1. Introduction
Immunotherapy with antibodies disrupting the PD-1/PD-L1 axis provides an important breakthrough in the treatment of cancer, as these antibodies have improved the survival outcomes of patients in many cancer types [
1]. For this reason, the use of antibodies against PD1/PDL1 alone or in combination with other drugs became a gold standard of first-line treatment in different advanced/metastatic tumor types. Furthermore, in addition to the growing FDA-approved indications (e.g., more than ten), a large number of clinical trials continue to investigate the efficiency of anti-PD-1 therapy alone or in combination with another anticancer therapy in various indications.
Prior to being involved in the regulation of the immune response and considered one of the most important negative immune checkpoints, PD-1 (Programmed cell Death Protein 1, UniProtKB reference: Q15116) was initially described as a gene associated with apoptosis during thymic T cell selection. Mechanistically, the canonical PD-1 pathway in T cells includes its binding with its ligands CD274/PD-L1 and CD273/PD-L2, the activation of the SHP1/2 signaling pathways, and inhibition of PI3K/AKT, MAPK, and mTOR pathways to deliver immune escape messages: suppression of T cell proliferation, cytokine production, and cytotoxic functions [
2]. Consequently, the blockade of the PD-1/PD-L1 or PD-L2 axis by antibodies such as Nivolumab (Opdivo
®), Pembrolizumab (Keytruda
®), or Cemiplimab (Libtayo
®) has emerged as a promising strategy to restore the anti-tumor immune response. However, some patients are completely refractory to anti-PD-1 therapy, and a large part of patients do not present durable remission.
To identify the molecular mechanisms responsible for these phenomena, most studies have focused on immune cells since PD-1 was initially described as being mainly expressed by these cells. Thus, reduced dendritic cells maturation, suboptimal T-cell activation, impaired T-cell trafficking and infiltration, stroma-dependent exclusion, additive negative immune checkpoint on immune cells, and presence of immunosuppressive cells have been identified as causes of anti-PD-1 therapy escape [
3].
In addition, several authors highlighted that the PD-1 blockade on the surface of tumor cells could also play a role in the resistance to anti-PD-1 therapy. Kleffel et al. (2015) [
4], Li et al. (2017) [
5], Du et al. (2018) [
6], Pu et al. (2019) [
7], and Wang et al. (2020) [
8] reported that melanoma, hepatocellular carcinoma, pancreatic ductal adenocarcinoma, and lung cancer cells expressed PD-1, respectively. As reported by Yao et al. (2018) [
9], several databases (TCGA, Human protein Atlas or Cancer Cell Line Encyclopedia (CCLE)) indicate that a large variety of cancer cells express PD-1. Based on these reports, it seems legitimate to question the effect of anti-PD-1 therapy on PD-1-expressing tumor cells. In the context of lung cancer, the literature reports that the PD-1 silencing or blockade promoted cell proliferation or colony formation in the cellular assay and tumor growth in in vivo assay (Du et al. (2018) [
6] and Wang et al. (2020) [
8]). Thus, the use of anti-PD-1 therapy appears paradoxical: on the one hand, anti-PD-1 therapy can provide an anti-tumor response by the immune system activation, and on the other hand, anti-PD-1 therapy can provide a pro-tumor response by increasing the proliferation of tumor cells.
Extracellular vesicles (EVs, i.e., exosomes and microvesicles) and their contents (miRNA, protein, mRNA, …) have been considered as candidate biomarkers for the diagnosis, the monitoring of disease progression and the prediction of response to cancer therapy [
10]. Numerous studies show correlations between the proteome and transcriptome of tumor cells and that of EVs circulating in the biological fluids (blood, urine, saliva, …) of patients with different cancers. Moreover, EVs are also considered actors of cell–cell communication via the transfer of the biological material they contain. Since Valadi et al. (2007) [
11] and the description that exosomes transfer mRNA and miRNA between mast cells, a large number of articles have reinforced the major role played by EVs and particularly exosomes in intercellular communication. Our laboratory also contributed to this by demonstrating that radiotherapy-induced overexpression of exosomal miRNA-378a-3p in cancer cells limits the cytotoxicity of natural killer cells [
12] and by reporting that anti-PD1 therapy induces the release of lymphocyte-derived exosomal miRNA-4315, thereby inhibiting Bim-mediated apoptosis of tumor cells [
13]. Moreover, in these two articles, it appears that the longitudinal study of the expression levels of exomiR-4315 and exomiR-378 during the administration of anticancer treatments can be used as a biomarker.
