Higher Content but No Specific Activity in Gelatinase B (MMP-9) Compared with Gelatinase A (MMP-2) in Human Renal Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Sample
2.2. Gelatin Zymography
2.3. Total Content Evaluation with ELISA
2.4. Western Blot
2.5. Gelatinase Activity
2.6. Protein Determination
2.7. Statistical Analysis
3. Results
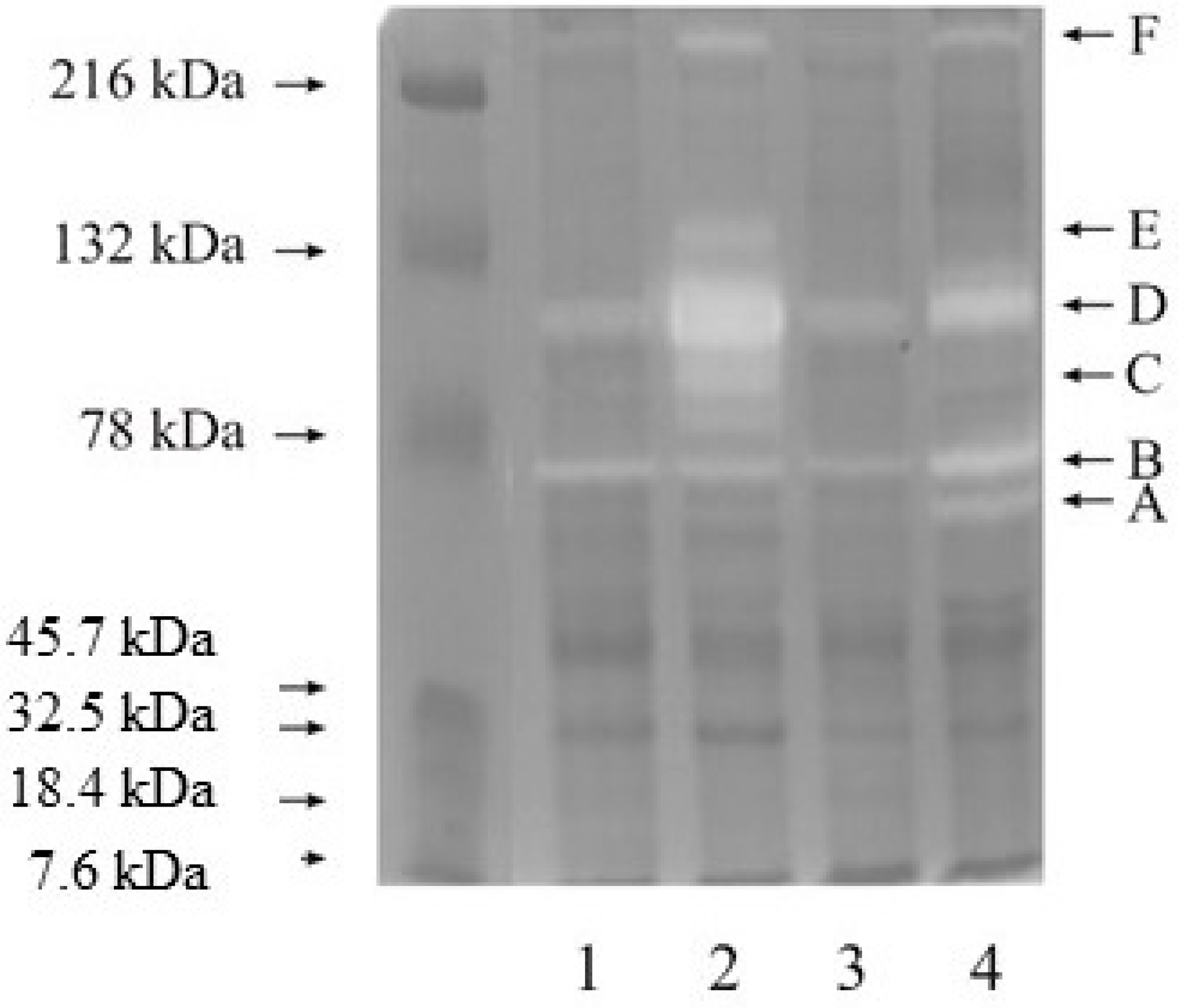
3.1. Zymography
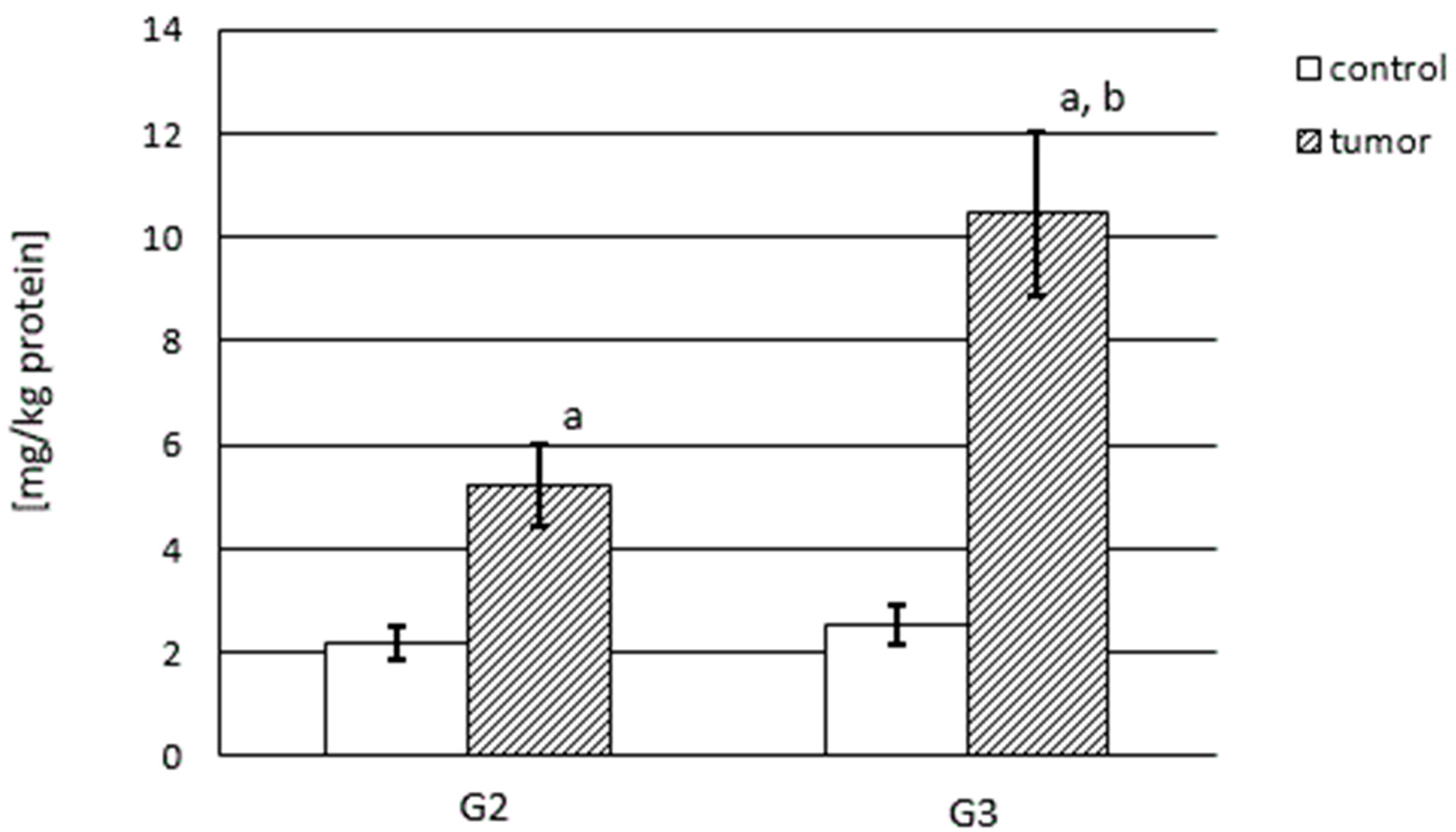
3.2. MMP-2 and MMP-9 Content
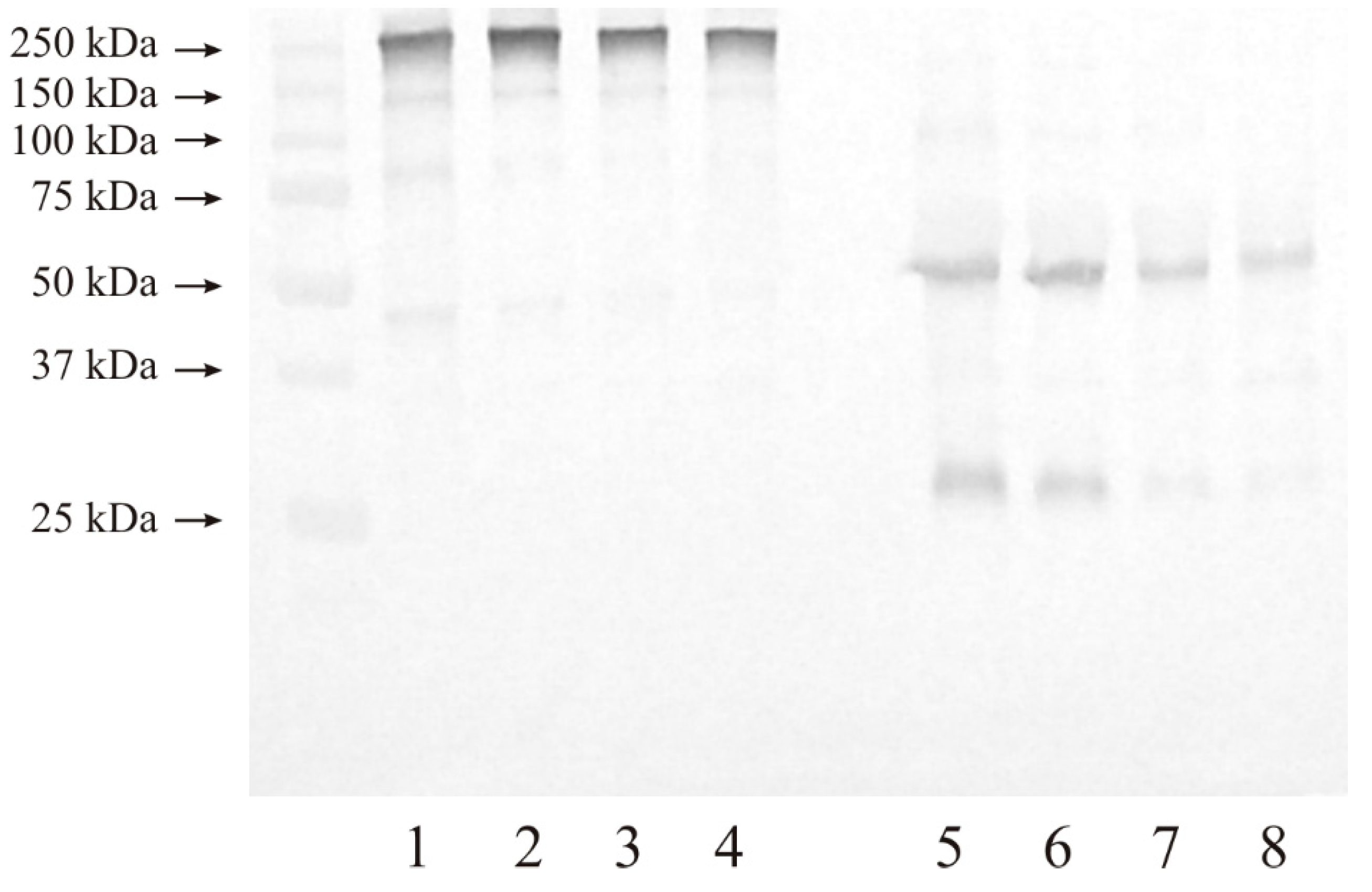
3.3. Western Blot Analysis of Investigated Gelatinases
3.3.1. Expression of MMP-2
3.3.2. Expression of MMP-9
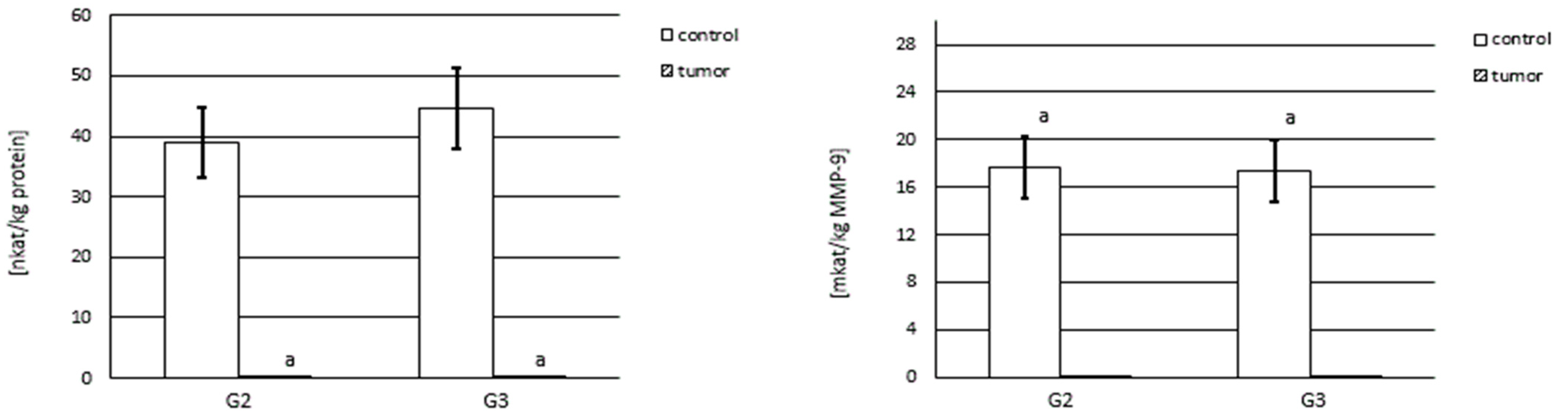
3.4. Activity of Gelatinase A and B
3.4.1. Actual and Specific Activity of MMP-2
3.4.2. Actual and Specific Activity of MMP-9
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Netter, F. Atlas of Human Anatomy, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Wallace, M.A. Anatomy and physiology of the kidney. AORN J. 1998, 68, 800, 803–816, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; Stamenkovic, I. Functional structure and composition of the extracellular matrix. J. Pathol. 