The Development and Role of Capmatinib in the Treatment of MET-Dysregulated Non-Small Cell Lung Cancer—A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
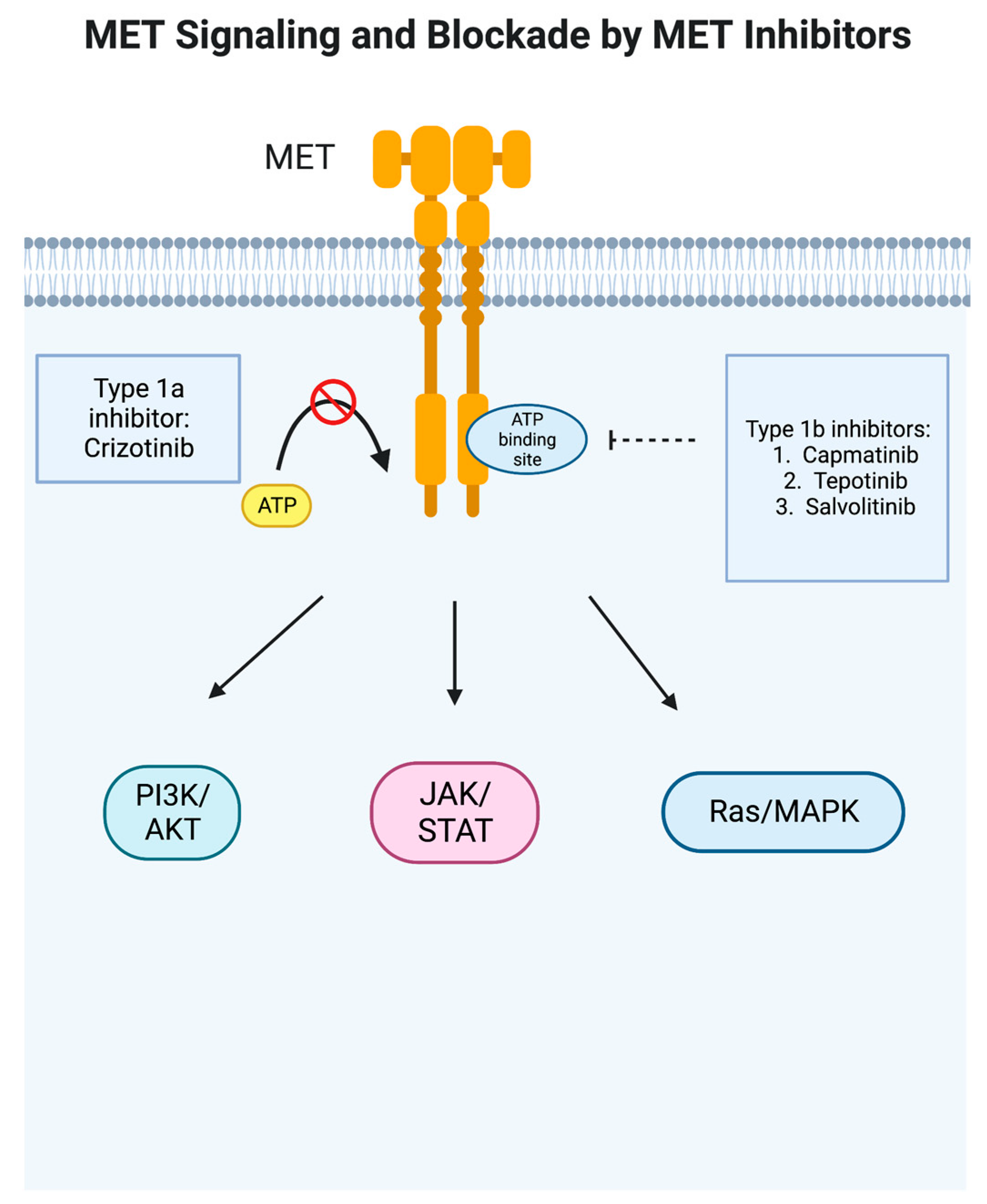
2. Crizotinib
3. Preclinical Studies
4. Pharmacodynamics/Pharmacokinetics
5. Phase I Clinical Trials
6. GEOMETRY Mono-1 Trial
7. Tepotinib and Savolitinib
8. Companion Diagnostic Assay
9. Toxicities
10. Discussion and Future Directions
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NSCLC | Non-small cell lung cancer |
| EGFR | Epidermal growth factor receptor |
| KRAS | Kirsten rat sarcoma virus |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| ALK | Anaplastic lymphoma kinase |
| ROS1 | Proto-oncogene tyrosine–protein kinase ROS |
| RET | Rearranged during transfection proto-oncogene |
| MET | Mesenchymal–epithelial transition |
| ERBB2 | erb-b2 receptor tyrosine kinase 2 |
| NTRK | Neurotrophic tyrosine receptor kinase |
| HGF | Hepatocyte growth factor |
| AMPK | AMP-activated protein kinase |
| LKB1 | Liver kinase B1 |
| GCN | Gain of copy number |
| FDA | U.S. Food and Drug Administration |
| EMA | European Medicines Agency |
| po | Oral |
| DLT | Drug limiting toxicity |
| b.i.d. | Twice a day |
| R2PD | Recommended Phase II dose |
| ORR | Overall response rate |
| PFS | Progression-free survival |
| DOR | Duration of response |
| IO | Immunotherapy |
| IHC | Immunohistochemistry |
| CEP7 | Chromosome 7 centromere |
| ctDNA | Circulating tumor DNA |
| cfDNA | Circulating-free DNA |
| CTCs | Circulating tumor cells |
| FFPE | Fresh-frozen paraffin-embedded |
| PPA | Positive percent agreement |
| NPA | Negative percent agreement |
| OA | Overall agreement |
| NCCN | National Comprehensive Cancer Network |
| Teliso-V | Telisotuzumab vedotin |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Smit, E.F.; Groen, H.J.M.; Mazieres, J.; Besse, B.; Helland, Å.; Giannone, V.; D’Amelio, A.M.; Zhang, P.; Mookerjee, B.; et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Riely, G.J.; Bang, Y.-J.; Kim, D.-W.; Camidge, D.R.; Solomon, B.J.; Varella-Garcia, M.; Iafrate, A.J.; Shapiro, G.I.; Usari, T.; et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): Updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M.G.; Shaw, A.T.; de Braud, F.; Rolfo, C.; Ahn, M.-J.; Wolf, J.; et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14–Mutated or MET -Amplified Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Yang, J.C.-H.; Lee, C.K.; Kurata, T.; Kim, D.-W.; John, T.; Nogami, N.; Ohe, Y.; Mann, H.; Rukazenov, Y.; et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 841–849. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakeley, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2 -Mutant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, M.; Jin, K.; Wang, S.; Wei, H.; Fan, C.; Wu, Y.; Li, X.; Li, X.; Li, G.; et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer 2018, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, F.; Rabe, D.C.; Bottaro, D.P. Targeting the HGF/Met signalling pathway in cancer. Eur. J. Cancer 2010, 46, 1260–1270. [Google Scholar] [CrossRef]
