Radiological Biomarkers in MRI directed Rectal Cancer Radiotherapy Volume Delineation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Tumour Demographics
2.2. Chemoradiotherapy Procedure
2.3. MRI Proforma
2.4. Target Volume Delineation and Data Collection
2.5. Statistical Analysis
3. Results
3.1. Initial Evaluation of Radiotherapy Treatment Volumes Using an Adjusted MRI Reporting Proforma
3.1.1. Patient and Tumour Demographics
3.1.2. Comparison of Volumes Using Adjusted MRI Reporting with Historical Radiotherapy Volumes
3.2. Re-Evaluation following Routine Use of Adjusted MRI Reporting
3.2.1. Patient Demographics
3.2.2. Retrospective Assessment of GTV Delineation by GI Radiologists
3.3. MRI and Histopathological Response Assessment following CRT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.F.; Collette, L.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Ollier, J.C.; et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006, 355, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.P.; Conroy, T.; Bonnetain, F.; Bouche, O.; Chapet, O.; Closon-Dejardin, M.T.; Untereiner, M.; Leduc, B.; Francois, E.; Maurel, J.; et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: Results of FFCD 9203. J. Clin. Oncol. 2006, 24, 4620–4625. [Google Scholar] [CrossRef]
- Maas, M.; Beets-Tan, R.G.; Lambregts, D.M.; Lammering, G.; Nelemans, P.J.; Engelen, S.M.; van Dam, R.M.; Jansen, R.L.; Sosef, M.; Leijtens, J.W.; et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J. Clin. Oncol. 2011, 29, 4633–4640. [Google Scholar] [CrossRef]
- Rödel, C.; Martus, P.; Papadoupolos, T.; Füzesi, L.; Klimpfinger, M.; Fietkau, R.; Liersch, T.; Hohenberger, W.; Raab, R.; Sauer, R.; et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J. Clin. Oncol. 2005, 23, 8688–8696. [Google Scholar] [CrossRef] [PubMed]
- de Campos-Lobato, L.F.; Stocchi, L.; da Luz Moreira, A.; Geisler, D.; Dietz, D.W.; Lavery, I.C.; Fazio, V.W.; Kalady, M.F. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann. Surg. Oncol. 2011, 18, 1590–1598. [Google Scholar] [CrossRef]
