CXC ELR-Positive Chemokines as Diagnostic and Prognostic Markers for Breast Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
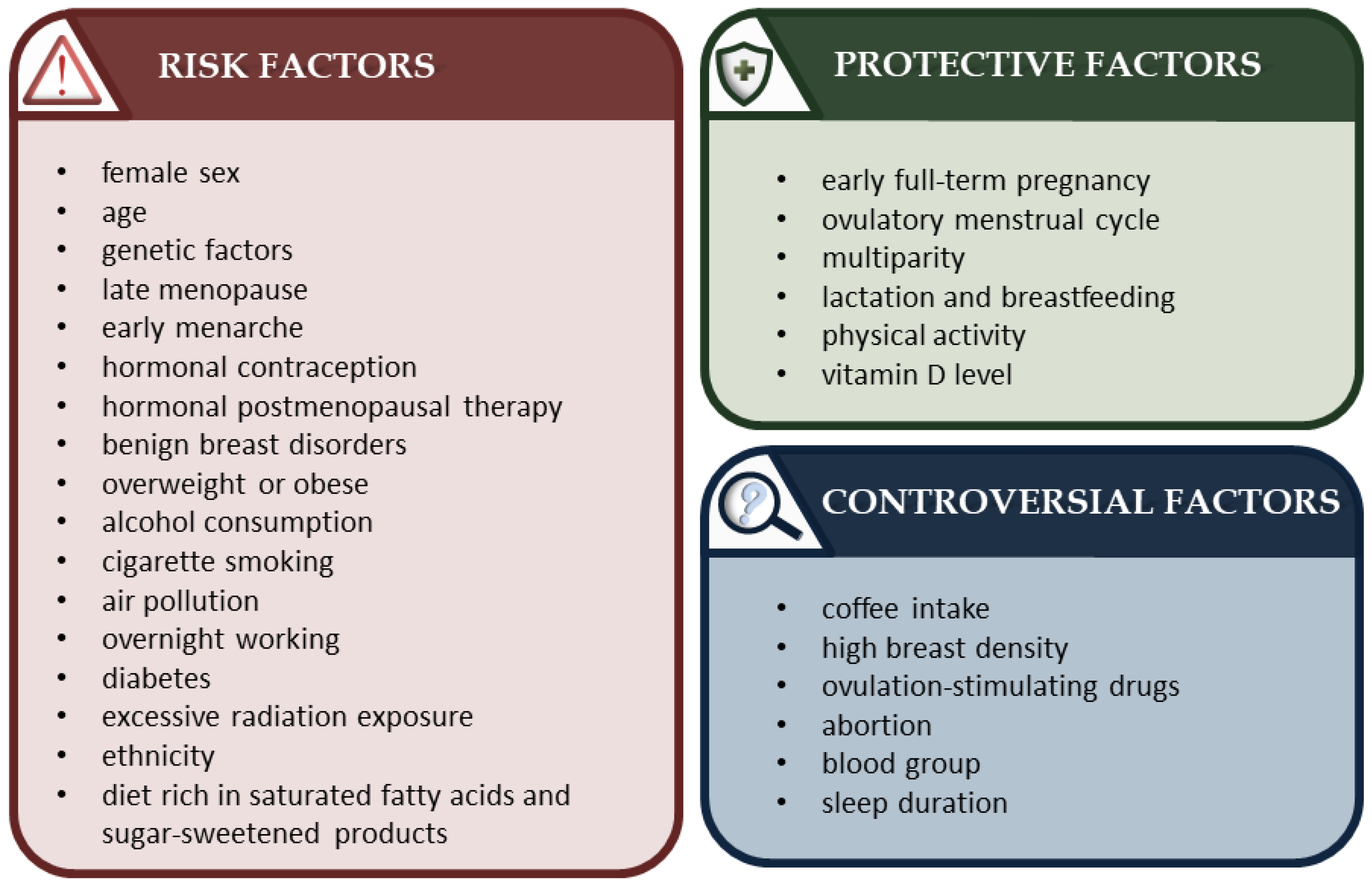
2. Breast Cancer: A Brief Overview
3. An Outline of the CXC Chemokine Group
- Control of angiogenesis—Allows tumor growth and metastasis by providing easy access to oxygen and nutrients.