- Pilotto, S.; Carbognin, L.; Karachaliou, N.; Ma, P.C.; Rosell, R.; Tortora, G.; Bria, E. Tracking MET de-addiction in lung cancer: A road towards the oncogenic target. Cancer Treat. Rev. 2017, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jain, P.; Wang, F.; Ma, P.C.; Borczuk, A.; Halmos, B. MET alterations and their impact on the future of non-small cell lung cancer (NSCLC) targeted therapies. Expert Opin. Ther. Targets 2021, 25, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Salgia, R. MET in Lung Cancer: Biomarker Selection Based on Scientific Rationale. Mol. Cancer Ther. 2017, 16, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-Y.; Cheng, F.-J.; Chen, C.-M.; Wang, S.-L.; Hsiao, Y.-C.; Chen, C.-H.; Tsia, T.-C.; He, Y.-H.; Wang, B.-W.; Hsieh, I.-S.; et al. Cigarette smoke enhances oncogene addiction to c-MET and desensitizes EGFR-expressing non-small cell lung cancer to EGFR TKIs. Mol. Oncol. 2018, 12, 705–723. [Google Scholar] [CrossRef]
- Cheng, F.-J.; Chen, C.-H.; Tsai, W.-C.; Wang, B.-W.; Yu, M.-C.; Hsia, T.-C.; Wei, Y.-L.; Hsiao, Y.-C.; Hu, D.-W.; Ho, C.-Y.; et al. Cigarette smoke-induced LKB1/AMPK pathway deficiency reduces EGFR TKI sensitivity in NSCLC. Oncogene 2021, 40, 1162–1175. [Google Scholar] [CrossRef]
- Hong, L.; Zhang, J.; Heymach, J.V.; Le, X. Current and future treatment options for MET exon 14 skipping alterations in non-small cell lung cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835921992976. [Google Scholar] [CrossRef]
- Cortot, A.B.; Kherrouche, Z.; Descarpentries, C.; Wislez, M.; Baldacci, S.; Furlan, A.; Tulasne, D. Exon 14 Deleted MET Receptor as a New Biomarker and Target in Cancers. JNCI J. Natl. Cancer Inst. 2017, 109, djw262. [Google Scholar] [CrossRef]
- Socinski, M.A.; Pennell, N.A.; Davies, K.D. MET Exon 14 Skipping Mutations in Non–Small-Cell Lung Cancer: An Overview of Biology, Clinical Outcomes, and Testing Considerations. JCO Precis. Oncol. 2021, 5, 653–663. [Google Scholar] [CrossRef]
- Tong, J.H.; Yeung, S.F.; Chan, A.W.H.; Chung, L.Y.; Chau, S.L.; Lung, R.W.M.; Tong, C.Y.; Chow, C.; Tin, E.K.Y.; Yu, Y.H.; et al. MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non-Small Cell Lung Carcinoma with Poor Prognosis. Clin. Cancer Res. 2016, 22, 3048–3056. [Google Scholar] [CrossRef] [PubMed]
- Schubart, C.; Stöhr, R.; Tögel, L.; Fuchs, F.; Sirbu, H.; Seitz, G.; Seggewiss-Bernhardt, R.; Leistner, R.; Sterlacci, W.; Vieth, M.; et al. MET Amplification in Non-Small Cell Lung Cancer (NSCLC)—A Consecutive Evaluation Using Next-Generation Sequencing (NGS) in a Real-World Setting. Cancers 2021, 13, 5023. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef]
- Chmielecki, J.; Mok, T.; Wu, Y.-L.; Han, J.-Y.; Ahn, M.; Ramalingam, S.S.; John, T.; Okamoto, I.; Yang, J.C.-H.; Shepherd, F.A.; et al. Analysis of acquired resistance mechanisms to osimertinib in patients with EGFR-mutated advanced non-small cell lung cancer from the AURA3 trial. Nat Commun. 2023, 14, 1071. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Yoda, S.; Lennerz, J.K.; Langenbucher, A.; Lin, J.J.; Rooney, M.M.; Prutisto-Chang, K.; Oh, A.; Adams, N.A.; Yeap, B.Y.; et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin. Cancer Res. 2020, 26, 2535–2545. [Google Scholar] [CrossRef]