In recent years, epitranscriptomic studies of miRNAs (i.e., the study of reversible chemical modifications occurring within microRNAs (whether mature or not)) are booming with the development of sequencing methods and/or the adaptation of DNA methylation analysis methods. Indeed, the work of Berulava et al. (2015) [
14] with a method coupling m6A-IP and sequencing, Konno et al. (2019) [
15] with the detection of methylated miRNA using MALDI-TOF-MS, Pandolfini et al. (2019) [
16] with a method coupling m7G-IP and sequencing, Cheray et al. (2020) [
17] with BS-miRNA-seq and the use of a method coupling m5C-IP and qPCR-Array, and Carissimi et al. (2021) [
18] with the use of BS-miRNA-seq and MAmBA methods have increased the knowledge in the field of epitranscriptomics of miRNA. Moreover, some of these works have highlighted that methylated miRNAs can hold a function of biomarker. For example, Konno et al. (2019) [
15] report that the adenosine methylation of miR-17-5p is a biomarker to distinguish early pancreatic cancer patients from healthy patients. Cheray et al. (2020) [
17] report that the high level of cytosine methylation of miR-181a-5p was associated with poor survival of GBM patients. Briand et al. (2020) [
19] report that the adenosine methylation of miR-200b-3p was associated with a prognosis of survival for GBM patients. Zhang et al. (2021) report that the level of serum circulating m6A miRNAs can be used as a biomarker for cancer detection and the development of non-invasive therapeutic monitoring [
20].
To further understand the molecular mechanisms involved in the effect of anti-PD-1 therapy on lung cancer cells, we propose here an original study aiming at defining whether an epitranscriptomic reprogramming of miRNAs (i.e., their adenosine, cytosine, and guanosine methylation, here) in lung cancer cells could account for the escape of the anti-PD-1 therapy.
2. Materials and Methods
2.1. Cell Culture
A549 (ATCC#CCL-185), H1975 (ATCC#CRL-5908), H358 (ATCC#CRL-5807), and H1650 (ATCC#CRL-5883) cells were cultured in a RPMI medium supplemented with 10% of fetal bovine serum, 1% penicillin–streptomycin, and 2 mM L-glutamine. All cells were cultivated in a 5% CO2 incubator at a temperature of 37 °C.
2.2. Cytometry Analysis
Directly-labeled monoclonal antibodies were used for flow cytometry APC-mouse IGg1K isotype control (#555751, BD Biosciences, Grenoble, France) and APC-mouse anti-human CD279 (#558694, BD Biosciences, Grenoble, France). Two hundred thousand cells of each cell line were labeled at room temperature in PBS/1% BSA, washed twice in PBS, and analyzed on a BD ACCURI C6 cytometer (Paris, France). Cancer lines were gated according to their forward-scatter and side-scatter properties and excluding debris and doublets.
2.3. PD-1 Receptor Occupancy (Saturation) Analysis
PD-1 receptor occupancy (saturation) by Nivolumab was investigated using the in-cell ELISA method. Tumor cells (4000 cells/well) were plated in 96 wells plates for 24 h. Nivolumab (#A1307, Biovision/CliniScience, Nanterre, France) was next incubated at the indicated concentration for 3 h at room temperature. Cells were then washed four times with 1× phosphate-buffered saline (PBS). Cells were fixed using an 8% paraformaldehyde solution (15 min, room temperature). After four extensive washes, anti-PD-1-HRP (0.5 μg/mL, #10377-MM07-H, Interchim, Montluçon, France) was incubated for 2 h at room temperature, and the signal was detected using Step Ultra TMB-ELISA substrate solution according to the manufacturer’s instructions (#34028, Thermo Fisher Scientific, Illkirch-Graffenstaden, France). The saturation percentage was calculated relative to the experiment performed with a control antibody (#DDXCH0P-100, Novus, Nantes, France).
2.4. miRNA Extraction
miRNA extractions were performed using miRNeasy mini kit (#217004, Qiagen, Paris, France) on a QIAcube instrument according to the manufacturer’s instructions (Qiagen, Paris, France).
2.5. qPCR of miRNA
For miRNA expression analysis and detection from products of RNA immunoprecipitation (RIP) performed with anti-m6A antibody, miRNA was reverse transcribed using a miScript II RT kit (#218161, Qiagen, Paris, France) and analyzed by qPCR with the miScript SYBR Green PCR kit (#218076, Qiagen, Paris, France) using the specific miScript primer assays (#218300, Qiagen, Paris, France) according to the manufacturer’s instructions. Rotor-Gene Q was used as a real-time thermocycler (Qiagen, Paris, France).
2.6. mRNA Extraction and qPCR
RNA extractions were performed using the RNeasy kit (#74116, Qiagen, France) and QIAcube according to the manufacturer’s instructions (Qiagen, Paris, France). Reverse transcription was then performed with a QuantiTect Reverse Transcription Kit (#205311, Qiagen, Paris, France), and PCR was performed with a QuantiFast SYBR Green PCR Kit (#204156, Qiagen, Paris, France) and Rotor-Gene Q (Qiagen, Paris, France). Primers used were the QuantiTect Primers assay (#249900, Qiagen, Paris, France) and, more precisely, RPLP0 (QT00075012), KHDRBS3 (QT00065296), METTL3 (QT00036540), and HuR (QT00037856).