- Immune regulation—Controls the influx of leukocytes into the tumor microenvironment.
- Modification of the functioning of cancer cells—Interacts with chemokine receptors and triggers intracellular signaling pathways.
4. CXC ELR-Positive Chemokines as Biomarkers of Breast Cancer
4.1. CXCL1
4.2. CXCL2 and CXCL3
4.3. CXCL5
4.4. CXCL6 and CXCL7
4.5. CXCL8
5. Conclusions
| CXCL1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | mRNA | Tissue protein | Blood circulating protein | ||||||
| Expression/Concentration towards HC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Refs. | [45,48] * | [51] | [45,46,47,48,49,50] | [43,44] | [49] | [36] | [35] ** | ||
| Expression/Concentration towards BLC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Ref. | [35] ** | ||||||||
| Differences of level between BC subtypes | Yes | No | Yes | No | Yes | No | |||
| Refs. | [45,48,50,51,52,54,55,56] | [47] | [43] | ||||||
| RFS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Ref. | [47], [48] ** | [48] ***, [50] | [43,45,55,57] | ||||||
| OS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Refs. | [47,48,49,50] | [45,55] | [33] | ||||||
| CXCL2 | |||||||||
| Source | mRNA | Tissue protein | Blood circulating protein | ||||||
| Expression/Expression/Concentration towards HC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Refs. | [39,47,48,50,58] | [49], | |||||||
| Expression/Concentration towards BLC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Ref. | |||||||||
| Differences of level between BC subtypes | Yes | No | Yes | No | Yes | No | |||
| Refs. | [47,48,50,52,57,58] | ||||||||
| RFS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Ref. | [47], [48] **, [50,58] | [48] *** | [58] | ||||||
| OS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Refs. | [48,49,58] | [47,50] | |||||||
| CXCL3 | |||||||||
| Source | mRNA | Tissue protein | Blood circulating protein | ||||||
| Expression/Concentration towards HC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Refs. | [39,47,48,49,50,58] | [49], | |||||||
| Expression/Concentration towards BLC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Refs. | |||||||||
| Differences of level between BC subtypes | Yes | No | Yes | No | Yes | No | |||
| Refs. | [48,50,52,57,58] | [47] | |||||||
| RFS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Refs. | [47], [58] ** [50] | [48] *** | [57] | ||||||
| OS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Refs. | [47,50] | [48,49] | |||||||
| CXCL5 | |||||||||
| Source | mRNA | Tissue protein | Blood circulating protein | ||||||
| Expression/Concentration towards HC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Refs. | [50], | [47,48,49] | [60] | [49] | [60] | ||||
| Expression/Concentration towards BLC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Ref. | [37] | ||||||||
| Differences of level between BC subtypes | Yes | No | Yes | No | Yes | No | |||
| Refs. | [48,50,55,57] | [47] | |||||||
| RFS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Refs. | [47,50] | [48,57] | |||||||
| OS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Refs. | [47,48,49,50] | ||||||||
| CXCL6 | |||||||||
| Source | mRNA | Tissue protein | Blood circulating protein | ||||||
| Expression/Concentration towards HC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Refs. | [48,49,57] | [47,50,58] | [49] | ||||||