- Awad, M.M.; Leonardi, G.C.; Kravets, S.; Dahlberg, S.E.; Drilon, A.; Noonan, S.A.; Camidge, D.R.; Ou, S. H-I; Costa, D.B.; Gadgeel, S.M.; et al. Impact of MET inhibitors on survival among patients with non-small cell lung cancer harboring MET exon 14 mutations: A retrospective analysis. Lung Cancer 2019, 133, 96–102. [Google Scholar] [CrossRef]
- Awad, M.M.; Oxnard, G.R.; Jackman, D.M.; Savukoski, D.O.; Hall, D.; Shivdasani, P.; Heng, J.C.; Dahlberg, S.E.; Janne, P.A.; Verma, S.; et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J. Clin. Oncol. 2016, 34, 721–730. [Google Scholar] [CrossRef]
- Bahcall, M.; Paweletz, C.P.; Kuang, Y.; Taus, L.J.; Sim, T.; Kim, N.D.; Dholakia, K.H.; Lau, C.J.; Gokhale, P.C.; Chopade, P.R.; et al. Combination of Type I and Type II MET Tyrosine Kinase Inhibitors as Therapeutic Approach to Prevent Resistance. Mol. Cancer Ther. 2022, 21, 322–335. [Google Scholar] [CrossRef]
- Cui, J.J. Targeting receptor tyrosine kinase MET in cancer: Small molecule inhibitors and clinical progress. J. Med. Chem. 2014, 57, 4427–4453. [Google Scholar] [CrossRef]
- Lu, S.; Fang, J.; Li, X.; Cao, L.; Zhou, J.; Guo, Q.; Liang, Z.; Cheng, Y.; Jiang, L.; Yang, N.; et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: A multicentre, single-arm, open-label, phase 2 study. Lancet Respir. Med. 2021, 9, 1154–1164. [Google Scholar] [CrossRef]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, M.; Massafra, M.; Gebbia, V.; D’Aquino, A.; Garipoli, C.; Altavilla, G.; Rosell, R. A narrative review of MET inhibitors in non-small cell lung cancer with MET exon 14 skipping mutations. Transl. Lung Cancer Res. 2021, 10, 1536–1556. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.B.; Um, S.L.; Peek, V.L.; Stephens, J.R.; Zeng, W.; Konicek, B.W.; Ling, L.; Manro, J.R.; Wacheck, W.; Walgren, R.A. MET-targeting antibody (emibetuzumab) and kinase inhibitor (merestinib) as single agent or in combination in a cancer model bearing MET exon 14 skipping. Investig. New Drugs 2018, 36, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Calles, A.; Kwiatkowski, N.; Cammarata, B.K.; Ercan, D.; Gray, N.S.; Jänne, P.A. Tivantinib (ARQ 197) efficacy is independent of MET inhibition in non-small-cell lung cancer cell lines. Mol. Oncol. 2015, 9, 260–269. [Google Scholar] [CrossRef]
- Klempner, S.J.; Borghei, A.; Hakimian, B.; Ali, S.M.; Ou, S.-H.I. Intracranial Activity of Cabozantinib in MET Exon 14-Positive NSCLC with Brain Metastases. J. Thorac. Oncol. 2017, 12, 152–156. [Google Scholar] [CrossRef]
- Scagliotti, G.; von Pawel, J.; Novello, S.; Ramlau, R.; Favaretto, A.; Barlesi, F.; Akerley, W.; Orlov, S.; Santoro, A.; Spigel, D.; et al. Phase III Multinational, Randomized, Double-Blind, Placebo-Controlled Study of Tivantinib (ARQ 197) Plus Erlotinib Versus Erlotinib Alone in Previously Treated Patients With Locally Advanced or Metastatic Nonsquamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2015, 33, 2667–2674. [Google Scholar] [CrossRef]
- Tabrecta. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tabrecta (accessed on 1 July 2023).
- Novartis. TABRECTA (Capmatinib): US Prescribing Information. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213591s000lbl.pdf (accessed on 8 March 2023).
