Comparison of Medical Management versus Parathyroidectomy in Patients with Mild Primary Hyperparathyroidism: A Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
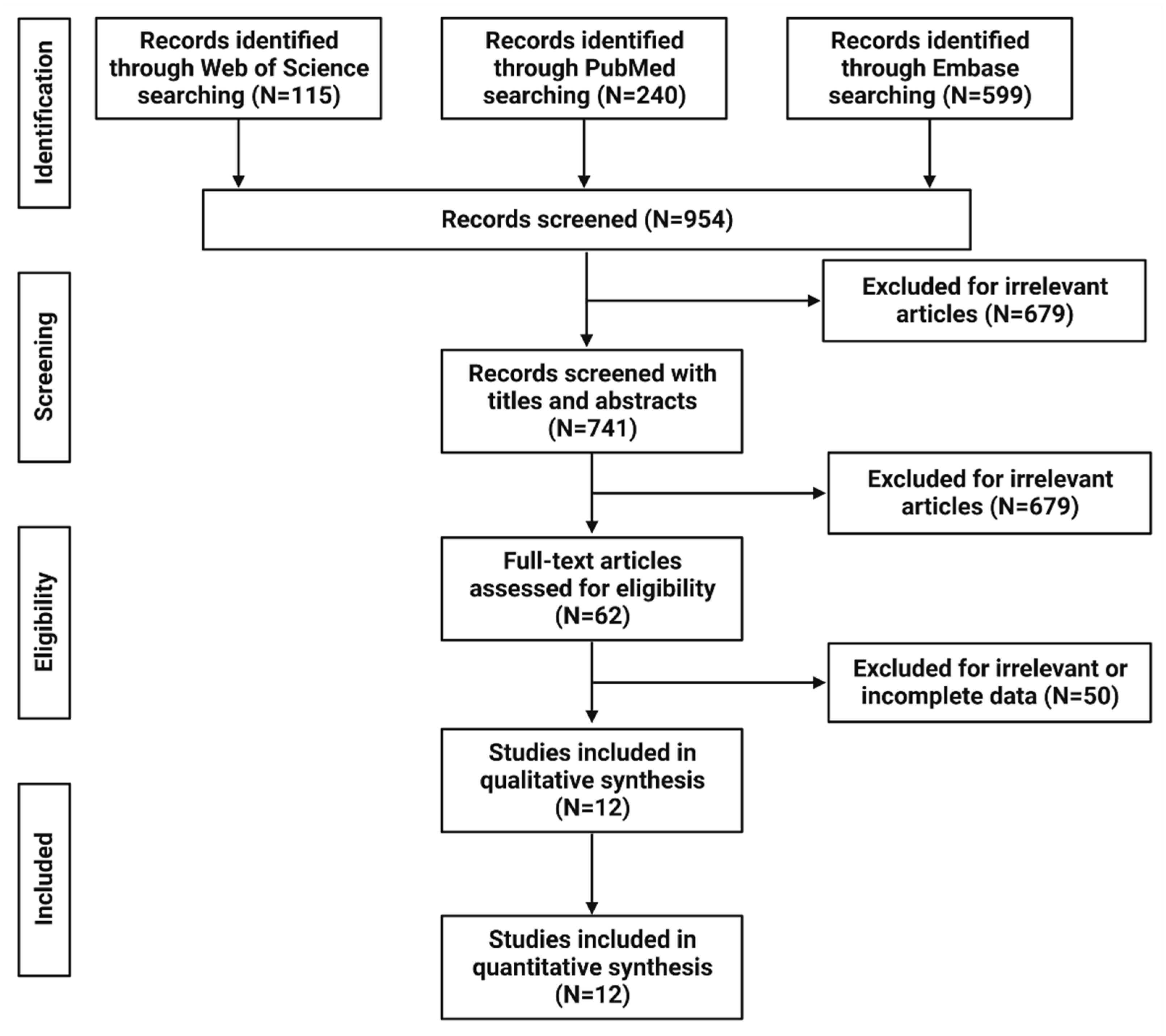
2. Methods
2.1. Study Design and Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Characteristics of the Study Population
3.3. Calcium Levels
3.4. Parathyroid Hormone (PTH) Levels
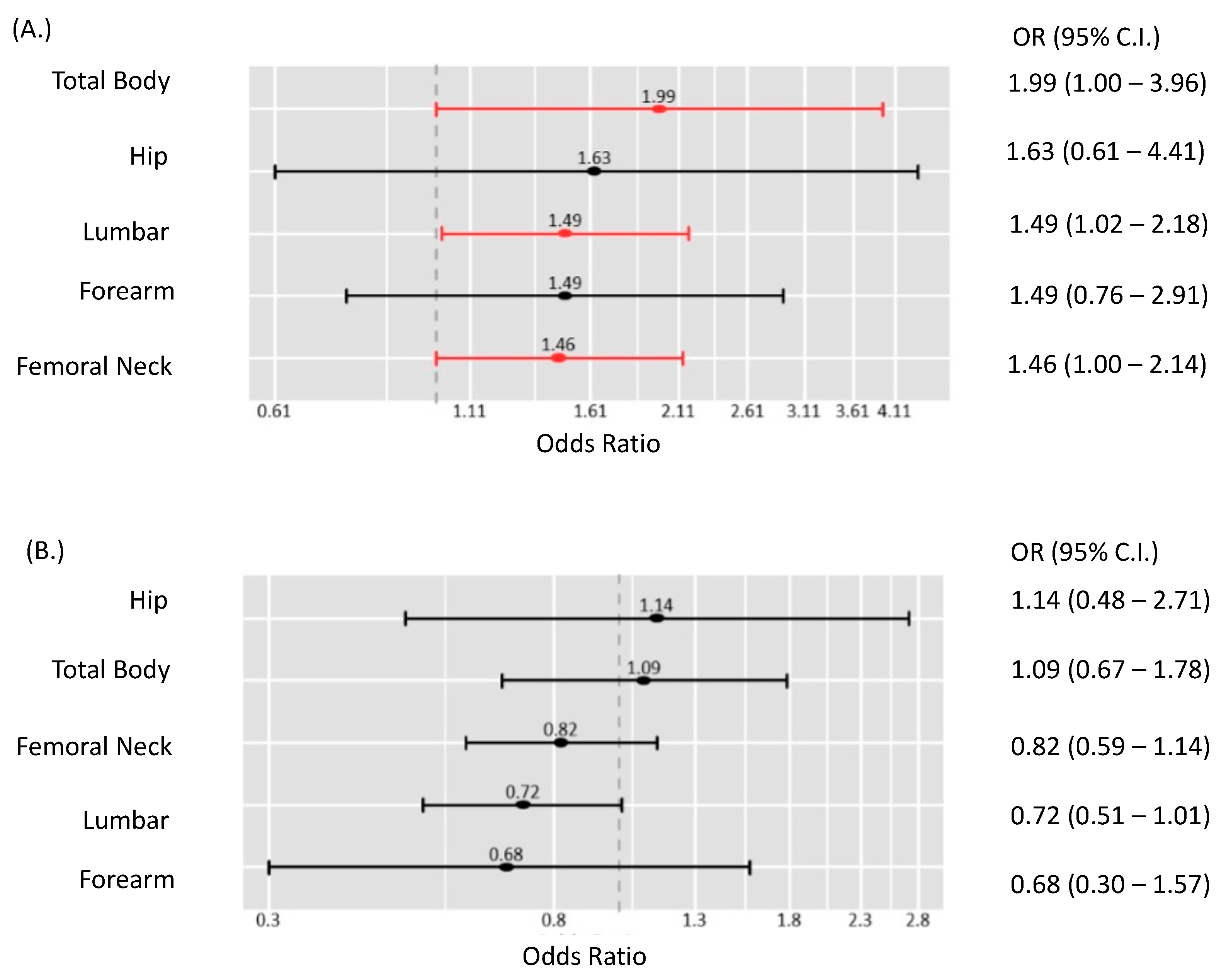
3.5. Bone Mineral Density (BMD) Measurements
3.6. Complications and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Adler, J.T.; Sippel, R.S.; Chen, H. New trends in parathyroid surgery. Curr. Probl. Surg. 2010, 47, 958–1017. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Silverberg, S.J. Primary hyperparathyroidism. Nat. Rev. Endocrinol. 2018, 14, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Stefenelli, T.; Mayr, H.; Bergler-Klein, J.; Globits, S.; Woloszczuk, W.; Niederle, B. Primary hyperparathyroidism: Incidence of cardiac abnormalities and partial reversibility after successful parathyroidectomy. Am. J. Med. 1993, 95, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, E.; Ljunghall, S.; Akerström, G.; Hetta, J.; Mallmin, H.; Rastad, J. Case-control study on symptoms and signs of “asymptomatic” primary hyperparathyroidism. Surgery 1998, 124, 980–985; discussion 985–986. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Wang, T.S.; Ruan, D.T.; Lee, J.A.; Asa, S.L.; Duh, Q.Y.; Doherty, G.M.; Herrera, M.F.; Pasieka, J.L.; Perrier, N.D.; et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016, 151, 959–968. [Google Scholar] [CrossRef]
- Applewhite, M.K.; Schneider, D.F. Mild primary hyperparathyroidism: A literature review. Oncologist 2014, 19, 919–929. [Google Scholar] [CrossRef]
- Alhefdhi, A.; Pinchot, S.N.; Davis, R.; Sippel, R.S.; Chen, H. The necessity and reliability of intraoperative parathyroid hormone (PTH) testing in patients with mild hyperparathyroidism and PTH levels in the normal range. World J. Surg. 2011, 35, 2006–2009. [Google Scholar] [CrossRef]
- Carneiro-Pla, D.M.; Irvin, G.L., 3rd; Chen, H. Consequences of parathyroidectomy in patients with “mild” sporadic primary hyperparathyroidism. Surgery 2007, 142, 795–799.e2. [Google Scholar] [CrossRef]
- Ghemigian, A.; Trandafir, A.I.; Petrova, E.; Carsote, M.; Valea, A.; Filipescu, A.; Oproiu, A.M.; Sandru, F. Primary hyperparathyroidism-related giant parathyroid adenoma (Review). Exp. Ther. Med. 2022, 23, 88. [Google Scholar] [CrossRef]
- Wermers, R.A.; Khosla, S.; Atkinson, E.J.; Achenbach, S.J.; Oberg, A.L.; Grant, C.S.; Melton, L.J., III. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993–2001: An update on the changing epidemiology of the disease. J. Bone Miner. Res. 2006, 21, 171–177. [Google Scholar] [CrossRef]
