Recent Advances in the Management of Clear Cell Renal Cell Carcinoma: Novel Biomarkers and Targeted Therapies
Simple Summary
Abstract
1. Introduction
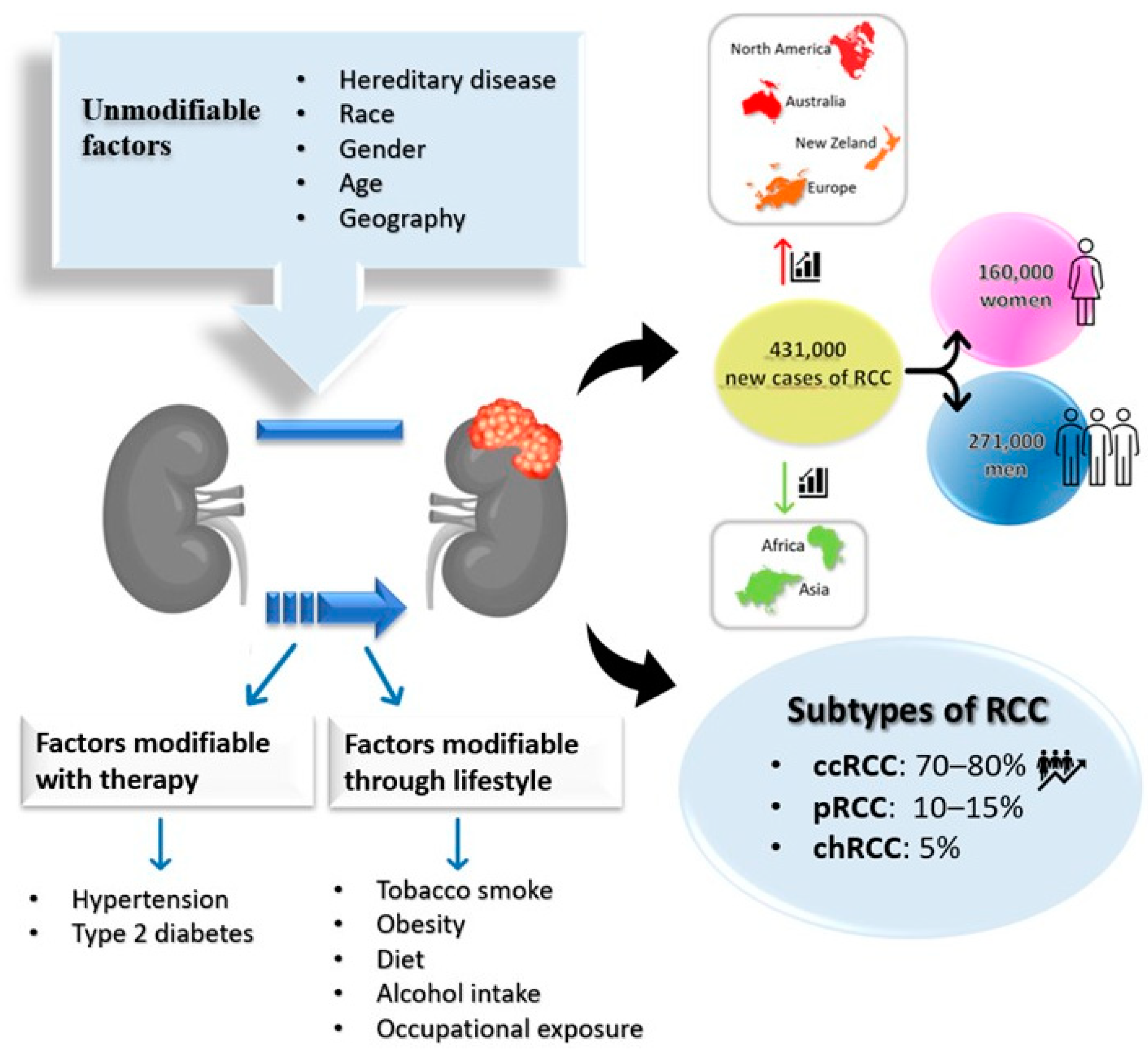
2. Epidemiology
3. Risk Factors and Prevention
4. Diagnosis
5. Prognostic Factors
6. Treatment
| Molecular Target | Drug | Reference |
|---|---|---|
| VEGFR | Sunitinib | [92] |
| Pazopanib | [92] | |
| Cabozantinib | [126] | |
| Lenvatinib | [126] | |
| Axitinib | [24] | |
| Sorafenib | [24] | |
| Bevacizumab | [127] | |
| Tivozanib | [128] | |
| HIF2α | PT2385 | [129] |
| Belzutifan (MK-6482) | [129] | |
| mTOR | Everolimus | [130] |
| Temsirolimus | [130] | |
| Checkpoint inhibitors (PD-1 or CTLA-4) | Nivolumab (PD-1) | [132] |
| Pembrolizumab (PD-1) | [133] | |
| Avelumab (PD-1) | [135] | |
| Ipilimumab (CTLA-4) | [126] |
7. Novel Strategies
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Ricketts, C.J.; De Cubas, A.A.; Fan, H.; Smith, C.C.; Lang, M.; Reznik, E.; Bowlby, R.; Gibb, E.A.; Akbani, R.; Beroukhim, R.; et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep. 2018, 23, 313–326. [Google Scholar] [CrossRef]
- Weaver, C.; Bin Satter, K.; Richardson, K.P.; Tran, L.K.H.; Tran, P.M.H.; Purohit, S. Diagnostic and Prognostic Biomarkers in Renal Clear Cell Carcinoma. Biomedicines 2022, 10, 2953. [Google Scholar] [CrossRef] [PubMed]
- Jonasch, E.; Walker, C.L.; Rathmell, W.K. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat. Rev. Nephrol. 2021, 17, 245–261. [Google Scholar] [CrossRef]
- Mattila, K.E.; Vainio, P.; Jaakkola, P.M. Prognostic Factors for Localized Clear Cell Renal Cell Carcinoma and Their Application in Adjuvant Therapy. Cancers 2022, 14, 239. [Google Scholar] [CrossRef]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef]
- Jonasch, E.; Gao, J.; Rathmell, W.K. Renal cell carcinoma. BMJ 2014, 349, g4797. [Google Scholar] [CrossRef]
- Studentova, H.; Rusarova, N.; Ondruskova, A.; Zemankova, A.; Student, V., Jr.; Skanderova, D.; Melichar, B. The Role of Cytoreductive Nephrectomy in Renal Cell Carcinoma with Sarcomatoid Histology: A Case Series and Review of the Literature. Curr. Oncol. 2022, 29, 5475–5488. [Google Scholar] [CrossRef] [PubMed]
- Shuch, B.; Zhang, J. Genetic Predisposition to Renal Cell Carcinoma: Implications for Counseling, Testing, Screening, and Management. J. Clin. Oncol. 2018, 36, 3560–3566. [Google Scholar] [CrossRef]
- Gerlinger, M.; Horswell, S.; Larkin, J.; Rowan, A.J.; Salm, M.P.; Varela, I.; Fisher, R.; McGranahan, N.; Matthews, N.; Santos, C.R.; et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet. 2014, 46, 225–233. [Google Scholar] [CrossRef]
- Bahadoram, S.; Davoodi, M.; Hassanzadeh, S.; Bahadoram, M.; Barahman, M.; Mafakher, L. Renal cell carcinoma: An overview of the epidemiology, diagnosis, and treatment. G. Ital. Nefrol. 2022, 39, 1–16. [Google Scholar]
- Linehan, W.M.; Rouault, T.A. Molecular Pathways: Fumarate hydratase-deficient kidney cancer—Targeting the warburg effect in cancer. Clin. Cancer Res. 2013, 19, 3345–3352. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.; Pantuck, A.J.; Said, J.W.; Seligson, D.B.; Rao, N.P.; LaRochelle, J.C.; Shuch, B.; Zisman, A.; Kabbinavar, F.F.; Belldegrun, A.S. Cytogenetic and molecular tumor profiling for type 1 and type 2 papillary renal cell carcinoma. Clin. Cancer Res. 2009, 15, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Uchida, K.; Kanai, M.; Asakawa, K.; Usui, M.; Murata, T. A case of primary renal oncocytic tumor: Chromophobe renal cell carcinoma or oncocytoma? IJU Case Rep. 2023, 6, 18–21. [Google Scholar] [CrossRef]
- Pavlovich, C.P.; Walther, M.M.; Eyler, R.A.; Hewitt, S.M.; Zbar, B.; Linehan, W.M.; Merino, M.J. Renal Tumors in the Birt-Hogg-Dubé Syndrome. Am. J. Surg. Pathol. 2002, 26, 1542–1552. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshizato, T.; Shiraishi, Y.; Maekawa, S.; Okuno, Y.; Kamura, T.; Shimamura, T.; Sato-Otsubo, A.; Nagae, G.; Suzuki, H.; et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat. Genet. 2013, 45, 860–867. [Google Scholar] [CrossRef]
- Mazzucchelli, R.; Marzioni, D.; Tossetta, G.; Pepi, L.; Montironi, R. Bladder Cancer Sample Handling and Reporting: Pathologist’s Point of View. Front. Surg. 2021, 8, 754741. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Gesuita, R.; Montironi, R.; Marzioni, D.; Mazzucchelli, R. AT-rich interactive domain 1A (ARID1A) cannot be considered a morphological marker for prostate cancer progression: A pilot study. Acta Histochem. 2022, 124, 151847. [Google Scholar] [CrossRef]
- Tossetta, G. Metformin Improves Ovarian Cancer Sensitivity to Paclitaxel and Platinum-Based Drugs: A Review of In Vitro Findings. Int. J. Mol. Sci. 2022, 23, 12893. [Google Scholar] [CrossRef]
- Altobelli, E.; Latella, G.; Morroni, M.; Licini, C.; Tossetta, G.; Mazzucchelli, R.; Profeta, V.F.; Coletti, G.; Leocata, P.; Castellucci, M.; et al. Low HtrA1 expression in patients with long-standing ulcerative colitis and colorectal cancer. Oncol. Rep. 2017, 38, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Pozzi, V.; Giorgini, S.; Morichetti, D.; Goteri, G.; Sartini, D.; Serritelli, E.N.; Emanuelli, M. Paraoxonase-2 is upregulated in triple negative breast cancer and contributes to tumor progression and chemoresistance. Hum. Cell 2023, 36, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef]
- Carmichael, J.W., Jr.; Bauer, J.D.; Hunter, J.R.; Labat, D.D.; Sevenair, J.P. An assessment of a premedical program in terms of its ability to serve black Americans. J. Natl. Med. Assoc. 1988, 80, 1094–1104. [Google Scholar] [PubMed]