- Moro-Sibilot, D.; Cozic, N.; Pérol, M.; Mazières, J.; Otto, J.; Souquet, P.J.; Bahleda, R.; Wislez, M.; Zalcman, G.; Guibert, S.D. Crizotinib in c-MET- or ROS1-positive NSCLC: Results of the AcSé phase II trial. Ann. Oncol. 2019, 30, 1985–1991. [Google Scholar] [CrossRef]
- Landi, L.; Chiari, R.; Tiseo, M.; D’Incà, F.; Dazzi, C.; Chella, A.; Delmonte, A.; Bonanno, L.; Giannarell, D.; Cortinovis, D.L.; et al. Crizotinib in MET -Deregulated or ROS1 -Rearranged Pretreated Non–Small Cell Lung Cancer (METROS): A Phase II, Prospective, Multicenter, Two-Arms Trial. Clin. Cancer Res. 2019, 25, 7312–7319. [Google Scholar] [CrossRef]
- Drilon, A.; Clark, J.W.; Weiss, J.; Ou, S.-H.I.; Camidge, D.R.; Solomon, B.J.; Otterson, G.A.; Villaruz, L.C.; Riely, G.J.; Heist, R.S.; et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat. Med. 2020, 26, 47–51. [Google Scholar] [CrossRef]
- Recondo, G.; Bahcall, M.; Spurr, L.F.; Che, J.; Ricciuti, B.; Leonardi, G.C.; Lo, Y.-C.; Li, Y.Y.; Lamberti, G.; Nguyen, T.; et al. Molecular Mechanisms of Acquired Resistance to MET Tyrosine Kinase Inhibitors in Patients with MET Exon 14-Mutant NSCLC. Clin. Cancer Res. 2020, 26, 2615–2625. [Google Scholar] [CrossRef]
- Xalkori (Crizotinib). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202570s021lbl.pdf (accessed on 1 July 2023).
- Xalkori, INN-Crizotinib. Available online: https://www.ema.europa.eu/en/documents/product-information/xalkori-epar-product-information_en.pdf (accessed on 1 July 2023).
- Liu, X.; Wang, Q.; Yang, G.; Marando, C.; Koblish, H.K.; Hall, L.M.; Fridman, J.S.; Behshad, E.; Wynn, R.; Li, Y.; et al. A Novel Kinase Inhibitor, INCB28060, Blocks c-MET–Dependent Signaling, Neoplastic Activities, and Cross-Talk with EGFR and HER-3. Clin. Cancer Res. 2011, 17, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Baltschukat, S.; Engstler, B.S.; Huang, A.; Hao, H.-X.; Tam, A.; Wang, H.Q.; Liang, J.; DiMare, M.T.; Bhang, H.-E.C.; Wang, Y.; et al. Capmatinib (INC280) Is Active Against Models of Non–Small Cell Lung Cancer and Other Cancer Types with Defined Mechanisms of MET Activation. Clin. Cancer Res. 2019, 25, 3164–3175. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Capmatinib: First Approval. Drugs 2020, 80, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Esaki, T.; Hirai, F.; Makiyama, A.; Seto, T.; Bando, H.; Naito, Y.; Yoh, K.; Ishihara, K.; Kakizume, T.; Natsume, K.; et al. Phase I dose-escalation study of capmatinib (INC280) in Japanese patients with advanced solid tumors. Cancer Sci. 2019, 110, 1340–1351. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Su, W.-C.; Schuler, M.; Nam, D.-H.; Lim, W.T.; Bauer, T.M.; Azaro, A.; Poon, R.T.P.; Hong, D.; Lin, C.-C.; et al. Phase 1 study of capmatinib in MET-positive solid tumor patients: Dose escalation and expansion of selected cohorts. Cancer Sci. 2020, 111, 536–547. [Google Scholar] [CrossRef]
- Schuler, M.; Berardi, R.; Lim, W.-T.; de Jonge, M.; Bauer, T.M.; Azaro, A.; Gottfried, M.; Han, J.-Y.; Lee, D.-H.; Wollner, M.; et al. Molecular correlates of response to capmatinib in advanced non-small-cell lung cancer: Clinical and biomarker results from a phase I trial. Ann. Oncol. 2020, 31, 789–797. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Zhang, L.; Kim, D.-W.; Liu, X.; Lee, D.H.; Yang, J.C.-H.; Ahn, M.-J.; Vansteenkiste, J.F.; Su, W.-C.; Felip, E.; et al. Phase Ib/II Study of Capmatinib (INC280) Plus Gefitinib After Failure of Epidermal Growth Factor Receptor (EGFR) Inhibitor Therapy in Patients With EGFR-Mutated, MET Factor-Dysregulated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3101–3109. [Google Scholar] [CrossRef]
- Vansteenkiste, J.F.; Smit, E.F.; Groen, H.J.M.; Garon, E.B.; Heist, R.S.; Hida, T.; Nishio, M.; Kokowski, K.; Grohe, C.; Reguart, N.; et al. 1285P Capmatinib in patients with METex14-mutated advanced non-small cell lung cancer who received prior immunotherapy: The phase II GEOMETRY mono-1 study. Ann. Oncol. 2020, 31, S830. [Google Scholar] [CrossRef]
- Wolf, J.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Gilloteau, I.; Le Mouhaer, S.; Hampe, M.; Cai, C.; Chassot-Agostinho, A.; Reynolds, M.; et al. Patient-reported outcomes in capmatinib-treated patients with METex14-mutated advanced NSCLC: Results from the GEOMETRY mono-1 study. Eur. J. Cancer 2023, 183, 98–108. [Google Scholar] [CrossRef]
- Seto, T.; Ohashi, K.; Sugawara, S.; Nishio, M.; Takeda, M.; Aoe, K.; Moizumi, S.; Nomura, S.; Tajima, T.; Hida, T. Capmatinib in Japanese patients with MET exon 14 skipping-mutated or MET-amplified advanced NSCLC: GEOMETRY mono-1 study. Cancer Sci. 2021, 112, 1556–1566. [Google Scholar] [CrossRef]
- Paik, P.K.; Goyal, R.K.; Cai, B.; Price, M.A.; Davis, K.L.; Ansquer, V.D.; Caro, N.; Saliba, T.R. Real-world outcomes in non-small-cell lung cancer patients with MET Exon 14 skipping mutation and brain metastases treated with capmatinib. Future Oncol. 2023, 19, 217–228. [Google Scholar] [CrossRef]
- Illini, O.; Fabikan, H.; Swalduz, A.; Vikström, A.; Krenbek, D.; Schumacher, M.; Dudnik, E.; Studnicka, M.; Ohman, R.; Wurm, R.; et al. Real-world experience with capmatinib in MET exon 14-mutated non-small cell lung cancer (RECAP): A retrospective analysis from an early access program. Ther. Adv. Med. Oncol. 2022, 14, 17588359221103206. [Google Scholar] [CrossRef]
- TEPMETKO, INN. Available online: https://www.ema.europa.eu/en/documents/product-information/tepmetko-epar-product-information_en.pdf (accessed on 1 July 2023).