| Expression/Concentration towards BLC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Ref. | |||||||||
| Differences of level between BC subtypes | Yes | No | Yes | No | Yes | No | |||
| Refs. | [48,50] | [47] | |||||||
| RFS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Refs. | [47,50] | [48] | |||||||
| OS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Refs. | [48,49] | [47,50] | |||||||
| CXCL7 | |||||||||
| Source | mRNA | Tissue protein | Blood circulating protein | ||||||
| Expression/Concentration towards HC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Refs. | [48,49] | [47,48,49,50] | [49] | [62] | |||||
| Expression/Concentration towards BLC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Ref. | [37] | ||||||||
| Differences of level between BC subtypes | Yes | No | Yes | No | Yes | No | |||
| Refs. | [47,48] | ||||||||
| RFS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Refs. | [47,50] | [48] | |||||||
| OS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Refs. | [47,48,49,50] | [61] | |||||||
| CXCL8 | |||||||||
| Source | mRNA | Tissue protein | Blood circulating protein | ||||||
| Expression/Concentration towards HC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Refs. | [47,51] | [47,48,49,50,51] | [50] | [49] | [35] **, [52,66,67,68,69] | ||||
| Expression/Concentration towards BLC | Up | No diff. | Down | Up | No diff. | Down | Up | No diff. | Down |
| Refs. | [37,68] | [35] **, [52] | |||||||
| Differences of level between BC subtypes | Yes | No | Yes | No | Yes | No | |||
| Refs. | [47,48,50,51,52,55,63] | [64] | [65] | [52,67], | [35] **, [66,68,70] | ||||
| RFS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Refs. | [48] ** | [47], [48] ***, [50] | [64] | [37,66] | |||||
| OS when upregulated | Longer | UA | Shorter | Longer | UA | Shorter | Longer | UA | Shorter |
| Ref. | [47,48,49,50,63] | [66,70] | |||||||
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Trieu, P.D.Y.; Mello-Thoms, C.R.; Barron, M.L.; Lewis, S.J. Look how far we have come: BREAST cancer detection education on the international stage. Front. Oncol. 2023, 12, 1023714. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, G.F.; Tegaw, E.M.; Abdisa, E.K. Diagnostic performance of mammography and ultrasound in breast cancer: A systematic review and meta-analysis. J. Ultrasound. 2023. advance online publication. [Google Scholar] [CrossRef]
- O’Grady, S.; Morgan, M.P. Microcalcifications in breast cancer: From pathophysiology to diagnosis and prognosis. Biochim. Biophys. Acta. Rev. Cancer 2018, 1869, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Seale, K.N.; Tkaczuk, K.H.R. Circulating Biomarkers in Breast Cancer. Clin. Breast Cancer 2022, 22, e319–e331. [Google Scholar] [CrossRef]
- Do, H.T.T.; Lee, C.H.; Cho, J. Chemokines and their Receptors: Multifaceted Roles in Cancer Progression and Potential Value as Cancer Prognostic Markers. Cancers 2020, 12, 287. [Google Scholar] [CrossRef]
