Changes in the Histology of Lung Cancer in Northern Italy: Impact on Incidence and Mortality
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Data Sources
2.3. Statistical Analysis
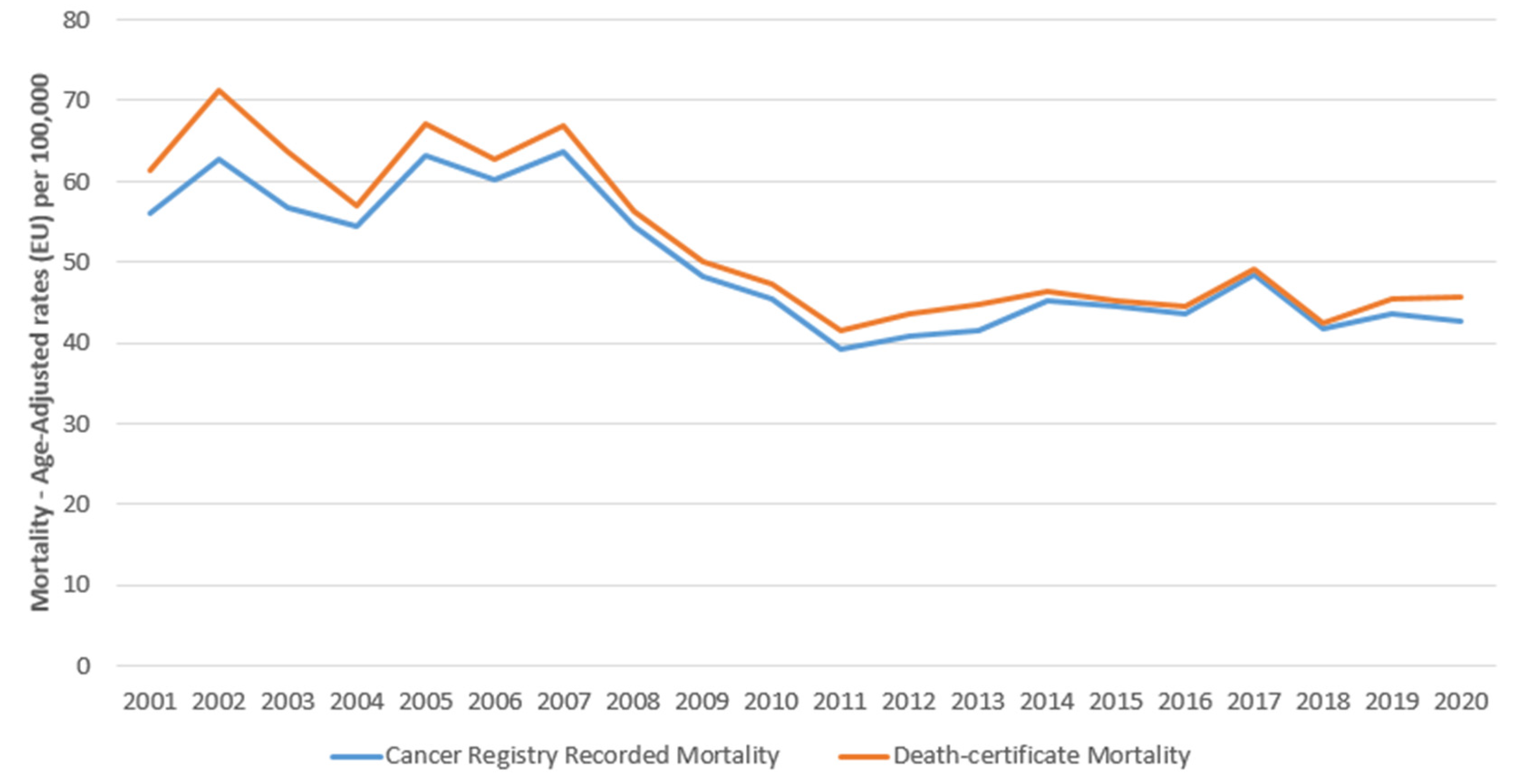
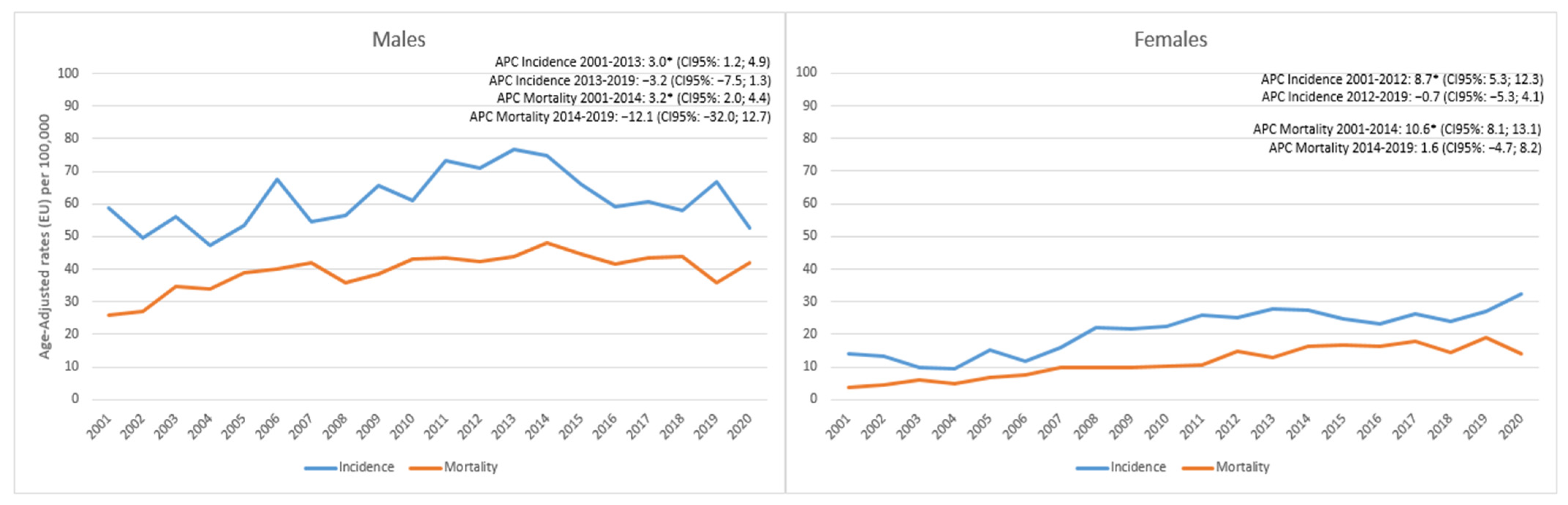
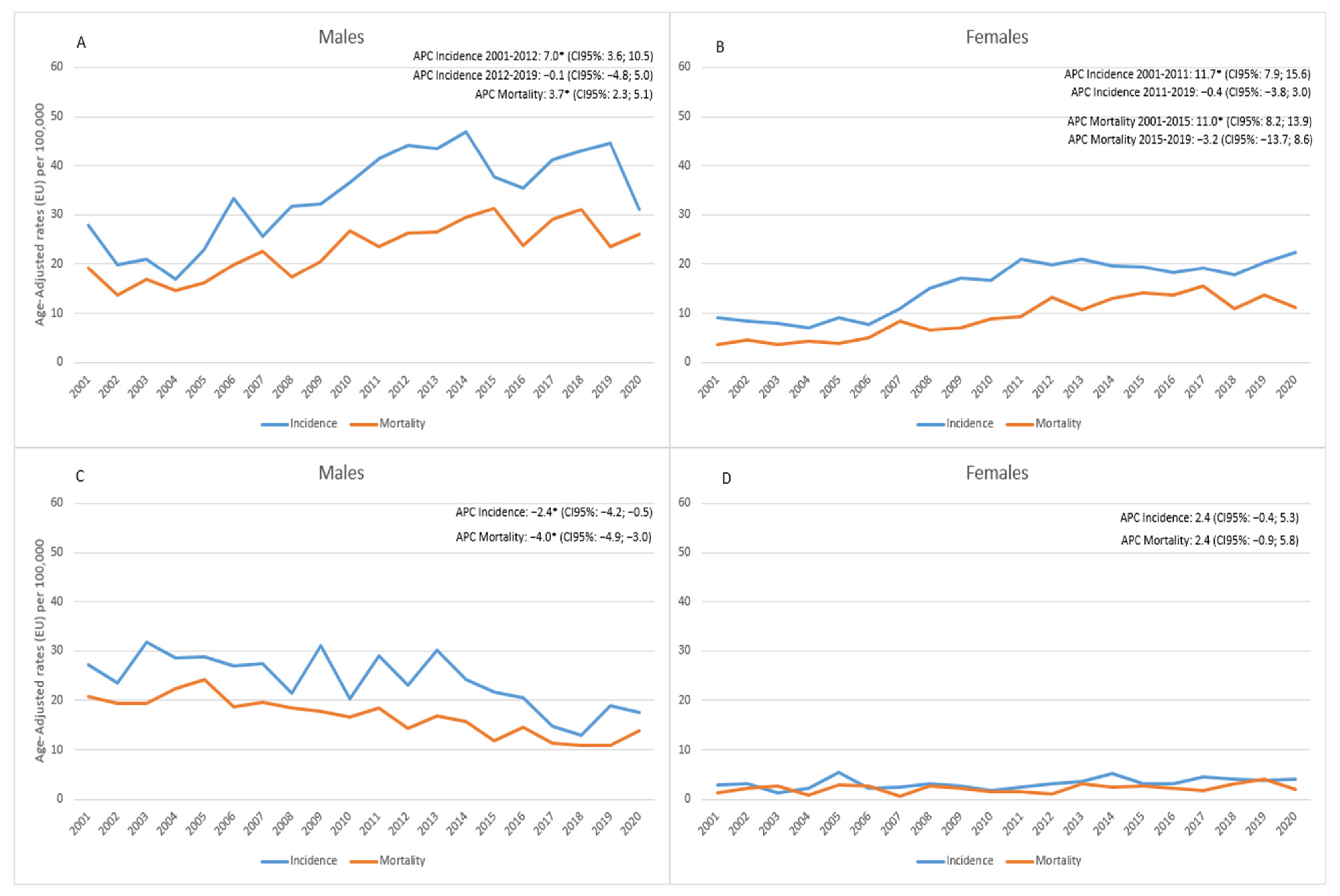
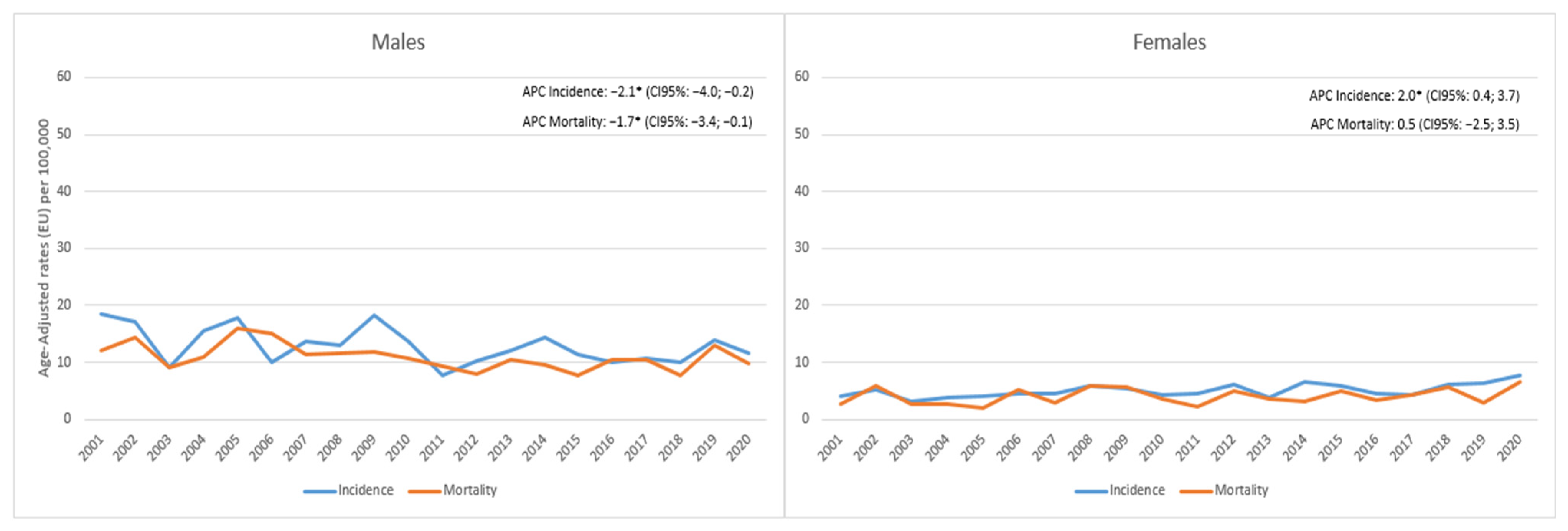
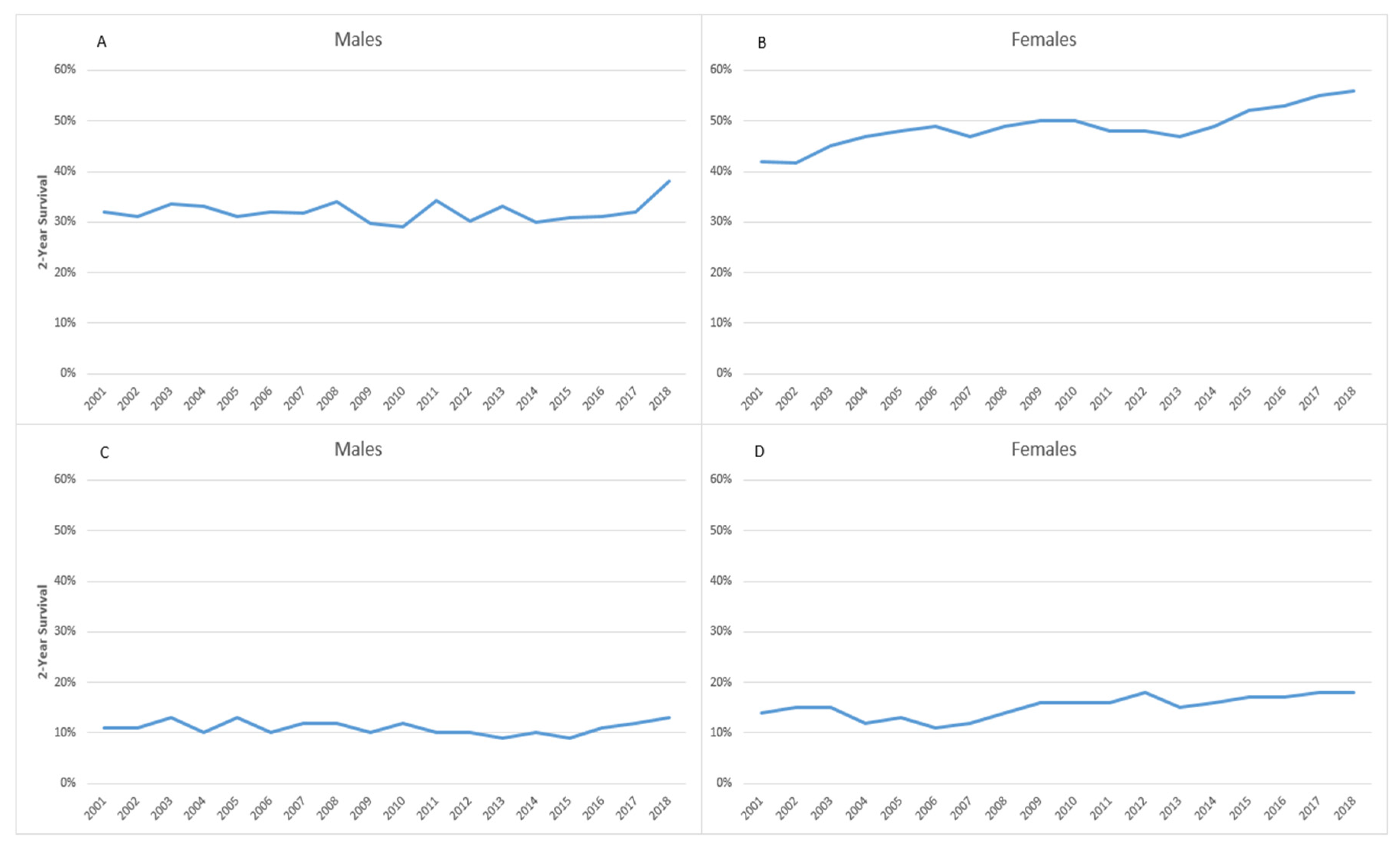
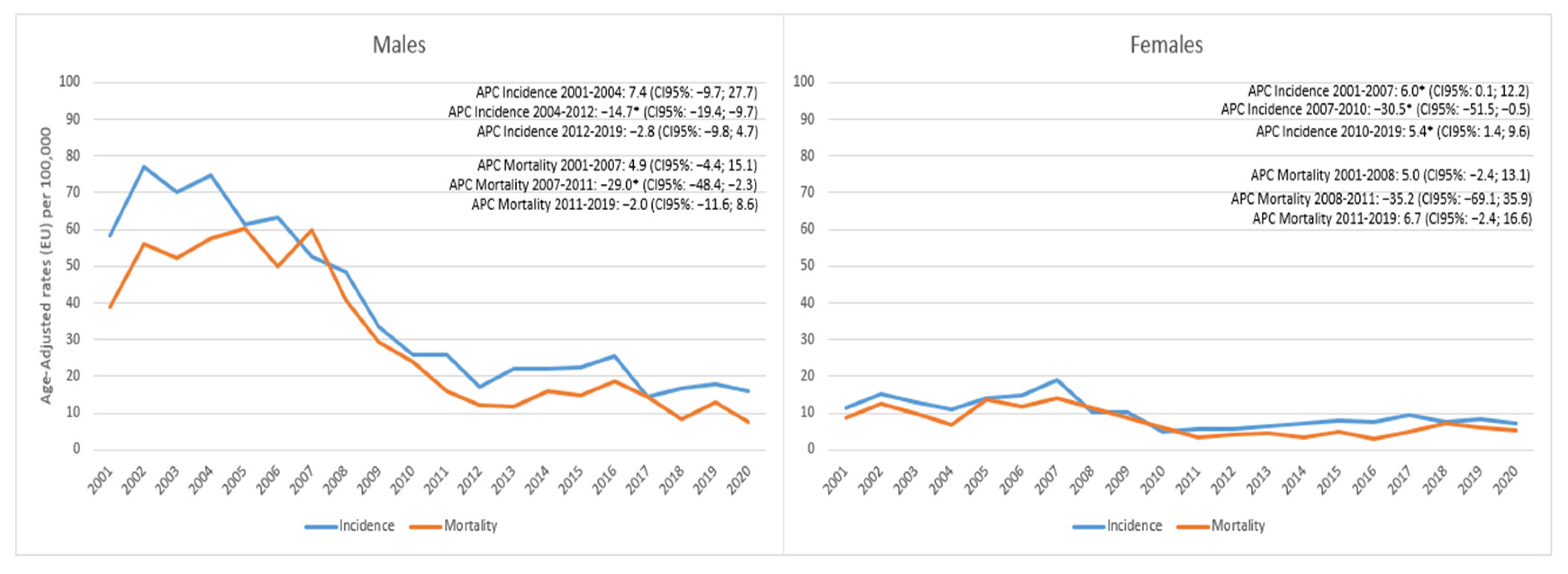