- Lo, C.-Y.; Chan, W.-F.; Kung, A.W.; Lam, K.-Y.; Tam, S.C.; Lam, K.S. Surgical treatment for primary hyperparathyroidism in Hong Kong: Changes in clinical pattern over 3 decades. Arch. Surg. 2004, 139, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Abood, A.; Vestergaard, P. Increasing incidence of primary hyperparathyroidism in Denmark. Dan. Med. J. 2013, 60, A4567. [Google Scholar] [PubMed]
- Pretorius, M.; Lundstam, K.; Heck, A.; Fagerland, M.W.; Godang, K.; Mollerup, C.; Fougner, S.L.; Pernow, Y.; Aas, T.; Hessman, O.; et al. Mortality and Morbidity in Mild Primary Hyperparathyroidism: Results From a 10-Year Prospective Randomized Controlled Trial of Parathyroidectomy Versus Observation. Ann. Intern. Med. 2022, 175, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Vaitsi, K.; Veneti, S.; Potoupni, V.; Kenanidis, E.; Tsiridis, E.; Papavramidis, T.S.; Goulis, D.G. Efficacy of parathyroidectomy compared with active surveillance in patients with mild asymptomatic primary hyperparathyroidism: A systematic review and meta-analysis of randomized-controlled studies. J. Endocrinol. Investig. 2021, 44, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bollerslev, J.; Jansson, S.; Mollerup, C.L.; Nordenström, J.; Lundgren, E.; Tørring, O.; Varhaug, J.-E.; Baranowski, M.; Aanderud, S.; Franco, C. Medical observation, compared with parathyroidectomy, for asymptomatic primary hyperparathyroidism: A prospective, randomized trial. J. Clin. Endocrinol. Metab. 2007, 92, 1687–1692. [Google Scholar] [CrossRef]
- Lundstam, K.; Heck, A.; Mollerup, C.; Godang, K.; Baranowski, M.; Pernow, Y.; Varhaug, J.E.; Hessman, O.; Rosén, T.; Nordenström, J.; et al. Effects of parathyroidectomy versus observation on the development of vertebral fractures in mild primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2015, 100, 1359–1367. [Google Scholar] [CrossRef]
- Lundstam, K.; Heck, A.; Godang, K.; Mollerup, C.; Baranowski, M.; Pernow, Y.; Aas, T.; Hessman, O.; Rosén, T.; Nordenström, J.; et al. Effect of Surgery Versus Observation: Skeletal 5-Year Outcomes in a Randomized Trial of Patients With Primary HPT (the SIPH Study). J. Bone Miner. Res. 2017, 32, 1907–1914. [Google Scholar] [CrossRef]
- Tzikos, G.; Chorti, A.; Evangelos, S.; Boura, E.; Manani, C.; Adamidou, F.; Tziatzios, I.; Zisi, A.; Economou, F.; Toulis, K.; et al. Quality of Life in Patients With Asymptomatic Primary Hyperparathyroidism After Parathyroidectomy: A 3-Year Longitudinal Study. Endocr. Pract. 2021, 27, 716–722. [Google Scholar] [CrossRef]