- Park, I.J.; You, Y.N.; Agarwal, A.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Eng, C.; Feig, B.W.; Das, P.; Krishnan, S.; Crane, C.H.; et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. Journal of clinical oncology. J. Clin. Oncol. 2012, 30, 1770–1776. [Google Scholar] [CrossRef]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rodel, C.; Kuo, L.J.; Calvo, F.A.; Garcia-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- O’Neill, B.D.; Brown, G.; Heald, R.J.; Cunningham, D.; Tait, D.M. Non-operative treatment after neoadjuvant chemoradiotherapy for rectal cancer. Lancet Oncol. 2007, 8, 625–633. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; Silva e Sousa, A.H.; Campos, F.G., Jr.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Beets, G.L. Critical appraisal of the ‘wait and see’ approach in rectal cancer for clinical complete responders after chemoradiation. Br. J. Surg. 2012, 99, 910. [Google Scholar] [CrossRef]
- Appelt, A.L.; Ploen, J.; Harling, H.; Jensen, F.S.; Jensen, L.H.; Jorgensen, J.C.; Lindebjerg, J.; Rafaelsen, S.R.; Jakobsen, A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: A prospective observational study. Lancet Oncol. 2015, 16, 919–927. [Google Scholar] [CrossRef]
- Hall, M.D.; Schultheiss, T.E.; Smith, D.D.; Fakih, M.G.; Wong, J.Y.; Chen, Y.J. Effect of increasing radiation dose on pathologic complete response in rectal cancer patients treated with neoadjuvant chemoradiation therapy. Acta Oncol. 2016, 55, 1392–1399. [Google Scholar] [CrossRef]
- Sanghera, P.; Wong, D.W.; McConkey, C.C.; Geh, J.I.; Hartley, A. Chemoradiotherapy for rectal cancer: An updated analysis of factors affecting pathological response. Clin. Oncol. 2008, 20, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Appelt, A.L.; Ploen, J.; Vogelius, I.R.; Bentzen, S.M.; Jakobsen, A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 74–80. [Google Scholar] [CrossRef]
- Chan, A.K.; Wong, A.O.; Langevin, J.; Jenken, D.; Heine, J.; Buie, D.; Johnson, D.R. Preoperative chemotherapy and pelvic radiation for tethered or fixed rectal cancer: A phase II dose escalation study. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Coco, C.; Cellini, N.; Picciocchi, A.; Fares, M.C.; Rosetto, M.E.; Mantini, G.; Morganti, A.G.; Barbaro, B.; Cogliandolo, S.; et al. Ten years of preoperative chemoradiation for extraperitoneal T3 rectal cancer: Acute toxicity, tumor response, and sphincter preservation in three consecutive studies. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, K.L.; Ward, I.G.; Swallow, C.; Oza, A.M.; Cummings, B.; Pond, G.R.; Catton, P.; Kim, J.; Ringash, J.; Wong, C.S.; et al. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: Effect of dose escalation on pathologic complete response, local recurrence-free survival, disease-free survival, and overall survival. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Arcadipane, F.; Trino, E.; Gallio, E.; Martini, S.; Iorio, G.C.; Piva, C.; Moretto, F.; Ruo Redda, M.G.; Verna, R.; et al. Variability of clinical target volume delineation for rectal cancer patients planned for neoadjuvant radiotherapy with the aid of the platform Anatom-e. Clin. Transl. Radiat. Oncol. 2018, 11, 33–39. [Google Scholar] [CrossRef]
- Rosa, C.; Caravatta, L.; Delli Pizzi, A.; Di Tommaso, M.; Cianci, R.; Gasparini, L.; Perrotti, F.; Solmita, J.; Sartori, S.; Zecca, I.A.L.; et al. Reproducibility of rectal tumor volume delineation using diffusion-weighted MRI: Agreement on volumes between observers. Cancer Radiother. 2019, 23, 216–221. [Google Scholar] [CrossRef]
- Owens, R.; Mukherjee, S.; Padmanaban, S.; Hawes, E.; Jacobs, C.; Weaver, A.; Betts, M.; Muirhead, R. Intensity-Modulated Radiotherapy with a Simultaneous Integrated Boost in Rectal Cancer. Clin. Oncol. 2020, 32, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Gambacorta, M.A.; Barbaro, B.; Chiloiro, G.; Coco, C.; Das, P.; Fanfani, F.; Joye, I.; Kachnic, L.; Maingon, P.; et al. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother. Oncol. 2016, 120, 195–201. [Google Scholar] [CrossRef]
- Iafrate, F.; Ciccarelli, F.; Masci, G.M.; Grasso, D.; Marruzzo, F.; De Felice, F.; Tombolini, V.; D’Ambrosio, G.; Magliocca, F.M.; Cortesi, E.; et al. Predictive role of diffusion-weighted MRI in the assessment of response to total neoadjuvant therapy in locally advanced rectal cancer. Eur. Radiol. 2023, 33, 854–862. [Google Scholar] [CrossRef]
- Ciolina, M.; Caruso, D.; De Santis, D.; Zerunian, M.; Rengo, M.; Alfieri, N.; Musio, D.; De Felice, F.; Ciardi, A.; Tombolini, V.; et al. Dynamic contrast-enhanced magnetic resonance imaging in locally advanced rectal cancer: Role of perfusion parameters in the assessment of response to treatment. Radiol. Med. 2019, 124, 331–338. [Google Scholar] [CrossRef]
- De Felice, F.; Magnante, A.L.; Musio, D.; Ciolina, M.; De Cecco, C.N.; Rengo, M.; Laghi, A.; Tombolini, V. Diffusion-weighted magnetic resonance imaging in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. European journal of surgical oncology. Eur. J. Surg. Oncol. 2017, 43, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Talbot, I.C.; Ritchie, S.; Leighton, M.H.; Hughes, A.O.; Bussey, H.J.; Morson, B.C. The clinical significance of invasion of veins by rectal cancer. Br. J. Surg. 1980, 67, 439–442. [Google Scholar] [CrossRef]