- Markham, A. Savolitinib: First Approval. Drugs 2021, 81, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.T.; Mollerup, J. Companion Diagnostics and Predictive Biomarkers for MET-Targeted Therapy in NSCLC. Cancers 2022, 14, 2150. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.; Hong, L.; Zhang, J.; Heymach, J.; Hong, D.; Le, X. Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non-small-cell lung cancer. ESMO Open 2021, 6, 100319. [Google Scholar] [CrossRef] [PubMed]
- Noonan, S.A.; Berry, L.; Lu, X.; Gao, D.; Barón, A.E.; Chesnut, P.; Sheren, J.; Aisner, D.L.; Merrick, D.; Doebele, R.C.; et al. Identifying the Appropriate FISH Criteria for Defining MET Copy Number-Driven Lung Adenocarcinoma through Oncogene Overlap Analysis. J. Thorac. Oncol. 2016, 11, 1293–1304. [Google Scholar] [CrossRef]
- Guo, R.; Offin, M.; Brannon, A.R.; Chang, J.; Chow, A.; Delasos, L.; Girshman, J.; Wilkins, O.; McCarthy, C.G.; Makhnin, A. MET Exon 14-altered Lung Cancers and MET Inhibitor Resistance. Clin. Cancer Res. 2021, 27, 799–806. [Google Scholar] [CrossRef]
- Ikeda, S.; Schwaederle, M.; Mohindra, M.; Fontes Jardim, D.L.; Kurzrock, R. MET alterations detected in blood-derived circulating tumor DNA correlate with bone metastases and poor prognosis. J. Hematol. Oncol. 2018, 11, 76. [Google Scholar] [CrossRef]
- Park, S.; Olsen, S.; Ku, B.M.; Lee, M.-S.; Jung, H.-A.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; Choi, Y.-L.; et al. High concordance of actionable genomic alterations identified between circulating tumor DNA–based and tissue-based next-generation sequencing testing in advanced non–small cell lung cancer: The Korean Lung Liquid Versus Invasive Biopsy Program. Cancer 2021, 127, 3019–3028. [Google Scholar] [CrossRef]
- Mondelo-Macía, P.; Rodríguez-López, C.; Valiña, L.; Aguín, S.; León-Mateos, L.; García-González, J.; Abalo, A.; Rapado-Gonzalez, O.; Suarez-Cunqueiro, M.; Díaz-Lagares, A.; et al. Detection of MET Alterations Using Cell Free DNA and Circulating Tumor Cells from Cancer Patients. Cells 2020, 9, 522. [Google Scholar] [CrossRef]
- Peng, H.; Lu, L.; Zhou, Z.; Liu, J.; Zhang, D.; Nan, K.; Zhao, X.; Li, F.; Tian, L.; Dong, H.; et al. CNV Detection from Circulating Tumor DNA in Late Stage Non-Small Cell Lung Cancer Patients. Genes 2019, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- FDA Summary of Safety and Effectiveness Data. FoundationOne CDx. 2020. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019S011B.pdf (accessed on 8 March 2023).
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.3.2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 15 June 2023).