- Masih, M.; Agarwal, S.; Kaur, R.; Gautam, P.K. Role of chemokines in breast cancer. Cytokine 2022, 155, 155909. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Breast Tumours. In WHO Classification of Tumours, 5th ed.; WHO Classification Editorial Board, Ed.; World Health Orgn: Lyon, France, 2019; Volume 2, pp. 5–12, 75–139. [Google Scholar]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. WHO Classification of Tumours Editorial Board. 2019 World Health Organization classification of tumours of the breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Nascimento, R.G.; Otoni, K.M. Histological and molecular classification of breast cancer: What do we know? Mastology 2020, 30, e20200024. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, X.; He, Y.; Liu, C.; Liu, H. Elevated Levels of Serum Tumor Markers CEA and CA15-3 Are Prognostic Parameters for Different Molecular Subtypes of Breast Cancer. PLoS ONE 2015, 10, e0133830. [Google Scholar] [CrossRef]
- Gaughran, G.; Aggarwal, N.; Shadbolt, B.; Stuart-Harris, R. The utility of the tumor markers CA15.3, CEA, CA-125 and CA19.9 in metastatic breast cancer. Breast Cancer Manag. 2020, 9, 4. [Google Scholar] [CrossRef]
- AL-Azzawi, H.S.K.; Rasheed, M.K.; AL-Naqqash, M. CA 27-29: A Valuable Marker for Breast Cancer Management in Correlation with CA 15-3. Indian J. Forensic Med. Toxicol. 2020, 14, 1615–1621. [Google Scholar]
- Huebner, H.; Häberle, L.; Müller, V.; Schrader, I.; Lorenz, R.; Forstbauer, H.; Fink, V.; Schochter, F.; Bekes, I.; Mahner, S.; et al. MUC1 (CA27.29) before and after Chemotherapy and Prognosis in High-Risk Early Breast Cancer Patients. Cancers 2022, 14, 1721. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Evoy, D.; McDermott, E.W. CA 15-3: Uses and limitation as a biomarker for breast cancer. Clin. Chim. Acta 2010, 411, 1869–1874. [Google Scholar] [CrossRef]
- Lucarelli, G.; Rutigliano, M.; Loizzo, D.; di Meo, N.A.; Lasorsa, F.; Mastropasqua, M.; Maiorano, E.; Bizzoca, C.; Vincenti, L.; Battaglia, M.; et al. MUC1 Tissue Expression and Its Soluble Form CA15-3 Identify a Clear Cell Renal Cell Carcinoma with Distinct Metabolic Profile and Poor Clinical Outcome. Int. J. Mol. Sci. 2022, 23, 13968. [Google Scholar] [CrossRef]
- Gomes, P.S.; Soares, M.R.; Marchenta, M.F.M.L.; Meirelles, G.S.P.; Ferreira, R.G.; Botelho, A.B.; Martins, R.B.; Pereira, C.A.C. Carbohydrate antigen 15-3 as a marker of disease severity in patients with chronic hypersensitivity pneumonitis. J. Bras. Pneumol. 2021, 47, e20200589. [Google Scholar] [CrossRef]
- Moll, S.A.; Wiertz, I.A.; Vorselaars, A.D.; Zanen, P.; Ruven, H.J.; van Moorsel, C.H.; Grutters, J.C. Serum biomarker CA 15-3 as predictor of response to antifibrotic treatment and survival in idiopathic pulmonary fibrosis. Biomark. Med. 2020, 14, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann. Coloproctol. 2019, 35, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M. Tumor markers of breast cancer: New prospectives. J. Oncol. Sci. 2017, 3, 5–11. [Google Scholar] [CrossRef]
- Admoun, C.; Mayrovitz, H.N. The Etiology of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK583809/ (accessed on 9 January 2021).
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Zlotnik, A.; Burkhardt, A.M.; Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 2011, 11, 597–606. [Google Scholar] [CrossRef]
- Legler, D.F.; Thelen, M. Chemokines: Chemistry, Biochemistry and Biological Function. Chimia 2016, 70, 856–859. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Raza, S.; Rajak, S.; Tewari, A.; Gupta, P.; Chattopadhyay, N.; Sinha, R.A.; Chakravarti, B. Multifaceted role of chemokines in solid tumors: From biology to therapy. Semin. Cancer Biol. 2022, 86, 1105–1121. [Google Scholar] [CrossRef]
