Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. NRF2 Modulation by Natural Compounds
2.1. Vegetable Substances
2.2. Vitamins
2.3. Trace Elements
2.4. Microbial Compounds
2.5. Hormones
2.6. Metabolic Compounds
3. NRF2 Modulation by Synthetic Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Gesuita, R.; Goteri, G.; Senzacqua, M.; Marcheggiani, F.; Tiano, L.; Marzioni, D.; Mazzucchelli, R. Ciliary Neurotrophic Factor Modulates Multiple Downstream Signaling Pathways in Prostate Cancer Inhibiting Cell Invasiveness. Cancers 2022, 14, 5917. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, M.A.; Lepor, H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 2007, 9 (Suppl. S1), S3–S8. [Google Scholar] [PubMed]
- Tossetta, G.; Fantone, S.; Gesuita, R.; Montironi, R.; Marzioni, D.; Mazzucchelli, R. AT-rich interactive domain 1A (ARID1A) cannot be considered a morphological marker for prostate cancer progression: A pilot study. Acta Histochem. 2022, 124, 151847. [Google Scholar] [CrossRef]
- Chinniah, S.; Stish, B.; Costello, B.A.; Pagliaro, L.; Childs, D.; Quevedo, F.; Lucien, F.; Bryce, A.; Park, S.S.; Orme, J.J. Radiation Therapy in Oligometastatic Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 684–692. [Google Scholar] [CrossRef]
- Leinonen, H.M.; Kansanen, E.; Polonen, P.; Heinaniemi, M.; Levonen, A.L. Role of the Keap1-Nrf2 pathway in cancer. Adv. Cancer Res. 2014, 122, 281–320. [Google Scholar] [CrossRef]
- Chaiswing, L.; Weiss, H.L.; Jayswal, R.D.; Clair, D.K.S.; Kyprianou, N. Profiles of Radioresistance Mechanisms in Prostate Cancer. Crit. Rev. Oncog. 2018, 23, 39–67. [Google Scholar] [CrossRef]
- Pejcic, T.; Todorovic, Z.; Durasevic, S.; Popovic, L. Mechanisms of Prostate Cancer Cells Survival and Their Therapeutic Targeting. Int. J. Mol. Sci. 2023, 24, 2939. [Google Scholar] [CrossRef]
- Oderda, M.; Bertetto, O.; Barbera, G.; Calleris, G.; Falcone, M.; Filippini, C.; Marquis, A.; Marra, G.; Montefusco, G.; Peretti, F.; et al. Appropriateness and complications of androgen deprivation therapy for prostate cancer: Can we do better? A retrospective observational analysis from a referral center. Urologia 2023, 90, 100–108. [Google Scholar] [CrossRef]
- Wang, L.; Stadlbauer, B.; Lyu, C.; Buchner, A.; Pohla, H. Shikonin enhances the antitumor effect of cabazitaxel in prostate cancer stem cells and reverses cabazitaxel resistance by inhibiting ABCG2 and ALDH3A1. Am. J. Cancer Res. 2020, 10, 3784–3800. [Google Scholar]
- Miyao, T.; Koike, H.; Sekine, Y.; Ohtsu, A.; Oka, D.; Suzuki, K. YM155 Reverses Cabazitaxel Resistance in Castration-resistant Prostate Cancer by Reducing Survivin Expression. Anticancer Res. 2020, 40, 5091–5095. [Google Scholar] [CrossRef] [PubMed]
- McPhaul, M.J. Mechanisms of prostate cancer progression to androgen independence. Best. Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Nadiminty, N.; Gao, A.C. Mechanisms of persistent activation of the androgen receptor in CRPC: Recent advances and future perspectives. World J. Urol. 2012, 30, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Song, Y.; Takeuchi, A.; Yokomizo, A.; Kashiwagi, E.; Kuroiwa, K.; Tatsugami, K.; Uchiumi, T.; Oda, Y.; Naito, S. Antioxidant therapy alleviates oxidative stress by androgen deprivation and prevents conversion from androgen dependent to castration resistant prostate cancer. J. Urol. 2012, 187, 707–714. [Google Scholar] [CrossRef]
- Shiota, M.; Yokomizo, A.; Naito, S. Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer. Free Radic. Biol. Med. 2011, 51, 1320–1328. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef]
- Tossetta, G.; Marzioni, D. Natural and synthetic compounds in Ovarian Cancer: A focus on NRF2/KEAP1 pathway. Pharmacol. Res. 2022, 183, 106365. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Montanari, E.; Marzioni, D.; Goteri, G. Role of NRF2 in Ovarian Cancer. Antioxidants 2022, 11, 663. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodriguez-Manas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G. Metformin Improves Ovarian Cancer Sensitivity to Paclitaxel and Platinum-Based Drugs: A Review of In Vitro Findings. Int. J. Mol. Sci. 2022, 23, 12893. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Vignini, A. NAD(+) Homeostasis and NAD(+)-Consuming Enzymes: Implications for Vascular Health. Antioxidants 2023, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Muti, N.D.; Salvio, G.; Ciarloni, A.; Perrone, M.; Tossetta, G.; Lazzarini, R.; Bracci, M.; Balercia, G. Can extremely low frequency magnetic field affect human sperm parameters and male fertility? Tissue Cell 2023, 82, 102045. [Google Scholar] [CrossRef] [PubMed]
- Marzioni, D.; Mazzucchelli, R.; Fantone, S.; Tossetta, G. NRF2 modulation in TRAMP mice: An in vivo model of prostate cancer. Mol. Biol. Rep. 2023, 50, 873–881. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Habibi-Rezaei, M.; Arese, M.; Saso, L.; Moosavi-Movahedi, A.A. Nrf2 Modulation in Breast Cancer. Biomedicines 2022, 10, 2668. [Google Scholar] [CrossRef]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef]
- Tew, K.D.; Townsend, D.M. Redox platforms in cancer drug discovery and development. Curr. Opin. Chem. Biol. 2011, 15, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Marzioni, D. Targeting the NRF2/KEAP1 pathway in cervical and endometrial cancers. Eur. J. Pharmacol. 2023, 941, 175503. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Mohammadi, A.T.; Gholami, M.H.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Hushmandi, K.; Makvandi, P.; Samec, M.; Liskova, A.; et al. Nrf2 signaling pathway in cisplatin chemotherapy: Potential involvement in organ protection and chemoresistance. Pharmacol. Res. 2021, 167, 105575. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Kunwar, A.; Sandur, S.K.; Pandey, B.N.; Chaubey, R.C. Differential response of DU145 and PC3 prostate cancer cells to ionizing radiation: Role of reactive oxygen species, GSH and Nrf2 in radiosensitivity. Biochim. Biophys. Acta 2014, 1840, 485–494. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Giannubilo, S.R.; Marzioni, D. The Multifaced Actions of Curcumin in Pregnancy Outcome. Antioxidants 2021, 10, 126. [Google Scholar] [CrossRef]