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Introduction to the 2015 World Health Organi-zation classification of tumors of the lung, pleura, thymus, and heart. J. Thorac. Oncol. 2015, 10, 1240–1242. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Linnoila, I. Pathology of non-small cell lung cancer. New diagnostic approaches. Hematol. Oncol. Clin. N. Am. 1990, 4, 1027–1051. [Google Scholar]
- Utada, M.; Yonehara, S.; Ozasa, K. Historical Changes in Histological Diagnosis of Lung Cancer. J. Epidemiol. 2019, 29, 238–240. [Google Scholar] [CrossRef]
- Guan, X.; Qin, T.; Qi, T. Precision Medicine in Lung Cancer Theranostics: Paving the Way from Traditional Technology to Advance Era. Cancer Control. 2022, 29, 10732748221077351. [Google Scholar] [CrossRef]
- De Sousa, V.M.L.; Carvalho, L. Heterogeneity in Lung Cancer. Pathobiology 2018, 85, 96–107. [Google Scholar] [CrossRef]
- Freedman, N.D.; Leitzmann, M.F.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Cigarette smoking and subsequent risk of lung cancer in men and women: Analysis of a prospective cohort study. Lancet Oncol. 2008, 9, 649–656. [Google Scholar] [CrossRef]
- Weber, M.F.; Sarich, P.E.A.; Vaneckova, P.; Wade, S.; Egger, S.; Ngo, P.; Joshy, G.; Goldsbury, D.E.; Yap, S.; Feletto, E.; et al. Cancer incidence and cancer death in relation to tobacco smoking in a population-based Australian cohort study. Int. J. Cancer 2021, 149, 1076–1088. [Google Scholar] [CrossRef]
- Jha, P. The hazards of smoking and the benefits of cessation: A critical summation of the epidemiological evidence in high-income countries. eLife 2020, 9, e49979. [Google Scholar] [CrossRef]
- Toll, B.A.; Rojewski, A.M.; Duncan, L.R.; Latimer-Cheung, A.E.; Fucito, L.M.; Boyer, J.L.; O’Malley, S.S.; Salovey, P.; Herbst, R.S. “Quitting Smoking Will Benefit Your Health”: The Evolution of Clinician Messaging to Encourage Tobacco Cessation. Clin. Cancer Res. 2014, 20, 301–309. [Google Scholar] [CrossRef]
- Available online: https://www.cdc.gov/tobacco/quit_smoking/how_to_quit/benefits/index.htm (accessed on 26 April 2023).