- Będkowska, G.E.; Gacuta, E.; Zbucka-Krętowska, M.; Ławicki, P.; Szmitkowski, M.; Lemancewicz, A.; Motyka, J.; Kobus, A.; Chorąży, M.; Paniczko, M.; et al. Plasma Levels and Diagnostic Utility of VEGF in a Three-Year Follow-Up of Patients with Breast Cancer. J. Clin. Med. 2021, 10, 5452. [Google Scholar] [CrossRef]
- Divella, R.; Daniele, A.; Savino, E.; Palma, F.; Bellizzi, A.; Giotta, F.; Simone, G.; Lioce, M.; Quaranta, M.; Paradiso, A.; et al. Circulating levels of transforming growth factor-βeta (TGF-β) and chemokine (C-X-C motif) ligand-1 (CXCL1) as predictors of distant seeding of circulating tumor cells in patients with metastatic breast cancer. Anticancer Res. 2013, 33, 1491–1497. [Google Scholar]
- Zajkowska, M.; Gacuta, E.; Kozłowska, S.; Lubowicka, E.; Głażewska, E.K.; Chrostek, L.; Szmitkowski, M.; Pawłowski, P.; Zbucka-Krętowska, M.; Ławicki, S. Diagnostic power of VEGF, MMP-9 and TIMP-1 in patients with breast cancer. A multivariate statistical analysis with ROC curve. Adv. Med. Sci. 2019, 64, 1–8. [Google Scholar] [CrossRef]
- Motyka, J.; Gacuta, E.; Kicman, A.; Kulesza, M.; Ławicki, P.; Ławicki, S. Plasma Levels of CXC Motif Chemokine 1 (CXCL1) and Chemokine 8 (CXCL8) as Diagnostic Biomarkers in Luminal A and B Breast Cancer. J. Clin. Med. 2022, 11, 6694. [Google Scholar] [CrossRef]
- Ma, K.; Yang, L.; Shen, R.; Kong, B.; Chen, W.; Liang, J.; Tang, G.; Zhang, B. Th17 cells regulate the production of CXCL1 in breast cancer. Int. Immunopharmacol. 2018, 56, 320–329. [Google Scholar] [CrossRef]
- Wang, J.; He, Q.; Shao, Y.G.; Ji, M. Chemokines fluctuate in the progression of primary breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 596–608. [Google Scholar]
- Piskór, B.M.; Przylipiak, A.; Dąbrowska, E.; Sidorkiewicz, I.; Niczyporuk, M.; Szmitkowski, M.; Ławicki, S. Plasma Concentrations of Matrilysins MMP-7 and MMP-26 as Diagnostic Biomarkers in Breast Cancer. J. Clin. Med. 2021, 10, 1436. [Google Scholar] [CrossRef]
- Kwon, M.J. Matrix metalloproteinases as therapeutic targets in breast cancer. Front Oncol. 2023, 12, 1108695. [Google Scholar] [CrossRef]
- Ławicki, P.; Malinowski, P.; Motyka, J.; Ławicki, M.; Kicman, A.; Kulesza, M.; Gacuta, E.; Guszczyn, T.; Januszkiewicz, M.; Zbucka-Krętowska, M.; et al. Plasma Levels of Metalloproteinase 3 (MMP-3) and Metalloproteinase 7 (MMP-7) as New Candidates for Tumor Biomarkers in Diagnostic of Breast Cancer Patients. J Clin Med. 2023, 12, 2618. [Google Scholar] [CrossRef]
- Allen, M.; Louise Jones, J. Jekyll and Hyde: The role of the microenvironment on the progression of cancer. J. Pathol. 2011, 223, 162–176. [Google Scholar] [CrossRef]
- Lin, H.J.; Liu, Y.; Lofland, D.; Lin, J. Breast Cancer Tumor Microenvironment and Molecular Aberrations Hijack Tumoricidal Immunity. Cancers 2022, 14, 285. [Google Scholar] [CrossRef]
- Zou, A.; Lambert, D.; Yeh, H.; Yasukawa, K.; Behbod, F.; Fan, F.; Cheng, N. Elevated CXCL1 expression in breast cancer stroma predicts poor prognosis and is inversely associated with expression of TGF-β signaling proteins. BMC Cancer 2014, 14, 781. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, S.; Oskarsson, T.; Vanharanta, S.; Malladi, S.; Kim, J.; Morris, P.G.; Manova-Todorova, K.; Leversha, M.; Hogg, N.; Seshan, V.E.; et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 2012, 150, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, W.; Zheng, Y.; Wang, S.; Yang, B.; Li, M.; Song, J.; Zhang, F.; Zhang, X.; Wang, Q.; et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling. Cell Death Dis. 2018, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.C.; Korkut, A.; Kanchi, R.S.; Hegde, A.M.; Lenoir, W.; Liu, W.; Liu, Y.; Fan, H.; Shen, H.; Ravikumar, V.; et al. A Comprehensive Pan-Cancer Molecular Study of Gynecologic and Breast Cancers. Cancer Cell 2018, 33, 690–705.e9. [Google Scholar] [CrossRef]