- Rejhova, A.; Opattova, A.; Cumova, A.; Sliva, D.; Vodicka, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Wozniak, M.; Krajewski, R.; Makuch, S.; Agrawal, S. Phytochemicals in Gynecological Cancer Prevention. Int. J. Mol. Sci. 2021, 22, 1219. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The Hallmarks of Flavonoids in Cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Licini, C.; Marzioni, D.; Mattioli-Belmonte, M. The multifaced role of HtrA1 in the development of joint and skeletal disorders. Bone 2022, 157, 116350. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Chainy, G.B.N. Hormone-linked redox status and its modulation by antioxidants. Vitam. Horm. 2023, 121, 197–246. [Google Scholar] [CrossRef] [PubMed]
- Dalleau, S.; Baradat, M.; Gueraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Ghanavat, M.; Shahrouzian, M.; Deris Zayeri, Z.; Banihashemi, S.; Kazemi, S.M.; Saki, N. Digging deeper through glucose metabolism and its regulators in cancer and metastasis. Life Sci. 2021, 264, 118603. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Khan, K.; Hafeez, A.; Irfan, M.; Armaghan, M.; Rahman, A.U.; Gurer, E.S.; Sharifi-Rad, J.; Butnariu, M.; Bagiu, I.C.; et al. Ursolic acid: A natural modulator of signaling networks in different cancers. Cancer Cell Int. 2022, 22, 399. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef]
- Kornel, A.; Nadile, M.; Retsidou, M.I.; Sakellakis, M.; Gioti, K.; Beloukas, A.; Sze, N.S.K.; Klentrou, P.; Tsiani, E. Ursolic Acid against Prostate and Urogenital Cancers: A Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2023, 24, 7414. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Punia, S.; Sharma, A.K. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent. Pat. Inflamm. Allergy Drug. Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef]
- Li, S.; Wu, R.; Wang, L.; Dina Kuo, H.C.; Sargsyan, D.; Zheng, X.; Wang, Y.; Su, X.; Kong, A.N. Triterpenoid ursolic acid drives metabolic rewiring and epigenetic reprogramming in treatment/prevention of human prostate cancer. Mol. Carcinog. 2022, 61, 111–121. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Sousa, A.S.; Reis, C.A.; Pintado, M.M. Phenylethyl Isothiocyanate: A Bioactive Agent for Gastrointestinal Health. Molecules 2022, 27, 794. [Google Scholar] [CrossRef]
- Hecht, S.S. Chemoprevention by isothiocyanates. J. Cell. Biochem. Suppl. 1995, 22, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Wright, S.E.; Kim, S.H.; Srivastava, S.K. Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochim. Biophys. Acta 2014, 1846, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yuan, X.; Pan, Z.; Shen, G.; Kim, J.H.; Yu, S.; Khor, T.O.; Li, W.; Ma, J.; Kong, A.N. Mechanism of action of isothiocyanates: The induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol. Cancer Ther. 2006, 5, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, R.; Owuor, E.D.; Kong, A.N. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch. Pharm. Res. 2000, 23, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Hebbar, V.; Nair, S.; Xu, C.; Li, W.; Lin, W.; Keum, Y.S.; Han, J.; Gallo, M.A.; Kong, A.N. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J. Biol. Chem. 2004, 279, 23052–23060. [Google Scholar] [CrossRef]
- Yu, R.; Lei, W.; Mandlekar, S.; Weber, M.J.; Der, C.J.; Wu, J.; Kong, A.N. Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J. Biol. Chem. 1999, 274, 27545–27552. [Google Scholar] [CrossRef]
- Wang, C.; Shu, L.; Zhang, C.; Li, W.; Wu, R.; Guo, Y.; Yang, Y.; Kong, A.N. Histone Methyltransferase Setd7 Regulates Nrf2 Signaling Pathway by Phenethyl Isothiocyanate and Ursolic Acid in Human Prostate Cancer Cells. Mol. Nutr. Food Res. 2018, 62, e1700840. [Google Scholar] [CrossRef]
- Barsyte-Lovejoy, D.; Li, F.; Oudhoff, M.J.; Tatlock, J.H.; Dong, A.; Zeng, H.; Wu, H.; Freeman, S.A.; Schapira, M.; Senisterra, G.A.; et al. (R)-PFI-2 is a potent and selective inhibitor of SETD7 methyltransferase activity in cells. Proc. Natl. Acad. Sci. USA 2014, 111, 12853–12858. [Google Scholar] [CrossRef]
- He, S.; Owen, D.R.; Jelinsky, S.A.; Lin, L.L. Lysine Methyltransferase SETD7 (SET7/9) Regulates ROS Signaling through mitochondria and NFE2L2/ARE pathway. Sci. Rep. 2015, 5, 14368. [Google Scholar] [CrossRef]
- Torella, D.; Salerno, N.; Cianflone, E. SETD7 methyltransferase is a key druggable target for effective cardioprotection from myocardial ischaemic injury. Cardiovasc. Res. 2023, 118, 3269–3271. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol. Sin. 2007, 28, 1343–1354. [Google Scholar] [CrossRef]
- Khurana, N.; Talwar, S.; Chandra, P.K.; Sharma, P.; Abdel-Mageed, A.B.; Mondal, D.; Sikka, S.C. Sulforaphane increases the efficacy of anti-androgens by rapidly decreasing androgen receptor levels in prostate cancer cells. Int. J. Oncol. 2016, 49, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Khurana, N.; Kim, H.; Chandra, P.K.; Talwar, S.; Sharma, P.; Abdel-Mageed, A.B.; Sikka, S.C.; Mondal, D. Multimodal actions of the phytochemical sulforaphane suppress both AR and AR-V7 in 22Rv1 cells: Advocating a potent pharmaceutical combination against castration-resistant prostate cancer. Oncol. Rep. 2017, 38, 2774–2786. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yao, X.D.; Li, W.; Geng, J.; Yan, Y.; Che, J.P.; Xu, Y.F.; Zheng, J.H. Nrf2 sensitizes prostate cancer cells to radiation via decreasing basal ROS levels. Biofactors 2015, 41, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar]
- Liu, X.; Wang, X. Recent advances on the structural modification of parthenolide and its derivatives as anticancer agents. Chin. J. Nat. Med. 2022, 20, 814–829. [Google Scholar] [CrossRef]