- Makino, T.; Kadomoto, S.; Izumi, K.; Mizokami, A. Epidemiology and Prevention of Renal Cell Carcinoma. Cancers 2022, 14, 4059. [Google Scholar] [CrossRef]
- Ricketts, C.J.; Linehan, W.M. Gender Specific Mutation Incidence and Survival Associations in Clear Cell Renal Cell Carcinoma (CCRCC). PLoS ONE 2015, 10, e0140257. [Google Scholar] [CrossRef]
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Y.; Luo, L.; Nong, S.; Li, T. circFOXO3 Induced by KLF16 Modulates Clear Cell Renal Cell Carcinoma Growth and Natural Killer Cell Cytotoxic Activity through Sponging miR-29a-3p and miR-122-5p. Dis. Markers 2022, 2022, 6062236. [Google Scholar] [CrossRef]
- Warren, A.Y.; Harrison, D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: Standards and controversies. World J. Urol. 2018, 36, 1913–1926. [Google Scholar] [CrossRef]
- Ding, R.; Wei, H.; Jiang, X.; Wei, L.; Deng, M.; Yuan, H. Prognosis and pain dissection of novel signatures in kidney renal clear cell carcinoma based on fatty acid metabolism-related genes. Front. Oncol. 2022, 12, 1094657. [Google Scholar] [CrossRef]
- Qu, G.; Liu, L.; Yi, L.; Tang, C.; Yang, G.; Chen, D.; Xu, Y. Prognostic prediction of clear cell renal cell carcinoma based on lipid metabolism-related lncRNA risk coefficient model. Front. Genet. 2022, 13, 1040421. [Google Scholar] [CrossRef]
- Xiao, Y.; Meierhofer, D. Glutathione Metabolism in Renal Cell Carcinoma Progression and Implications for Therapies. Int. J. Mol. Sci. 2019, 20, 3672. [Google Scholar] [CrossRef]
- Kase, A.M.; George, D.J.; Ramalingam, S. Clear Cell Renal Cell Carcinoma: From Biology to Treatment. Cancers 2023, 15, 665. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, D.; Song, N.; Xu, Y.; Ge, H.; Peng, Z. Prognosis of clear cell renal cell carcinoma patients stratified by age: A research relied on SEER database. Front. Oncol. 2022, 12, 975779. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Kallam, A. Clear Cell Renal Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Haggstrom, C.; Rapp, K.; Stocks, T.; Manjer, J.; Bjorge, T.; Ulmer, H.; Engeland, A.; Almqvist, M.; Concin, H.; Selmer, R.; et al. Metabolic factors associated with risk of renal cell carcinoma. PLoS ONE 2013, 8, e57475. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, O. Diseases of Hereditary Renal Cell Cancers. Urol. Clin. N. Am. 2023, 50, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.S.; Sayegh, N.; Prajapati, S.; Chan, E.; Pal, S.K.; Chehrazi-Raffle, A. An Update on the Treatment of Papillary Renal Cell Carcinoma. Cancers 2023, 15, 565. [Google Scholar] [CrossRef]
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef]
- Batai, K.; Harb-De la Rosa, A.; Lwin, A.; Chaus, F.; Gachupin, F.C.; Price, E.; Lee, B.R. Racial and Ethnic Disparities in Renal Cell Carcinoma: An Analysis of Clinical Characteristics. Clin. Genitourin. Cancer 2019, 17, e195–e202. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.N.; Yedjou, C.G.; Abugri, D.; Payton, M.; Turner, T.; Miele, L.; Tchounwou, P.B. Racial Disparities and Preventive Measures to Renal Cell Carcinoma. Int. J. Environ. Res. Public Health 2018, 15, 1089. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Higgins, P.J.; Samarakoon, R. Downstream Targets of VHL/HIF-α Signaling in Renal Clear Cell Carcinoma Progression: Mechanisms and Therapeutic Relevance. Cancers 2023, 15, 1316. [Google Scholar] [CrossRef]
- Rai, K.; Pilarski, R.; Cebulla, C.M.; Abdelrahman, M.H. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin. Genet. 2016, 89, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.J.; Hes, O.; Papathomas, T.; Sedivcova, M.; Tan, P.H.; Agaimy, A.; Andresen, P.A.; Kedziora, A.; Clarkson, A.; Toon, C.W.; et al. Succinate dehydrogenase (SDH)-deficient renal carcinoma: A morphologically distinct entity: A clinicopathologic series of 36 tumors from 27 patients. Am. J. Surg. Pathol. 2014, 38, 1588–1602. [Google Scholar] [CrossRef] [PubMed]
- van der Tuin, K.; Tops, C.M.J.; Adank, M.A.; Cobben, J.-M.; Hamdy, N.A.T.; Jongmans, M.C.; Menko, F.H.; van Nesselrooij, B.P.M.; Netea-Maier, R.T.; Oosterwijk, J.C.; et al. CDC73-Related Disorders: Clinical Manifestations and Case Detection in Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2017, 102, 4534–4540. [Google Scholar] [CrossRef]
- Carlo, M.I.; Hakimi, A.A.; Stewart, G.D.; Bratslavsky, G.; Brugarolas, J.; Chen, Y.B.; Linehan, W.M.; Maher, E.R.; Merino, M.J.; Offit, K.; et al. Familial Kidney Cancer: Implications of New Syndromes and Molecular Insights. Eur. Urol. 2019, 76, 754–764. [Google Scholar] [CrossRef]
- Courthod, G.; Tucci, M.; Di Maio, M.; Scagliotti, G.V. Papillary renal cell carcinoma: A review of the current therapeutic landscape. Crit. Rev. Oncol. Hematol. 2015, 96, 100–112. [Google Scholar] [CrossRef]
- Ooi, A. Advances in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) research. Semin. Cancer Biol. 2020, 61, 158–166. [Google Scholar] [CrossRef]
- Ross, R.K.; Paganini-Hill, A.; Landolph, J.; Gerkins, V.; Henderson, B.E. Analgesics, cigarette smoking, and other risk factors for cancer of the renal pelvis and ureter. Cancer Res 1989, 49, 1045–1048. [Google Scholar]
- Hunt, J.D.; van der Hel, O.L.; McMillan, G.P.; Boffetta, P.; Brennan, P. Renal cell carcinoma in relation to cigarette smoking: Meta-analysis of 24 studies. Int. J. Cancer 2005, 114, 101–108. [Google Scholar] [CrossRef]
- Patel, N.H.; Attwood, K.M.; Hanzly, M.; Creighton, T.T.; Mehedint, D.C.; Schwaab, T.; Kauffman, E.C. Comparative Analysis of Smoking as a Risk Factor among Renal Cell Carcinoma Histological Subtypes. J. Urol. 2015, 194, 640–646. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Yang, Z. Treatment strategies for clear cell renal cell carcinoma: Past, present and future. Front. Oncol. 2023, 13, 1133832. [Google Scholar] [CrossRef] [PubMed]
