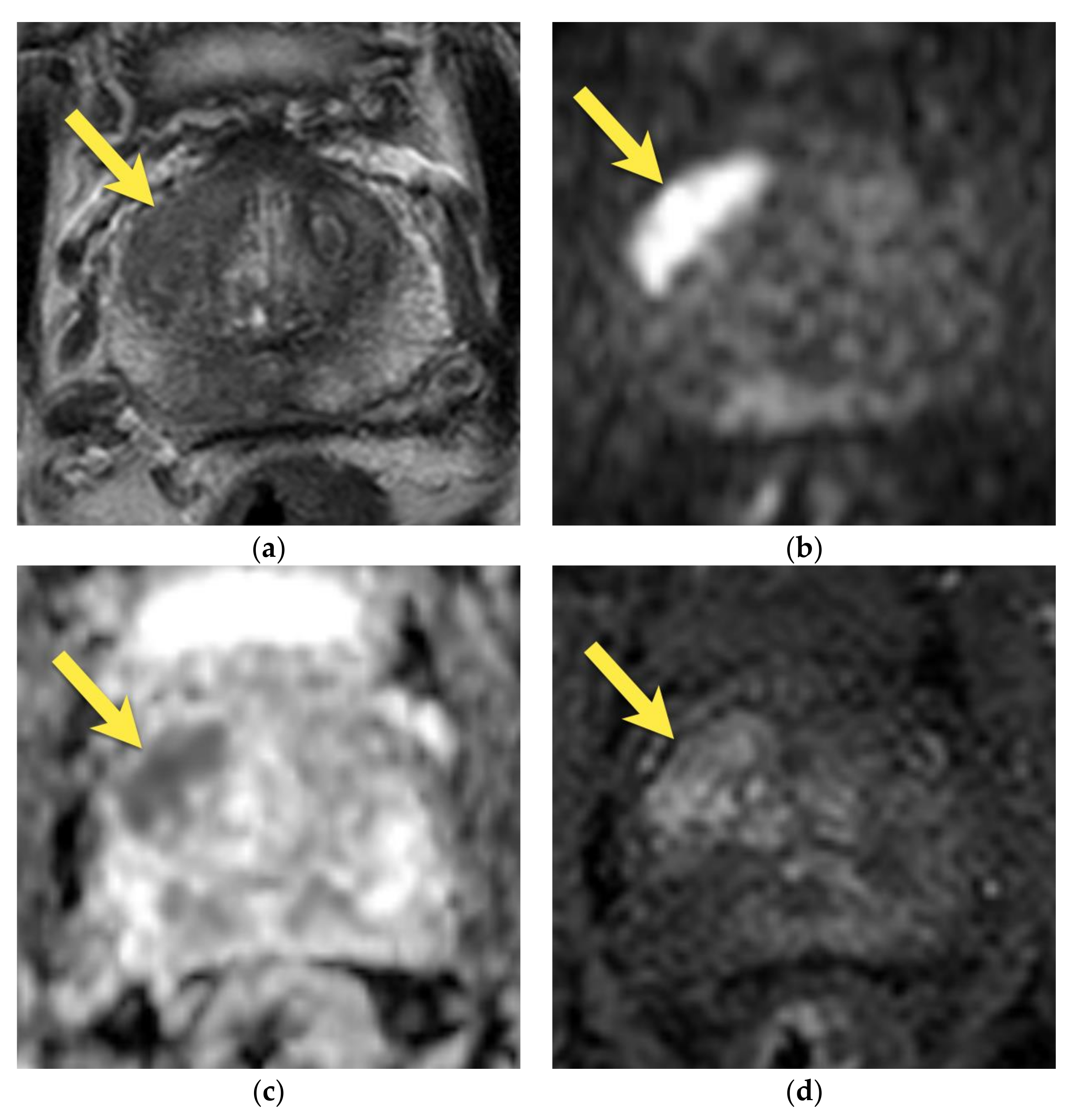
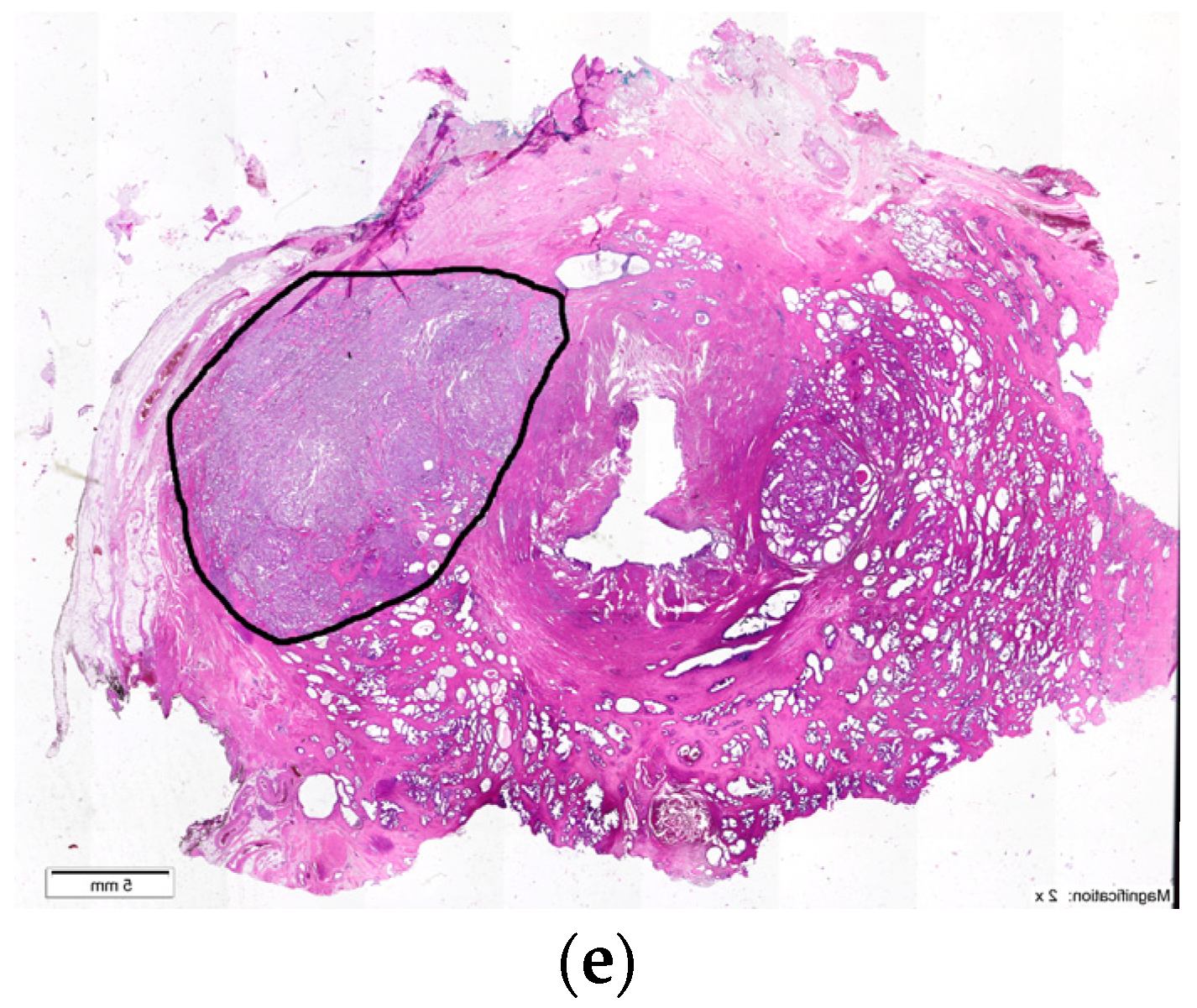
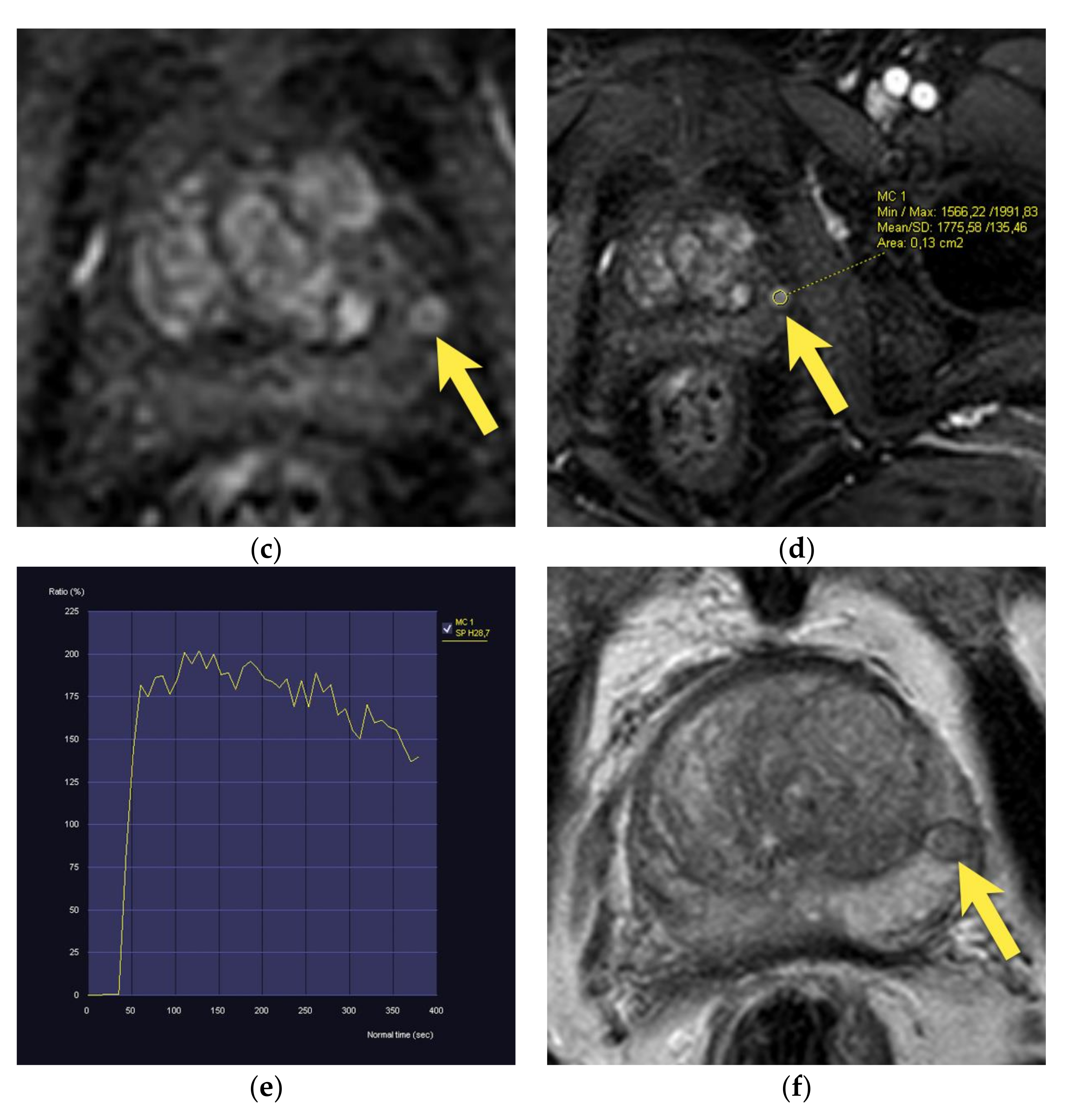
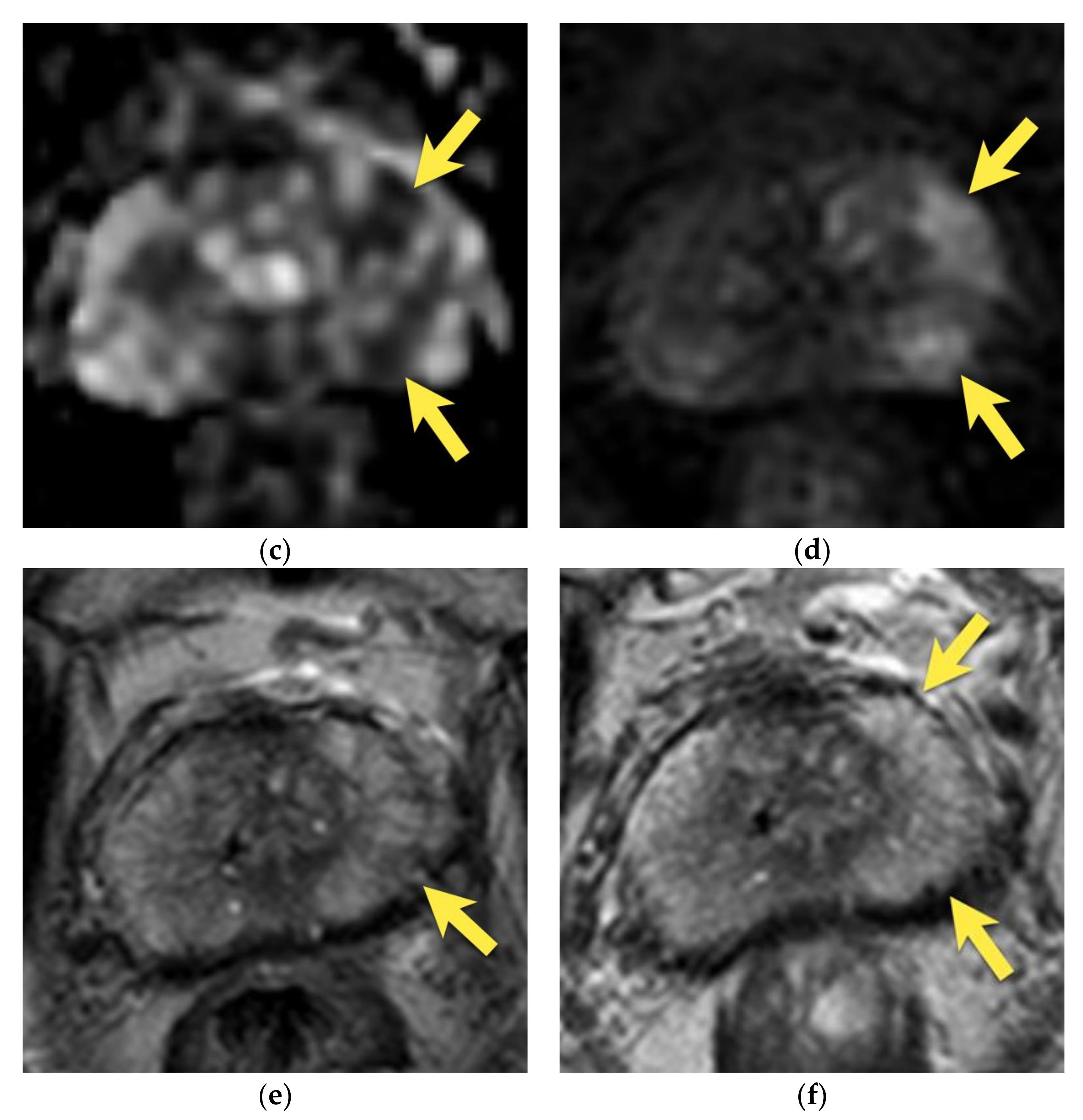
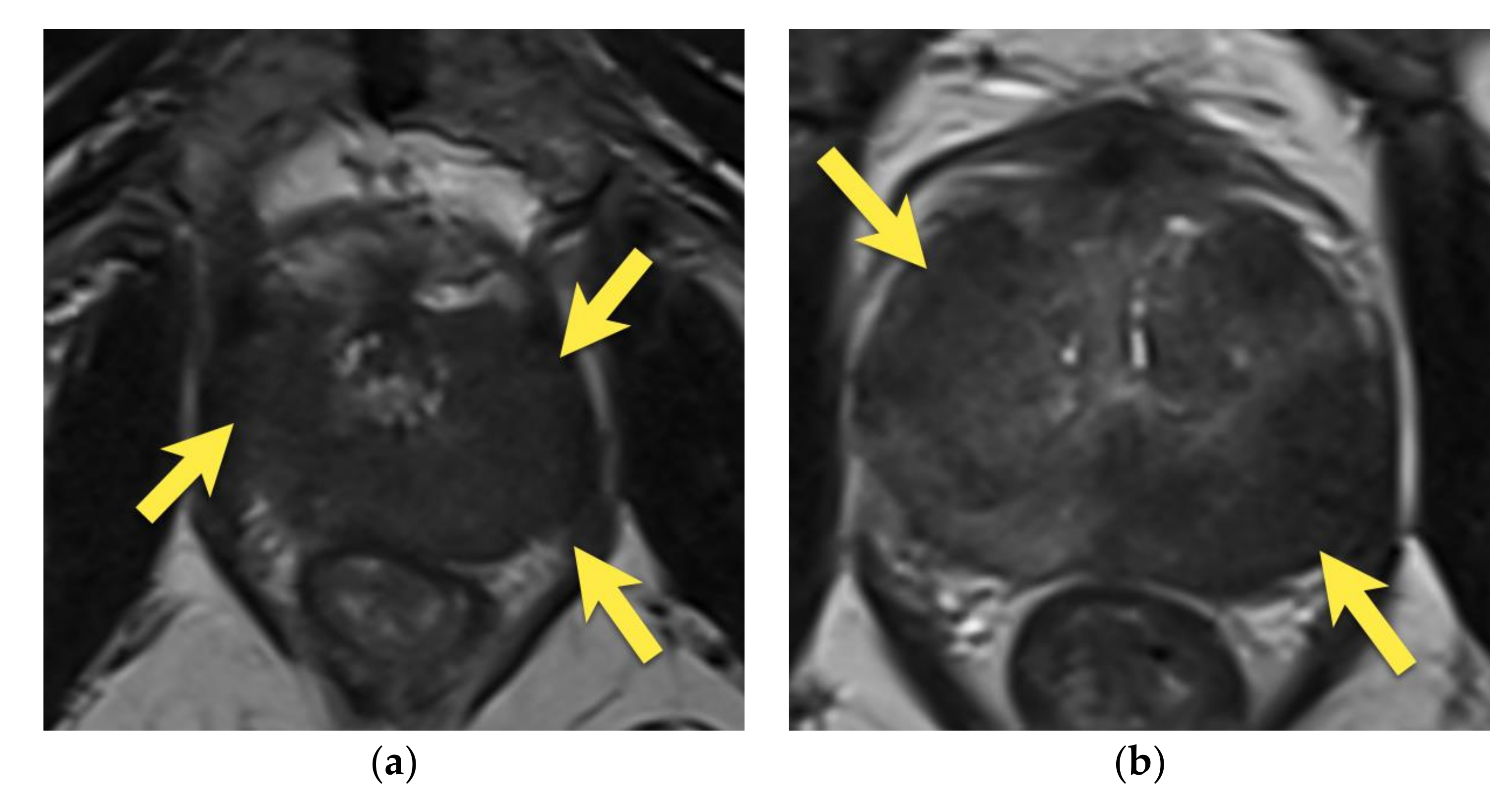
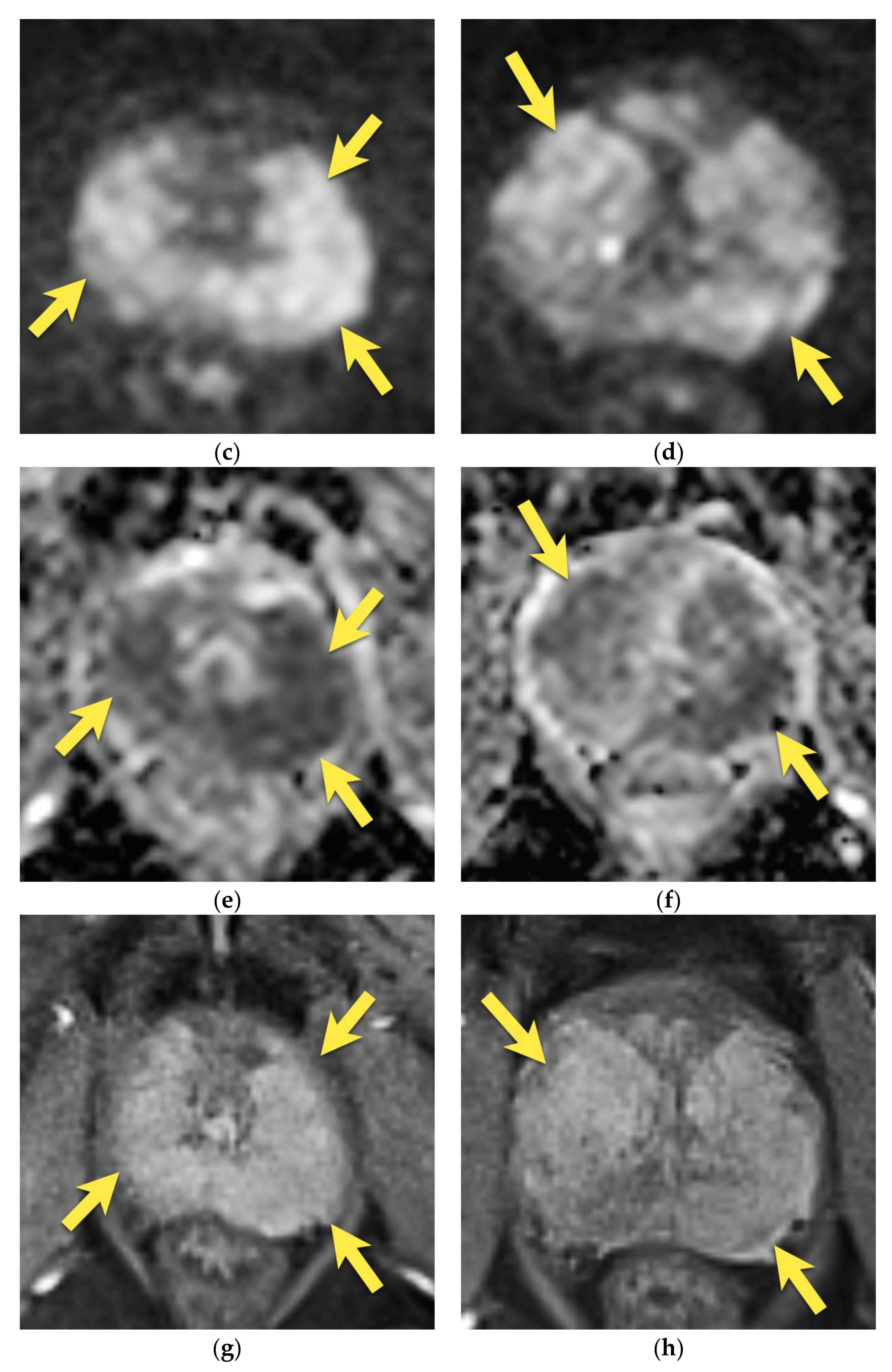
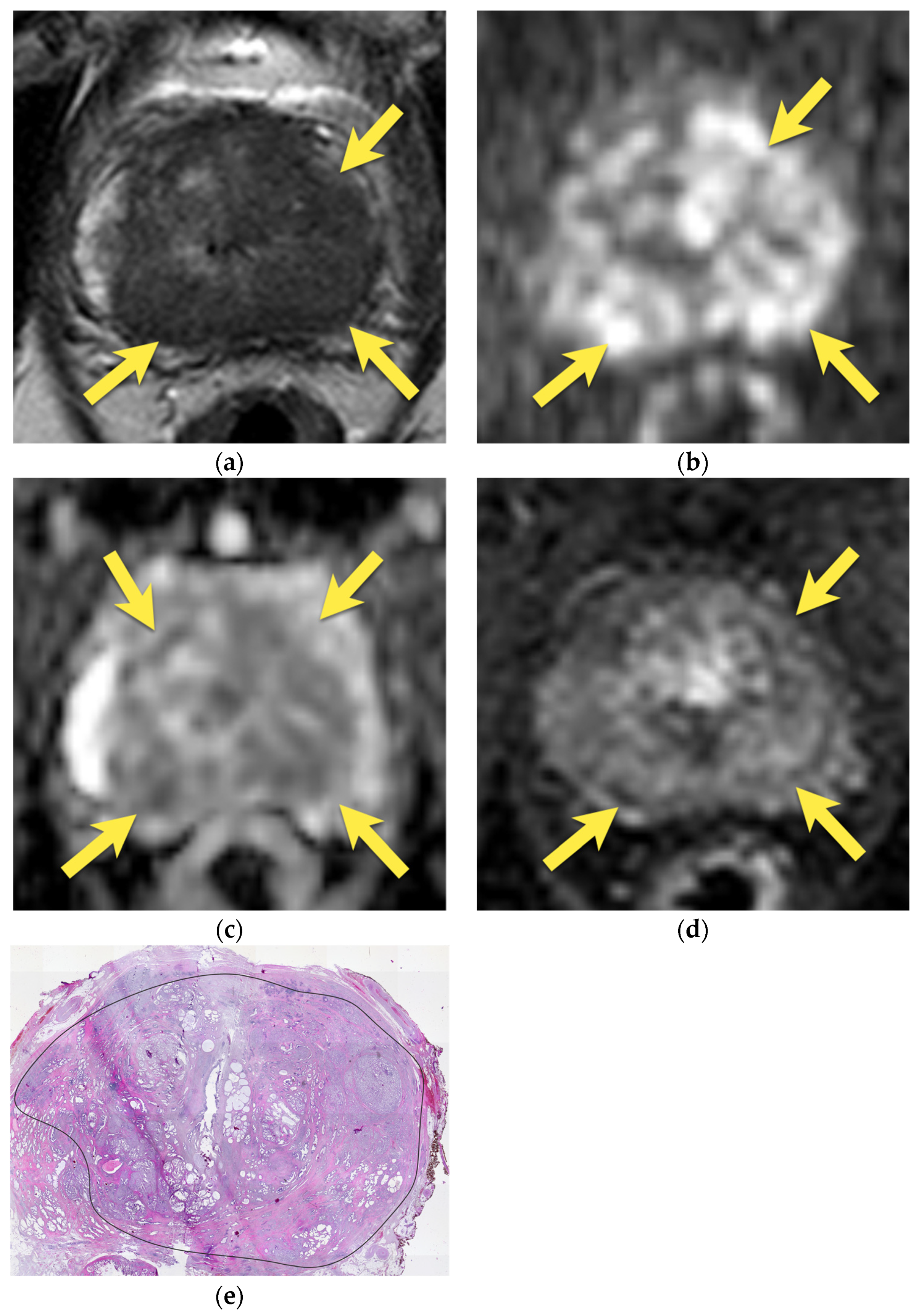
6. Transition Zone—Benign Prostatic Hyperplasia
7. Ectopic BPH Nodules
8. Post-Biopsy Hemorrhage
9. Prostatitis
10. Prostatic Abscess
11. Quality of Images