- Lo, S.C.; Hannink, M. PGAM5, a Bcl-XL-interacting protein, is a novel substrate for the redox-regulated Keap1-dependent ubiquitin ligase complex. J. Biol. Chem. 2006, 281, 37893–37903. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, F.; Miriyala, S.; Crooks, P.A.; Oberley, T.D.; Chaiswing, L.; Noel, T.; Holley, A.K.; Zhao, Y.; Kiningham, K.K.; et al. KEAP1 is a redox sensitive target that arbitrates the opposing radiosensitive effects of parthenolide in normal and cancer cells. Cancer Res. 2013, 73, 4406–4417. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Das, D.; Das, S.; Jha, N.K.; Pal, M.; Kolesarova, A.; Kesari, K.K.; Kalita, J.C.; Slama, P. Clinical Potential of Himalayan Herb Bergenia ligulata: An Evidence-Based Study. Molecules 2022, 27, 7039. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, N.; Banerjee, P.; Gajbhiye, R.L.; Sareng, H.R.; Kapse, P.; Pal, S.; Burdelya, L.; Mandal, N.C.; Ravichandiran, V.; et al. Induction of monoamine oxidase A-mediated oxidative stress and impairment of NRF2-antioxidant defence response by polyphenol-rich fraction of Bergenia ligulata sensitizes prostate cancer cells in vitro and in vivo. Free Radic. Biol. Med. 2021, 172, 136–151. [Google Scholar] [CrossRef]
- Wang, D.; Bu, T.; Li, Y.; He, Y.; Yang, F.; Zou, L. Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin. Antioxidants 2022, 11, 2121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, L.; Wang, M. Effects of puerarin on chronic inflammation: Focus on the heart, brain, and arteries. Aging Med. 2021, 4, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, J.; Shi, J. Management of Diabetes Mellitus with Puerarin, a Natural Isoflavone From Pueraria lobata. Am. J. Chin. Med. 2018, 46, 1771–1789. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiong, C.; Xu, P.; Luo, Q.; Zhang, R. Puerarin induces apoptosis in prostate cancer cells via inactivation of the Keap1/Nrf2/ARE signaling pathway. Bioengineered 2021, 12, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Barranco, W.T.; Eckhert, C.D. Boric acid inhibits human prostate cancer cell proliferation. Cancer Lett. 2004, 216, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Barranco, W.T.; Hudak, P.F.; Eckhert, C.D. Evaluation of ecological and in vitro effects of boron on prostate cancer risk (United States). Cancer Causes Control 2007, 18, 71–77. [Google Scholar] [CrossRef]
- Cui, Y.; Winton, M.I.; Zhang, Z.F.; Rainey, C.; Marshall, J.; De Kernion, J.B.; Eckhert, C.D. Dietary boron intake and prostate cancer risk. Oncol. Rep. 2004, 11, 887–892. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Zhang, D.; Hannink, M.; Arvisais, E.; Kaufman, R.J.; Diehl, J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003, 23, 7198–7209. [Google Scholar] [CrossRef]
- Yamada, K.E.; Eckhert, C.D. Boric Acid Activation of eIF2α and Nrf2 Is PERK Dependent: A Mechanism that Explains How Boron Prevents DNA Damage and Enhances Antioxidant Status. Biol. Trace Elem. Res. 2019, 188, 2–10. [Google Scholar] [CrossRef]
- Bacherikov, V.A. Total Synthesis, Mechanism of Action, and Antitumor Efficacy of Camptothecin and Some of its Analogues. Anticancer Agents Med. Chem. 2022, 22, 3438–3465. [Google Scholar] [CrossRef]
- Zhou, L.; Du, L.; Chen, X.; Li, X.; Li, Z.; Wen, Y.; Li, Z.; He, X.; Wei, Y.; Zhao, X.; et al. The antitumor and antimetastatic effects of N-trimethyl chitosan-encapsulated camptothecin on ovarian cancer with minimal side effects. Oncol. Rep. 2010, 24, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, J.; Wang, S.; Zhao, G.; Jiao, X.; Zhu, L. Overexpression of Smad7 suppressed ROS/MMP9-dependent collagen synthesis through regulation of heme oxygenase-1. Mol. Biol. Rep. 2013, 40, 5307–5314. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, R.G.; Park, S.R.; Choi, Y.H.; Hyun, J.W.; Chang, W.Y.; Kim, G.Y. Camptothecin suppresses expression of matrix metalloproteinase-9 and vascular endothelial growth factor in DU145 cells through PI3K/Akt-mediated inhibition of NF-kappaB activity and Nrf2-dependent induction of HO-1 expression. Environ. Toxicol. Pharmacol. 2015, 39, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Balaji Raghavendra Rao, H.; Sathivel, A.; Devaki, T. Antihepatotoxic nature of Ulva reticulata (Chlorophyceae) on acetaminophen-induced hepatoxicity in experimental rats. J. Med. Food 2004, 7, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.Q.; Lim, C.S.; Sung, J.Y.; Choi, H.G.; Ha, I.; Han, J.S. Ulva conglobata, a marine algae, has neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells. Neurosci. Lett. 2006, 402, 154–158. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef]
- Wang, R.; Paul, V.J.; Luesch, H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Free Radic. Biol. Med. 2013, 57, 141–153. [Google Scholar] [CrossRef]
- Ratnayake, R.; Liu, Y.; Paul, V.J.; Luesch, H. Cultivated sea lettuce is a multiorgan protector from oxidative and inflammatory stress by enhancing the endogenous antioxidant defense system. Cancer Prev. Res. 2013, 6, 989–999. [Google Scholar] [CrossRef]
- van Die, M.D.; Bone, K.M.; Williams, S.G.; Pirotta, M.V. Soy and soy isoflavones in prostate cancer: A systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014, 113, E119–E130. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Soy isoflavones and cancer prevention. Cancer Investig. 2003, 21, 744–757. [Google Scholar] [CrossRef]
- Sivonova, M.K.; Kaplan, P.; Tatarkova, Z.; Lichardusova, L.; Dusenka, R.; Jurecekova, J. Androgen receptor and soy isoflavones in prostate cancer. Mol. Clin. Oncol. 2019, 10, 191–204. [Google Scholar] [CrossRef]
- Boutas, I.; Kontogeorgi, A.; Dimitrakakis, C.; Kalantaridou, S.N. Soy Isoflavones and Breast Cancer Risk: A Meta-analysis. In Vivo 2022, 36, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Pudenz, M.; Roth, K.; Gerhauser, C. Impact of soy isoflavones on the epigenome in cancer prevention. Nutrients 2014, 6, 4218–4272. [Google Scholar] [CrossRef]
- Kim, I.S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef]
- Barve, A.; Khor, T.O.; Nair, S.; Lin, W.; Yu, S.; Jain, M.R.; Chan, J.Y.; Kong, A.N. Pharmacogenomic profile of soy isoflavone concentrate in the prostate of Nrf2 deficient and wild-type mice. J. Pharm. Sci. 2008, 97, 4528–4545. [Google Scholar] [CrossRef]
- Thompson, M.D.; Cooney, R.V. The Potential Physiological Role of gamma-Tocopherol in Human Health: A Qualitative Review. Nutr. Cancer 2020, 72, 808–825. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E and metabolites-regulation of cancer cell death and underlying mechanisms. IUBMB Life 2019, 71, 495–506. [Google Scholar] [CrossRef]