2.7. Methylation Level of miRNAs
For RNA immunoprecipitation (RIP), two rounds using 5 μg of anti-m6A (#ab208577, Abcam, Paris, France), anti-m7G (#6655, Biovison, Milpitas, CA, USA), and anti-m5C (#61255, Active Motif, Nantes, France) antibodies and 5 μg of small RNA were performed. The reaction was carried out using a Dynabeads protein G IP kit with some modifications (#10007D, Thermo Fisher Scientific, Paris, France) such as described by Berulava et al. (2015) [
14] and Briand et al. (2021) [
19]. As a control, IP was performed using IgG (#ab18443, Abcam, Paris, France) instead of an anti-m6A antibody. miRNAs obtained from RIP were reverse transcribed using a miScript II RT kit (#218161, Qiagen, Paris, France) and analyzed using the miScript miRNA PCR array human cancer pathway kit (#331221, Qiagen, Paris, France) according to the manufacturer’s instructions. Fold enrichment was next calculated using the Ct value obtained from qRT-PCR performed with input miR, IP-IgG, IP-m6A, and the 2
−ΔΔCt formula.
2.8. siRNA Transfection
siRNA directed against METTL3, KHDRBS3, and HuR (#sc-92172, #sc-40922, and sc-35619 Santa Cruz, Morzine, France) were used in this article. siRNA-A (#sc-37007, Santa Cruz, Morzine, France), i.e., a scrambled sequence devoid of specific degradation of any cellular message, was used as control. In a six wells culture plate, 2.105 cells were incubated for 24 h at 37 °C in a CO2 incubator. Then, 60 pmol of siRNA mixture (prepared with siRNA Transfection reagent, #sc-29528, Santa Cruz, France) was incubated on cells for 7 h at 37 °C in a CO2 incubator. Without removing the siRNA mixture, we next added 1 mL of normal growth medium containing two times the normal serum and antibiotics concentration for 24 h. Then, cells were expanded for 48 h in a normal culture medium. Thus, analyses were realized about 72 h after the siRNA incubation.
2.9. Immunoprecipitation
After two cold PBS 1× washes (10 mL), three 100 mm dish cultures at confluence 75% (2.105 cells) were incubated with 500 μL of lysis buffer (20 mM TrisHcl pH 7.4, 150 mM de NaCl, 1 mM EDTA, 1% Triton + Protease cocktail inhibitor) for 5 min on ice. Then, cells were scraped and centrifuged in a 1.5 mL Eppendorf tube (4 °C, 15 min, 14,000× g). After a transfer in a new tube, the supernatant is used for protein dosage (#23225, Pierce BCA Protein Assay Kit, Thermo Scientific, Paris, France). At this step, the supernatant can be stored at −80 °C. Then, we performed of pre-clean step. For that, 20 μL of magnetic beads (#10004D, DynaBeads, Thermo Scientific, Paris, France) were washed twice with 1 mL of lysis buffer in a 1.5 mL Eppendorf tube. After the last wash, 500 μg of lysate was added to magnetic beads for 2 h at 4 °C on a revolver tube mixer (Labnet International, Issy-les-Moulineaux, France) to saturate aspecific interactions. After magnetic separation, the lysate is then collected to be incubated (overnight, 4 °C, revolver tube mixer) with the antibody (4 μg of anti-METTL3 (#ab195352, Abcam, France) or IgG as control (#2729S, Cell Signaling/Ozyme, Paris, France). Then, the lysate-antibody mixture was incubated (2 h, 4 °C, revolver tube mixer) with 20 μL of saturated magnetic beads (as previously described). Four washes were next performed (two with lysis buffer and two lysis buffers devoid of triton). Finally, beads were resuspended in 100 µL of Buffer NH4HCO3 at 50 mM pH = 8, and a supernatant (containing immunoprecipitation products) was stored for future use.
2.10. miRNA Pull-Down Assay
Cellular extracts were performed by incubating 10 min on ice 2.106 cells in cell lysis buffer (85 nM KCl, 0.5% NP40, 5 mM HEPES, pH 7.4, three volumes of buffer for one cell volume). After incubation, the mixture was sonicated in a Bioruptor/Diagenode (15 cycles, high, 30 s on/off). After centrifugation (10 min, 14,000× g, 4 °C), the supernatant was transferred to a fresh tube to be quantified (Qubit Method, Thermo Fisher, Paris, France).