- Callahan, C.L.; Hofmann, J.N.; Corley, D.A.; Zhao, W.K.; Shuch, B.; Chow, W.-H.; Purdue, M.P. Obesity and renal cell carcinoma risk by histologic subtype: A nested case-control study and meta-analysis. Cancer Epidemiol. 2018, 56, 31–37. [Google Scholar] [CrossRef]
- Macleod, L.C.; Hotaling, J.M.; Wright, J.L.; Davenport, M.T.; Gore, J.L.; Harper, J.; White, E. Risk factors for renal cell carcinoma in the VITAL study. J. Urol. 2013, 190, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.E.; Boffetta, P.; Karami, S.; Brennan, P.; Stewart, P.S.; Hung, R.; Zaridze, D.; Matveev, V.; Janout, V.; Kollarova, H.; et al. Occupational trichloroethylene exposure and renal carcinoma risk: Evidence of genetic susceptibility by reductive metabolism gene variants. Cancer Res 2010, 70, 6527–6536. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Je, Y.; Cho, E. Analgesic use and the risk of kidney cancer: A meta-analysis of epidemiologic studies. Int. J. Cancer 2014, 134, 384–396. [Google Scholar] [CrossRef]
- Colt, J.S.; Schwartz, K.; Graubard, B.I.; Davis, F.; Ruterbusch, J.; DiGaetano, R.; Purdue, M.; Rothman, N.; Wacholder, S.; Chow, W.-H. Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology 2011, 22, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Du, X.; Zou, S.Y.; Shi, B.M. Blood pressure and kidney cancer risk: Meta-analysis of prospective studies. J. Hypertens. 2017, 35, 1333–1344. [Google Scholar] [CrossRef]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar] [CrossRef]
- Tossetta, G.; Marzioni, D. Targeting the NRF2/KEAP1 pathway in cervical and endometrial cancers. Eur. J. Pharmacol. 2023, 941, 175503. [Google Scholar] [CrossRef]
- Campagna, R.; Mateuszuk, L.; Wojnar-Lason, K.; Kaczara, P.; Tworzydło, A.; Kij, A.; Bujok, R.; Mlynarski, J.; Wang, Y.; Sartini, D.; et al. Nicotinamide N-methyltransferase in endothelium protects against oxidant stress-induced endothelial injury. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119082. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Marzioni, D. Natural and synthetic compounds in Ovarian Cancer: A focus on NRF2/KEAP1 pathway. Pharmacol. Res. 2022, 183, 106365. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, R.; Mathai, M.; Zulli, A. Cytoprotective remedies for ameliorating nephrotoxicity induced by renal oxidative stress. Life Sci. 2023, 318, 121466. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Montanari, E.; Marzioni, D.; Goteri, G. Role of NRF2 in Ovarian Cancer. Antioxidants 2022, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Ishikane, S.; Takahashi-Yanaga, F. The role of angiotensin II in cancer metastasis: Potential of renin-angiotensin system blockade as a treatment for cancer metastasis. Biochem. Pharmacol. 2018, 151, 96–103. [Google Scholar] [CrossRef]
- Graff, R.E.; Sanchez, A.; Tobias, D.K.; Rodríguez, D.; Barrisford, G.W.; Blute, M.L.; Li, Y.; Sun, Q.; Preston, M.A.; Wilson, K.M.; et al. Type 2 Diabetes in Relation to the Risk of Renal Cell Carcinoma Among Men and Women in Two Large Prospective Cohort Studies. Diabetes Care 2018, 41, 1432–1437. [Google Scholar] [CrossRef]
- Bao, C.; Yang, X.; Xu, W.; Luo, H.; Xu, Z.; Su, C.; Qi, X. Diabetes mellitus and incidence and mortality of kidney cancer: A meta-analysis. J. Diabetes Its Complicat. 2013, 27, 357–364. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, P.; Tian, R.; He, J.; Han, Q.; Fan, L. Metabolic Syndrome is an Independent Risk Factor for Fuhrman Grade and TNM Stage of Renal Clear Cell Carcinoma. Int. J. Gen. Med. 2022, 15, 143–150. [Google Scholar] [CrossRef]
- Morrissey, J.J.; Kharasch, E.D. The Specificity of Urinary Aquaporin 1 and Perilipin 2 to Screen for Renal Cell Carcinoma. J. Urol. 2013, 189, 1913–1920. [Google Scholar] [CrossRef]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A.; ESMO Guidelines Committee. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, H.; Hu, X.; Liu, A.; Huang, D.; Xu, Y.; Chen, L.; Xu, D. Development and validation of a metastasis-associated prognostic signature based on single-cell RNA-seq in clear cell renal cell carcinoma. Aging 2019, 11, 10183–10202. [Google Scholar] [CrossRef] [PubMed]
- Dudani, S.; de Velasco, G.; Wells, J.C.; Gan, C.L.; Donskov, F.; Porta, C.; Fraccon, A.; Pasini, F.; Lee, J.L.; Hansen, A.; et al. Evaluation of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma Metastasis Sites and Association with Survival. JAMA Netw. Open 2021, 4, e2021869. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.H.; Prezzi, D.; Kelly-Morland, C.; Goh, V. Imaging for the diagnosis and response assessment of renal tumours. World J. Urol. 2018, 36, 1927–1942. [Google Scholar] [CrossRef]
- Volpe, A.; Kachura, J.R.; Geddie, W.R.; Evans, A.J.; Gharajeh, A.; Saravanan, A.; Jewett, M.A. Techniques, Safety and Accuracy of Sampling of Renal Tumors by Fine Needle Aspiration and Core Biopsy. J. Urol. 2007, 178, 379–386. [Google Scholar] [CrossRef]
- Ballman, K.V. Biomarker: Predictive or Prognostic? J. Clin. Oncol. 2015, 33, 3968–3971. [Google Scholar] [CrossRef]
- Klatte, T.; Rossi, S.H.; Stewart, G.D. Prognostic factors and prognostic models for renal cell carcinoma: A literature review. World J. Urol. 2018, 36, 1943–1952. [Google Scholar] [CrossRef]
- Ficarra, V.; Galfano, A.; Mancini, M.; Martignoni, G.; Artibani, W. TNM staging system for renal-cell carcinoma: Current status and future perspectives. Lancet Oncol. 2007, 8, 554–558. [Google Scholar] [CrossRef]