- Khuder, S.A.; Mutgi, A.B. Effect of Smoking Cessation on Major Histologic Types of Lung Cancer. Chest 2001, 120, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Dubey, A.; Saini, D.; Singh, M.; Prasad, C.P.; Roy, S.; Bharati, S.J.; Rinki, M.; Singh, N.; Seth, T.; et al. Environmental and occupational determinants of lung cancer. Transl. Lung Cancer Res. 2019, 8 (Suppl. S1), S31–S49. [Google Scholar] [CrossRef]
- Available online: https://www.iarc.who.int/ (accessed on 26 April 2023).
- Loomis, D.; Huang, W.; Chen, G. The International Agency for Research on Cancer (IARC) evaluation of the carcinogenicity of outdoor air pollution: Focus on China. Chin. J. Cancer 2014, 33, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- IARC Monographs: Vol. 109: Outdoor Air Pollution. Available online: http://monographs.iarc.fr/ENG/Monographs/vol.109/mono109.pdf (accessed on 26 April 2023).
- AIOM-AIRTUM-SIAPEC-IAP. The Numbers of Cancer in Italy, 2018; Intermedia Editore: Brescia, Italy, 2018. [Google Scholar]
- De Marinis, F.; Attili, I.; Gridelli, C.; Cecere, F.; Curcio, C.; Facciolo, F.; Spaggiari, L. Incorporating atezolizumab in the adjuvant setting of non-small cell lung cancer: Key discussion points from an expert multidisciplinary panel by Italian Association of Thoracic Oncology. Front. Oncol. 2022, 12, 971042. [Google Scholar] [CrossRef]
- Jing, Z.; Zhou, R.; Zhang, N. Achievement of long-term local control after radiation and anti-PD-1 immuno-therapy in locally advanced non-small cell lung cancer. Ther. Adv. Chronic. Dis. 2021, 12, 20406223211047306. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.R.; Di Muzio, J.; Badellino, S.; Mantovani, C.; Ricardi, U. Locally-advanced non-small cell lung cancer: Shall immunotherapy be a new chance? J. Thorac. Dis. 2018, 10 (Suppl. S13), S1461–S1467. [Google Scholar] [CrossRef]
- García, B.; Neninger, E.; de la Torre, A.; Leonard, I.; Martínez, R.; Viada, C.; González, G.; Mazorra, Z.; Lage, A.; Crombet, T. Effective Inhibition of the Epidermal Growth Factor/Epidermal Growth Factor Receptor Binding by Anti–Epidermal Growth Factor Antibodies Is Related to Better Survival in Advanced Non–Small-Cell Lung Cancer Patients Treated with the Epidermal Growth Factor Cancer Vaccine. Clin. Cancer Res. 2008, 14, 840–846. [Google Scholar] [CrossRef]
- López-Castro, R.; García-Peña, T.; Mielgo-Rubio, X.; Riudavets, M.; Teixidó, C.; Vilariño, N.; Couñago, F.; Mezquita, L. Targeting molecular alterations in non-small-cell lung cancer: What’s next? Per. Med. 2022, 19, 341–359. [Google Scholar] [CrossRef]
- Michelotti, A.; de Scordilli, M.; Bertoli, E.; De Carlo, E.; Del Conte, A.; Bearz, A. NSCLC as the Paradigm of Preci-sion Medicine at Its Finest: The Rise of New Druggable Molecular Targets for Advanced Disease. Int. J. Mol. Sci. 2022, 23, 6748. [Google Scholar] [CrossRef]
- Hui, R.; Garon, E.B.; Goldman, J.W.; Leighl, N.B.; Hellmann, M.D.; Patnaik, A.; Gandhi, L.; Eder, J.P.; Ahn, M.J.; Horn, L.; et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: A phase 1 trial. Ann. Oncol. 2017, 28, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, Z.; Zhang, X.; Chen, C.; Zhao, H.; Hong, S.; Zhang, L. First-line treatment for patients with advanced non-small cell lung carcinoma and high PD-L1 expression: Pembrolizumab or pembrolizumab plus chemo-therapy. J. Immunother. Cancer 2019, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Singh, P.K.; Malik, P.S.; Singh, N. Immunotherapy Alone or in Combination with Chemotherapy as First-Line Treatment of Non-Small Cell Lung Cancer. Curr Treat Options Oncol. 2020, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.R.; Check, D.P.; Caporaso, N.E.; Travis, W.D.; Devesa, S.S. US lung cancer trends by histologic type. Cancer 2014, 120, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Meza, R.; Meernik, C.; Jeon, J.; Cote, M.L. Lung Cancer Incidence Trends by Gender, Race and Histology in the United States, 1973–2010. PLoS ONE 2015, 10, e0121323. [Google Scholar] [CrossRef]