Streptavidin M280 Dynabeads (#11205D, Thermo Fisher, Paris, France) were washed three times in binding/washing buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 2 M NaCl), twice with 50 μL of 0.1 NaOH/0.05 M NaCl buffer, once with 50 μL of 0.1 M NaCl. Beads were finally resuspended in 50 μL of 0.1 M NaCl and transferred in a new DNA low-binding tube. Then, 800 μg of protein was incubated with 50 μL of beads for 15 min at room temperature.
Then, the tube was placed on a magnetic stand for 2–3 min, and the supernatant was collected for future use.
In parallel, 500 pmol of biotinylated mimic miRNA was then incubated with beads for 15 min at room temperature under gentle rotation. Then, the tube was placed on a magnetic stand for 2–3 min to collect miRNA-immobilized beads. After two washes in binding/washing buffer, miRNA-immobilized beads were resuspended in 100 μL of Protein binding buffer (20 mM Hepes (pH 7.3), 50 mM KCL, 10% glycerol, and 5 mM MgCl2. Add the following reagents before use: 1/1000 vol. of 1 M Dithiothreitol (DTT), Phosphatase inhibitor (100×), Proteinase inhibitor (100×), and RNase Inhibitor (25~1000 U/mL, for RNA application).
The supernatant was then added, and the mixture was incubated for 30 min at room temperature.
The tube was next placed on a magnetic stand for 1 min, and the supernatant was discarded. After three washes, elution was performed using 20 μL of 4× Laemmli buffer (#1610747, BioRad, Marnes-la-Coquette, France). After incubation for 10 min at 95 °C, samples were loaded onto an SDS-PAGE gel, and proteins were detected by western blotting.
2.11. Western Blot
After a denaturation step (dry-bath/95 °C/10 min), samples are placed in the wells of a 4–15% Mini-PROTEAN electrophoresis gel (#4568084, Bio-Rad, Marnes-la-Coquette, France), and the electrophoresis (90 V) is performed in the presence of Tris/Glycine/SDS Electrophoresis Buffer (#1610732EDU, Bio-Rad, Marnes-la-Coquette, France). A transfer (30 V, 90 min RT) is performed on a PVDF membrane (Trans-Blot Turbo Mini PVDF, #1704156, Bio-Rad, Marnes-la-Coquette, France) in the presence of a cold Tris/Glycine transfer buffer (#1610734EDU, BioRad, Marnes-la-Coquette, France). The membrane is then saturated with the saturation solution (5% milk, PBS 1×). Primary and secondary antibodies (METTL3#MA5-27527, Thermo Fischer, Paris, France; KHDRBS3#ab68515, Abcam, Paris, France, RBMX# PA5-99433, Thermo Fischer, Paris, France; HuR# 39-0600, Thermo Fischer, Paris, France; HRP-anti-Rabbit IgG (#111-035-006, Jackson Immunology, West Grove, PA, USA) and HRP-anti-Mouse IgG (#115-036-75, Jackson Immunology)) are incubated in milk1%-PBS 1×. The HRP signal is revealed using the ChemiDoc imager (Bio-Rad, Marnes-la-Coquette, France) and the ECL-Clarity kit (#1705061, Bio-Rad, Marnes-la-Coquette, France).
2.12. Chromatin Immunoprecipitation (ChIP) Analyses
ChIP was performed using the ChIP-IT Express kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s instructions. The cross-linking step was performed by treating the cells with 37% formaldehyde solution for 15 min at room temperature. Sonication was performed with the Bioruptor Plus (8 cycles 30 s ON/90 s OFF) (Diagenode, France). The QuantiFast SYBR Green PCR Kit and Rotor-Gene Q (Qiagen, Paris, France) were used to perform the qPCR. Antibodies used were: Anti-IgG (#ab2410, Abcam, Paris, France) and anti-ELK1 (sc365876x, Santa Cruz, Morzine, France). The primers used here are gagagcatttgctgggttg and ttgtcatcccgacttgctcct.