- Chen, E.; Qin, X.; Peng, K.; Xu, X.; Li, W.; Cheng, X.; Tang, C.; Cui, Y.; Wang, Z.; Liu, T. Identification of Potential Therapeutic Targets Among CXC Chemokines in Breast Tumor Microenvironment Using Integrative Bioinformatics Analysis. Cell Physiol. Biochem. 2018, 45, 1731–1746. [Google Scholar] [CrossRef]
- Li, Y.; Liang, M.; Lin, Y.; Lv, J.; Chen, M.; Zhou, P.; Fu, F.; Wang, C. Transcriptional Expressions of CXCL9/10/12/13 as Prognosis Factors in Breast Cancer. J. Oncol. 2020, 2020, 4270957. [Google Scholar] [CrossRef]
- Li, L.; Yao, W.; Yan, S.; Dong, X.; Lv, Z.; Jing, Q.; Wang, Q.; Ma, B.; Hao, C.; Xue, D.; et al. Pan-Cancer Analysis of Prognostic and Immune Infiltrates for CXCs. Cancers 2021, 13, 4153. [Google Scholar] [CrossRef]
- Hozhabri, H.; Moghaddam, M.M.; Moghaddam, M.M.; Mohammadian, A. A comprehensive bioinformatics analysis to identify potential prognostic biomarkers among CC and CXC chemokines in breast cancer. Sci. Rep. 2022, 12, 10374. [Google Scholar] [CrossRef]
- Bild, A.H.; Yao, G.; Chang, J.T.; Wang, Q.; Potti, A.; Chasse, D.; Joshi, M.B.; Harpole, D.; Lancaster, J.M.; Berchuck, A.; et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006, 439, 353–357. [Google Scholar] [CrossRef]
- Narita, D.; Seclaman, E.; Anghel, A.; Ilina, R.; Cireap, N.; Negru, S.; Sirbu, I.O.; Ursoniu, S.; Marian, C. Altered levels of plasma chemokines in breast cancer and their association with clinical and pathological characteristics. Neoplasma 2016, 63, 141–149. [Google Scholar] [CrossRef]
- Pawitan, Y.; Bjöhle, J.; Amler, L.; Borg, A.L.; Egyhazi, S.; Hall, P.; Han, X.; Holmberg, L.; Huang, F.; Klaar, S.; et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: Derived and validated in two population-based cohorts. Breast Cancer Res. 2005, 7, R953–R964. [Google Scholar] [CrossRef]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- SenGupta, S.; Hein, L.E.; Xu, Y.; Zhang, J.; Konwerski, J.R.; Li, Y.; Johnson, C.; Cai, D.; Smith, J.L.; Parent, C.A. Triple-Negative Breast Cancer Cells Recruit Neutrophils by Secreting TGF-β and CXCR2 Ligands. Front. Immunol. 2021, 12, 659996. [Google Scholar] [CrossRef]
- Yang, C.; Yu, H.; Chen, R.; Tao, K.; Jian, L.; Peng, M.; Li, X.; Liu, M.; Liu, S. CXCL1 stimulates migration and invasion in ER negative breast cancer cells via activation of the ERK/MMP2/9 signaling axis. Int. J. Oncol. 2019, 55, 684–696. [Google Scholar] [CrossRef]
- Bièche, I.; Chavey, C.; Andrieu, C.; Busson, M.; Vacher, S.; Le Corre, L.; Guinebretière, J.M.; Burlinchon, S.; Lidereau, R.; Lazennec, G. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr.-Relat. Cancer 2007, 14, 1039–1052. [Google Scholar] [CrossRef]
- An, J.X.; Chen, Y.Y.; Ma, Z.S.; Yu, W.J.; Hu, J.X.; Cao, F.L. CXCL2 as a Prognostic Marker in Breast Cancer is Associated with Immune Infiltration and Regulated by miR-215. 14 July 2020, Preprint, 1st Version. Available online: https://www.researchsquare.com/article/rs-41001/v1 (accessed on 20 January 2021).