- Yu, M.; Feuer, E.J.; Cronin, K.A.; Caporaso, N.E. Use of multiple imputation to correct for bias in lung cancer in-cidence trends by histologic subtype. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1546–1558. [Google Scholar] [CrossRef]
- The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Haiman, C.A.; Stram, D.O.; Wilkens, L.R.; Pike, M.C.; Kolonel, L.N.; Henderson, B.E.; Le Marchand, L. Ethnic and Racial Differences in the Smoking-Related Risk of Lung Cancer. New Engl. J. Med. 2006, 354, 333–342. [Google Scholar] [CrossRef]
- A Kenfield, S.; Wei, E.K.; Stampfer, M.J.; A Rosner, B.; A Colditz, G. Comparison of aspects of smoking among the four histological types of lung cancer. Tob. Control. 2008, 17, 198–204. [Google Scholar] [CrossRef]
- Mangone, L.; Borciani, E.; Michiara, M.; Vicentini, M.; Carrozzi, G.; Mancuso, P.; Sacchettini, C.; Giorgi Rossi, P. I Tumori Nelle Province dell’Area Vasta Emilia Nord: Piacenza, Parma, Reggio Emilia e Modena: Anni 2013–2014; Associazione Italiana Registri Tumori: Modena, Italy, 2015; pp. 1–124. Available online: https://www.ausl.mo.it/flex/cm/pages/ServeAttachment.php/L/IT/D/3%252F3%252F4%252FD.c0a5c9fc018e517fa340/P/BLOB%3AID%3D31942/E/pdf?mode=download (accessed on 27 April 2023).
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.; Whelan, S. (Eds.) International Classification of Disease for Oncology, 3rd ed.; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Dickman, P.W.; Coviello, E. Estimating and Modeling Relative Survival. Stata J. 2015, 15, 186–215. [Google Scholar] [CrossRef]
- National Cancer Institute. Joinpoint Regression Program; Version 4.9.0.0.; March 2021; Statistical Research and Applications Branch, National Cancer Institute: Bethesda, MD, USA, 2021. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 26 April 2023).
- O’Neil, M.E.; Henley, S.J.; Rohan, E.A.; Ellington, T.D.; Gallaway, M.S. Lung Cancer Incidence in Nonmetropolitan and Metropolitan Counties—United States, 2007–2016. MMWR Morb Mortal Wkly Rep. 2019, 68, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chang, Z.; Wu, J.; Li, W. Air pollution and lung cancer incidence in China: Who are faced with a great-er effect? Environ. Int. 2019, 132, 105077. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.I.; McKinley, M.; Cheng, I.; Haile, R.; Wakelee, H.; Gomez, S.L. Lung cancer incidence trends in California by race/ethnicity, histology, sex, and neighborhood socioeconomic status: An analysis spanning 28 years. Lung Cancer 2017, 108, 140–149. [Google Scholar] [CrossRef]
- Ramírez-Tirado, L.A.; Uribe-Ortíz, C.E.; Arrieta, O.; Tirado-Gómez, L.L. Lung cancer mortality and municipal marginalization in Mexico, 1998–2016. Salud Publica Mex. 2019, 61, 249–256. [Google Scholar] [CrossRef]
- Jung, K.J.; Jeon, C.; Jee, S.H. The effect of smoking on lung cancer: Ethnic differences and the smoking paradox. Epidemiol. Health 2016, 38, e2016060. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, J.-S.; Huang, H.-Y.; Shi, J.-F.; Li, N.; Zhang, Y.; Dai, M. International trends in lung cancer incidence from 1973 to 2007. Cancer Med. 2018, 7, 1479–1489. [Google Scholar] [CrossRef]
- Lim, W.-Y.; Tan, C.S.; Loy, E.Y.; Prasad, R.O.; Seow, A.; Chia, K.S. Lung cancer incidence in Singapore: Ethnic and gender differences. Lung Cancer 2014, 84, 23–30. [Google Scholar] [CrossRef]
- Henley, S.J.; Richards, T.B.; Underwood, J.M.; Eheman, C.R.; Plescia, M.; McAfee, T.A.; Centers for Disease Control and Prevention (CDC). Lung cancer incidence trends among men and women--United States, 2005–2009. MMWR Morb. Mortal Wkly Rep. 2014, 63, 1–5, Erratum in: MMWR Morb. Mortal Wkly. Rep. 2014, 63, 45. [Google Scholar]
- Xia, W.; Yu, X.; Mao, Q.; Xia, W.; Wang, A.; Dong, G.; Chen, B.; Ma, W.; Xu, L.; Jiang, F. Improvement of survival for non-small cell lung cancer over time. OncoTargets Ther. 2017, 10, 4295–4303. [Google Scholar] [CrossRef]
- Available online: https://www.epicentro.iss.it/passi/ (accessed on 26 April 2023).