- Constantinou, C.; Charalambous, C.; Kanakis, D.; Kolokotroni, O.; Constantinou, A.I. Update on the Anti-Cancer Potency of Tocotrienols and α-Tocopheryl Polyethylene Glycol 1000 Succinate on Leukemic Cell Lines. Nutr. Cancer 2021, 73, 1302–1308. [Google Scholar] [CrossRef]
- Khadangi, F.; Azzi, A. Vitamin E—The Next 100 Years. IUBMB Life 2019, 71, 411–415. [Google Scholar] [CrossRef]
- Bellezza, I.; Grottelli, S.; Gatticchi, L.; Mierla, A.L.; Minelli, A. α-Tocopheryl succinate pre-treatment attenuates quinone toxicity in prostate cancer PC3 cells. Gene 2014, 539, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Tucci, A.; Galli, F.; Grottelli, S.; Mierla, A.L.; Pilolli, F.; Minelli, A. Inhibition of NF-kappaB nuclear translocation via HO-1 activation underlies α-tocopheryl succinate toxicity. J. Nutr. Biochem. 2012, 23, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Mohd Idris, R.A.; Ahmed, N.; Ahmad, S.; Murtadha, A.H.; Tengku Din, T.; Yean, C.Y.; Wan Abdul Rahman, W.F.; Mat Lazim, N.; Uskokovic, V.; et al. High-Dose Vitamin C for Cancer Therapy. Pharmaceuticals 2022, 15, 711. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczynski, A. Role of Vitamin C in Selected Malignant Neoplasms in Women. Nutrients 2022, 14, 882. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Calderaro, A.; Patane, G.T.; Navarra, M.; Barreca, D.; Cirmi, S.; Felice, M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int. J. Mol. Sci. 2023, 24, 2952. [Google Scholar] [CrossRef]
- Shabir, I.; Kumar Pandey, V.; Shams, R.; Dar, A.H.; Dash, K.K.; Khan, S.A.; Bashir, I.; Jeevarathinam, G.; Rusu, A.V.; Esatbeyoglu, T.; et al. Promising bioactive properties of quercetin for potential food applications and health benefits: A review. Front. Nutr. 2022, 9, 999752. [Google Scholar] [CrossRef]
- Abbasi, A.; Mostafavi-Pour, Z.; Amiri, A.; Keshavarzi, F.; Nejabat, N.; Ramezani, F.; Sardarian, A.; Zal, F. Chemoprevention of Prostate Cancer Cells by Vitamin C plus Quercetin: Role of Nrf2 in Inducing Oxidative Stress. Nutr. Cancer 2021, 73, 2003–2013. [Google Scholar] [CrossRef]
- Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Wlodarek, D.; Gromadzka-Ostrowska, J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients 2021, 13, 496. [Google Scholar] [CrossRef]
- Willett, W.C.; Polk, B.F.; Morris, J.S.; Stampfer, M.J.; Pressel, S.; Rosner, B.; Taylor, J.O.; Schneider, K.; Hames, C.G. Prediagnostic serum selenium and risk of cancer. Lancet 1983, 2, 130–134. [Google Scholar] [CrossRef]
- Vogt, T.M.; Ziegler, R.G.; Graubard, B.I.; Swanson, C.A.; Greenberg, R.S.; Schoenberg, J.B.; Swanson, G.M.; Hayes, R.B.; Mayne, S.T. Serum selenium and risk of prostate cancer in U.S. blacks and whites. Int. J. Cancer 2003, 103, 664–670. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M.; Lippman, S.M.; Goodman, P.J.; Albanes, D.; Taylor, P.R.; Coltman, C. SELECT: The selenium and vitamin E cancer prevention trial. Urol. Oncol. 2003, 21, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Terazawa, R.; Garud, D.R.; Hamada, N.; Fujita, Y.; Itoh, T.; Nozawa, Y.; Nakane, K.; Deguchi, T.; Koketsu, M.; Ito, M. Identification of organoselenium compounds that possess chemopreventive properties in human prostate cancer LNCaP cells. Bioorg. Med. Chem. 2010, 18, 7001–7008. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Turovsky, E.A. The Main Cytotoxic Effects of Methylseleninic Acid on Various Cancer Cells. Int. J. Mol. Sci. 2021, 22, 6614. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, M.C.; Park, J.M.; Chung, A.S. Antitumor Effects of Selenium. Int. J. Mol. Sci. 2021, 22, 11844. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, C. Selenium and cancer chemoprevention: Hypotheses integrating the actions of selenoproteins and selenium metabolites in epithelial and non-epithelial target cells. Antioxid. Redox Signal. 2005, 7, 1715–1727. [Google Scholar] [CrossRef]
- Jackson, M.I.; Combs, G.F., Jr. Selenium and anticarcinogenesis: Underlying mechanisms. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 718–726. [Google Scholar] [CrossRef]
- Singh, C.K.; Ndiaye, M.A.; Siddiqui, I.A.; Nihal, M.; Havighurst, T.; Kim, K.; Zhong, W.; Mukhtar, H.; Ahmad, N. Methaneseleninic acid and gamma-Tocopherol combination inhibits prostate tumor growth in Vivo in a xenograft mouse model. Oncotarget 2014, 5, 3651–3661. [Google Scholar] [CrossRef]
- Naujokat, C.; Fuchs, D.; Opelz, G. Salinomycin in cancer: A new mission for an old agent. Mol. Med. Rep. 2010, 3, 555–559. [Google Scholar] [CrossRef]
- Huczynski, A.; Janczak, J.; Antoszczak, M.; Wietrzyk, J.; Maj, E.; Brzezinski, B. Antiproliferative activity of salinomycin and its derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 7146–7150. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, Y.; Li, S.; Meng, P. Salinomycin triggers prostate cancer cell apoptosis by inducing oxidative and endoplasmic reticulum stress via suppressing Nrf2 signaling. Exp. Ther. Med. 2021, 22, 946. [Google Scholar] [CrossRef] [PubMed]
- Vis, A.N.; Schroder, F.H. Key targets of hormonal treatment of prostate cancer. Part 1: The androgen receptor and steroidogenic pathways. BJU Int. 2009, 104, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.A.; Hagan, S.S.; Datta, A.; Zhang, Y.; Freeman, M.L.; Sikka, S.C.; Abdel-Mageed, A.B.; Mondal, D. Nrf1 and Nrf2 transcription factors regulate androgen receptor transactivation in prostate cancer cells. PLoS ONE 2014, 9, e87204. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Bohmann, D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci. Signal. 2010, 3, re3. [Google Scholar] [CrossRef]
- Landis, M.D.; Lehmann, B.D.; Pietenpol, J.A.; Chang, J.C. Patient-derived breast tumor xenografts facilitating personalized cancer therapy. Breast Cancer Res. 2013, 15, 201. [Google Scholar] [CrossRef]
- Rodriguez-Santana, C.; Florido, J.; Martinez-Ruiz, L.; Lopez-Rodriguez, A.; Acuna-Castroviejo, D.; Escames, G. Role of Melatonin in Cancer: Effect on Clock Genes. Int. J. Mol. Sci. 2023, 24, 1919. [Google Scholar] [CrossRef]
- Franco, P.I.R.; do Carmo Neto, J.R.; Milhomem, A.C.; Machado, J.R.; Miguel, M.P. Antitumor effect of melatonin on breast cancer in experimental models: A systematic review. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188838. [Google Scholar] [CrossRef]