- Sun, M.; Shariat, S.F.; Cheng, C.; Ficarra, V.; Murai, M.; Oudard, S.; Pantuck, A.J.; Zigeuner, R.; Karakiewicz, P.I. Prognostic Factors and Predictive Models in Renal Cell Carcinoma: A Contemporary Review. Eur. Urol. 2011, 60, 644–661. [Google Scholar] [CrossRef]
- Fuhrman, S.A.; Lasky, L.C.; Limas, C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 1982, 6, 655–664. [Google Scholar] [CrossRef]
- Delahunt, B.; Srigley, J.R.; Egevad, L.; Montironi, R.; International Society for Urological Pathology. International Society of Urological Pathology Grading and Other Prognostic Factors for Renal Neoplasia. Eur. Urol. 2014, 66, 795–798. [Google Scholar] [CrossRef]
- Delahunt, B.; McKenney, J.K.; Lohse, C.M.; Leibovich, B.C.; Thompson, R.H.; Boorjian, S.A.; Cheville, J.C. A Novel Grading System for Clear Cell Renal Cell Carcinoma Incorporating Tumor Necrosis. Am. J. Surg. Pathol. 2013, 37, 311–322. [Google Scholar] [CrossRef]
- Bedke, J.; Heide, J.; Ribback, S.; Rausch, S.; de Martino, M.; Scharpf, M.; Haitel, A.; Zimmermann, U.; Pechoel, M.; Alkhayyat, H.; et al. Microvascular and lymphovascular tumour invasion are associated with poor prognosis and metastatic spread in renal cell carcinoma: A validation study in clinical practice. BJU Int. 2018, 121, 84–92. [Google Scholar] [CrossRef]
- Cheaib, J.G.; Patel, H.D.; Johnson, M.H.; Gorin, M.A.; Haut, E.R.; Canner, J.K.; Allaf, M.E.; Pierorazio, P.M. Stage-specific conditional survival in renal cell carcinoma after nephrectomy. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 6.e1–6.e7. [Google Scholar] [CrossRef]
- Delahunt, B.; Cheville, J.C.; Martignoni, G.; Humphrey, P.A.; Magi-Galluzzi, C.; McKenney, J.; Egevad, L.; Algaba, F.; Moch, H.; Grignon, D.J.; et al. The International Society of Urological Pathology (ISUP) Grading System for Renal Cell Carcinoma and Other Prognostic Parameters. Am. J. Surg. Pathol. 2013, 37, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Leibovich, B.C.; Lohse, C.M.; Cheville, J.C.; Zaid, H.B.; Boorjian, S.A.; Frank, I.; Thompson, R.H.; Parker, W.P. Predicting Oncologic Outcomes in Renal Cell Carcinoma After Surgery. Eur. Urol. 2018, 73, 772–780. [Google Scholar] [CrossRef]
- Motzer, R.J.; Mazumdar, M.; Bacik, J.; Berg, W.; Amsterdam, A.; Ferrara, J. Survival and Prognostic Stratification of 670 Patients With Advanced Renal Cell Carcinoma. J. Clin. Oncol. 1999, 17, 2530. [Google Scholar] [CrossRef] [PubMed]
- Guida, A.; Le Teuff, G.; Alves, C.; Colomba, E.; Di Nunno, V.; Derosa, L.; Flippot, R.; Escudier, B.; Albiges, L. Identification of international metastatic renal cell carcinoma database consortium (IMDC) intermediate-risk subgroups in patients with metastatic clear-cell renal cell carcinoma. Oncotarget 2020, 11, 4582–4592. [Google Scholar] [CrossRef] [PubMed]
- Köhn, L.; Svenson, U.; Ljungberg, B.; Roos, G. Specific Genomic Aberrations Predict Survival, But Low Mutation Rate in Cancer Hot Spots, in Clear Cell Renal Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 334–342. [Google Scholar] [CrossRef]
- Klatte, T.; Rao, P.N.; de Martino, M.; LaRochelle, J.; Shuch, B.; Zomorodian, N.; Said, J.; Kabbinavar, F.F.; Belldegrun, A.S.; Pantuck, A.J. Cytogenetic Profile Predicts Prognosis of Patients With Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2009, 27, 746–753. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Kaelin, W.G., Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat. Med. 2020, 26, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Miikkulainen, P.; Högel, H.; Seyednasrollah, F.; Rantanen, K.; Elo, L.L.; Jaakkola, P.M. Hypoxia-inducible factor (HIF)-prolyl hydroxylase 3 (PHD3) maintains high HIF2A mRNA levels in clear cell renal cell carcinoma. J. Biol. Chem. 2019, 294, 3760–3771. [Google Scholar] [CrossRef] [PubMed]
- Gayed, B.A.; Youssef, R.F.; Bagrodia, A.; Darwish, O.M.; Kapur, P.; Sagalowsky, A.; Lotan, Y.; Margulis, V. Ki67 is an independent predictor of oncological outcomes in patients with localized clear-cell renal cell carcinoma. BJU Int. 2014, 113, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-G.; Li, S.-Q.; Li, Z.-J.; Zhu, X.-J.; Xu, P.; Liu, G.; Shi, Z.-G.; Li, S.-Q.; Li, Z.-J.; Zhu, X.-J.; et al. Expression of vimentin and survivin in clear cell renal cell carcinoma and correlation with p53. Clin. Transl. Oncol. 2015, 17, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wei, J.; Tian, X.; Li, Y.; Li, X. Prognostic role of PPAR-gamma and PTEN in the renal cell carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 12668–12677. [Google Scholar] [PubMed]
- Brannon, A.R.; Reddy, A.; Seiler, M.; Arreola, A.; Moore, D.T.; Pruthi, R.S.; Wallen, E.M.; Nielsen, M.E.; Liu, H.; Nathanson, K.L.; et al. Molecular Stratification of Clear Cell Renal Cell Carcinoma by Consensus Clustering Reveals Distinct Subtypes and Survival Patterns. Genes Cancer 2010, 1, 152–163. [Google Scholar] [CrossRef]
- Brooks, S.A.; Brannon, A.R.; Parker, J.S.; Fisher, J.C.; Sen, O.; Kattan, M.W.; Hakimi, A.A.; Hsieh, J.J.; Choueiri, T.K.; Tamboli, P.; et al. ClearCode34: A Prognostic Risk Predictor for Localized Clear Cell Renal Cell Carcinoma. Eur. Urol. 2014, 66, 77–84. [Google Scholar] [CrossRef]
- Terry, S.; Dalban, C.; Rioux-Leclercq, N.; Adam, J.; Meylan, M.; Buart, S.; Bougoüin, A.; Lespagnol, A.; Dugay, F.; Moreno, I.C.; et al. Association of AXL and PD-L1 Expression with Clinical Outcomes in Patients with Advanced Renal Cell Carcinoma Treated with PD-1 Blockade. Clin. Cancer Res. 2021, 27, 6749–6760. [Google Scholar] [CrossRef]