2.13. Cell Cytotoxicity Assay
This assay permits estimating the lysis of desired target cells (H1975 here) via an effector immune cell of choice (PBMC activated by IL2, here). In this assay, the lysis of H1975 cells is determined by labeling these cells with fluorescent molecules, co-incubating them with IL2-activated PBMC, then measuring the release of the labeled molecule in the supernatant. DELFIA EuTDA Cell Cytotoxicity (#AD0116, Perkin Elmer, Villebon-sur-Yvette, France) reagents were used as previously described (Briand et al. 2019) [
21]. For PBMC isolation, whole blood was diluted with an equal amount of 1× phosphate-buffered saline (PBS) and placed on top of Ficoll-Paque PLUS (#17-1440-02, VWR, Rosny-sous-Bois, France) in tubes for centrifugation at room temperature for 20 min at 1000×
g. PBMCs were next carefully collected from the interface layer between the blood plasma and Ficoll solution. The collected PBMCs were twice washed with 1× PBS, then cultured in an RPMI1640 medium containing 10% FBS, 0.1 nM human IL-2 (Diaclone Research, Besançon, France), and 55 μM β-mercaptoethanol for 24 h. BATDA-labeled target cells and PBMC effector cells (effector to target cell ratio: 10:1) were incubated (2 h, 37 °C, 5% CO
2) together to allow redirected lysis. The supernatant containing TDA released by dead cells was incubated with Europium solution to allow the stable formation of a highly fluorescent Europium-TDA chelate (EuTDA). The amount of EuTDA was quantified by analysis of the relative fluorescence intensity (FL
sample) at 520 nm. Spontaneous fluorescence intensity was determined with target cells without effector cells. The fluorescence intensity of maximal lysis (FL
max) was determined using target cells and DELFIA
® lysis buffer. The percentage of specific cell lysis was calculated as 100 × (FL
sample − FL
Spon)/(FL
max − FL
Spon).
2.14. RT-qPCR Analysis
RNA extract is performed using RNeasy Mini QIAcube Kit and QIAcube (#1038703, Qiagen, Paris, France). RT-qPCRs are performed using the QuantiTect Reverse Transcription Kit (#205313, Qiagen, Paris, France), QuantiFast SYBR Green PCR Kit (#204057, Qiagen, France), QuantiTect Primer Assays (#249900, Qiagen, Paris, France), and Rotor-Gene Q as a real-time thermocycler (Qiagen, Paris, France). Reference gene RPLP0 was used with the 2−∆∆Ct relative quantification method.
2.15. CLIP
CLIP assays were performed using a RIP-assay kit (#RN1005 CliniScience, Nanterre, France) from 10 million per sample of UV-crosslinked cells (150 mJ/cm2 of UVA (Bio-link, Vilber, Marne-la-Vallée, France)) according to the manufacturer’s instructions. IP assays were performed in the presence of 15 μg of anti-GW182 (#RNP033P, CliniScience, Nanterre, France) overnight at 4 °C.
2.16. Isolating Exosomal miRNA from Blood
From the blood sample collected in K + EDTA tubes, 4–5 mL of blood was centrifugated for 10 min/1900× g/4 °C. Supernatants were carefully transferred into a new centrifuge tube and centrifuged a second time at 16,000× g for 10 min at 4 °C to remove cellular debris. Plasmas were aliquoted in 2 mL tubes and were frozen at −80 °C until use. A total of 1 mL of plasma was processed for the isolation of miRNA using the ExomiRNeasy Midi kit (#77144, Qiagen, Paris, France) according to the manufacturer’s instructions.
2.17. Exosome Isolation
As described in our previous articles [
12,
13], ExoQuick kits (#EXOTC10A1 and #EXOCG50A1, Ozyme, Saint-Cyr-l’École, France) were used according to the manufacturer’s instructions. Exosome total protein concentration was determined using the Bradford assay (Bio-Rad Laboratories, Marnes-la-Coquette, France), and exosomes were stored at −80 °C until use. As already mentioned, the Nanosight experiment was performed to validate the use of the “exosome” term due to the isolation of EVs with a size predominantly between 80 and 120 nm, i.e., values commonly characterizing exosomes. Consequently, the term “exosome” is used in this article.
2.18. Patients Data
Plasma was collected from patients treated at the “Institut de Cancérologie de l’Ouest” (ICO,
http://www.ico-cancer.fr, accessed on 4 May 2023). All patients recruited gave signed informed consent. All the samples collected and the associated clinical information were registered in the database (N° DC-2018-3321) and validated by the French research ministry. Biological resources were stored at the “Centre de Ressources Biologiques-Tumorothèque (CRB)” (Institut de Cancérologie de l’Ouest, Saint-Herblain, France) [
22]. All clinical and radiologic data were collected from the electronic medical records stored at the Institut de Cancérologie de l’Ouest.
2.19. Statistical Analysis and Results
Except when indicated, data are representative of the mean and standard deviation calculated from three independent experiments. The significance of the differences in means ± standard deviations was calculated using the Student t-test. p < 0.05 was used as a criterion for statistical significance.