- Bokey, E.L.; Chapuis, P.H.; Dent, O.F.; Newland, R.C.; Koorey, S.G.; Zelas, P.J.; Stewart, P.J. Factors affecting survival after excision of the rectum for cancer: A multivariate analysis. Dis. Colon Rectum 1997, 40, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Betge, J.; Pollheimer, M.J.; Lindtner, R.A.; Kornprat, P.; Schlemmer, A.; Rehak, P.; Vieth, M.; Hoefler, G.; Langner, C. Intramural and extramural vascular invasion in colorectal cancer: Prognostic significance and quality of pathology reporting. Cancer 2012, 118, 628–638. [Google Scholar] [CrossRef]
- Chand, M.; Bhangu, A.; Wotherspoon, A.; Stamp, G.W.H.; Swift, R.I.; Chau, I.; Tekkis, P.P.; Brown, G. EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann. Oncol. 2014, 25, 858–863. [Google Scholar] [CrossRef]
- Siddiqui, M.R.S.; Simillis, C.; Hunter, C.; Chand, M.; Bhoday, J.; Garant, A.; Vuong, T.; Artho, G.; Rasheed, S.; Tekkis, P.; et al. A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases. Br. J. Cancer 2017, 116, 1513–1519. [Google Scholar] [CrossRef]
- Myerson, R.J.; Garofalo, M.C.; El Naqa, I.; Abrams, R.A.; Apte, A.; Bosch, W.R.; Das, P.; Gunderson, L.L.; Hong, T.S.; Kim, J.J.; et al. Elective clinical target volumes for conformal therapy in anorectal cancer: A radiation therapy oncology group consensus panel contouring atlas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 824–830. [Google Scholar] [CrossRef] [PubMed]
- The Royal College of Radiologists. National Rectal Cancer Intensity-Modulated Radiotherapy (IMRT) Guidance. Clinical Oncology. 2021. Available online: https://www.rcr.ac.uk/publication/national-rectal-cancer-intensity-modulated-radiotherapy-imrt-guidance (accessed on 13 October 2023).
- Sclafani, F.; Brown, G.; Cunningham, D.; Wotherspoon, A.; Mendes, L.S.T.; Balyasnikova, S.; Evans, J.; Peckitt, C.; Begum, R.; Tait, D.; et al. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br. J. Cancer 2017, 117, 1478–1485. [Google Scholar] [CrossRef] [PubMed]


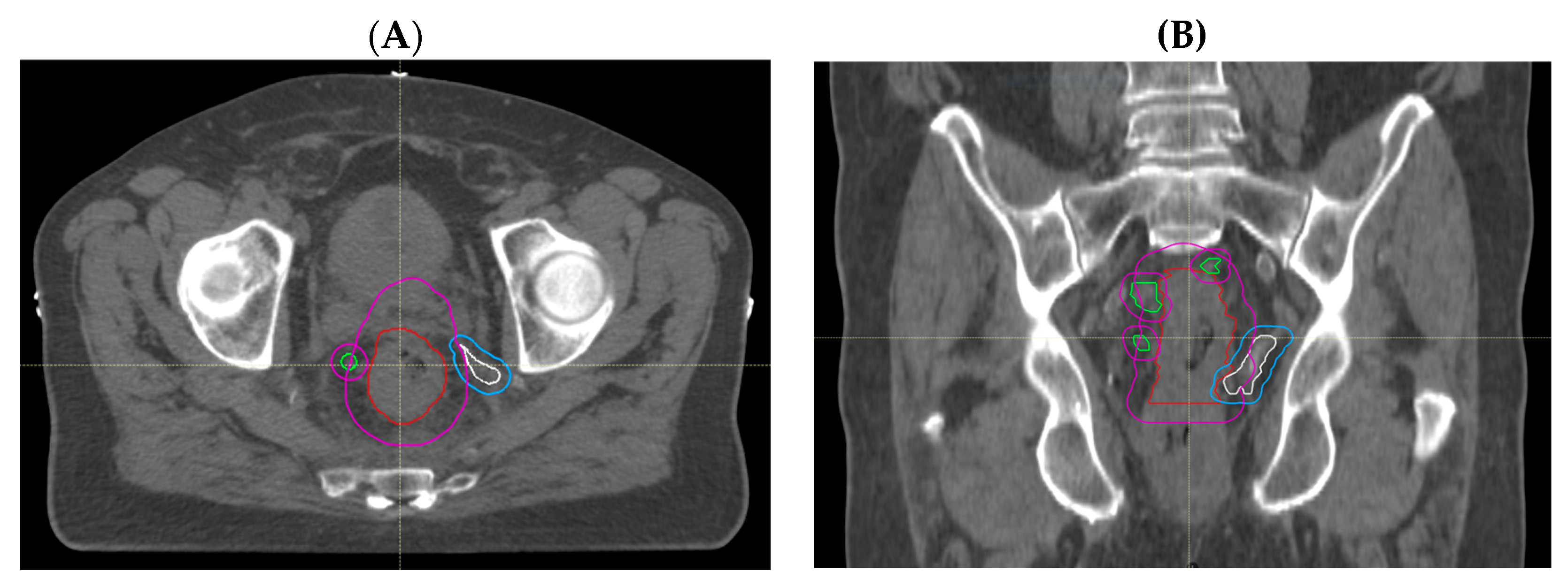
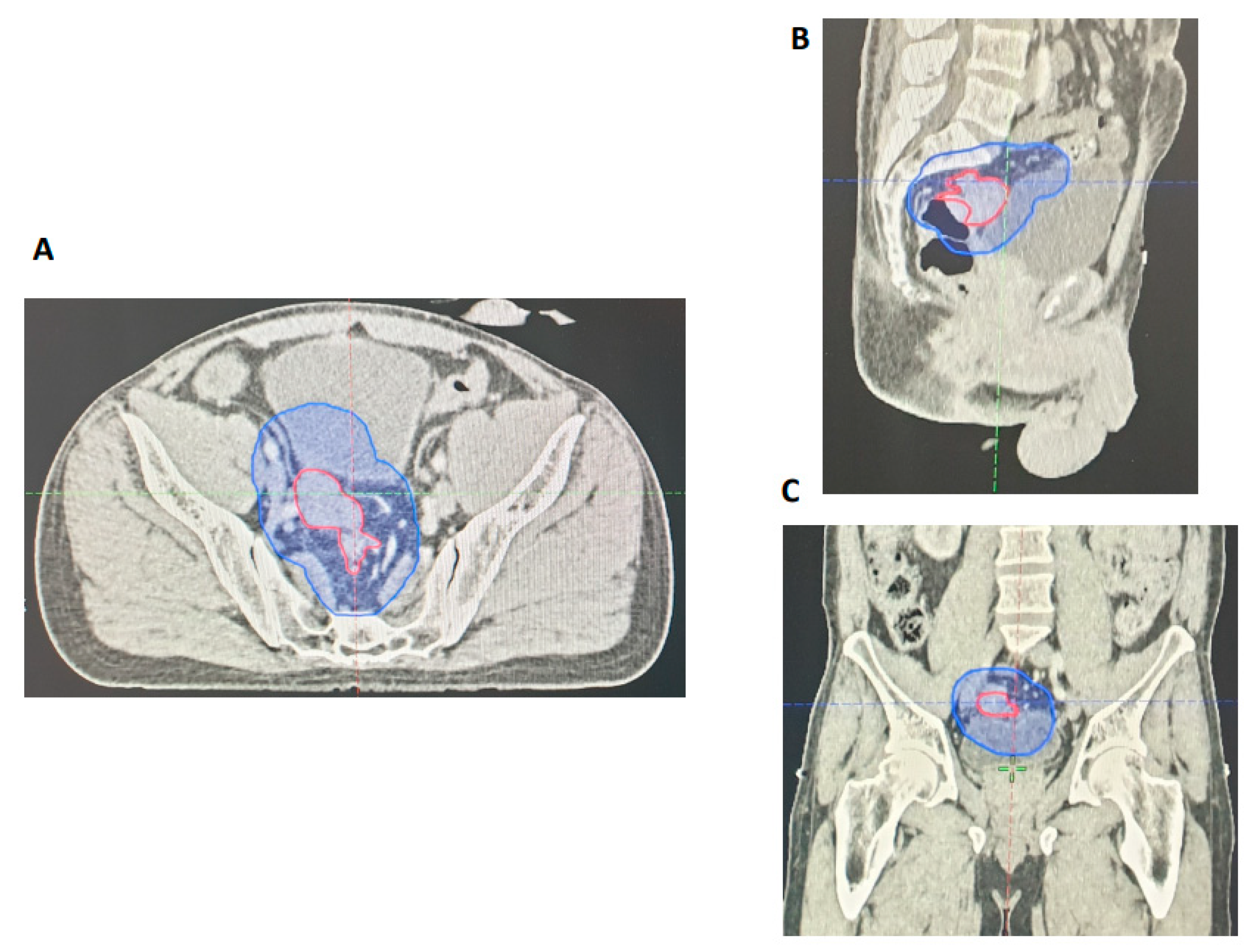
| Demographic or Clinical Characteristic | All Treated Patients (n = 27) N (%) | CRM Involved (n = 27) N (%) | CRM Negative (n = 5) N (%) |