- Souquet, P.; Kim, S.; Solomon, B.; Vansteenkiste, J.; Carbini, M.; Kenny, S.; Glaser, S.; Chassot Agostinho, A.; Wolf, J. P47.17 Capmatinib vs Docetaxel in Pretreated Patients With MET Exon 14 Skipping–mutated Stage IIIB/IIIC or IV NSCLC (GeoMETry-III). J. Thorac. Oncol. 2021, 16, S1104. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Moonsamy, P.; Gainor, J.F.; Lennerz, J.K.; Piotrowska, Z.; Lin, J.J.; Lenne, I.T.; Sequist, L.V.; Shaw, A.T.; Goodwin, K.; et al. A Phase 2 Study of Capmatinib in Patients With MET-Altered Lung Cancer Previously Treated With a MET Inhibitor. J. Thorac. Oncol. 2021, 16, 850–859. [Google Scholar] [CrossRef]
- Lee, J.M.; Awad, M.M.; Saliba, T.R.; Caro, N.; Banerjee, H.; Kelly, K. Neoadjuvant and adjuvant capmatinib in resectable non–small cell lung cancer with MET exon 14 skipping mutation or high MET amplification: GEOMETRY-N trial. J. Clin. Oncol. 2022, 40, TPS8590. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR -Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Sabari, J.K.; Leonardi, G.C.; Shu, C.A.; Umeton, R.; Montecalvo, J.; Ni, A.; Chen, R.; Dienstag, J.; Mrad, C.; Bergagnini, I.; et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann. Oncol. 2018, 29, 2085–2091. [Google Scholar] [CrossRef]
- Wolf, J.; Heist, R.; Kim, T.M.; Nishio, M.; Dooms, C.; Kanthala, R.R.; Leo, E.; Giorgetti, E.; Mardjuadi, F.I.; Corto, A. 994P Efficacy and safety of capmatinib plus spartalizumab in treatment-naïve patients with advanced NSCLC harboring MET exon 14 skipping mutation. Ann. Oncol. 2022, 33, S1007–S1008. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Cortinovis, D.L.; Majem, M.; Johnson, M.L.; Mardjuadi, F.I.; Zhao, X.; Siripurapu, S.V.; Jiang, Z.; Wolf, J. Efficacy and safety of capmatinib plus pembrolizumab in treatment (tx)-naïve patients with advanced non–small cell lung cancer (NSCLC) with high tumor PD-L1 expression: Results of a randomized, open-label, multicenter, phase 2 study. J. Clin. Oncol. 2022, 40, 9118. [Google Scholar] [CrossRef]
- Dempke, W.C.M.; Fenchel, K. Has programmed cell death ligand-1 MET an accomplice in non-small cell lung cancer?—A narrative review. Transl. Lung Cancer Res. 2021, 10, 2667–2682. [Google Scholar] [CrossRef]
- Sequist, L.V.; Han, J.-Y.; Ahn, M.-J.; Cho, B.C.; Yu, H.; Kim, S.-W.; Yang, J.C.-H.; Lee, J.S.; Su, W.-C.; Kowalski, D.; et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: Interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020, 21, 373–386. [Google Scholar] [CrossRef]
- Ou, S.-H.I.; Young, L.; Schrock, A.B.; Johnson, A.; Klempner, S.J.; Zhu, V.W.; Miller, V.A.; Ali, S.M. Emergence of Preexisting MET Y1230C Mutation as a Resistance Mechanism to Crizotinib in NSCLC with MET Exon 14 Skipping. J. Thorac. Oncol. 2017, 12, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Suda, K.; Koga, T.; Hamada, A.; Ohara, S.; Chiba, M.; Shimoji, M.; Takemoto, T.; Soh, J.; Mitsudomi, T. Foretinib can overcome common on-target resistance mutations after capmatinib/tepotinib treatment in NSCLCs with MET exon 14 skipping mutation. J. Hematol. Oncol. 2022, 15, 79. [Google Scholar] [CrossRef]
- Bahcall, M.; Sim, T.; Paweletz, C.P.; Patel, J.D.; Alden, R.S.; Kuang, Y.; Sacher, A.G.; Kim, N.D.; Lydon, C.A.; Awad, M.M.; et al. Acquired METD1228V Mutation and Resistance to MET Inhibition in Lung Cancer. Cancer Discov. 2016, 6, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.; Spira, A.I.; Cho, B.C.; Besse, B.; Goldman, J.W.; Janne, P.A.; Ma, Z.; Mansfield, A.S.; Minchom, A.R.; Ou, S.-H.I.; et al. Amivantamab in patients with NSCLC with MET exon 14 skipping mutation: Updated results from the CHRYSALIS study. J. Clin. Oncol. 2022, 40, 9008. [Google Scholar] [CrossRef]
- Shu, C.A.; Goto, K.; Ohe, Y.; Besse, B.; Lee, S.-H.; Wang, Y.; Griesinger, F.; Yang, J.C.-H.; Felip, E.; Sanborn, R.E.; et al. Amivantamab and lazertinib in patients with EGFR-mutant non–small cell lung (NSCLC) after progression on osimertinib and platinum-based chemotherapy: Updated results from CHRYSALIS-2. J. Clin. Oncol. 2022, 40, 9006. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Camidge, D.R.; Bar, J.; Horinouchi, H.; Goldman, J.W.; Moiseenko, F.V.; Filippova, E.; Cicin, I.; Bradbury, P.A.; Daaboul, N.; Tomasini, P.; et al. Telisotuzumab vedotin (Teliso-V) monotherapy in patients (pts) with previously treated c-Met–overexpressing (OE) advanced non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2022, 40, 9016. [Google Scholar] [CrossRef]