4. Discussion
PD-1 signaling pathway characterization in cancer and immune cells has provided meaningful progress in understanding the resistance to PD-1 therapy but also gave rise to a paradox. Indeed, blockade, invalidation, or inhibition of PD-1 in melanoma (Kleffel et al.) [
4] and HCC (Li et al. [
5]) reduced tumor growth, while the blockade of PD-1 in lung and colon cancer cells promoted tumor growth (Du et al. [
6], Wang et al. [
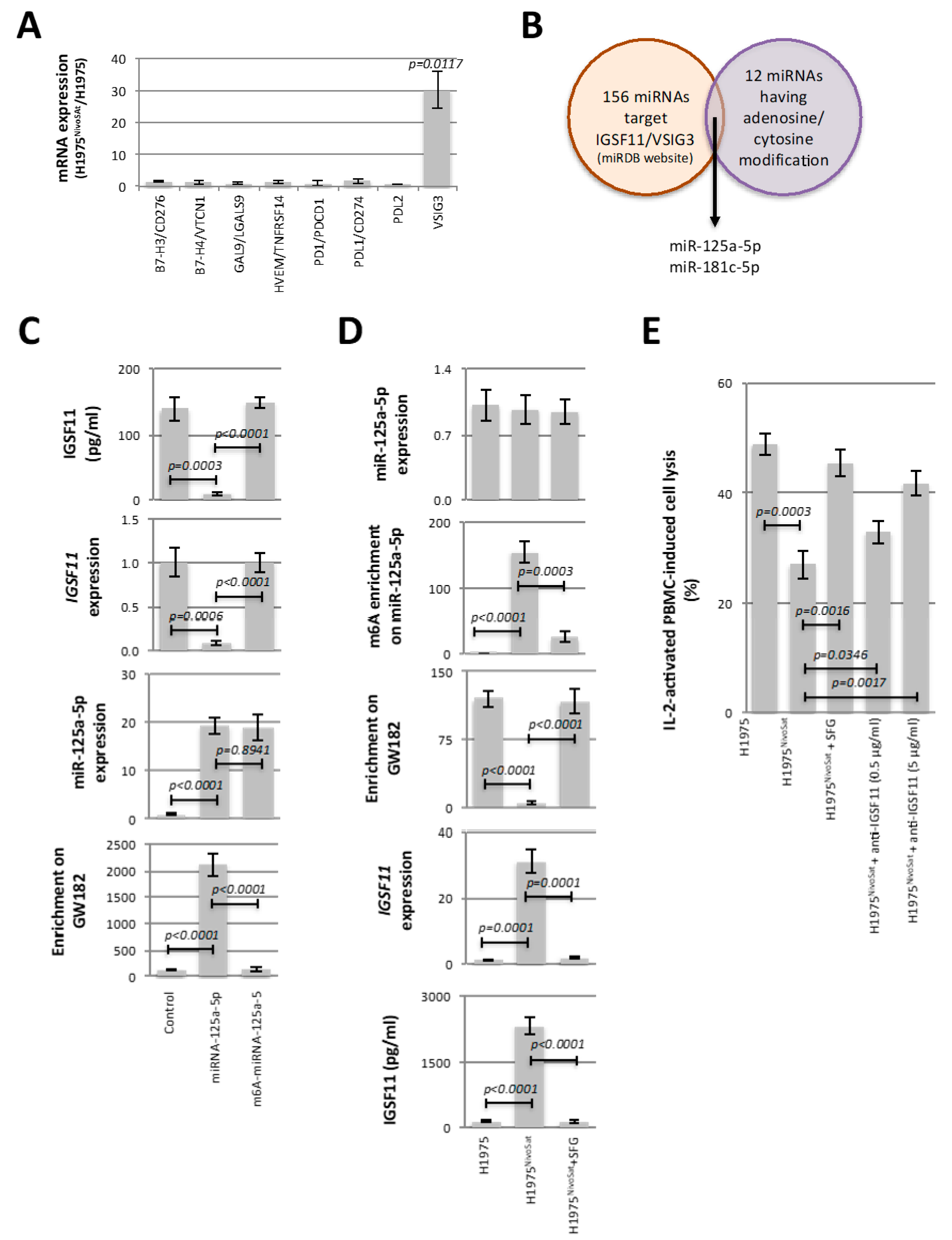
8]). In addition, these articles underline the fact that the PI3K/AKT, MAPK/ERK, and/or mTOR signaling pathways play a crucial role, via their activation or inhibition, in the pro- or anti-tumor role played by the PD-1 inhibition/blockade. Our work adds a new pathway of resistance to PD-1 therapy by identifying the METTL3-mediated adenosine methylation of miR-125a-5p and the regulation of IGSF11 expression as the two central points of the PBMC-induced lysis escape phenomenon.