|---|---|---|---|
| Sex | |||
| Men | 21 (78) | 18 (82) | 3 (60) |
| Women | 6 (22) | 4 (18) | 2 (40) |
| Age, years | |||
| Median | 69 | 68.5 | 69 |
| Range | 54–83 | 54–82 | 57–83 |
| ECOG Performance Status | |||
| 0 | 13 (48) | 11 (50) | 2 (40) |
| 1 | 13 (48) | 11 (50) | 2 (40) |
| 2 | 1 (4) | 0 (0) | 1 (20) |
| MR TNM Stage | |||
| T2 | 1 (4) | 1 (5) | 0 (0) |
| T3b-d | 20 (74) | 15 (68) | 5 (100) |
| T4 | 6 (22) | 6 (27) | 0 (0) |
| N0 | 5 (19) | 5 (23) | 0 (0) |
| N1 | 20 (74) | 16 (60) | 4 (80) |
| N2 | 2 (7) | 1 (4) | 1 (20) |
| M0 | 25 (93) | 20 (91) | 5 (100) |
| M1 | 2 (liver) (7) | 2 (liver) (9) | 0 (0) |
| MR EMVI | |||
| Negative | 7 (26) | 5 (23) | 2 (40) |
| Positive | 20 (74) | 17 (77) | 3 (60) |
| Location * | |||
| Low | 14 (52) | 12 (55) | 2 (40) |
| Mid | 11 (41) | 8 (36) | 3 (60) |
| High | 2 (7) | 2 (9) | 0 (0) |
| Histopathology (Differentiation Grade) | |||
| Moderate | 23 (85) | 18 (82) | 5 (100) |
| Poor | 2 (7) | 2 (9) | 0 (0) |
| Not available | 2 (7) | 2 (9) | 0 (0) |
| Surgery performed | |||
| Yes | 18 (67) | 14 (64) | 4 (80) |
| No | 9 (33) Died during RT (n = 1) Declined surgery (n = 2) Deferral of surgery non-intervention arm of trial (n = 2) Disease progression (n = 1) Unfit for surgery (n = 1) CRT delivered for recurrence (n = 2) | 8 (36) Died during RT (n = 1) Declined surgery (n = 2) Deferral of surgery non-intervention arm of trial (n = 1) Disease progression (n = 1) Unfit for surgery (n = 1) CRT delivered for recurrence (n = 2) | 1 (20) Deferral of surgery non-intervention arm of trial (n = 1) |
| Demographic or Clinical Characteristic | All Treated Patients (n = 27) N (%) | CRM Involved (n = 23) N (%) | CRM Negative (n = 4) N (%) |
|---|---|---|---|
| Sex | |||
| Men | 15 (56) | 13 (57) | 2 (50) |
| Women | 12 (44) | 10 (43) | 2 (50) |
| Age, years | |||
| Median | 56 | 54 | 72.5 |
| Range | 33–83 | 33–83 | 63–79 |
| ECOG Performance Status | |||
| 0 | 18 (67) | 17 (74) | 1 (25) |
| 1 | 8 (30) | 5 (22) | 3 (75) |
| 2 | 1 (3) | 1 (4) | 0 (0) |
| MR TNM Stage | |||
| T2 | 1 (4) | 1 (5) | 0 (0) |
| T3b-d | 17 (63) | 13 (57) | 4 (100) |
| T4 | 9 (33) | 9 (39) | 0 (0) |
| N0 | 2 (7) | 2 (9) | 0 (0) |
| N1 | 25 (93) | 21 (91) | 4 (100) |
| N2 | 0 (0) | 0 (0) | 0 (0) |
| M0 | 26 (96) | 22 (96) | 4 (100) |
| M1 | 1 (liver) (4) | 1 (4) | 0 (0) |
| MR EMVI | |||
| Negative | 3 (11) | 3 (13) | 0 (0) |
| Positive | 24 (89) | 20 (87) | 4 (100) |