- Khan, R.; Martin, J.; Das, G. The Impact of Observation Versus Parathyroidectomy on Bone Mineral Density and Fracture Risk Determined by FRAX Tool in Patients With Primary Hyperparathyroidism. J. Clin. Densitom. 2021, 24, 571–580. [Google Scholar] [CrossRef]
- Ramos, L.; Piedra, M.; Muñoz, P.; Vázquez, L.A.; García-Unzueta, M.T.; Montalbán, C.; Amado, J.A. Bone mineral density evolution and incidence of fractures in a cohort of patients with primary hyperparathyroidism treated with parathyroid surgery vs active surveillance during 6 years of follow-up. Endocrinol. Diabetes Nutr. 2019, 66, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Perrier, N.D.; Balachandran, D.; Wefel, J.S.; Jimenez, C.; Busaidy, N.; Morris, G.S.; Dong, W.; Jackson, E.; Weaver, S.; Gantela, S.; et al. Prospective, randomized, controlled trial of parathyroidectomy versus observation in patients with “asymptomatic” primary hyperparathyroidism. Surgery 2009, 146, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Ambrogini, E.; Cetani, F.; Cianferotti, L.; Vignali, E.; Banti, C.; Viccica, G.; Oppo, A.; Miccoli, P.; Berti, P.; Bilezikian, J.P.; et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: A prospective, randomized clinical trial. J. Clin. Endocrinol. Metab. 2007, 92, 3114–3121. [Google Scholar] [CrossRef] [PubMed]
- Hagström, E.; Lundgren, E.; Mallmin, H.; Rastad, J.; Hellman, P. Positive effect of parathyroidectomy on bone mineral density in mild asymptomatic primary hyperparathyroidism. J. Intern. Med. 2006, 259, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.S.; Phillips, E.R.; Divine, G.W.; Talpos, G.B. Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2004, 89, 5415–5422. [Google Scholar] [CrossRef]
- Rao, D.S.; Wallace, E.A.; Antonelli, R.F.; Talpos, G.B.; Ansari, M.R.; Jacobsen, G.; Divine, G.W.; Parfitt, A.M. Forearm bone density in primary hyperparathyroidism: Long-term follow-up with and without parathyroidectomy. Clin. Endocrinol. 2003, 58, 348–354. [Google Scholar] [CrossRef]
- Yeh, M.W.; Ituarte, P.H.; Zhou, H.C.; Nishimoto, S.; Amy Liu, I.L.; Harari, A.; Haigh, P.I.; Adams, A.L. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J. Clin. Endocrinol. Metab. 2013, 98, 1122–1129. [Google Scholar] [CrossRef]
- Cameron, J.L.; Cameron, A.M. Current Surgical Therapy E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Bilezikian, J.P.; Khan, A.A.; Potts, J.T., Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: Summary statement from the third international workshop. J. Clin. Endocrinol. Metab. 2009, 94, 335–339. [Google Scholar] [CrossRef]