2003, 200, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Chou, C.J.; Affolter, M.; Kussmann, M. A nutrigenomics view of protein intake: Macronutrient, bioactive peptides, and protein turnover. Prog. Mol. Biol. Transl. Sci. 2012, 108, 51–74. [Google Scholar]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Boor, P.; Sebekova, K.; Ostendorf, T.; Floege, J. Treatment targets in renal fibrosis. Nephrol. Dial. Transplant. 2007, 22, 3391–3407. [Google Scholar] [CrossRef]
- Catania, J.M.; Chen, G.; Parrish, A.R. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol. Renal. Physiol. 2007, 292, F905–F911. [Google Scholar] [CrossRef]
- Pardo, A.; Selman, M. Matrix metalloproteases in aberrant fibrotic tissue remodelling. Proc. Am. Thorac. Soc. 2006, 3, 383–388. [Google Scholar] [CrossRef]
- Maskos, K.; Bode, W. Structural basis of matrix metalloproteinases and tissue inhibitors of metalloproteinases. Mol. Biotechnol. 2003, 25, 241–266. [Google Scholar] [CrossRef]
- Zitka, O.; Kukacka, J.; Krizkova, S.; Huska, D.; Adam, V.; Masarik, M.; Prusa, R.; Kizek, R. Matrix Metalloproteinases. Curr. Med. Chem. 2010, 17, 3751–3768. [Google Scholar] [CrossRef]
- Allan, J.A.; Docherty, A.J.; Barker, P.J.; Huskisson, N.S.; Reynolds, J.J.; Murphy, G. Binding of gelatinases A and B to type I collagen and other matrix components. Biochem. J. 1995, 309, 299–306. [Google Scholar] [CrossRef]