- Licini, C.; Avellini, C.; Picchiassi, E.; Mensà, E.; Fantone, S.; Ramini, D.; Tersigni, C.; Tossetta, G.; Castellucci, C.; Tarquini, F.; et al. Pre-eclampsia predictive ability of maternal miR-125b: A clinical and experimental study. Transl. Res. 2021, 228, 13–27. [Google Scholar] [CrossRef]
- Avellini, C.; Licini, C.; Lazzarini, R.; Gesuita, R.; Guerra, E.; Tossetta, G.; Castellucci, C.; Giannubilo, S.R.; Procopio, A.; Alberti, S.; et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget 2017, 8, 58642–58653. [Google Scholar] [CrossRef]
- Marzioni, D.; Mazzucchelli, R.; Fantone, S.; Tossetta, G. NRF2 modulation in TRAMP mice: An in vivo model of prostate cancer. Mol. Biol. Rep. 2023, 50, 873–881. [Google Scholar] [CrossRef]
- Nuzzo, P.V.; Berchuck, J.E.; Korthauer, K.; Spisak, S.; Nassar, A.H.; Abou Alaiwi, S.; Chakravarthy, A.; Shen, S.Y.; Bakouny, Z.; Boccardo, F.; et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat. Med. 2020, 26, 1041–1043. [Google Scholar] [CrossRef]
- Minardi, D.; Lucarini, G.; Filosa, A.; Milanese, G.; Zizzi, A.; Di Primio, R.; Montironi, R.; Muzzonigro, G. Prognostic role of global DNA-methylation and histone acetylation in pT1a clear cell renal carcinoma in partial nephrectomy specimens. J. Cell. Mol. Med. 2009, 13, 2115–2121. [Google Scholar] [CrossRef]
- Tito, C.; De Falco, E.; Rosa, P.; Iaiza, A.; Fazi, F.; Petrozza, V.; Calogero, A. Circulating microRNAs from the Molecular Mechanisms to Clinical Biomarkers: A Focus on the Clear Cell Renal Cell Carcinoma. Genes 2021, 12, 1154. [Google Scholar] [CrossRef]
- Iwamoto, H.; Kanda, Y.; Sejima, T.; Osaki, M.; Okada, F.; Takenaka, A. Serum miR-210 as a potential biomarker of early clear cell renal cell carcinoma. Int. J. Oncol. 2014, 44, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, F.G.; Tolkach, Y.; Deng, M.; Schmidt, D.; Perner, S.; Kristiansen, G.; Müller, S.C.; Ellinger, J. Serum miR-122-5p and miR-206 expression: Non-invasive prognostic biomarkers for renal cell carcinoma. Clin. Epigenet. 2018, 10, 11. [Google Scholar] [CrossRef]
- Sun, X.; Lou, L.; Zhong, K.; Wan, L. MicroRNA-451 regulates chemoresistance in renal cell carcinoma by targeting ATF-2 gene. Exp. Biol. Med. 2017, 242, 1299–1305. [Google Scholar] [CrossRef]
- Petrozza, V.; Pastore, A.L.; Palleschi, G.; Tito, C.; Porta, N.; Ricci, S.; Marigliano, C.; Costantini, M.; Simone, G.; Di Carlo, A.; et al. Secreted miR-210-3p as non-invasive biomarker in clear cell renal cell carcinoma. Oncotarget 2017, 8, 69551–69558. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.; Eburn, E. Patients’ and nurses’ perceptions of symptom distress in cancer. J. Adv. Nurs. 1989, 14, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Cochetti, G.; Cari, L.; Nocentini, G.; Maula, V.; Suvieri, C.; Cagnani, R.; Rossi De Vermandois, J.A.; Mearini, E. Detection of urinary miRNAs for diagnosis of clear cell renal cell carcinoma. Sci. Rep. 2020, 10, 21290. [Google Scholar] [CrossRef]
- Kubiliute, R.; Jarmalaite, S. Epigenetic Biomarkers of Renal Cell Carcinoma for Liquid Biopsy Tests. Int. J. Mol. Sci. 2021, 22, 8846. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.-Q.; Weng, W.-W.; Zhang, Q.-Y.; Yang, X.-Q.; Gan, H.-L.; Yang, Y.-S.; Zhang, P.-P.; Sun, M.-H.; Xu, M.-D.; et al. A serum-circulating long noncoding RNA signature can discriminate between patients with clear cell renal cell carcinoma and healthy controls. Oncogenesis 2016, 5, e192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, J.; Ma, S.; Zhang, S. Long Noncoding RNA MALAT-1 Can Predict Metastasis and a Poor Prognosis: A Meta-Analysis. Pathol. Oncol. Res. 2015, 21, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Li, Z.; Wei, D.; Chen, H.; Yang, C. LncRNA, NEAT1 is a prognosis biomarker and regulates cancer progression via epithelial-mesenchymal transition in clear cell renal cell carcinoma. Cancer Biomarkers 2017, 19, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Ao, J.; Zhao, F.; Xu, G.; Chen, H.; Gao, C. The roles of long non-coding RNAs in renal cell carcinoma (Review). Mol. Clin. Oncol. 2023, 18, 4. [Google Scholar] [CrossRef]
- Su, H.; Sun, T.; Wang, H.; Shi, G.; Zhang, H.; Sun, F.; Ye, D. Decreased TCL6 expression is associated with poor prognosis in patients with clear cell renal cell carcinoma. Oncotarget 2017, 8, 5789–5799. [Google Scholar] [CrossRef]
- Qiao, H.-P.; Gao, W.-S.; Huo, J.-X.; Yang, Z.-S. Long Non-coding RNA GAS5 Functions as a Tumor Suppressor in Renal Cell Carcinoma. Asian Pac. J. Cancer Prev. 2013, 14, 1077–1082. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, F.; Wei, D.; Liu, B.; Chen, C.; Bao, Y.; Wu, Z.; Wu, D.; Tan, H.; Li, J.; et al. Long noncoding RNA-SRLR elicits intrinsic sorafenib resistance via evoking IL-6/STAT3 axis in renal cell carcinoma. Oncogene 2017, 36, 1965–1977. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, Z.; Cheng, X.; Yang, H.; Gong, B.; Zhou, X.; Zhang, C.; Wang, X.; Wang, G. Downregulation of RAB17 have a poor prognosis in kidney renal clear cell carcinoma and its expression correlates with DNA methylation and immune infiltration. Cell. Signal. 2023, 109, 110743. [Google Scholar] [CrossRef]
- Flammia, R.S.; Tufano, A.; Proietti, F.; Gerolimetto, C.; DE Nunzio, C.; Franco, G.; Leonardo, C. Renal surgery for kidney cancer: Is preoperative proteinuria a predictor of functional and survival outcomes after surgery? A systematic review of the literature. Minerva Urol. Nephrol. 2022, 74, 255–264. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, J.; Zabell, J.; Remer, E.; Li, J.; Campbell, J.; Dong, W.; Palacios, D.A.; Patel, T.; Demirjian, S.; et al. Proteinuria in Patients Undergoing Renal Cancer Surgery: Impact on Overall Survival and Stability of Renal Function. Eur. Urol. Focus 2016, 2, 616–622. [Google Scholar] [CrossRef]