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Rouvière, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef]
- Drost, F.H.; Osses, D.F.; Nieboer, D.; Steyerberg, E.W.; Bangma, C.H.; Roobol, M.J.; Schotts, I.G. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst. Rev. 2019, 4, CD012663. [Google Scholar] [CrossRef]
- van der Leest, M.; Cornel, E.; Israël, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Setiasti, A.Y.; Veltman, J.; et al. Head-to-head Comparison of Transrectal Ultrasound-guided Prostate Biopsy Versus Multiparametric Prostate Resonance Imaging with Subsequent Magnetic Resonance-guided Biopsy in Biopsy-naïve Men with Elevated Prostate-specific Antigen: A Large Prospective Mu. Eur. Urol. 2019, 75, 570–578. [Google Scholar] [CrossRef]
- Klotz, L.; Chin, J.; Black, P.C.; Finelli, A.; Anidjar, M.; Bladou, F.; Mercado, A.; Levental, M.; Ghai, S.; Chang, S.D.; et al. Comparison of Multiparametric Magnetic Resonance Imaging-Targeted Biopsy with Systematic Transrectal Ultrasonography Biopsy for Biopsy-Naive Men at Risk for Prostate Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 534–542. [Google Scholar] [CrossRef]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Expert Patient Advocate (European Prostate Cancer Coalition/Europa UOMO); Eberli, D.; De Meerleer, G.; De Santis, M.; Gillessen, S.; Grummet, J.; et al. Guidelines on Prostate Cancer. EAU Guidel. EAU Guidel Edn Present EAU Annu Congr Milan 2023. 2023. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 30 April 2022).