- Targhazeh, N.; Reiter, R.J.; Rahimi, M.; Qujeq, D.; Yousefi, T.; Shahavi, M.H.; Mir, S.M. Oncostatic activities of melatonin: Roles in cell cycle, apoptosis, and autophagy. Biochimie 2022, 202, 34–48. [Google Scholar] [CrossRef]
- Anim-Koranteng, C.; Shah, H.E.; Bhawnani, N.; Ethirajulu, A.; Alkasabera, A.; Onyali, C.B.; Mostafa, J.A. Melatonin-A New Prospect in Prostate and Breast Cancer Management. Cureus 2021, 13, e18124. [Google Scholar] [CrossRef]
- Paroni, R.; Terraneo, L.; Bonomini, F.; Finati, E.; Virgili, E.; Bianciardi, P.; Favero, G.; Fraschini, F.; Reiter, R.J.; Rezzani, R.; et al. Antitumour activity of melatonin in a mouse model of human prostate cancer: Relationship with hypoxia signalling. J. Pineal Res. 2014, 57, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Terraneo, L.; Bianciardi, P.; Virgili, E.; Finati, E.; Samaja, M.; Paroni, R. Transdermal administration of melatonin coupled to cryopass laser treatment as noninvasive therapy for prostate cancer. Drug Deliv. 2017, 24, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xi, X.; Mei, Y.; Zhao, X.; Zhou, L.; Ma, M.; Liu, S.; Zha, X.; Yang, Y. High-glucose induces retinal pigment epithelium mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ROS/PINK1/Parkin signal pathway. Biomed. Pharmacother. 2019, 111, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, A.; Yamada, M.; Shida, H.; Nakazawa, D.; Kusunoki, Y.; Nakamura, A.; Miyoshi, H.; Tomaru, U.; Atsumi, T.; Ishizu, A. Circulating Neutrophil Extracellular Trap Levels in Well-Controlled Type 2 Diabetes and Pathway Involved in Their Formation Induced by High-Dose Glucose. Pathobiology 2016, 83, 243–251. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, F.B.; Xu, H.; Xu, L.F.; Chen, D.; Liu, W.H.; Mu, X.; Wen, Y.Q. High glucose promotes prostate cancer cells apoptosis via Nrf2/ARE signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 192–200. [Google Scholar] [CrossRef]
- De La Pinta, C. Radiotherapy in Prostate Brain Metastases: A Review of the Literature. Anticancer Res. 2023, 43, 311–315. [Google Scholar] [CrossRef]
- Solodyannikova, O.I.; Shypko, A.F.; Danilenko, V.V.; Sukach, G.G. Radionuclide Therapy for Bone Lesions in Castration-Resistant Prostate Cancer (State-of-the-Art Literature Review). Probl. Radiac. Med. Radiobiol. 2022, 27, 131–137. [Google Scholar] [CrossRef]
- Lutz, S.Z.; Todenhofer, T.; Wagner, R.; Hennenlotter, J.; Ferchl, J.M.; Scharpf, M.O.; Martus, P.; Staiger, H.; Fritsche, A.; Stenzl, A.; et al. Higher prevalence of lymph node metastasis in prostate cancer in patients with diabetes. Endocr. Relat. Cancer 2018, 25, L19–L22. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Gesuita, R.; Di Renzo, G.C.; Meyyazhagan, A.; Tersigni, C.; Scambia, G.; Di Simone, N.; Marzioni, D. HtrA1 in Gestational Diabetes Mellitus: A Possible Biomarker? Diagnostics 2022, 12, 2705. [Google Scholar] [CrossRef]
- Di Pietrantonio, N.; Di Tomo, P.; Mandatori, D.; Formoso, G.; Pandolfi, A. Diabetes and Its Cardiovascular Complications: Potential Role of the Acetyltransferase p300. Cells 2023, 12, 431. [Google Scholar] [CrossRef]
- De Oliveira, M.S.; Pellenz, F.M.; de Souza, B.M.; Crispim, D. Blueberry Consumption and Changes in Obesity and Diabetes Mellitus Outcomes: A Systematic Review. Metabolites 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Pizzimenti, S.; Dianzani, M.U. Lipid peroxidation: Control of cell proliferation, cell differentiation and cell death. Mol. Aspects Med. 2008, 29, 1–8. [Google Scholar] [CrossRef]
- McGrath, C.E.; Tallman, K.A.; Porter, N.A.; Marnett, L.J. Structure-activity analysis of diffusible lipid electrophiles associated with phospholipid peroxidation: 4-hydroxynonenal and 4-oxononenal analogues. Chem. Res. Toxicol. 2011, 24, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Cipak, A.; Mrakovcic, L.; Ciz, M.; Lojek, A.; Mihaylova, B.; Goshev, I.; Jaganjac, M.; Cindric, M.; Sitic, S.; Margaritoni, M.; et al. Growth suppression of human breast carcinoma stem cells by lipid peroxidation product 4-hydroxy-2-nonenal and hydroxyl radical-modified collagen. Acta Biochim. Pol. 2010, 57, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Pettazzoni, P.; Ciamporcero, E.; Medana, C.; Pizzimenti, S.; Dal Bello, F.; Minero, V.G.; Toaldo, C.; Minelli, R.; Uchida, K.; Dianzani, M.U.; et al. Nuclear factor erythroid 2-related factor-2 activity controls 4-hydroxynonenal metabolism and activity in prostate cancer cells. Free Radic. Biol. Med. 2011, 51, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Kawai, M.; Hoshi, M.; Segawa, J.; Fujita, M.; Matsukawa, T.; Fujimoto, N.; Matsunaga, T.; Ikari, A. Targeting Nrf2-antioxidant signalling reverses acquired cabazitaxel resistance in prostate cancer cells. J. Biochem. 2021, 170, 89–96. [Google Scholar] [CrossRef]
- Mondal, D.; Narwani, D.; Notta, S.; Ghaffar, D.; Mardhekar, N.; Quadri, S.S.A. Oxidative stress and redox signaling in CRPC progression: Therapeutic potential of clinically-tested Nrf2-activators. Cancer Drug. Resist. 2021, 4, 96–124. [Google Scholar] [CrossRef]
- Yamazaki, T.; Mimura, I.; Tanaka, T.; Nangaku, M. Treatment of Diabetic Kidney Disease: Current and Future. Diabetes Metab. J. 2021, 45, 11–26. [Google Scholar] [CrossRef]
- Nakagami, Y. Nrf2 Is an Attractive Therapeutic Target for Retinal Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7469326. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yang, Y.X.; Zhe, H.; He, Z.X.; Zhou, S.F. Bardoxolone methyl (CDDO-Me) as a therapeutic agent: An update on its pharmacokinetic and pharmacodynamic properties. Drug Des. Devel. Ther. 2014, 8, 2075–2088. [Google Scholar] [CrossRef]