- O’donnell, K.; Tourojman, M.; Tobert, C.M.; Kirmiz, S.W.; Riedinger, C.B.; Demirjian, S.; Lane, B.R. Proteinuria is a Predictor of Renal Functional Decline in Patients with Kidney Cancer. J. Urol. 2016, 196, 658–663. [Google Scholar] [CrossRef]
- Larcher, A.; Wallis, C.J.D.; Bex, A.; Blute, M.L.; Ficarra, V.; Mejean, A.; Karam, J.A.; Van Poppel, H.; Pal, S.K. Individualised Indications for Cytoreductive Nephrectomy: Which Criteria Define the Optimal Candidates? Eur. Urol. Oncol. 2019, 2, 365–378. [Google Scholar] [CrossRef]
- Christensen, M.; Hannan, R. The Emerging Role of Radiation Therapy in Renal Cell Carcinoma. Cancers 2022, 14, 4693. [Google Scholar] [CrossRef] [PubMed]
- Serzan, M.T.; Atkins, M.B. Current and emerging therapies for first line treatment of metastatic clear cell renal cell carcinoma. J. Cancer Metastasis Treat. 2021, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Pluzanska, A.; Koralewski, P.; Ravaud, A.; Bracarda, S.; Szczylik, C.; Chevreau, C.; Filipek, M.; Melichar, B.; Bajetta, E.; et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet 2007, 370, 2103–2111. [Google Scholar] [CrossRef]
- Chatzkel, J.; Ramnaraign, B.; Sonpavde, G. Tivozanib for the treatment of advanced renal cell carcinoma. Expert Opin. Pharmacother. 2022, 23, 1135–1142. [Google Scholar] [CrossRef]
- Fallah, J.; Weinstock, C.; Mehta, G.U.; Brave, M.H.; Pierce, W.F.; Pazdur, R.; Nair, A.; Suzman, D.L.; Amiri-Kordestani, L. FDA Approval Summary: Belzutifan for VHL Disease Tumors—Response. Clin. Cancer Res. 2023, 29, 685. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Klapper, J.A.; Downey, S.G.; Smith, F.O.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Sherry, R.M.; Royal, R.E.; Steinberg, S.M.; Rosenberg, S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 2008, 113, 293–301. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Plimack, E.R.; Puzanov, I.; Fishman, M.N.; McDermott, D.F.; Cho, D.C.; Vaishampayan, U.; George, S.; Olencki, T.E.; Tarazi, J.C.; et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: A non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018, 19, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Larkin, J.; Oya, M.; Thistlethwaite, F.; Martignoni, M.; Nathan, P.; Powles, T.; McDermott, D.; Robbins, P.B.; Chism, D.D.; et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): An open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018, 19, 451–460. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Z.; Liu, L.; Xuan, B. Assessment of the prognostic value of SPOCK1 in clear cell renal cell carcinoma: A bioinformatics analysis. Transl. Androl. Urol. 2022, 11, 509–518. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Wen, Y.-C.; Hsiao, C.-H.; Lai, F.-R.; Yang, S.-F.; Yang, Y.-C.; Ho, K.-H.; Hsieh, F.-K.; Hsiao, M.; Lee, W.-J.; et al. Proteoglycan SPOCK1 as a Poor Prognostic Marker Promotes Malignant Progression of Clear Cell Renal Cell Carcinoma via Triggering the Snail/Slug-MMP-2 Axis-Mediated Epithelial-to-Mesenchymal Transition. Cells 2023, 12, 352. [Google Scholar] [CrossRef]
- Goteri, G.; Altobelli, E.; Tossetta, G.; Zizzi, A.; Avellini, C.; Licini, C.; Lorenzi, T.; Castellucci, M.; Ciavattini, A.; Marzioni, D. High temperature requirement A1, transforming growth factor beta1, phosphoSmad2 and Ki67 in eutopic and ectopic endometrium of women with endometriosis. Eur. J. Histochem. 2015, 59, 2570. [Google Scholar] [CrossRef]
- Tossetta, G.; Avellini, C.; Licini, C.; Giannubilo, S.R.; Castellucci, M.; Marzioni, D. High temperature requirement A1 and fibronectin: Two possible players in placental tissue remodelling. Eur. J. Histochem. 2016, 60, 2724. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Licini, C.; Marzioni, D.; Mattioli-Belmonte, M. The multifaced role of HtrA1 in the development of joint and skeletal disorders. Bone 2022, 157, 116350. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Busilacchi, E.M.; Di Simone, N.; Giannubilo, S.R.; Scambia, G.; Giordano, A.; Marzioni, D. Modulation of matrix metalloproteases by ciliary neurotrophic factor in human placental development. Cell Tissue Res. 2022, 390, 113–129. [Google Scholar] [CrossRef]
- Chrabanska, M.; Rynkiewicz, M.; Kiczmer, P.; Drozdzowska, B. Does the Immunohistochemical Expression of CD44, MMP-2, and MMP-9 in Association with the Histopathological Subtype of Renal Cell Carcinoma Affect the Survival of Patients with Renal Cancer? Cancers 2023, 15, 1202. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Kim, J.M.; Lee, H.J.; Seong, I.-O.; Kim, K.-H. Immunohistochemical expression of CD44, matrix metalloproteinase2 and matrix metalloproteinase9 in renal cell carcinomas. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, H.; Lu, Y.; Huang, Y.; Xuan, Y.; Li, X.; Guo, T.; Wang, C.; Lai, D.; Wu, S.; et al. GTSE1 promotes tumor growth and metastasis by attenuating of KLF4 expression in clear cell renal cell carcinoma. Lab. Investig. 2022, 102, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Min, P.; Zhao, S.; Liu, L.; Zhang, Y.; Ma, Y.; Zhao, X.; Wang, Y.; Song, Y.; Zhu, C.; Jiang, H.; et al. MICAL-L2 potentiates Cdc42-dependent EGFR stability and promotes gastric cancer cell migration. J. Cell. Mol. Med. 2019, 23, 4475–4488. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, H.; Yang, J.; Hu, J. Micall2 Is Responsible for the Malignancy of Clear Cell Renal Cell Carcinoma. Yonago Acta Medica 2023, 66, 171–179. [Google Scholar] [CrossRef]