| Location * | |||
| Low | 13 (48) | 12 (52) | 1(25) |
| Mid | 10 (37) | 8 (35) | 2 (50) |
| High | 4 (15) | 3 (13) | 1 (25) |
| Histopathology (Differentiation Grade) | |||
| Moderate | 20 (74) | 17 (74) | 3 (75) |
| Poor | 7 (26) | 6 (26) | 1 (25) |
| Surgery Performed | |||
| Yes | 22 (81) | 18 (78) | 4 (100) |
| No | 5 (19) Declined surgery (n = 1) Solitary liver metastasis RR (n = 1) Awaiting completion of adjuvant chemotherapy (n = 1) TRIGGER (deferral of surgery arm) (n = 2) | 5 (22) Declined surgery (n = 1) Solitary liver metastases RR (n = 1) Awaiting completion of adjuvant chemotherapy (n = 1) TRIGGER (deferral of surgery arm) (n = 2) | 0 (0) |
| TRG Grading | 2012–2014 (n = 17) N (%) | 2016–2020 (n = 21) N (%) |
|---|---|---|
| TRG 1: No residual cancer | 5 (29) | 2 (10) |
| TRG 2: Rare residual cancer cells | 4 (24) | 8 (38) |
| TRG 3: Fibrosis outgrowing residual cancer | 7 (41) | 11 (52) |
| TRG 4: Residual cancer outgrowing fibrosis | 1 (6) | 0 (0) |
| TRG 5: Absence of regressive changes | 0 (0) | 0 (0) |
| TRG Grading | 2012–2014 (n = 24) N (%) | 2016–2020 (n = 25) N (%) |
|---|---|---|
| TRG 1: Complete radiological response | 1 (4) | 3 (12) |
| TRG 2: Good response | 7 (29) | 2 (8) |
| TRG 3: Moderate response | 10 (42) | 11 (44) |
| TRG 4: Slight response | 5 (21) | 9 (36) |
| TRG 5: No response | 1 (4) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan Wah Hak, C.; Balyasnikova, S.; Withey, S.; Tait, D.; Brown, G.; Chong, I. Radiological Biomarkers in MRI directed Rectal Cancer Radiotherapy Volume Delineation. Cancers 2023, 15, 5176. https://doi.org/10.3390/cancers15215176
Chan Wah Hak C, Balyasnikova S, Withey S, Tait D, Brown G, Chong I. Radiological Biomarkers in MRI directed Rectal Cancer Radiotherapy Volume Delineation. Cancers. 2023; 15(21):5176. https://doi.org/10.3390/cancers15215176
Chicago/Turabian StyleChan Wah Hak, Charleen, Svetlana Balyasnikova, Samuel Withey, Diana Tait, Gina Brown, and Irene Chong. 2023. "Radiological Biomarkers in MRI directed Rectal Cancer Radiotherapy Volume Delineation" Cancers 15, no. 21: 5176. https://doi.org/10.3390/cancers15215176
APA StyleChan Wah Hak, C., Balyasnikova, S., Withey, S., Tait, D., Brown, G., & Chong, I. (2023). Radiological Biomarkers in MRI directed Rectal Cancer Radiotherapy Volume Delineation. Cancers, 15(21), 5176. https://doi.org/10.3390/cancers15215176