- Khurana, N.; Chandra, P.K.; Kim, H.; Abdel-Mageed, A.B.; Mondal, D.; Sikka, S.C. Bardoxolone-Methyl (CDDO-Me) Suppresses Androgen Receptor and Its Splice-Variant AR-V7 and Enhances Efficacy of Enzalutamide in Prostate Cancer Cells. Antioxidants 2020, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.L.; Bostwick, D.G.; Brawley, O.W.; Gomella, L.G.; Marberger, M.; Montorsi, F.; Pettaway, C.A.; Tammela, T.L.; Teloken, C.; Tindall, D.J.; et al. Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med. 2010, 362, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Miller, G.J.; Ford, L.G.; Lieber, M.M.; Cespedes, R.D.; Atkins, J.N.; Lippman, S.M.; et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 2003, 349, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.K.; Lee, J.; Keum, Y.S. Finasteride Increases the Expression of Hemoxygenase-1 (HO-1) and NF-E2-Related Factor-2 (Nrf2) Proteins in PC-3 Cells: Implication of Finasteride-Mediated High-Grade Prostate Tumor Occurrence. Biomol. Ther. 2013, 21, 49–53. [Google Scholar] [CrossRef]
- Gao, W.; Bohl, C.E.; Dalton, J.T. Chemistry and structural biology of androgen receptor. Chem. Rev. 2005, 105, 3352–3370. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, C.A.; Chang, C. Androgen receptor in prostate cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L. Hormone therapy for patients with prostate carcinoma. Cancer 2000, 88, 3009–3014. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Busilacchi, E.M.; Di Simone, N.; Giannubilo, S.R.; Scambia, G.; Giordano, A.; Marzioni, D. Modulation of matrix metalloproteases by ciliary neurotrophic factor in human placental development. Cell Tissue Res. 2022, 390, 113–129. [Google Scholar] [CrossRef]
- Perugini, J.; Di Mercurio, E.; Tossetta, G.; Severi, I.; Monaco, F.; Reguzzoni, M.; Tomasetti, M.; Dani, C.; Cinti, S.; Giordano, A. Biological Effects of Ciliary Neurotrophic Factor on hMADS Adipocytes. Front. Endocrinol. 2019, 10, 768. [Google Scholar] [CrossRef]
- Pantiora, P.; Furlan, V.; Matiadis, D.; Mavroidi, B.; Perperopoulou, F.; Papageorgiou, A.C.; Sagnou, M.; Bren, U.; Pelecanou, M.; Labrou, N.E. Monocarbonyl Curcumin Analogues as Potent Inhibitors against Human Glutathione Transferase P1-1. Antioxidants 2022, 12, 63. [Google Scholar] [CrossRef]
- Lavian, S.; Mardaneh, P.; Bagherniya, M.; Emami, S.A.; Butler, A.E.; Sahebkar, A. The effect of synthetic curcumin analogues on obesity, diabetes and cardiovascular Disease: A literature review. Curr. Med. Chem. 2023, 30, 3979–3992. [Google Scholar] [CrossRef]
- Wu, L.; Xu, G.; Li, N.; Zhu, L.; Shao, G. Curcumin Analog, HO-3867, Induces Both Apoptosis and Ferroptosis via Multiple Mechanisms in NSCLC Cells with Wild-Type p53. Evid. Based Complement. Alternat. Med. 2023, 2023, 8378581. [Google Scholar] [CrossRef] [PubMed]
- Mladenov, M.; Bogdanov, J.; Bogdanov, B.; Hadzi-Petrushev, N.; Kamkin, A.; Stojchevski, R.; Avtanski, D. Efficacy of the monocarbonyl curcumin analog C66 in the reduction of diabetes-associated cardiovascular and kidney complications. Mol. Med. 2022, 28, 129. [Google Scholar] [CrossRef]
- Weber, W.M.; Hunsaker, L.A.; Roybal, C.N.; Bobrovnikova-Marjon, E.V.; Abcouwer, S.F.; Royer, R.E.; Deck, L.M.; Vander Jagt, D.L. Activation of NFkappaB is inhibited by curcumin and related enones. Bioorg. Med. Chem. 2006, 14, 2450–2461. [Google Scholar] [CrossRef]
- Fajardo, A.M.; MacKenzie, D.A.; Ji, M.; Deck, L.M.; Vander Jagt, D.L.; Thompson, T.A.; Bisoffi, M. The curcumin analog ca27 down-regulates androgen receptor through an oxidative stress mediated mechanism in human prostate cancer cells. Prostate 2012, 72, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Tokar, E.J.; Qu, W.; Waalkes, M.P. Arsenic, stem cells, and the developmental basis of adult cancer. Toxicol. Sci. 2011, 120 (Suppl. S1), S192–S203. [Google Scholar] [CrossRef] [PubMed]
- Bardach, A.E.; Ciapponi, A.; Soto, N.; Chaparro, M.R.; Calderon, M.; Briatore, A.; Cadoppi, N.; Tassara, R.; Litter, M.I. Epidemiology of chronic disease related to arsenic in Argentina: A systematic review. Sci. Total Environ. 2015, 538, 802–816. [Google Scholar] [CrossRef]
- Benbrahim-Tallaa, L.; Waalkes, M.P. Inorganic arsenic and human prostate cancer. Environ. Health Perspect. 2008, 116, 158–164. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Nam, H.J. Autophagy Modulators in Cancer: Focus on Cancer Treatment. Life 2021, 11, 839. [Google Scholar] [CrossRef]
- Lau, A.; Zheng, Y.; Tao, S.; Wang, H.; Whitman, S.A.; White, E.; Zhang, D.D. Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner. Mol. Cell. Biol. 2013, 33, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes. Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Hu, W.Y.; Hu, D.P.; Shi, G.; Li, Y.; Yang, J.; Prins, G.S. Effects of Inorganic Arsenic on Human Prostate Stem-Progenitor Cell Transformation, Autophagic Flux Blockade, and NRF2 Pathway Activation. Environ. Health Perspect. 2020, 128, 67008. [Google Scholar] [CrossRef] [PubMed]
- Oberley-Deegan, R.E.; Steffan, J.J.; Rove, K.O.; Pate, K.M.; Weaver, M.W.; Spasojevic, I.; Frederick, B.; Raben, D.; Meacham, R.B.; Crapo, J.D.; et al. The antioxidant, MnTE-2-PyP, prevents side-effects incurred by prostate cancer irradiation. PLoS ONE 2012, 7, e44178. [Google Scholar] [CrossRef]
- Chatterjee, A.; Zhu, Y.; Tong, Q.; Kosmacek, E.A.; Lichter, E.Z.; Oberley-Deegan, R.E. The Addition of Manganese Porphyrins during Radiation Inhibits Prostate Cancer Growth and Simultaneously Protects Normal Prostate Tissue from Radiation Damage. Antioxidants 2018, 7, 21. [Google Scholar] [CrossRef]
- Chatterjee, A.; Kosmacek, E.A.; Shrishrimal, S.; McDonald, J.T.; Oberley-Deegan, R.E. MnTE-2-PyP, a manganese porphyrin, reduces cytotoxicity caused by irradiation in a diabetic environment through the induction of endogenous antioxidant defenses. Redox Biol. 2020, 34, 101542. [Google Scholar] [CrossRef]
- Shrishrimal, S.; Chatterjee, A.; Kosmacek, E.A.; Davis, P.J.; McDonald, J.T.; Oberley-Deegan, R.E. Manganese porphyrin, MnTE-2-PyP, treatment protects the prostate from radiation-induced fibrosis (RIF) by activating the NRF2 signaling pathway and enhancing SOD2 and sirtuin activity. Free Radic. Biol. Med. 2020, 152, 255–270. [Google Scholar] [CrossRef]
- Wang, S.; Hannafon, B.N.; Zhou, J.; Ding, W.Q. Clofibrate induces heme oxygenase 1 expression through a PPARα-independent mechanism in human cancer cells. Cell Physiol. Biochem. 2013, 32, 1255–1264. [Google Scholar] [CrossRef]
- De Sa Bacelar, T.; da Silva, A.J.; Costa, P.R.; Rumjanek, V.M. The pterocarpanquinone LQB 118 induces apoptosis in tumor cells through the intrinsic pathway and the endoplasmic reticulum stress pathway. Anticancer Drugs 2013, 24, 73–83. [Google Scholar] [CrossRef]