- Duhachek-Muggy, S.; Qi, Y.; Wise, R.; Alyahya, L.; Li, H.; Hodge, J.; Zolkiewska, A. Metalloprotease-disintegrin ADAM12 actively promotes the stem cell-like phenotype in claudin-low breast cancer. Mol. Cancer 2017, 16, 32. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Jiang, J.; Yin, C.; Shi, B. ADAM12 promotes clear cell renal cell carcinoma progression and triggers EMT via EGFR/ERK signaling pathway. J. Transl. Med. 2023, 21, 56. [Google Scholar] [CrossRef]
- Lingui, X.; Weifeng, L.; Yufei, W.; Yibin, Z. High SPATA18 Expression and its Diagnostic and Prognostic Value in Clear Cell Renal Cell Carcinoma. Med. Sci. Monit. 2023, 29, e938474. [Google Scholar] [CrossRef]
- Meng, K.; Hu, Y.; Wang, D.; Li, Y.; Shi, F.; Lu, J.; Wang, Y.; Cao, Y.; Zhang, C.Z.; He, Q.-Y. EFHD1, a novel mitochondrial regulator of tumor metastasis in clear cell renal cell carcinoma. Cancer Sci. 2023, 114, 2029–2040. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Xu, J.; Ang, X.; Ge, N.; Xu, M.; Pei, C. Identify AGAP2 as prognostic biomarker in clear cell renal cell carcinoma based on bioinformatics and IHC staining. Heliyon 2023, 9, e13543. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Shen, D.; Hu, X.; Ke, Z.; Ehrich Lister, I.N.; Sihombing, B. Prognostic significance of CKAP2L expression in patients with clear cell renal cell carcinoma. Front. Genet. 2022, 13, 873884. [Google Scholar] [CrossRef]
- Yao, H.; Lyu, F.; Ma, J.; Sun, F.; Tang, G.; Wu, J.; Zhou, Z. PIMREG is a prognostic biomarker involved in immune microenvironment of clear cell renal cell carcinoma and associated with the transition from G1 phase to S phase. Front. Oncol. 2023, 13, 1035321. [Google Scholar] [CrossRef]
- Campagna, R.; Vignini, A. NAD(+) Homeostasis and NAD(+)-Consuming Enzymes: Implications for Vascular Health. Antioxidants 2023, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Roberti, A.; Fernández, A.F.; Fraga, M.F. Nicotinamide N-methyltransferase: At the crossroads between cellular metabolism and epigenetic regulation. Mol. Metab. 2021, 45, 101165. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Zhai, B.; Pissios, P. Nicotinamide N-Methyltransferase Interacts with Enzymes of the Methionine Cycle and Regulates Methyl Donor Metabolism. Biochemistry 2018, 57, 5775–5779. [Google Scholar] [CrossRef]
- Togni, L.; Mascitti, M.; Sartini, D.; Campagna, R.; Pozzi, V.; Salvolini, E.; Offidani, A.; Santarelli, A.; Emanuelli, M. Nicotinamide N-Methyltransferase in Head and Neck Tumors: A Comprehensive Review. Biomolecules 2021, 11, 1594. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, J.; Wu, W.; Xie, S.; Yu, H.; Li, G.; Zhu, T.; Li, F.; Lu, J.; Wang, G.Y.; et al. Nicotinamide N-methyltransferase enhances chemoresistance in breast cancer through SIRT1 protein stabilization. Breast Cancer Res. 2019, 21, 51. [Google Scholar] [CrossRef]
- Pozzi, V.; Campagna, R.; Sartini, D.; Emanuelli, M. Nicotinamide N-Methyltransferase as Promising Tool for Management of Gastrointestinal Neoplasms. Biomolecules 2022, 12, 1173. [Google Scholar] [CrossRef]
- Li, J.; You, S.; Zhang, S.; Hu, Q.; Wang, F.; Chi, X.; Zhao, W.; Xie, C.; Zhang, C.; Yu, Y.; et al. Elevated N-methyltransferase expression induced by hepatic stellate cells contributes to the metastasis of hepatocellular carcinoma via regulation of the CD44v3 isoform. Mol. Oncol. 2019, 13, 1993–2009. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zou, Y.; Cai, Z.; Huang, J.; Hong, X.; Liang, Q.; Jin, W. Overexpression of NNMT in Glioma Aggravates Tumor Cell Progression: An Emerging Therapeutic Target. Cancers 2022, 14, 3538. [Google Scholar] [CrossRef]
- Sartini, D.; Molinelli, E.; Pozzi, V.; Campagna, R.; Salvolini, E.; Rubini, C.; Goteri, G.; Simonetti, O.; Campanati, A.; Offidani, A.; et al. Immunohistochemical expression of nicotinamide N-methyltransferase in lymph node metastases from cutaneous malignant melanoma. Hum. Cell 2023, 36, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Lei, Y.; Chu, Y.; Yu, X.; Tong, Q.; Zhu, T.; Yu, H.; Fang, S.; Li, G.; et al. NNMT contributes to high metastasis of triple negative breast cancer by enhancing PP2A/MEK/ERK/c-Jun/ABCA1 pathway mediated membrane fluidity. Cancer Lett. 2022, 547, 215884. [Google Scholar] [CrossRef]
- Yao, M.; Tabuchi, H.; Nagashima, Y.; Baba, M.; Nakaigawa, N.; Ishiguro, H.; Hamada, K.; Inayama, Y.; Kishida, T.; Hattori, K.; et al. Gene expression analysis of renal carcinoma: Adipose differentiation-related protein as a potential diagnostic and prognostic biomarker for clear-cell renal carcinoma. J. Pathol. 2005, 205, 377–387. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, X.-Y.; Yang, S.-W.; Wang, J.; He, C. Nicotinamide N-methyltransferase protein expression in renal cell cancer. J. Zhejiang Univ. Sci. B 2010, 11, 136–143. [Google Scholar] [CrossRef]
- Kim, D.S.; Choi, Y.P.; Kang, S.; Gao, M.Q.; GIL Kim, B.; Park, H.R.; Choi, Y.D.; Lim, J.-B.; Na, H.J.; Kim, H.K.; et al. Panel of Candidate Biomarkers for Renal Cell Carcinoma. J. Proteome Res. 2010, 9, 3710–3719. [Google Scholar] [CrossRef]
- Su Kim, D.; Choi, Y.D.; Moon, M.; Kang, S.; Lim, J.-B.; Kim, K.M.; Park, K.M.; Cho, N.H. Composite Three-Marker Assay for Early Detection of Kidney Cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Ham, W.S.; Jang, W.S.; Cho, K.S.; Choi, Y.D.; Kang, S.; Kim, B.; Kim, K.J.; Lim, E.J.; Rha, S.Y.; et al. Scale-Up Evaluation of a Composite Tumor Marker Assay for the Early Detection of Renal Cell Carcinoma. Diagnostics 2020, 10, 750. [Google Scholar] [CrossRef]