- Stolk, T.T.; de Jong, I.J.; Kwee, T.C.; Luiting, H.B.; Mahesh, S.V.K.; Doornweerd, B.H.J.; Willemse, P.P.M.; Yakar, D. False positives in PIRADS (V2) 3, 4, and 5 lesions: Relationship with reader experience and zonal location. Abdom. Radiol. 2019, 44, 1044–1051. [Google Scholar] [CrossRef]
- Mehralivand, S.; Bednarova, S.; Shih, J.H.; Mertan, F.V.; Gaur, S.; Merino, M.J.; Wood, B.J.; Pinto, P.A.; Choyke, P.L.; Turkbey, B. Prospective Evaluation of PI-RADSTM Version 2 Using the International Society of Urological Pathology Prostate Cancer Grade Group System. J. Urol. 2017, 198, 583–590. [Google Scholar] [CrossRef]
- Mcneal, J.E. The Zonal Anatomy of The prostate. Prostate 1981, 2, 35–49. [Google Scholar] [CrossRef]
- ACR. PI-RADS ® v2.1 PI-RADS ® Prostate Imaging-Reporting and Data System 2019 Version 2.1 PI-RADS ® Prostate Imaging-Reporting and Data System 2019 Version 2.1 ACKNOWLEDGEMENTS. Available online: http://www.acr.org/-/media/ACR/Files/RADS/Pi-RADS/PIRADS-V2-1.pdf?la=en (accessed on 1 April 2022).
- Cohen, R.J.; Shannon, B.A.; Phillips, M.; Moorin, R.E.; Wheeler, T.M.; Garrett, K.L. Central Zone Carcinoma of the Prostate Gland: A Distinct Tumor Type With Poor Prognostic Features. J. Urol. 2008, 179, 1762–1767. [Google Scholar] [CrossRef]
- Vargas, H.A.; Akin, O.; Franiel, T.; Goldman, D.A.; Udo, K.; Touijer, K.A.; Reuter, V.E.; Hricak, H. Normal central zone of the prostate and central zone involvement by prostate cancer: Clinical and mr imaging implications. Radiology 2012, 262, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, V.; Giganti, F.; Kitzing, Y.X.; Cornud, F.; Campa, R.; De Rubeis, G.; Ciardi, A.; Catalano, C.; Villeirs, G. An update of pitfalls in prostate mpMRI: A practical approach through the lens of PI-RADS v. 2 guidelines. Insights Imaging 2018, 9, 87–101. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Taneja, S.S. Radiologist, be aware: Ten pitfalls that confound the interpretation of multiparametric prostate MRI. Am. J. Roentgenol. 2014, 202, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Thomas, S.; Oto, A. Prostate MR: Pitfalls and benign lesions. Abdom. Radiol. 2020, 45, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, J.C.; Joan, C.; Catalá, V.; Algaba, F.; Laucirica, O. Atlas of Multiparametric Prostate MRI: With PI-RADS Approach and Anatomic-MRI-Pathological Correlation; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef]
- Kiyoshima, K.; Yokomizo, A.; Yoshida, T.; Tomita, K.; Yonemasu, H.; Nakamura, M.; Oda, Y.; Naito, S.; Hasegawa, Y. Anatomical features of periprostatic tissue and its surroundings: A histological analysis of 79 radical retropubic prostatectomy specimens. Jpn. J. Clin. Oncol. 2004, 34, 463–468. [Google Scholar] [CrossRef]