The involvement of the METTL3-mediated adenosine methylation of miR-125a-5p and the IGSF11 expression in the PBMC-induced lysis escape phenomenon provides two distinct strategies to limit the acquisition of this phenomenon. Indeed, our work supports the idea that the PBMC-induced lysis escape phenomenon observed in H1975
NivoSat can be abrogated using either anti-IGSF11 antibody-based immunotherapy or targeted therapy based on inhibition of adenosine methylation of miR-125a-5p. In addition to SFG, we also observed that UZH1a, a potent and selective METTL3 inhibitor [
25], and siRNA directed against METTL3 reduced the adenosine methylation level of miR-125a-5p and decreased the IGSF11 expression in H1975
NivoSat cells (
Supplementary Figure S11). Our work is not the only one to focus on these two therapeutic areas, which are IGSF11 antibody-based immunotherapy and METTL3 inhibitor-based therapy. Several studies support the idea that IGSF11 is a promising new target for the immunotherapy of gastrointestinal and hepatocellular carcinomas (Watanebe et al. [
26]) and glioma (Ghouzlani et al. [
27]). In this regard, iOmx therapeutics claims to have an anti-IGSF11 antibody in development (
https://iomx.com/pipeline, accessed on 4 May 2023). The search for METTL3 inhibitors is also a growing field, especially since the recent acceleration of epitranscriptomics with the characterization of methylations of small non-coding RNAs such as miRNAs [
14,
16,
17,
28]. Concerning METTL3, the main actor of the adenosine methylation of miRNAs, several inhibitors have been described, such as UZH1a [
25] and others [
29,
30]). In this regard, STORM Therapeutics develops STM2457, a small-molecule inhibiting METTL3 presenting a potential therapeutic interest in the fight against leukemia [
31] (
https://www.stormtherapeutics.com/science/pipeline/, accessed on 4 May 2023).
By identifying the overexpression of the negative immune checkpoint IGSF11 as a player in the loss of PBMC-induced lysis, our work echoes the idea that resistance to anti-PD-1 therapy may result from the overexpression of other negative immune checkpoints. In this respect, the work of Koyama et al. [
32] shows that resistance to anti-PD-1 therapy may be due to the overexpression of the negative immune checkpoint TIM-3. In addition, by showing that anti-IGSF11 increased the PBMC-mediated lysis of H1975
NivoSat, our work contributes to the idea that combining several immunotherapies may be more efficient than using single immunotherapy and/or could limit the apparition of PD-1 therapy resistance [
33].
In many cases, resistance to anticancer treatment is favored by changes in DNA methylation patterns. By identifying a gain of cytosine and adenosine methylation in seven and five miRNAs, respectively, our results show that changes in miRNA methylation patterns are also a molecular event associated with resistance to anticancer treatment. The role of METTL3 and adenosine RNA methylation in response to anti-PD-1 therapy has already been demonstrated in the literature. Indeed, Wang et al. [
8] have recently shown that adenosine methylation of the 3’UTR regions of STAT1 and IRF1 transcripts influences the response to anti-PD-1 therapy.
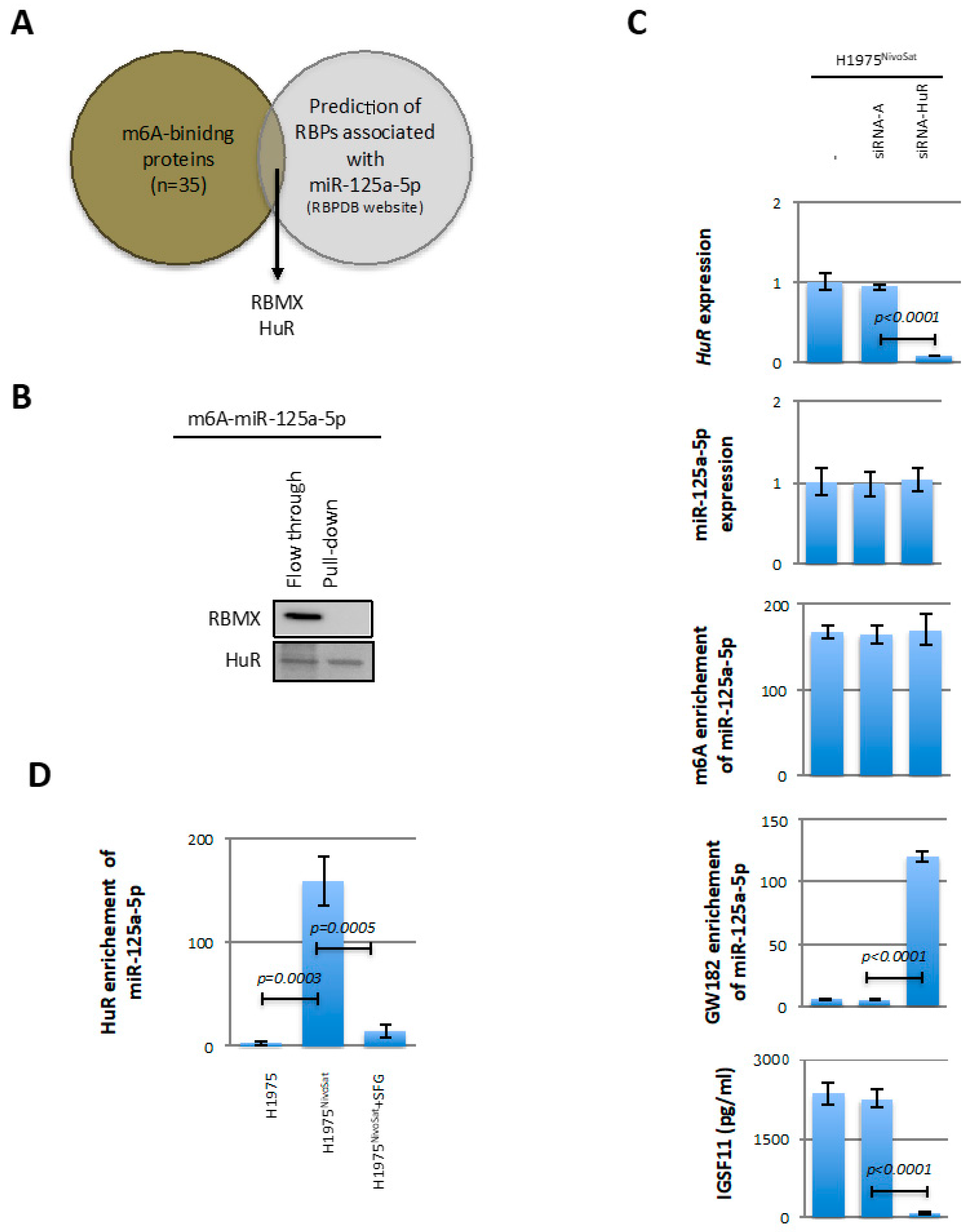