- See, A.L.; Chong, P.K.; Lu, S.Y.; Lim, Y.P. CXCL3 is a potential target for breast cancer metastasis. Curr. Cancer Drug Targets 2014, 14, 294–309. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Gong, T.; Lei, X.; Hu, T.; Tian, M.; Ding, F.; Ma, F.; Chen, H.; Liu, Z. A S100A14-CCL2/CXCL5 signaling axis drives breast cancer metastasis. Theranostics 2020, 10, 5687–5703. [Google Scholar] [CrossRef]
- Wang, Y.H.; Shen, C.Y.; Lin, S.C.; Kuo, W.H.; Kuo, Y.T.; Hsu, Y.L.; Wang, W.C.; Lin, K.T.; Wang, L.H. Monocytes secrete CXCL7 to promote breast cancer progression. Cell. Death Dis. 2021, 12, 1090. [Google Scholar] [CrossRef]
- Kosir, M.A.; Ju, D. Serum chemokine CXCL7 activity after resection of breast cancer. J. Clin. Oncol. 2013, 31, e22182. [Google Scholar] [CrossRef]
- Fang, Q.I.; Wang, X.; Luo, G.; Yu, M.; Zhang, X.; Xu, N. Increased CXCL8 Expression Is Negatively Correlated with the Overall Survival of Patients with ER-Negative Breast Cancer. Anticancer Res. 2017, 37, 4845–4852. [Google Scholar]
- Milovanovic, J.; Todorovic-Rakovic, N.; Abu Rabi, Z. The prognostic role of interleukin-8 (IL-8) and matrix metalloproteinases -2 and -9 in lymph node-negative untreated breast cancer patients. J. BUON 2013, 18, 866–873. [Google Scholar] [PubMed]
- Kamalakar, A.; Bendre, M.S.; Washam, C.L.; Fowler, T.W.; Carver, A.; Dilley, J.D.; Bracey, J.W.; Akel, N.S.; Margulies, A.G.; Skinner, R.A.; et al. Circulating interleukin-8 levels explain breast cancer osteolysis in mice and humans. Bone 2014, 61, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Benoy, I.H.; Salgado, R.; Van Dam, P.; Geboers, K.; Van Marck, E.; Scharpé, S.; Vermeulen, P.B.; Dirix, L.Y. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin. Cancer Res. 2004, 10, 7157–7162. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ren, Y.; Dai, Z.J.; Wu, C.J.; Ji, Y.H.; Xu, J. IL-6, IL-8 and TNF-α levels correlate with disease stage in breast cancer patients. Adv. Clin. Exp. Med. 2017, 26, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.F.; Al-naqqash, M.A.; Alawn, N.A.; Ad’hiah, A.H. Biomarker Significance of Serum CXCL8, CXCL10 and CXCL16 in Breast Tumors of Iraqi Patients. Baghdad Sci. J. 2020, 17, 0199. [Google Scholar] [CrossRef]
- Celik, B.; Yalcin, A.D.; Genc, G.E.; Bulut, T.; Kuloglu Genc, S.; Gumuslu, S. CXCL8, IL-1β and sCD200 are pro-inflammatory cytokines and their levels increase in the circulation of breast carcinoma patients. Biomed. Rep. 2016, 5, 259–263. [Google Scholar] [CrossRef]
- Tiainen, L.; Hämäläinen, M.; Luukkaala, T.; Tanner, M.; Lahdenperä, O.; Vihinen, P.; Jukkola, A.; Karihtala, P.; Moilanen, E.; Kellokumpu-Lehtinen, P.L. Low Plasma IL-8 Levels During Chemotherapy Are Predictive of Excellent Long-Term Survival in Metastatic Breast Cancer. Clin. Breast Cancer 2019, 19, e522–e533. [Google Scholar] [CrossRef]