- Rica, I.G.; Netto, C.D.; Renno, M.N.; Abreu, P.A.; Costa, P.R.R.; da Silva, A.J.M.; Cavalcante, M.C.M. Anti-inflammatory properties of pterocarpanquinone LQB-118 in mice. Bioorg. Med. Chem. 2016, 24, 4415–4423. [Google Scholar] [CrossRef]
- Lima, E.A.; Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; Netto, C.D.; Costa, P.R.R.; Rodrigues-Mascarenhas, S. The pterocarpanquinone LQB 118 inhibits inflammation triggered by zymosan in vivo and in vitro. Int. Immunopharmacol. 2020, 83, 106399. [Google Scholar] [CrossRef] [PubMed]
- Hancio, T.; Mazzoccoli, L.; Guimaraes, G.; Robaina, M.; Mendonca, B.D.S.; Nestal De Moraes, G.; Monte-Mor, B.; Mayumi Gutiyama, L.; De Carvalho, L.O.; Netto, C.D.; et al. The pterocarpanquinone LQB-118 compound induces apoptosis of cytarabine-resistant acute myeloid leukemia cells. Int. J. Oncol. 2021, 58, 24. [Google Scholar] [CrossRef] [PubMed]
- Martino, T.; Kudrolli, T.A.; Kumar, B.; Salviano, I.; Mencalha, A.; Coelho, M.G.P.; Justo, G.; Costa, P.R.R.; Sabino, K.C.C.; Lupold, S.E. The orally active pterocarpanquinone LQB-118 exhibits cytotoxicity in prostate cancer cell and tumor models through cellular redox stress. Prostate 2018, 78, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine receptors: Expression, function and regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Abbracchio, M.P.; Burnstock, G.; Daly, J.W.; Harden, T.K.; Jacobson, K.A.; Leff, P.; Williams, M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994, 46, 143–156. [Google Scholar]
- Jacobson, K.A.; Hoffmann, C.; Cattabeni, F.; Abbracchio, M.P. Adenosine-induced cell death: Evidence for receptor-mediated signalling. Apoptosis 1999, 4, 197–211. [Google Scholar] [CrossRef]
- Minelli, A.; Bellezza, I.; Tucci, A.; Rambotti, M.G.; Conte, C.; Culig, Z. Differential involvement of reactive oxygen species and nucleoside transporters in cytotoxicity induced by two adenosine analogues in human prostate cancer cells. Prostate 2009, 69, 538–547. [Google Scholar] [CrossRef]
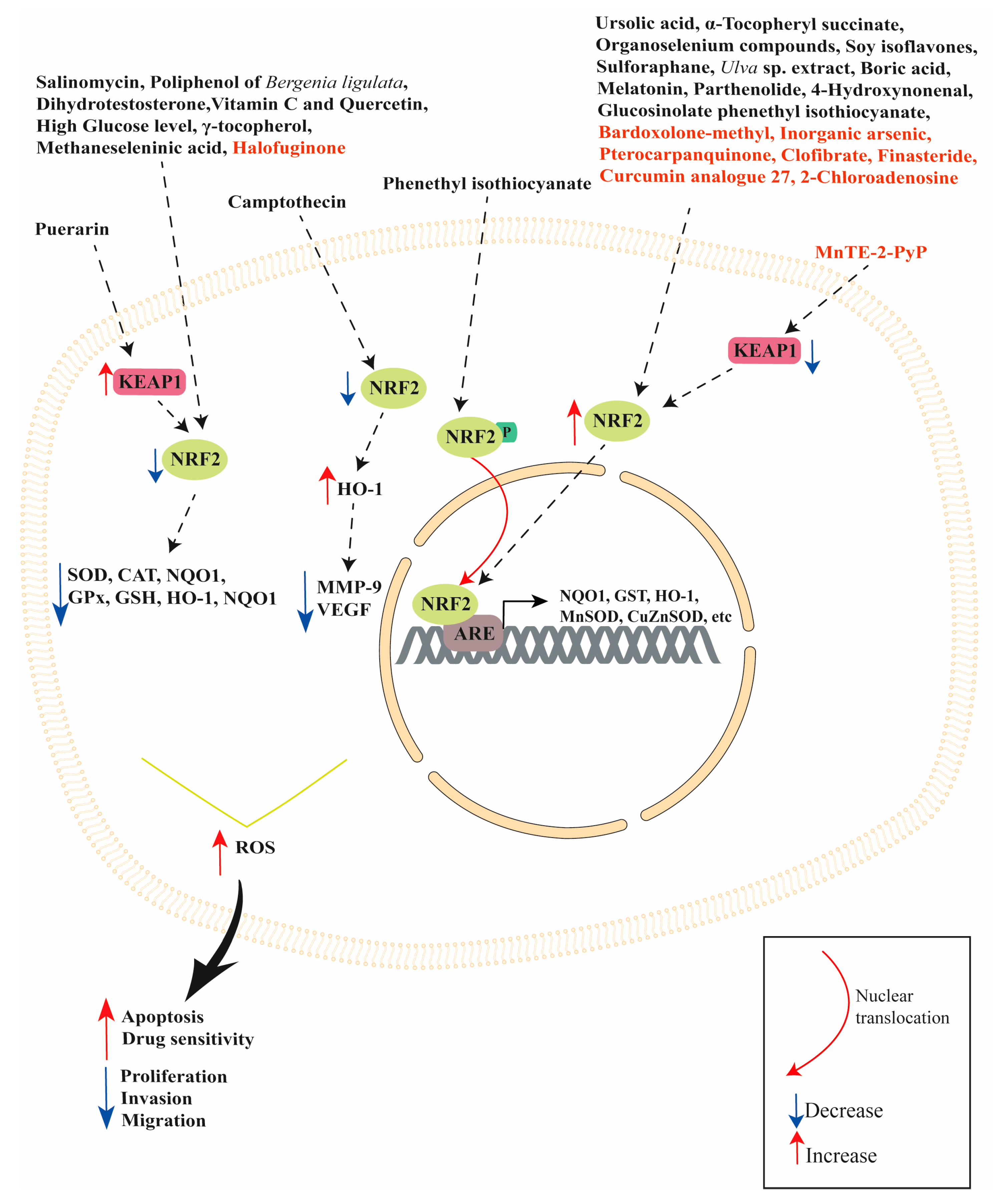
| Modulator | Model Studied | Effect | Reference |
|---|---|---|---|
| Ursolic acid (UA) | VCaP cells | UA activated NRF2 pathway | [49] |
| Phenethyl isothiocyanate (PEITC) | PC3 cells | PEITC increased ERK1/2 and JNK1/2 phosphorylation. Phenethyl isothiocyanate induced NRF2 phosphorylation favoring its translocation into the nucleus, which increased HO-1 expression. This effect was attenuated when ERK and JNK signaling was inhibited. Both ERK2 and JNK1 can directly phosphorylate NRF2. | [53] |
| PEITC and UA | LNCaP and PC3 cells | PEITC and UA treatment induced SETD7 expression activating NRF2 signaling pathway leading to increased NQO1 and GSTT2 mRNA expression, protecting DNA from oxidative damage. | [57] |
| Sulforaphane | 22RV1 cells | Sulforaphane inhibited both AR-FL and AR-V7 expression. NRF2 induction (through the NRF2 activator bardoxolone methyl) significantly decreased both AR-FL and AR-V7 levels. | [63] |
| Sulforaphane | 22RV1 cells | Sulforaphane increased NRF2, HO-1, NQO1 and Trx6 expression, decreasing basal ROS levels and sensitizing cells to radiation. | [64] |
| Parthenolide | LNCaP, PC3, DU145, PZ and RWPE-1 cell lines | Parthenolide increased ROS levels in LNCaP, PC3 and DU145 prostate cancer cells, leading to apoptosis. Parthenolide increased oxidation of KEAP1 in normal prostate PZ and RWPE-1 cell lines, increasing NRF2, MnSOD and CuZnSOD expression. | [68] |
| Polyphenol-rich fraction of Bergenia ligulata (PFBL) | LNCaP, PC3, DU145 and TRUMP-C1 cells | PFBL treatments induces cell apoptosis enhancing catalytic activity of MAO-A, consequently upregulating ROS production. PFBL inhibited NRF2, GPx, SOD1 and catalase expression, promoting PC3 cell death. | [70] |
| Puerarin | androgen-independent (DU145 and PC-3) and androgen-dependent LNCaP cells | Puerarin decreased cell viability in DU145, PC-3 and LNCaP cells. DU145 and PC-3 exposure to puerarin increased apoptosis, intracellular ROS and LDH production. Puerarin increased KEAP1 protein expression and decreased NRF2, HO-1 and NQO1 protein expression in DU145 and PC3 cells. | [74] |
| Boric Acid | DU145, MEFs wild type and MEFs PERK−/− | Boric acid induced NRF2 translocation into the nucleus in DU-145 and wild type MEFs, but not in the MEFs PERK-/- cells. Boric acid increased the expression of HO-1, NQO1 and GCLC in DU-145 cells, and HO-1 and GCLC in MEF WT cells. | [79] |
| Camptothecin | DU145 cells | Camptothecin inhibited cell proliferation and invasion. Camptothecin inhibited PMA-induced MMP-9 and VEGF expression by NRF2 activation and HO-1 expression, which directly attenuates MMP-9 and VEGF production. | [83] |
| Ulva sp. extract | LNCaP | Ulva sp. extract treatment activated NRF2 pathway increasing NQO1 mRNA expression. | [88] |
| Soy isoflavones | NRF2 knockout and wildtype mice. | Soy isoflavones induced NRF2 expression while NRF2 knockout altered the expression of genes involved in electron transport, phase II metabolizing enzymes, cell growth and differentiation, apoptosis, cell cycle, transcription factors, transport, mRNA processing and carbohydrate homeostasis. | [96] |
| α-Tocopheryl succinate | PC3 cells | α-Tocopheryl succinate reduced cell viability increasing ROS production and intracellular GSH content. α-Tocopheryl succinate increased NRF2 nuclear translocation and HO-1 expression, while decreasing NF-κB nuclear translocation. α-Tocopheryl succinate inhibited NF-κB activation upregulating HO-1 expression. α-Tocopheryl increased glutathione intracellular content when exposed to the oxidant paraquat. α-tocopheryl succinate did not alter NQO1 expression, but NQO1 silencing or its activity inhibition by dicumarol counteracted the α-tocopheryl succinate-induced adaptive response. | [101,102] |
| Vitamin C Quercetin | PC3 and DU145 cells | Vitamin C and Quercetin cotreatment reduced NRF2 mRNA and protein expression accompanied by a decrease in GPx, GR and NQO1 enzymatic activity. | [107] |
| Organoselenium compounds | LNCaP cells | Organoselenium compounds activated NRF2 (increased expression in the nuclei) increasing HO-1 expression, reducing ROS levels and cell growth. | [114] |