- Schneider, D.F.; Burke, J.F.; Ojomo, K.A.; Clark, N.; Mazeh, H.; Sippel, R.S.; Chen, H. Multigland Disease and Slower Decline in Intraoperative PTH Characterize Mild Primary Hyperparathyroidism. Ann. Surg. Oncol. 2013, 20, 4205–4211. [Google Scholar] [CrossRef]
- Calvi, L.M.; Sims, N.; Hunzelman, J.; Knight, M.; Giovannetti, A.; Saxton, J.; Kronenberg, H.; Baron, R.; Schipani, E. Activated parathyroid hormone/parathyroid hormone–related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J. Clin. Investig. 2001, 107, 277–286. [Google Scholar] [CrossRef]
- Amaral, L.M.; Queiroz, D.C.; Marques, T.F.; Mendes, M.; Bandeira, F. Normocalcemic versus hypercalcemic primary hyperparathyroidism: More stone than bone? J. Osteoporos. 2012, 2012, 128352. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.L.; Wang, T.S.; Wade, T.J.; Yen, T.W. Normal PTH levels in primary hyperparathyroidism: Still the same disease? Ann. Surg. Oncol. 2011, 18, 3437–3742. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.T.; Sippel, R.S.; Schaefer, S.; Chen, H. Surgery improves quality of life in patients with “mild” hyperparathyroidism. Am. J. Surg. 2009, 197, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Lindh, E.; Lind, L.; Persson, I.; Ljunghall, S. Increased fracture risk in hypercalcemia. Bone mineral content measured in hyperparathyroidism. Acta Orthop. Scand. 1989, 60, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Cordellat, I.M. Hyperparathyroidism: Primary or secondary disease? Reumatol. Clin. 2012, 8, 287–291. [Google Scholar] [CrossRef]
- Yu, N.; Donnan, P.T.; Leese, G.P. A record linkage study of outcomes in patients with mild primary hyperparathyroidism: The Parathyroid Epidemiology and Audit Research Study (PEARS). Clin. Endocrinol. 2011, 75, 169–176. [Google Scholar] [CrossRef]
- Silverberg, S.J.; Shane, E.; Jacobs, T.P.; Siris, E.; Bilezikian, J.P. A 10-Year Prospective Study of Primary Hyperparathyroidism with or without Parathyroid Surgery. N. Engl. J. Med. 1999, 341, 1249–1255. [Google Scholar] [CrossRef]
- Wallace, L.B.; Parikh, R.T.; Ross, L.V.; Mazzaglia, P.J.; Foley, C.; Shin, J.J.; Mitchell, J.C.; Berber, E.; Siperstein, A.E.; Milas, M. The phenotype of primary hyperparathyroidism with normal parathyroid hormone levels: How low can parathyroid hormone go? Surgery 2011, 150, 1102–1112. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef]
- Macfarlane, D.P.; Yu, N.; Leese, G.P. Subclinical and asymptomatic parathyroid disease: Implications of emerging data. Lancet Diabetes Endocrinol. 2013, 1, 329–340. [Google Scholar] [CrossRef]