- Tang, S.-W.; Yang, T.-C.; Lin, W.-C.; Chang, W.-H.; Wang, C.-C.; Lai, M.-K.; Lin, J.-Y. Nicotinamide N -methyltransferase induces cellular invasion through activating matrix metalloproteinase-2 expression in clear cell renal cell carcinoma cells. Carcinogenesis 2011, 32, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Song, J.; Lee, H.; Kim, E.-Y.; Lee, K.; Lee, S.K.; Kim, S. Design, Synthesis, and Biological Activity of Sulfonamide Analogues of Antofine and Cryptopleurine as Potent and Orally Active Antitumor Agents. J. Med. Chem. 2015, 58, 7749–7762. [Google Scholar] [CrossRef]
- Campagna, R.; Pozzi, V.; Spinelli, G.; Sartini, D.; Milanese, G.; Galosi, A.B.; Emanuelli, M. The Utility of Nicotinamide N-Methyltransferase as a Potential Biomarker to Predict the Oncological Outcomes for Urological Cancers: An Update. Biomolecules 2021, 11, 1214. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Nguyen, T.H.; Kim, H.S.; Dao, T.T.P.; Moon, Y.; Seo, M.; Kang, S.; Mai, V.-H.; An, Y.J.; Jung, C.-R.; et al. GPX8 regulates clear cell renal cell carcinoma tumorigenesis through promoting lipogenesis by NNMT. J. Exp. Clin. Cancer Res. 2023, 42, 42. [Google Scholar] [CrossRef] [PubMed]
- Reustle, A.; Menig, L.S.; Leuthold, P.; Hofmann, U.; Stühler, V.; Schmees, C.; Becker, M.; Haag, M.; Klumpp, V.; Winter, S.; et al. Nicotinamide-N-methyltransferase is a promising metabolic drug target for primary and metastatic clear cell renal cell carcinoma. Clin. Transl. Med. 2022, 12, e883. [Google Scholar] [CrossRef] [PubMed]
- Barrows, R.D.; Jeffries, D.E.; Vishe, M.; Tukachinsky, H.; Zheng, S.-L.; Li, F.; Ma, Z.; Li, X.; Jin, S.; Song, H.; et al. Potent Uncompetitive Inhibitors of Nicotinamide N-Methyltransferase (NNMT) as In Vivo Chemical Probes. J. Med. Chem. 2022, 65, 14642–14654. [Google Scholar] [CrossRef] [PubMed]
- van Haren, M.J.; Zhang, Y.; Thijssen, V.; Buijs, N.; Gao, Y.; Mateuszuk, L.; Fedak, F.A.; Kij, A.; Campagna, R.; Sartini, D.; et al. Macrocyclic peptides as allosteric inhibitors of nicotinamide N-methyltransferase (NNMT). RSC Chem. Biol. 2021, 2, 1546–1555. [Google Scholar] [CrossRef]
- Hayashi, K.; Uehara, S.; Yamamoto, S.; Cary, D.R.; Nishikawa, J.; Ueda, T.; Ozasa, H.; Mihara, K.; Yoshimura, N.; Kawai, T.; et al. Macrocyclic Peptides as a Novel Class of NNMT Inhibitors: A SAR Study Aimed at Inhibitory Activity in the Cell. ACS Med. Chem. Lett. 2021, 12, 1093–1101. [Google Scholar] [CrossRef]
- Gao, Y.; van Haren, M.J.; Buijs, N.; Innocenti, P.; Zhang, Y.; Sartini, D.; Campagna, R.; Emanuelli, M.; Parsons, R.B.; Jespers, W.; et al. Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics. J. Med. Chem. 2021, 64, 12938–12963. [Google Scholar] [CrossRef]
- van Haren, M.J.; Gao, Y.; Buijs, N.; Campagna, R.; Sartini, D.; Emanuelli, M.; Mateuszuk, L.; Kij, A.; Chlopicki, S.; Escude Martinez de Castilla, P.; et al. Esterase-Sensitive Prodrugs of a Potent Bisubstrate Inhibitor of Nicotinamide N-Methyltransferase (NNMT) Display Cellular Activity. Biomolecules 2021, 11, 1357. [Google Scholar] [CrossRef]
- Ye, S.; Li, S.; Qin, L.; Zheng, W.; Liu, B.; Li, X.; Ren, Z.; Zhao, H.; Hu, X.; Ye, N.; et al. GBP2 promotes clear cell renal cell carcinoma progression through immune infiltration and regulation of PD-L1 expression via STAT1 signaling. Oncol. Rep. 2023, 49, 49. [Google Scholar] [CrossRef]
- Getu, A.A.; Tigabu, A.; Zhou, M.; Lu, J.; Fodstad, O.; Tan, M. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol. Cancer 2023, 22, 43. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Y.; Guo, C.; Liu, T.; Fei, X.; Chang, C. Androgen receptor regulates ASS1P3/miR-34a-5p/ASS1 signaling to promote renal cell carcinoma cell growth. Cell Death Dis. 2019, 10, 339. [Google Scholar] [CrossRef]
- Huang, Q.; Sun, Y.; Ma, X.; Gao, Y.; Li, X.; Niu, Y.; Zhang, X.; Chang, C. Androgen receptor increases hematogenous metastasis yet decreases lymphatic metastasis of renal cell carcinoma. Nat. Commun. 2017, 8, 918. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Sun, Y.; Guo, C.; Hu, G.; Wang, M.; Zheng, J.; Lin, W.; Huang, Q.; Li, G.; Zheng, J.; et al. LncRNA-SARCC suppresses renal cell carcinoma (RCC) progression via altering the androgen receptor(AR)/miRNA-143-3p signals. Cell Death Differ. 2017, 24, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavoni, V.; Campagna, R.; Pozzi, V.; Cecati, M.; Milanese, G.; Sartini, D.; Salvolini, E.; Galosi, A.B.; Emanuelli, M. Recent Advances in the Management of Clear Cell Renal Cell Carcinoma: Novel Biomarkers and Targeted Therapies. Cancers 2023, 15, 3207. https://doi.org/10.3390/cancers15123207
Schiavoni V, Campagna R, Pozzi V, Cecati M, Milanese G, Sartini D, Salvolini E, Galosi AB, Emanuelli M. Recent Advances in the Management of Clear Cell Renal Cell Carcinoma: Novel Biomarkers and Targeted Therapies. Cancers. 2023; 15(12):3207. https://doi.org/10.3390/cancers15123207
Chicago/Turabian StyleSchiavoni, Valentina, Roberto Campagna, Valentina Pozzi, Monia Cecati, Giulio Milanese, Davide Sartini, Eleonora Salvolini, Andrea Benedetto Galosi, and Monica Emanuelli. 2023. "Recent Advances in the Management of Clear Cell Renal Cell Carcinoma: Novel Biomarkers and Targeted Therapies" Cancers 15, no. 12: 3207. https://doi.org/10.3390/cancers15123207
APA StyleSchiavoni, V., Campagna, R., Pozzi, V., Cecati, M., Milanese, G., Sartini, D., Salvolini, E., Galosi, A. B., & Emanuelli, M. (2023). Recent Advances in the Management of Clear Cell Renal Cell Carcinoma: Novel Biomarkers and Targeted Therapies. Cancers, 15(12), 3207. https://doi.org/10.3390/cancers15123207