- Oh, S.Y.; Lee, Y.W.; Lee, E.J.; Kim, J.H.; Park, Y.; Heo, S.G.; Yu, M.R.; Hong, M.H.; DaSilva, J.; Daly, C.; et al. Preclinical Study of a Biparatopic METxMET Antibody–Drug Conjugate, REGN5093-M114, Overcomes MET-driven Acquired Resistance to EGFR TKIs in EGFR-mutant NSCLC. Clin. Cancer Res. 2023, 29, 221–232. [Google Scholar] [CrossRef]
- Goodwin, K.; Ledezma, B.; Heist, R.; Garon, E. MO01.04 Management of Selected Adverse Events With Capmatinib: Institutional Experiences From the GEOMETRY Mono-1 Trial. J. Thorac. Oncol. 2021, 16, S16–S17. [Google Scholar] [CrossRef]

| Publication | n | Indication | R2PD | ORR |
|---|---|---|---|---|
| Esaki et al. [48] | 44 (15 NSCLC) | Advanced solid tumors | 400 mg po bid | |
| Bang et al. [49] | 38 (1 NSCLC) | Advanced solid tumors | 600 mg po bid (capsule)/400 mg po bid (tablet) | |
| Schuler et al. [50] | 55 | Advanced NSCLC | 600 mg po bid (capsule)/400 mg po bid (tablet) | 47% |
| Wu et al. [51] | 61 Phase Ib/100 Phase II | Advanced NSCLC in patients with acquired EGFR TKI resistance | 400 mg po b.i.d. plus gefitinib 250 mg po daily | 27% (47% in patients with MET GCN ≥ 6) |
| Response | NSCLC with MET Exon 14 Skipping Mutation | NSCLC with MET Amplification | |||||
|---|---|---|---|---|---|---|---|
| Best Response—No (%) | Cohort 4 n = 69, any GCN with 1–2 Lines of Therapy | Cohort 5b n = 28, any GCN with No Previous Therapy | Cohort 1a n = 69, GCN ≥ 10 with 1–2 Lines of Therapy | Cohort 5a n = 15, GCN ≥ 10 with No Previous Therapy | Cohort 1b n = 42, GCN 6–9 with 1–2 Lines of Therapy | Cohort 2 n = 54, GCN 4 or 5 with 1–2 Lines of Therapy | Cohort 3 n = 30, GCN < 4 with 1–2 Lines of Therapy |
| Complete response | 0 | 1 (4) | 1 (1) | 0 | 0 | 0 | 0 |
| Partial Response | 28 (41) | 18 (64) | 19 (28) | 6 (40) | 5 (12) | 5 (9) | 2 (7) |
| Stable disease | 25 (36) | 7 (25) | 28 (41) | 4 (27) | 17 (40) | 20 (37) | 14 (47) |
| Incomplete response or nonprogressive disease | 1 (1) | 1 (4) | 1 (1) | 0 | 1 (2) | 0 | 0 |
| Unknown or could not be evaluated | 9 (13) | 0 | 8 (12) | 1 (7) | 4 (10) | 8 (15) | 8 (27) |
| Overall response | |||||||
| No. of patients with overall response | 28 | 19 | 20 | 6 | 5 | 5 | 2 |
| Percent of patients (95% CI) | 41 (29–53) | 68 (48–84) | 29 (19–41) | 40 (16–68) | 12 (4–26) | 9 (3–20) | 7 (1–22) |
| Disease control | |||||||
| No. of patients with disease control | 54 | 27 | 49 | 10 | 23 | 25 | 16 |
| Percent of patients (95% CI) | 78 (67–87) | 96 (82–100) | 71 (59–81) | 67 (38–88) | 55 (39–70) | 46 (33–60) | 53 (34–72) |
| Duration of Response | |||||||
| No. of events/No. of patients with response | 23/28 | 11/19 | 15/20 | 6/6 | 3/5 | 4/5 | 2/2 |
| Median duration of response (95% CI)—mo | 9.7 (5.6–13.0) | 12.6 (5.6–NE) | 8.3 (4.2–15.4) | 7.5 (2.6–14.3) | 24.9 (2.7–24.9) | 9.7 (4.2–NE) | 4.2 (4.2–4.2) |
| Progression-free survival | |||||||
| Progression or death—No. of patients | 60 | 17 | 58 | 15 | 34 | 50 | 22 |
| Median progression-free survival (95% CI)—mo | 5.4 (4.2–7.0) | 12.4 (8.2–NE) | 4.1 (2.9–4.8) | 4.2 (1.4–6.9) | 2.7 (1.4–3.1) | 2.7 (1.4–4.1) | 3.6 (2.2–4.2) |
| Capmatinib [6] | Tepotinib [31] | Savolitinib [30] | |
|---|---|---|---|
| N (with MET exon 14 skipping mutation) | 97 | 152 (99 evaluable) | 84 (70 evaluable) |
| Overall response rate (%) (95% CI) | 68% (48–84) in untreated patients (n = 28) and 41 (29–53) in previously treated patients (n = 69) | 46 (36–57); 44.2% (29.1–60.1) in untreated patients (n = 43) and 48.2 (34.7–62.0) in previously treated patients (n = 56) | 42.9 (31.1–53.3); 46.4 (27.5–66.1) in untreated patients (n = 28) and 40.5 (25.6–56.7) in previously treated patients (n = 42) |
| Duration of response mo (95% CI) | 12.6 (5.6—NE) in untreated patients and 9.7 (5.6–13.0) in previously treated patients | 11.1 (7.2—NE) | 8.3 (5.3–16.6); 5.6 (4.1–9.6) in untreated patients and 9.7 (4.9—NE) In previously treated patients |