- Silverberg, S.J.; Lewiecki, E.M.; Mosekilde, L.; Peacock, M.; Rubin, M.R. Presentation of asymptomatic primary hyperparathyroidism: Proceedings of the third international workshop. J. Clin. Endocrinol. Metab. 2009, 94, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Bargren, A.E.; Repplinger, D.; Chen, H.; Sippel, R.S. Can biochemical abnormalities predict symptomatology in patients with primary hyperparathyroidism? J. Am. Coll. Surg. 2011, 213, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, R.P.; Kearns, A.E.; Vickers, K.S.; Grant, C.; Ryu, E.; Wermers, R.A. Depression in primary hyperparathyroidism: Prevalence and benefit of surgery. J. Clin. Endocrinol. Metab. 2011, 96, E1737–E1745. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Eberle, J.; Messelhäuser, U.; Schiffmann, L.; Nies, C.; Schabram, J.; Zielke, A.; Holzer, K.; Rottler, E.; Henne-Bruns, D.; et al. Parathyroidectomy, elevated depression scores, and suicidal ideation in patients with primary hyperparathyroidism: Results of a prospective multicenter study. JAMA Surg. 2013, 148, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.F.; Mazeh, H.; Chen, H.; Sippel, R.S. Predictors of recurrence in primary hyperparathyroidism: An analysis of 1386 cases. Ann. Surg. 2014, 259, 563. [Google Scholar] [CrossRef]
- Barczynski, M.; Konturek, A.; Hubalewska-Dydejczyk, A.; Cichon, S.; Nowak, W. Evaluation of Halle, Miami, Rome, and Vienna intraoperative iPTH assay criteria in guiding minimally invasive parathyroidectomy. Langenbecks Arch. Surg. 2009, 394, 843–849. [Google Scholar] [CrossRef]
- Kelly, K.J.; Chen, H.; Sippel, R.S. Primary hyperparathyroidism. Cancer Treat. Res. 2010, 153, 87–103. [Google Scholar]
- Edafe, O.; Balasubramanian, S.P. Incidence, prevalence and risk factors for post-surgical hypocalcaemia and hypoparathyroidism. Gland. Surg. 2017, 6 (Suppl. 1), S59–S68. [Google Scholar] [CrossRef]
- Udelsman, R.; Lin, Z.; Donovan, P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann. Surg. 2011, 253, 585–591. [Google Scholar] [CrossRef]
- Brown, B.C.; McKenna, S.P.; Siddhi, K.; McGrouther, D.A.; Bayat, A. The hidden cost of skin scars: Quality of life after skin scarring. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 1049–1058. [Google Scholar] [CrossRef]
- Kandil, E.; Akkera, M.; Shalaby, H.; Munshi, R.; Attia, A.; Elnahla, A.; Shalaby, M.; Abdelgawad, M.; Grace, L.; Kang, S.W. A Single Surgeon’s 10-Year Experience in Remote-Access Thyroid and Parathyroid Surgery. Am. Surg. 2021, 87, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Garstka, M.E.; Terris, D.J.; Kandil, E. Alternative Remote Access Approaches to Endocrine and Neck Surgery. In Transoral Neck Surgery; Russell, J.O., Inabnet Iii, W.B., Tufano, R.P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 29–39. [Google Scholar]