| Methaneseleninic acid and γ-tocopherol | 22Rν1 cells implanted in nude Nu/J mice | Methaneseleninic acid and γ-tocopherol decreased tumor volume/weight and serum PSA. γ-tocopherol alone and in combination with methaneseleninic acid increased apoptosis and decreased NRF2 expression. | [119] |
| Salinomycin | DU145 and PC-3 cells | Salinomycin inhibited cell viability and induced apoptosis in both cell lines increasing ROS and decreasing NRF2, HO-1, NQO1 and GCL expression. Salinomycin decreased the activity of SOD, CAT, and GSH-Px enzymes. | [122] |
| Dihydrotestosterone (DHT) | LNCaP and LNCaP C4-2B cells | DHT treatment induced ARE transactivation in both cell lines, but it was greater in LNCaP C4-2B than in LNCaP cells. DHT-induced androgen receptor transactivation was associated with higher nuclear translocation of p65-NRF1 in LNCaP C4-2B cells compared to LNCaP cells. p65-NRF1 silencing attenuated androgen receptor transactivation, while p65-NRF1 overexpression enhanced androgen receptor transactivation. Conversely, DHT treatment completely suppressed NRF2 expression in LNCaP C4-2B cells, while NRF2 was significantly increased in LNCaP cells. p65-NRF1 and p120-NRF1 isoforms, but not NRF2, physically interacts with androgen receptor enhancing its DNA-binding activity. p65-NRF1 has an activator function on androgen receptor, while p120-NRF1 had an inhibitory effect on androgen receptor transactivation. NRF2 exerted a suppressive effect on androgen receptor transactivation by increasing nuclear p120-NRF1 levels. | [124] |
| Melatonin | LNCaP xenografted in nude mice | Increased NRF2 expression in xenograft. | [131,132] |
| High glucose concentrations | LNCaP | High glucose levels reduced cell proliferation increasing apoptosis, ROS, LDH and interleukin-6 (IL-6), but decreased the content of IL-10. High glucose levels lowered NRF2, HO-1 and γ-GCS expression. | [135] |
| 4-Hydroxynonenal | PC3, LNCaP and DU145 cells. | PC3 and LNCaP cells are more sensitive to 4-Hydroxynonenal compared to DU145 cells. Different from PC3 and LNCaP cells, 4-Hydroxynonenal did not induce ROS production, cause DNA damage or generate a lower amount of 4-Hydroxynonenal-protein adducts and did not induce apoptosis in DU145 cells. DU145 cells had lower KEAP1 expression and an increased expression of NRF2 with greater GSH and GST A4 content compared to PC3 and LNCaP cells. NRF2 silencing reduced GST A4 expression and GS-HNE formation, increasing the antiproliferative and proapoptotic activity of 4-Hydroxynonenal in DU145 cells. | [145] |
| Modulator | Model Studied | Effect | Reference |
|---|---|---|---|
| Halofuginone | cabazitaxel-resistant 22Rv1/Cab-R and cabazitaxel-sensitive 22Rv1 cells | Halofuginone increased cabazitaxel sensitivity, suppressing NRF2 and its downstream enzymes γ-GCS, CBR1, NQO1 and HO-1 expression in 22Rv1/Cab-R cells. | [146] |
| Bardoxolone-methyl | 22RV1 cells | Bardoxolone-methyl increased NRF2 expression, decreasing ROS production. | [151] |
| Finasteride | DU-145, PC-3 and LNCaP cells | Finasteride increased NRF2 and HO-1 expression. Basal expression level of NRF2 protein was higher in androgen-refractory prostate cancer cells (DU-145 and PC-3 cells) compared to androgen-responsive prostate cancer cells (LNCaP cells). Finasteride selectively induced the expression of NRF2 in DU-145 and PC-3 cells, but not in LNCaP cells. | [154] |
| Curcumin analogue 27 (ca27) | LNCaP, LNCaP C4-2, and LAPC-4 cells. | ca27 decreased androgen receptor expression and induced ROS formation, but also induced NRF2 activation, NQO1 and AKR1C1 expression. ROS production preceded androgen receptor protein loss and its down-regulation decreased when cells were treated with N-acetyl cysteine (an antioxidant). | [165] |
| Inorganic arsenic | normal human prostate stem-progenitor cells (PrSPCs) | Inorganic arsenic increased self-renewal and suppressed differentiation, activating the NRF2/KEAP1 pathway by inhibiting p62 degradation and increasing the expression of its downstream enzymes, such as NQO1, TXNRD1, GPX2, AKR1C3, HO-1 and UCHL1. | [173] |
| MnTE-2-PyP | normal human prostate fibroblasts | MnTE-2-PyP treatment of human prostate fibroblast cells in an irradiated hyperglycaemic environment protected against hyperglycaemia-induced cell death after radiation through the increase in NRF2 and NQO1 expression. | [176] |
| MnTE-2-PyP | mouse primary prostate fibroblast cells | MnTE-2-PyP increased NRF2, SOD2 and NQO1 expression, downregulating KEAP1 expression. The NRF2 pathway activation was essential to prevent myofibroblast formation and fibrosis during radiation exposure. | [177] |
| Clofibrate | DU145 cells | Clofibrate increased HO-1 expression, activating NRF2 signaling pathway, but not PPARα pathway. NRF2 silencing attenuated clofibrate-induced HO-1 gene transcription, while PPARα silencing had no effect on clofibrate-induced HO-1 expression. | [178] |
| Pterocarpanquinone | PC3, LNCaP, and LAPC4 | Pterocarpanquinone increased apoptosis inducing ROS production and lipid peroxidation. It increased NRF2, NQO1 and SOD expression. | [183] |
| 2-Chloroadenosine | PC3 and LNCaP cells | 2-Chloroadenosine reduced viability and increased apoptosis. 2-Chloroadenosine reduced GSH content and increased ROS levels in PC3, whereas only ROS production was increased in LNCaP cells. 2-Chloroadenosine treatment increased NRF2 nuclear translocation in PC3 cells. | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Cancers 2023, 15, 3037. https://doi.org/10.3390/cancers15113037
Tossetta G, Fantone S, Marzioni D, Mazzucchelli R. Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Cancers. 2023; 15(11):3037. https://doi.org/10.3390/cancers15113037
Chicago/Turabian StyleTossetta, Giovanni, Sonia Fantone, Daniela Marzioni, and Roberta Mazzucchelli. 2023. "Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer" Cancers 15, no. 11: 3037. https://doi.org/10.3390/cancers15113037
APA StyleTossetta, G., Fantone, S., Marzioni, D., & Mazzucchelli, R. (2023). Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Cancers, 15(11), 3037. https://doi.org/10.3390/cancers15113037