- Panebianco, V.; Barchetti, F.; Barentsz, J.; Ciardi, A.; Cornud, F.; Futterer, J.; Villeirs, G. Pitfalls in Interpreting mp-MRI of the Prostate: A Pictorial Review with Pathologic Correlation. Insights Imaging 2015, 6, 611–630. [Google Scholar] [CrossRef]
- Bouyé, S.; Potiron, E.; Puech, P.; Leroy, X.; Lemaitre, L.; Villers, A. Transition zone and anterior stromal prostate cancers: Zone of origin and intraprostatic patterns of spread at histopathology. Prostate 2009, 69, 105–113. [Google Scholar] [CrossRef]
- Poon, P.Y.; Bronskill, M.J.; Poon, C.S.; McCallum, R.W.; Bruce, A.W.; Henkelman, R.M. Identification of the periprostatic venous plexus by mr imaging. J. Comput. Assist. Tomogr. 1991, 15, 265–268. [Google Scholar] [CrossRef]
- Allen, K.S.; Kressel, H.Y.; Arger, P.H.; Pollack, H.M. Age-related changes of the prostate: Evaluation by MR imaging. Am. J. Roentgenol. 1989, 152, 77–81. [Google Scholar] [CrossRef]
- Kitzing, Y.X.; Prando, A.; Varol, C.; Karczmar, G.S.; Maclean, F.; Oto, A. Benign conditions that mimic prostate carcinoma: MR imaging features with histopathologic correlation. Radiographics 2016, 36, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Hansford, B.G.; Karademir, I.; Peng, Y.; Jiang, Y.; Karczmar, G.; Thomas, S.; Yousuf, A.; Antic, T.; Eggener, S.; Oto, A. Dynamic contrast-enhanced MR imaging features of the normal central zone of the prostate. Acad Radiol. 2014, 21, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Grossfeld, G.D.; Coakley, F.V. Benign prostatic hyperplasia: Clinical overview and value of diagnostic imaging. Radiol. Clin. N. Am. 2000, 38, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Gallan, A.J.; He, D.; Fan, X.; Mustafi, D.; Yousuf, A.; Antic, T.; Karczmar, G.S.; Oto, A. Revisiting quantitative multi-parametric MRI of benign prostatic hyperplasia and its differentiation from transition zone cancer. Abdom. Radiol. 2019, 44, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Turkbey, B.; Muradyan, N.; Rajesh, A.; Cornud, F.; Haider, M.A.; Choyke, P.L.; Harisinghani, M. Overview of Dynamic Contrast-Enhanced MRI in Prostate Cancer Diagnosis and Management. Am. J. Roentgenol. 2012, 198, 1277–1288. [Google Scholar] [CrossRef]
- Eusebi, L.; Carpagnano, F.A.; Sortino, G.; Bartelli, F.; Guglielmi, G. Prostate Multiparametric MRI: Common Pitfalls in Primary Diagnosis and How to Avoid Them. Curr. Radiol. Rep. 2021, 9, 3. [Google Scholar] [CrossRef]
- Sharif-Afshar, A.R.; Feng, T.; Koopman, S.; Nguyen, C.; Li, Q.; Shkolyar, E.; Saouaf, R.; Kim, H.L. Impact of post prostate biopsy hemorrhage on multiparametric magnetic resonance imaging. Can. J. Urol. 2015, 22, 7698–7702. [Google Scholar]