| Progression-free survival mo (95% CI) | 12.4 (8.2—NE) in untreated patients and 5.4 (4.2–7.0) in previously treated patients | 8.5 (5.1–11.0) | 6.8 (4.2–9.6); 5.6 (4.1–9.6) in untreated patients and 6.9 (4.1–9.3) in previously treated patients |
| Publication | Drug | Method | Biomarker | N | ORR% |
|---|---|---|---|---|---|
| Moro-Sibilot et al. [39] | Crizotinib | FISH | MET GCN ≥ 6 | 25 | 16 |
| NGS | MET exon 14 skip | 25 | 12 | ||
| Landi et al. [40] | Crizotinib | FISH | MET/CEP7 > 2.2 | 16 | 31 |
| NGS | MET exon 14 skip | 10 | 20 | ||
| Drilon et al. [41] | Crizotinib | NGS | MET exon 14 skip | 65 | 32 |
| Schuler et al. [50] | Capmatinib | FISH | MET GCN < 4 | 17 | 6 |
| MET GCN 4–6 | 12 | 25 | |||
| MET GCN ≥ 6 | 15 | 47 | |||
| MET/CEP7 > 2.0 | 9 | 44 | |||
| MET/CEP7 < 2.0 | 32 | 22 | |||
| IHC | MET IHC 2+ | 14 | 14 | ||
| MET IHC 3_ | 37 | 27 | |||
| Wu et al. [51] | Capmatinib with gefitinib | FISH | MET GCN < 4 | 41 | 12 |
| MET GCN 4–6 | 18 | 22 | |||
| MET GCN ≥ 6 | 36 | 47 | |||
| IHC | MET IHC 2+ | 16 | 19 | ||
| MET IHC 3+_ | 37 | 27 | |||
| Wolf et al. [6] | Capmatinib | NGS | MET exon 14 skip (Previously treated) | 69 | 41 |
| MET exon 14 skip (Untreated) | 28 | 64 | |||
| NGS | MET GCN < 4 (Previously treated) | 30 | 7 | ||
| MET GCN 4–5 (Previously treated) | 54 | 9 | |||
| MET GCN > 6–9 (Previously treated) | 42 | 12 | |||
| MET GCN ≥ 10 (Previously treated) | 69 | 28 | |||
| MET GCN ≥ 10 (Untreated) | 15 | 40 | |||
| Paik et al. [31] | Tepotinib | NGS | MET exon 14 skip | 99 | 46 |
| Adverse Event | Total | Grade 3 or 4 |
|---|---|---|
| Any event—No. (%) | 355 (98) | 244 (67) |
| Most common events—No. (%) | ||
| Peripheral edema | 186 (51) | 33 (9) |
| Nausea | 163 (45) | 9 (2) |
| Vomiting | 102 (28) | 9 (2) |
| Blood creatinine increased | 89 (24) | 0 |
| Dyspnea | 84 (23) | 24 (7) |
| Fatigue | 80 (22) | 16 (4) |
| Decreased appetite | 76 (21) | 3 (1) |
| Constipation | 66 (18) | 3 (1) |
| Diarrhea | 64 (18) | 2 (1) |
| Cough | 58 (16) | 2 (1) |
| Back Pain | 54 (15) | 3 (1) |
| Pyrexia | 50 (14) | 3 (1) |
| ALT increased | 48 (13) | 23 (6) |
| Asthenia | 42 (12) | 13 (4) |
| Pneumonia | 39 (11) | 17 (5) |
| Weight loss | 36 (10) | 2 (1) |
| Noncardiac chest pain | 35 (10) | 4 (1) |
| Serious adverse event—No. (%) | 184 (51) | 152 (42) |
| Event leading to discontinuation—No. (%) | 56 (15) | 35 (10) |
| Clinical Trial Number | Phase | Purpose |
|---|---|---|
| NCT04427072 | Phase III | Previously treated advanced NSCLC patients with MET exon 14 skipping mutation treated with capmatinib versus docetaxel |
| NCT04926831 | Phase II | Efficacy and safety of neoadjuvant and adjuvant capmatinib |
| NCT05435846 | Phase I/Ib | Capmatinib plus trametinib in patients with MET exon 14 skipping mutation |
| NCT04677595 | Phase II | Chinese patients who are EGFR wt and ALK rearrangement negative with MET exon 14 skipping mutation |
| NCT05110196 | Phase IV | Indian patients with MET exon 14 skipping mutation |
| NCT05488314 | Phase I/II | Combination therapy of capmatinib and amivantamab in unresectable Stage IV NSCLC in patients with MET exon 14 skipping mutations or MET amplification |
| NCT05642572 | Phase II | Combination therapy of capmatinib with osimertinib +/− ramucirumab in EGFR mutant, MET-amplified, Stage IV or recurrent NSCLC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, R.; Benjamin, D.J.; Nagasaka, M. The Development and Role of Capmatinib in the Treatment of MET-Dysregulated Non-Small Cell Lung Cancer—A Narrative Review. Cancers 2023, 15, 3561. https://doi.org/10.3390/cancers15143561
Hsu R, Benjamin DJ, Nagasaka M. The Development and Role of Capmatinib in the Treatment of MET-Dysregulated Non-Small Cell Lung Cancer—A Narrative Review. Cancers. 2023; 15(14):3561. https://doi.org/10.3390/cancers15143561
Chicago/Turabian StyleHsu, Robert, David J. Benjamin, and Misako Nagasaka. 2023. "The Development and Role of Capmatinib in the Treatment of MET-Dysregulated Non-Small Cell Lung Cancer—A Narrative Review" Cancers 15, no. 14: 3561. https://doi.org/10.3390/cancers15143561
APA StyleHsu, R., Benjamin, D. J., & Nagasaka, M. (2023). The Development and Role of Capmatinib in the Treatment of MET-Dysregulated Non-Small Cell Lung Cancer—A Narrative Review. Cancers, 15(14), 3561. https://doi.org/10.3390/cancers15143561