- König, A.; Vilsmaier, T.; Rack, B.; Friese, K.; Janni, W.; Jeschke, U.; Andergassen, U.; Trapp, E.; Jückstock, J.; Jäger, B.; et al. Determination of Interleukin-4, -5, -6, -8 and -13 in Serum of Patients with Breast Cancer Before Treatment and its Correlation to Circulating Tumor Cells. Anticancer Res. 2016, 36, 3123–3130. [Google Scholar]
- Zhuang, X.; Wang, J. Correlations of MRP1 gene with serum TGF-β1 and IL-8 in breast cancer patients during chemotherapy. J. BUON 2018, 23, 1302–1308. [Google Scholar]
- Salcedo, R.; Martins-Green, M.; Gertz, B.; Oppenheim, J.J.; Murphy, W.J. Combined administration of antibodies to human interleukin 8 and epidermal growth factor receptor results in increased antimetastatic effects on human breast carcinoma xenografts. Clin. Cancer. Res. 2002, 8, 2655. [Google Scholar]
- Kerbel, R.S.; Yu, J.; Tran, J.; Man, S.; Viloria-Petit, A.; Klement, G.; Coomber, B.L.; Rak, J. Possible mechanisms of acquired resistance to anti-angiogenic drugs: Implications for the use of combination therapy approaches. Cancer Metastasis Rev. 2001, 20, 79–86. [Google Scholar] [CrossRef]
- Ruffini, P.A. The CXCL8-CXCR1/2 Axis as a Therapeutic Target in Breast Cancer Stem-Like Cells. Front Oncol. 2019, 9, 40. [Google Scholar] [CrossRef]
- Nasiri, F.; Kazemi, M.; Mirarefin, S.M.J.; Mahboubi Kancha, M.; Ahmadi Najafabadi, M.; Salem, F.; Dashti Shokoohi, S.; Evazi Bakhshi, S.; Safarzadeh Kozani, P.; Safarzadeh Kozani, P. CAR-T cell therapy in triple-negative breast cancer: Hunting the invisible devil. Front Immunol. 2022, 13, 1018786. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motyka, J.; Kicman, A.; Kulesza, M.; Ławicki, S. CXC ELR-Positive Chemokines as Diagnostic and Prognostic Markers for Breast Cancer Patients. Cancers 2023, 15, 3118. https://doi.org/10.3390/cancers15123118
Motyka J, Kicman A, Kulesza M, Ławicki S. CXC ELR-Positive Chemokines as Diagnostic and Prognostic Markers for Breast Cancer Patients. Cancers. 2023; 15(12):3118. https://doi.org/10.3390/cancers15123118
Chicago/Turabian StyleMotyka, Joanna, Aleksandra Kicman, Monika Kulesza, and Sławomir Ławicki. 2023. "CXC ELR-Positive Chemokines as Diagnostic and Prognostic Markers for Breast Cancer Patients" Cancers 15, no. 12: 3118. https://doi.org/10.3390/cancers15123118
APA StyleMotyka, J., Kicman, A., Kulesza, M., & Ławicki, S. (2023). CXC ELR-Positive Chemokines as Diagnostic and Prognostic Markers for Breast Cancer Patients. Cancers, 15(12), 3118. https://doi.org/10.3390/cancers15123118