- Qayyum, A.; Coakley, F.V.; Lu, Y.; Olpin, J.D.; Wu, L.; Yeh, B.M.; Carroll, P.R.; Kurhanewicz, J. Organ-confined prostate cancer: Effect of prior transrectal biopsy on endorectal MRI and MR spectroscopic imaging. Am. J. Roentgenol. 2004, 183, 1079–1083. [Google Scholar] [CrossRef]
- Purysko, A.S.; Herts, B.R. Prostate MRI: The hemorrhage exclusion sign. J. Urol. 2012, 188, 1946–1947. [Google Scholar] [CrossRef]
- Barrett, T.; Vargas, H.A.; Akin, O.; Goldman, D.A.; Hricak, H. Value of the hemorrhage exclusion sign on T1-weighted prostate MR images for the detection of prostate cancer. Radiology 2012, 263, 751–757. [Google Scholar] [CrossRef]
- Tamada, T.; Sone, T.; Jo, Y.; Yamamoto, A.; Yamashita, T.; Egashira, N.; Imai, S.; Fukunaga, M. Prostate cancer: Relationships between postbiopsy hemorrhage and tumor detectability at MR diagnosis. Radiology 2008, 248, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Coker, T.J.; Dierfeldt, D.M. Acute bacterial prostatitis: Diagnosis and management. Am. Fam. Physician 2016, 93, 114–120. [Google Scholar] [PubMed]
- Lipsky, B.A.; Byren, I.; Hoey, C.T. Treatment of bacterial prostatitis. Clin. Infect. Dis. 2010, 50, 1641–1652. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Salinas, R.C. Prostatitis: Acute and Chronic. Prim. Care Clin. Off. Pract. 2010, 37, 547–563. [Google Scholar] [CrossRef]
- Holt, J.D.; Garrett, W.A.; McCurry, T.K.; Teichman, J.M.H. Common questions about chronic prostatitis. Am. Fam. Physician 2016, 93, 290–296. [Google Scholar]
- Rourke, E.; Sunnapwar, A.; Mais, D.; Kukkar, V.; Digiovanni, J.; Kaushik, D.; Liss, M.A. Inflammation appears as high prostate imaging—Reporting and data system scores on prostate magnetic resonance imaging (MRI) leading to false positive mri fusion biopsy. Investig. Clin. Urol. 2019, 60, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.L.; Parameshwar, P.S.; Anger, J.T. Diagnosis and treatment of patients with prostatic abscess in the post-antibiotic era. Int. J. Urol. 2018, 25, 103–110. [Google Scholar] [CrossRef]
- Ren, J.; Huang, X.; Wang, H.; Liu, H.; Ren, F.; Zhang, Z.; Yang, Y.; Yin, H.; Huan, Y. Prostatic abscess and seminal vesicle abscess: MRI findings and quantitative analysis of apparent diffusion coefficient values. Radiol. Infect. Dis. 2015, 2, 27–32. [Google Scholar] [CrossRef]
- Stabile, A.; Giganti, F.; Rosenkrantz, A.B.; Taneja, S.S.; Villeirs, G.; Gill, I.S.; Allen, C.; Emberton, M.; Moore, C.M.; Kasivisvanathan, V. Multiparametric MRI for prostate cancer diagnosis: Current status and future directions. Nat. Rev. Urol. 2020, 17, 41–61. [Google Scholar] [CrossRef]