- Di Carlo, A. Matrix metalloproteinase-2 and -9 and tissue inhibitor of metalloproteinase-1 and -2 in sera and urine of patients with renal carcinoma. Oncol. Lett. 2014, 7, 621–626. [Google Scholar] [CrossRef]
- Lu, H.; Cao, X.; Zhang, H.; Sun, G.; Fan, G.; Chen, L.; Wang, S. Imbalance between MMP-2, 9 and TIMP-1 promote the invasion and metastasis of renal cell carcinoma via SKP2 signalling pathways. Tumour. Biol. 2014, 35, 9807–9813. [Google Scholar] [CrossRef]
- Qiao, Z.K.; Li, Y.L.; Lu, H.T.; Wang, K.L.; Xu, W.H. Expression of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in renal cell carcinoma. World J. Surg. Oncol. 2013, 11, 1. [Google Scholar] [CrossRef]
- Bhuvarahamurthy, V.; Kristiansen, G.O.; Johannsen, M.; Loening, S.A.; Schnorr, D.; Jung, K.; Staack, A. In situ gene expression and localization of MMP1, MMP2, MMP3, MMP9 and their inhibitors TIMP1 and TIMP2 in human renal cell carcinoma. Oncol. Rep. 2006, 15, 1379–1384. [Google Scholar] [CrossRef]