At the level of fundamental knowledge of miRNA epitranscriptomic regulation mechanisms, this paper introduces two new concepts. Indeed, through the demonstration of the role played by METTL3/KHDRBS3 in the adenosine methylation of miR-125a-5p, our work introduces, for the first time, the notion of RBP-directed miRNA methylation, i.e., a mechanism by which an RBP (here KHDRBS3) addresses a miRNA methyltransferase (here METTL3) on a miRNA to methylate it. Interestingly, this mechanism is reminiscent of the TF-directed DNA methylation mechanism in which a transcription factor (TF) addresses a DNMT to DNA to methylate it.
Through the demonstration of the role played by HuR in blocking the recruitment of m6A-miR-125a-5p by GW182, our work introduces, for the first time, the notion of a blocker of miR-mediated gene silencing via the blockage of adenosine–methylated miRNA recruitment by GW182. Interestingly, this mechanism is reminiscent of the mechanism of transcription blocking via the recruitment of methylated-binding proteins (MeCP2, Kaiso, …) on methylated DNA [
34,
35,
36].
Finally, our data technically provide a first proof of concept that the adenosine methylation level of miR-125a-5p can be studied using miRNA encapsulated in circulating blood exosomes. Of course, the prognosis character of the evolution of the adenosine methylation of exomiR-125a-5p will require evaluation in large cohorts of patients treated with anti-PD-1 therapy. However, based on the promising results obtained in the two patients reported here, the study of the evolution of the adenosine methylation of exomiR-125a-5p during anti-PD-1 therapy represents an attractive alternative approach to select the right time when the right patient is eligible for personalized medicine combining anti-PD-1 therapy with other immunotherapies (such as the one targeting IGSF11) or therapy targeting METTL3. Moreover, the methylation level of miR-125a-5p could be used as an early marker of response to anti-PD-1 therapy and thus serve to guide the realization of other more invasive examinations (and/or more costly in technical and human resources) to evaluate the response to anti-PD-1 therapy. In other terms, our article suggests that the analysis of the methylation level of exomiR-125a-5p is part of a context of precision and personalized medicine: “precision” in terms of the use of therapy targeting IGSF11 or METTL3 and “personalized” in terms of the patient via the possible detection of the right time to treat the right patient with the right therapy.
Our paper differs from the one of Konno et al. (2019) [
15] in that we are talking here about a biomarker of response to therapy and not a diagnostic biomarker. Moreover, our work relates to an exosomal miRNA and not to a tissue miRNA (such as in Konno et al. [
15]). Interestingly, the present study complements the study of Guyon et al. (2020) [
13], showing that exomiR-4315 from lymphocytes exposed to anti-PD-1 therapy is a source of resistance in tumor cells receiving this exomiR via regulation of the pro-apoptotic protein Bim. Indeed, these two studies highlight the existence of two exomiRs (exomiR-125a-5p and exomiR-4315) having different cellular origins (tumor cells and lymphocytes), different mechanisms of action (inhibition of apoptosis and immune escape) but both being potential biomarkers of resistance to anti-PD-1 therapy. This point is a current line of research in our laboratory via the constitution of a prospective cohort of patients with lung cancer receiving anti-PD-1.