- Russell, J.O.; Razavi, C.R.; Garstka, M.E.; Chen, L.W.; Vasiliou, E.; Kang, S.W.; Tufano, R.P.; Kandil, E. Remote-Access Thyroidectomy: A Multi-Institutional North American Experience with Transaxillary, Robotic Facelift, and Transoral Endoscopic Vestibular Approaches. J. Am. Coll. Surg. 2019, 228, 516–522. [Google Scholar] [CrossRef]
- Baek, J.H.; Lee, J.H.; Sung, J.Y.; Bae, J.I.; Kim, K.T.; Sim, J.; Baek, S.M.; Kim, Y.S.; Shin, J.H.; Park, J.S.; et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: A multicenter study. Radiology 2012, 262, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Kandil, E.; Omar, M.; Aboueisha, M.; Attia, A.S.; Ali, K.M.; Abu Alhuda, R.F.; Issa, P.P.; Wolfe, S.; Omari, S.; Buti, Y.; et al. Efficacy and Safety of Radiofrequency Ablation of Thyroid Nodules: A Multi-institutional Prospective Cohort Study. Ann. Surg. 2022, 276, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cheng, L.; Zhang, W.; He, W. Ultrasound-guided thermal ablation for hyperparathyroidism: Current status and prospects. Int. J. Hyperth. 2022, 39, 466–474. [Google Scholar] [CrossRef]
- Tordjman, K.M.; Greenman, Y.; Osher, E.; Shenkerman, G.; Stern, N. Characterization of normocalcemic primary hyperparathyroidism. Am. J. Med. 2004, 117, 861–863. [Google Scholar] [CrossRef]
| Author | Year | Design | Sample Size | Female, % | Age, Years (mean ± SD) | Follow-Up, Month (mean) |
|---|---|---|---|---|---|---|
| Pretorius et al. [13] | 2022 | RCT | 191 | 86.4 | 63.1 ± 7.8 | 60 |
| Tzikos et al. [19] | 2021 | Prospective | 38 | 89.5 | NR | 36 |
| Khan et al. [20] | 2021 | Retrospective | 60 | 85.0 | 68.2 ± 8.9 | NR |
| Ramos et al. [21] | 2019 | Retrospective | 170 | 92.4 | 64.6 ± 11.6 | 72 |
| Lundstam et al. [18] | 2017 | RCT | 145 | 86.9 | 62.5 ± 7.5 | 60 |
| Lundstam et al. [17] | 2015 | RCT | 145 | 86.9 | 62.5 ± 7.5 | 60 |
| Perrier et al. [22] | 2009 | RCT | 18 | 83.3 | 63 ± 17 | 6 |
| Bollerslev et al. [16] | 2007 | RCT | 191 | 86.4 | 64.1 ± 7.4 | 24 |
| Ambrogini et al. [23] | 2007 | RCT | 50 | 92.0 | 64.5 ± 6 | 12 |
| Hagström et al. [24] | 2006 | Prospective | 69 | 100.0 | 65.9 ± 5.7 | 60 |
| Rao et al. [25] | 2004 | RCT | 53 | 79.2 | 64.9 ± 7 | 12 |
| Rao et al. [26] | 2003 | Retrospective | 216 | 77.8 | 57.5 ± 11 | 49 |
| Demographic Data | Group | Studies | Effect Size | Heterogeneity | ||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | I2 | p-Value | |||
| Age, years | Medical | 9 | 65.12 | 64.28–65.96 | 93% | <0.01 * |
| Surgery | 9 | 62.56 | 61.77–63.34 | 92% | <0.01 * | |
| Sex: (female) | Medical | 9 | 86% | 80–91% | 42% | 0.09 $ |
| Surgery | 9 | 86% | 90–91% | 39% | 0.11 $ | |