- Kawata, N.; Nagane, Y.; Igarashi, T.; Hirakata, H.; Ichinose, T.; Hachiya, T.; Takimoto, Y.; Takahashi, S. Strong significant correlation between MMP-9 and systemic symptoms in patients with localized renal cell carcinoma. Urology 2006, 68, 523–527. [Google Scholar] [CrossRef]
- Kallakury, B.V.; Karikehalli, S.; Haholu, A.; Sheehan, C.E.; Azumi, N.; Ross, J.S. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin. Cancer Res. 2001, 7, 3113–3119. [Google Scholar]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer Publishing Company: New York, NY, USA, 2017; pp. 849–854. [Google Scholar]
- Cannon, G.M., Jr.; Getzenburg, R.H. Urinary matrix metalloproteinases activity is not significantly altered in patients with renal cell carcinoma. Urology 2006, 67, 8450–8484. [Google Scholar] [CrossRef]
- Chen, Y.M.; Miner, J.H. Glomerular basement membrane and related glomerular disease. Translational Research. J. Lab. Clin. Med. 2012, 160, 291–297. [Google Scholar]
- Genovese, F.; Manresa, A.A.; Leeming, D.J.; Karsdal, M.A.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair 2014, 7, 4. [Google Scholar] [CrossRef]
- Kudelski, J.; Młynarczyk, G.; Gudowska-Sawczuk, M.; Mroczko, B.; Darewicz, B.; Bruczko-Goralewska, M.; Romanowicz, L. Higher content but not the activity of stromelysin-2 (MMP-10) in comparison to stromelysin-1 (MMP-3) in human renal carcinoma. Int. J. Environ. Res. Public Health 2022, 2, 12613. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, G.; Kudelski, J.; Darewicz, B.; Bruczko-Goralewska, M.; Romanowicz, L. Supressed Expression but not activity of collagenases MMP-1 and MMP-13 in human renal carcinoma. Pathobiology 2019, 86, 201–207. [Google Scholar] [CrossRef]
- Unemori, E.N.; Werb, Z. Reorganization of polymerized actin: A possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J. Cell Biol. 1989, 103, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.D.; Ji, X.Y.; Qazi, H.; Tarbell, J.M. Interstitial flow promotes vascular fibroblast, myofibroblast, and smooth muscle cell motility in 3-D collagen I via upregulation of MMP-1. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1225–H1234. [Google Scholar] [CrossRef]
- Watkins, G.A.; Fung Jones, E.; Scott Shell, M.; VanBrocklin, H.F.; Pan, M.-H.; Hanrahan, S.M.; Feng, J.J.; He, J.; Sounni, N.E.; Dill, K.A.; et al. Development of an optimized activatable MMP-14 targeted SPECT imaging probe. Bioorg. Med. Chem. 2009, 17, 653–659. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Available online: http://www.uniprot.org/uniprot/P45452 (accessed on 1 November 1995).