- Giganti, F.; Kirkham, A.; Kasivisvanathan, V.; Papoutsaki, M.V.; Punwani, S.; Emberton, M.; Moore, C.M.; Allen, C. Understanding PI-QUAL for prostate MRI quality: A practical primer for radiologists. Insights Imaging 2021, 12, 59. [Google Scholar] [CrossRef]
- Purysko, A.S.; Mielke, N.; Bullen, J.; Nachand, D.; Rizk, A.; Stevens, E.; Ward, R.D.; Klein, E.A.; Rosenkrantz, A.B.; Ream, J.M. Influence of Enema and Dietary Restrictions on Prostate MR Image Quality: A Multireader Study. Acad Radiol. 2022, 29, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.L.; Lazarakis, S.; Lee, A.; Tan, T.H.; Chin, K.Y.; Oon, S.F. Do antispasmodics or rectal enemas improve image quality on multiparametric prostate MRI? An ‘Evidence-Based Practice’ review of the literature. Abdom. Radiol. 2021, 46, 2770–2778. [Google Scholar] [CrossRef] [PubMed]
- Caglic, I.; Hansen, N.L.; Slough, R.A.; Patterson, A.J.; Barrett, T. Evaluating the effect of rectal distension on prostate multiparametric MRI image quality. Eur. J. Radiol. 2017, 90, 174–180. [Google Scholar] [CrossRef] [PubMed]






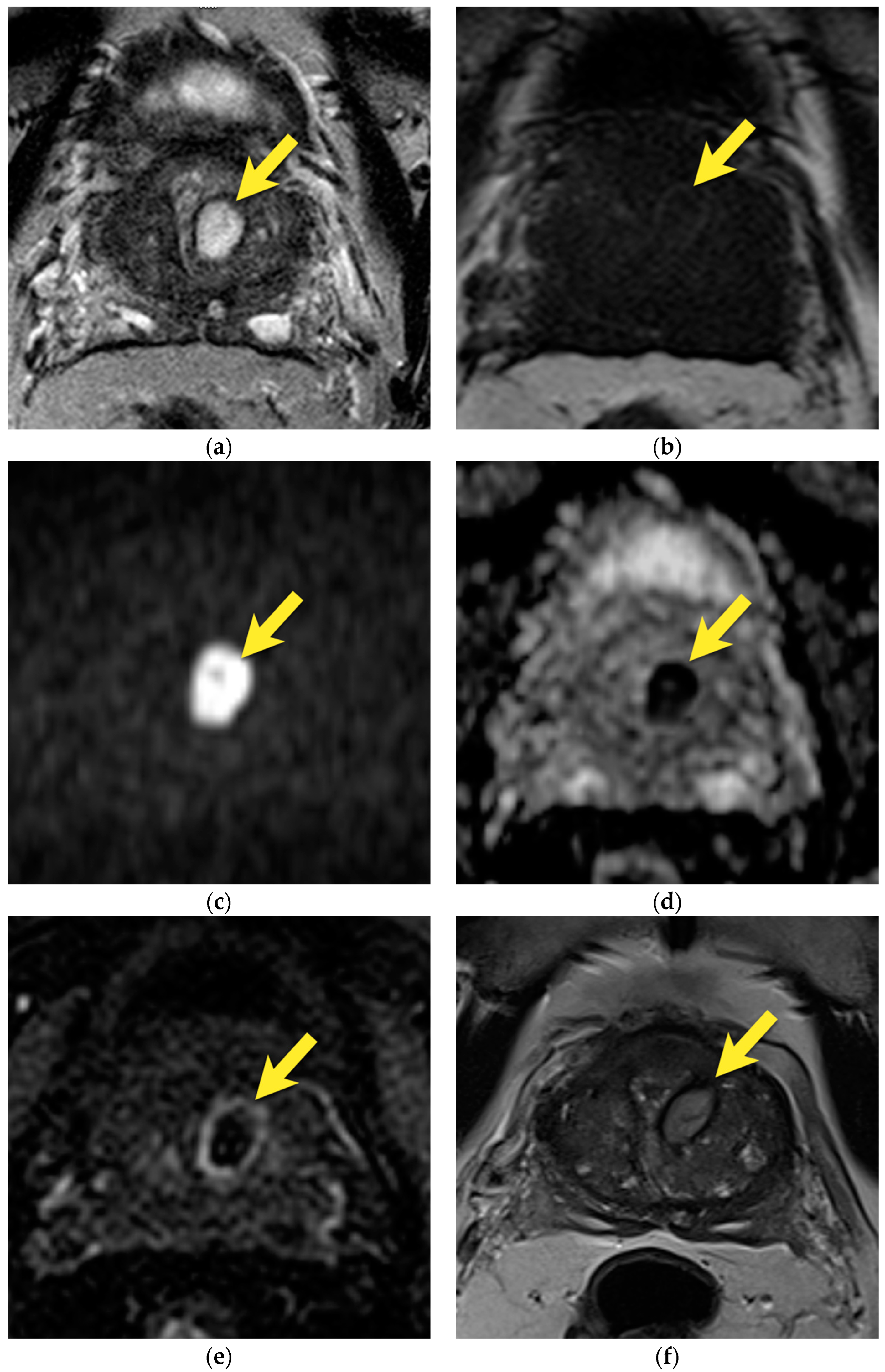
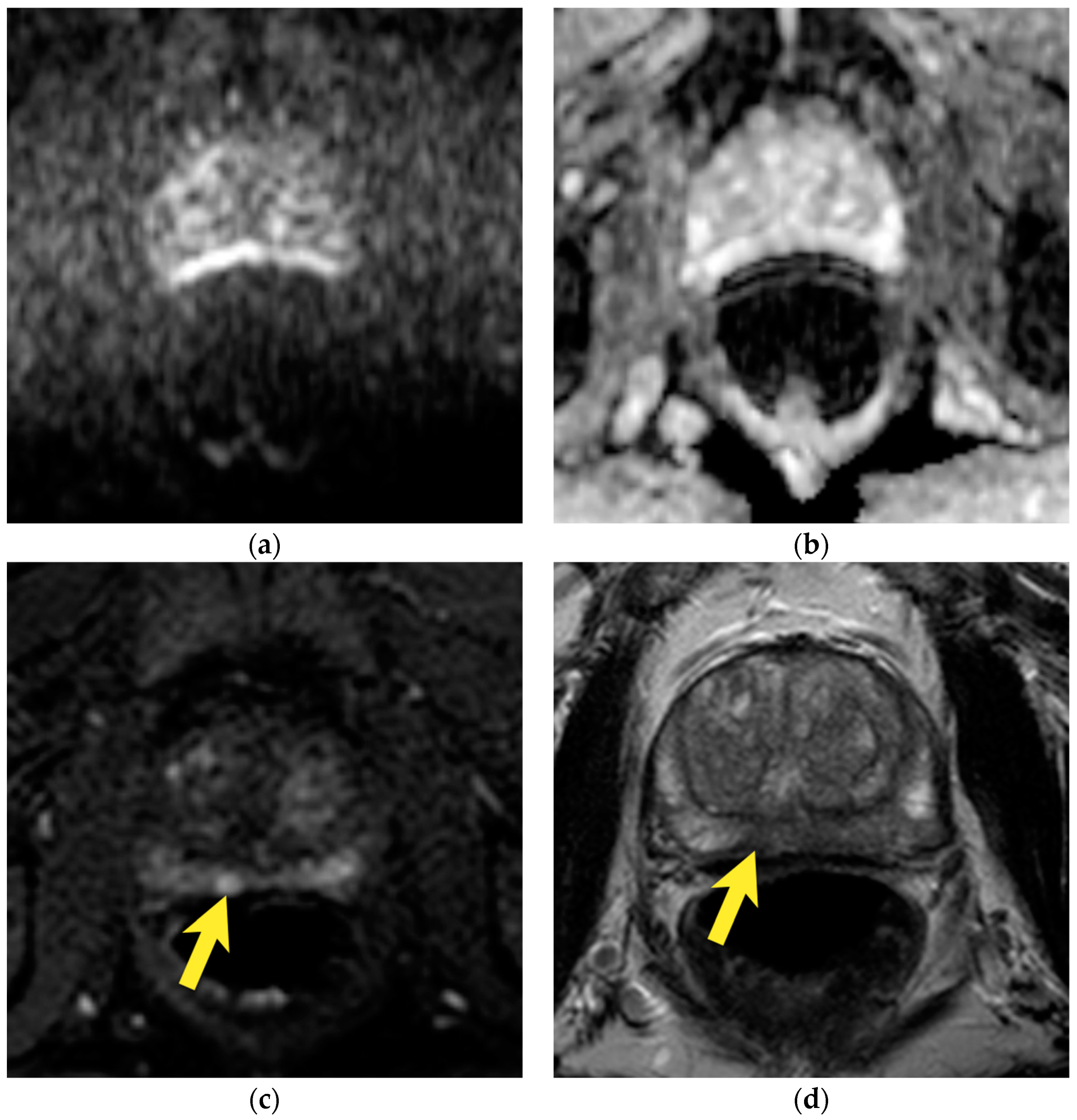
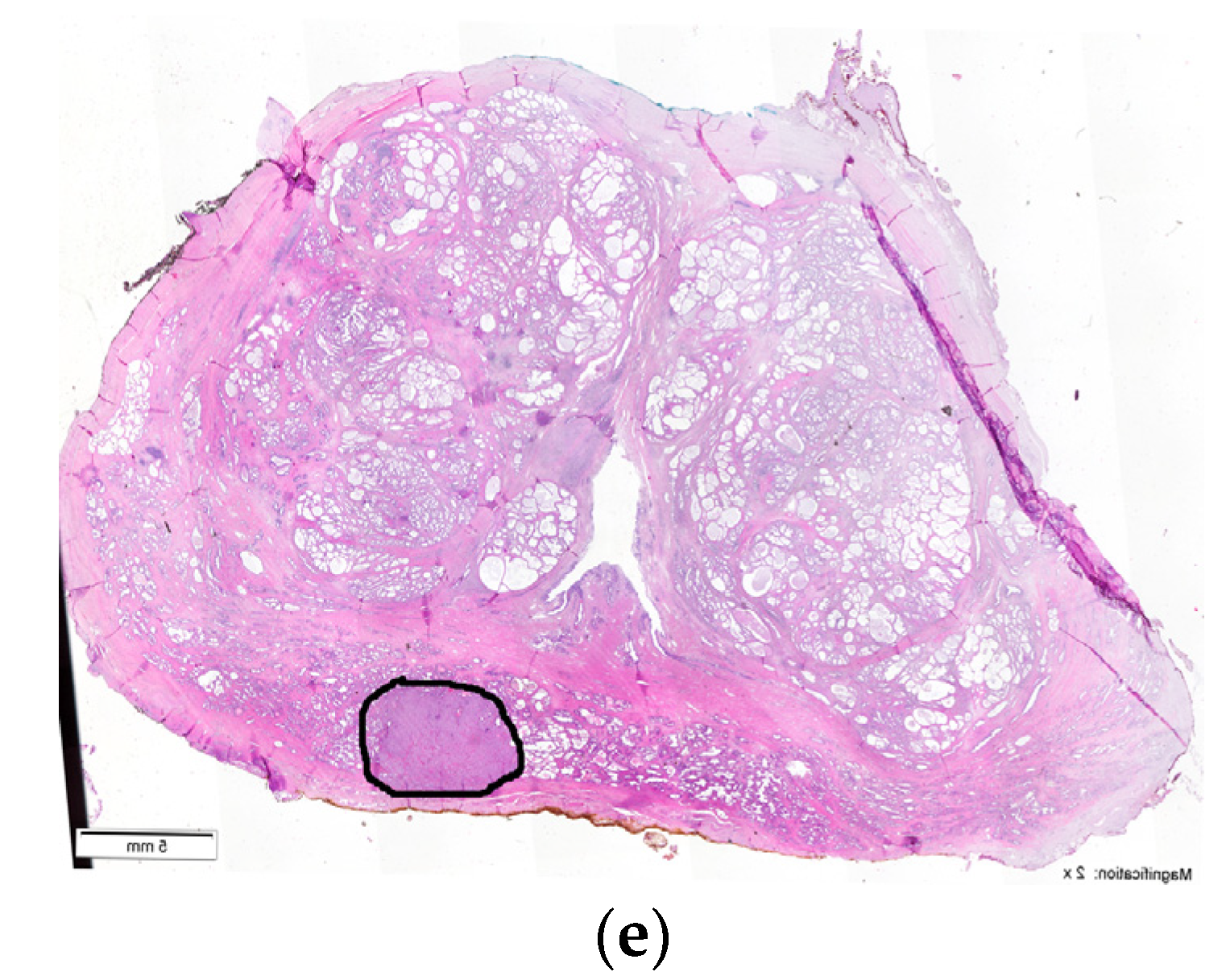
| Type | Location | |
|---|---|---|
| Anatomic structures | Anterior fibromuscular stroma | Anterior prostate |
| Central zone | Base of the prostate and posterior middle peripheral zone | |
| Periprostatic venous plexus | Peripheral zone | |
| Benign pathology | Stromal BPH nodule | Transition zone and occasionally the peripheral zone (as ectopic BPH nodule) |
| Ectopic BPH nodule | Peripheral zone | |
| Diffuse and focal prostatitis | Peripheral zone is more often involved, adjacent transition zone may also be affected | |
| Abscess | Peripheral or transition zone | |
| Hemorrhage | Peripheral or transition zone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żurowska, A.; Pęksa, R.; Bieńkowski, M.; Skrobisz, K.; Sowa, M.; Matuszewski, M.; Biernat, W.; Szurowska, E. Prostate Cancer and Its Mimics—A Pictorial Review. Cancers 2023, 15, 3682. https://doi.org/10.3390/cancers15143682
Żurowska A, Pęksa R, Bieńkowski M, Skrobisz K, Sowa M, Matuszewski M, Biernat W, Szurowska E. Prostate Cancer and Its Mimics—A Pictorial Review. Cancers. 2023; 15(14):3682. https://doi.org/10.3390/cancers15143682
Chicago/Turabian StyleŻurowska, Anna, Rafał Pęksa, Michał Bieńkowski, Katarzyna Skrobisz, Marek Sowa, Marcin Matuszewski, Wojciech Biernat, and Edyta Szurowska. 2023. "Prostate Cancer and Its Mimics—A Pictorial Review" Cancers 15, no. 14: 3682. https://doi.org/10.3390/cancers15143682
APA StyleŻurowska, A., Pęksa, R., Bieńkowski, M., Skrobisz, K., Sowa, M., Matuszewski, M., Biernat, W., & Szurowska, E. (2023). Prostate Cancer and Its Mimics—A Pictorial Review. Cancers, 15(14), 3682. https://doi.org/10.3390/cancers15143682