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Available online: https://www.epicentro.iss.it/passi/dati/SmettereFumo?tab-container-1=tab1 (accessed on 31 May 2023).
- Di Noia, V.; D’aveni, A.; D’argento, E.; Rossi, S.; Ghirardelli, P.; Bortolotti, L.; Vavassori, V.; Bria, E.; Ceresoli, G. Treating disease progression with osimertinib in EGFR-mutated non-small-cell lung cancer: Novel targeted agents and combination strategies. ESMO Open 2021, 6, 100280. [Google Scholar] [CrossRef] [PubMed]
| Small Cell | Non-Small Cell | Others | Total | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Overall | 876 (12.2) | 4038 (56.1) | 2283 (31.7) | 7197 (100.0) |
| Sex | ||||
| Males | 596 (68.0) | 2867 (71.0) | 1641 (71.9) | 5104 (70.9) |
| Females | 280 (32.0) | 1171 (29.0) | 642 (28.1) | 2093 (29.1) |
| Age at diagnosis | ||||
| 15–54 | 62 (7.1) | 332 (8.2) | 101 (4.4) | 495 (6.9) |
| 55–64 | 175 (20.0) | 847 (21.0) | 242 (10.6) | 1264 (17.6) |
| 65–74 | 330 (37.6) | 1489 (36.9) | 537 (23.5) | 2356 (32.7) |
| 75+ | 309 (35.3) | 1370 (33.9) | 1403 (61.5) | 3082 (42.8) |
| Year of diagnosis | ||||
| 2001–2005 | 213 (24.3) | 690 (17.1) | 866 (37.9) | 1769 (24.6) |
| 2006–2010 | 219 (25.0) | 937 (23.2) | 652 (28.6) | 1808 (25.1) |
| 2011–2015 | 211 (24.1) | 1244 (30.8) | 379 (16.6) | 1834 (25.5) |
| 2016–2020 | 233 (26.6) | 1167 (28.9) | 386 (16.9) | 1786 (24.8) |
| Method of diagnosis | ||||
| Histological | 684 (78.1) | 3373 (83.5) | 375 (16.4) | 4432 (61.6) |
| Cytological | 192 (21.9) | 660 (16.4) | 425 (18.6) | 1277 (17.7) |
| Clinical/instrumental | 0 (0.0) | 5 (0.1) | 1483 (65.0) | 1488 (20.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangone, L.; Marinelli, F.; Bisceglia, I.; Zambelli, A.; Zanelli, F.; Pagano, M.; Alberti, G.; Morabito, F.; Pinto, C. Changes in the Histology of Lung Cancer in Northern Italy: Impact on Incidence and Mortality. Cancers 2023, 15, 3187. https://doi.org/10.3390/cancers15123187
Mangone L, Marinelli F, Bisceglia I, Zambelli A, Zanelli F, Pagano M, Alberti G, Morabito F, Pinto C. Changes in the Histology of Lung Cancer in Northern Italy: Impact on Incidence and Mortality. Cancers. 2023; 15(12):3187. https://doi.org/10.3390/cancers15123187
Chicago/Turabian StyleMangone, Lucia, Francesco Marinelli, Isabella Bisceglia, Alessandro Zambelli, Francesca Zanelli, Maria Pagano, Giulia Alberti, Fortunato Morabito, and Carmine Pinto. 2023. "Changes in the Histology of Lung Cancer in Northern Italy: Impact on Incidence and Mortality" Cancers 15, no. 12: 3187. https://doi.org/10.3390/cancers15123187
APA StyleMangone, L., Marinelli, F., Bisceglia, I., Zambelli, A., Zanelli, F., Pagano, M., Alberti, G., Morabito, F., & Pinto, C. (2023). Changes in the Histology of Lung Cancer in Northern Italy: Impact on Incidence and Mortality. Cancers, 15(12), 3187. https://doi.org/10.3390/cancers15123187