| BMI, kg/m2 | Medical | 5 | 29.14 | 27.37–30.91 | 64% | 0.03 * |
| Surgery | 5 | 28.16 | 26.90–29.42 | 62% | 0.03 * | |
| Biochemical Data | Group | Studies | Effect Size | Heterogeneity | ||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | I2 | p-Value | |||
| Baseline calcium (mg/dL) | Medical | 6 | 10.58 | 10.54–10.63 | 95% | <0.01 |
| Surgery | 6 | 10.68 | 10.63–10.73 | 94% | <0.01 | |
| Post-treatment calcium (mg/dL) | Medical | 4 | 10.46 | 10.39–10.54 | 92% | <0.01 |
| Surgery | 5 | 9.39 | 9.27–9.52 | 80% | <0.01 | |
| Baseline PTH (pmol/L) | Medical | 8 | 83.84 | 80.06–87.63 | 94% | <0.01 |
| Surgery | 8 | 98.17 | 83.19–113.15 | 94% | <0.01 | |
| Post-treatment PTH (pmol/L) | Medical | 5 | 106.14 | 94.44–117.85 | 72% | <0.01 |
| Surgery | 6 | 43.25 | 39.03–47.47 | 74% | <0.01 | |
| Complications | Studies | Effect Size | Heterogeneity | ||
|---|---|---|---|---|---|
| RR | 95% CI | I2 | p-Value | ||
| Nephrolithiasis | 3 | 2.08 | 0.60–7.30 | 0% | 0.84 |
| All fractures | 3 | 1.20 | 0.53–2.75 | 91% | <0.01 |
| Non-vertebral fracture | 3 | 1.22 | 0.58–2.59 | 81% | <0.01 |
| Vertebral fracture | 3 | 1.37 | 0.69–2.72 | 42% | 0.18 |
| Cardiovascular death | 1 | 0.99 | 0.43–2.27 | NA | NA |
| Disease-related mortality | 1 | 0.87 | 0.33–2.29 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cironi, K.A.; Issa, P.P.; Albuck, A.L.; McCarthy, C.; Rezvani, L.; Hussein, M.; Luo, X.; Shama, M.; Toraih, E.; Kandil, E. Comparison of Medical Management versus Parathyroidectomy in Patients with Mild Primary Hyperparathyroidism: A Meta-Analysis. Cancers 2023, 15, 3085. https://doi.org/10.3390/cancers15123085
Cironi KA, Issa PP, Albuck AL, McCarthy C, Rezvani L, Hussein M, Luo X, Shama M, Toraih E, Kandil E. Comparison of Medical Management versus Parathyroidectomy in Patients with Mild Primary Hyperparathyroidism: A Meta-Analysis. Cancers. 2023; 15(12):3085. https://doi.org/10.3390/cancers15123085
Chicago/Turabian StyleCironi, Katherine A., Peter P. Issa, Aaron L. Albuck, Christina McCarthy, Leely Rezvani, Mohammad Hussein, Xinyi Luo, Mohamed Shama, Eman Toraih, and Emad Kandil. 2023. "Comparison of Medical Management versus Parathyroidectomy in Patients with Mild Primary Hyperparathyroidism: A Meta-Analysis" Cancers 15, no. 12: 3085. https://doi.org/10.3390/cancers15123085
APA StyleCironi, K. A., Issa, P. P., Albuck, A. L., McCarthy, C., Rezvani, L., Hussein, M., Luo, X., Shama, M., Toraih, E., & Kandil, E. (2023). Comparison of Medical Management versus Parathyroidectomy in Patients with Mild Primary Hyperparathyroidism: A Meta-Analysis. Cancers, 15(12), 3085. https://doi.org/10.3390/cancers15123085