- Available online: https://www.wikigenes.org/e/gene/e/4322.html (accessed on 1 August 2008).
- Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=MMP2&keywords=gelatinases (accessed on 4 October 2023).
- Lee, D.G.; Yang, K.E.; Hwang, J.W.; Kang, H.S.; Lee, S.Y.; Choi, S.; Shin, J.; Jang, I.S.; An, H.J.; Chung, H.; et al. Degradation of Kidney and Psoas Muscle Proteins as Indicators of Post-Mortem Interval in a Rat Model, with Use of Lateral Flow Technology. PLoS ONE 2016, 11, e0160557. [Google Scholar] [CrossRef]



| GRADE 2 | GRADE 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient Number | Sex | Age (Years) | BMI | Tumor Size (cm) | Patient Number | Sex | Age (Years) | BMI | Tumor Size (cm) |
| 1 | F | 73 | 23.5 | 5.0 | 1 | M | 78 | 26.5 | 12.0 |
| 2 | F | 64 | 24.8 | 5.0 | 2 | M | 51 | 28.9 | 3.5 |
| 3 | M | 48 | 24.1 | 3.5 | 3 | M | 62 | 31.6 | 7.0 |
| 4 | M | 70 | 27.7 | 4.0 | 4 | M | 53 | 29.9 | 4.0 |
| 5 | M | 68 | 26.6 | 5.5 | 5 | M | 77 | 25.3 | 9.5 |
| 6 | M | 63 | 24.2 | 5.5 | 6 | F | 82 | 24.9 | 5.0 |
| 7 | F | 75 | 25.9 | 4.5 | 7 | M | 62 | 31.3 | 8.0 |
| 8 | M | 53 | 29.6 | 5.5 | 8 | M | 72 | 28.6 | 6.5 |
| 9 | F | 67 | 23.1 | 8.5 | 9 | M | 73 | 24.2 | 10.5 |
| 10 | M | 74 | 27.8 | 6.0 | 10 | F | 53 | 28.9 | 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarczyk, G.; Gudowska-Sawczuk, M.; Mroczko, B.; Bruczko-Goralewska, M.; Romanowicz, L.; Tokarzewicz, A. Higher Content but No Specific Activity in Gelatinase B (MMP-9) Compared with Gelatinase A (MMP-2) in Human Renal Carcinoma. Cancers 2023, 15, 5475. https://doi.org/10.3390/cancers15225475
Młynarczyk G, Gudowska-Sawczuk M, Mroczko B, Bruczko-Goralewska M, Romanowicz L, Tokarzewicz A. Higher Content but No Specific Activity in Gelatinase B (MMP-9) Compared with Gelatinase A (MMP-2) in Human Renal Carcinoma. Cancers. 2023; 15(22):5475. https://doi.org/10.3390/cancers15225475
Chicago/Turabian StyleMłynarczyk, Grzegorz, Monika Gudowska-Sawczuk, Barbara Mroczko, Marta Bruczko-Goralewska, Lech Romanowicz, and Anna Tokarzewicz. 2023. "Higher Content but No Specific Activity in Gelatinase B (MMP-9) Compared with Gelatinase A (MMP-2) in Human Renal Carcinoma" Cancers 15, no. 22: 5475. https://doi.org/10.3390/cancers15225475
APA StyleMłynarczyk, G., Gudowska-Sawczuk, M., Mroczko, B., Bruczko-Goralewska, M., Romanowicz, L., & Tokarzewicz, A. (2023). Higher Content but No Specific Activity in Gelatinase B (MMP-9) Compared with Gelatinase A (MMP-2) in Human Renal Carcinoma. Cancers, 15(22), 5475. https://doi.org/10.3390/cancers15225475








