Endoscopic Resection of Early Gastric Cancer and Pre-Malignant Gastric Lesions
Abstract
Simple Summary
Abstract
1. Introduction
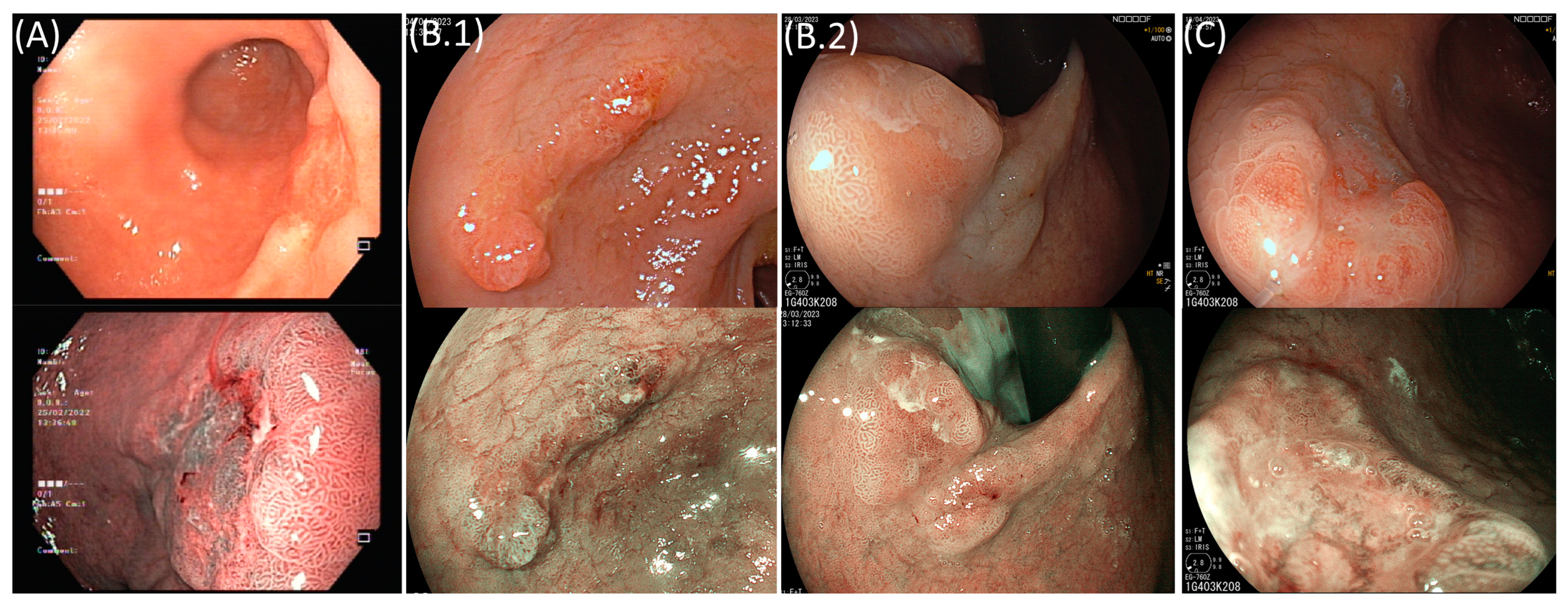
2. Superficial Gastric Lesions
3. Indications for Endoscopic Resection: Pre-Procedural Evaluation
4. Endoscopic Resection
5. Endoscopic Resection versus Surgery
| Author, Year | Type of Resection | Operation Time (in Minutes) | in-Hospital Stay (in Days) | Overall Postoperative Complication | Recurrence | Synchronous Lesions | Metachronous Lesions |
|---|---|---|---|---|---|---|---|
| Abdelfatah MM, 2019 [61] | ESD | ND | ND | ND | 40/2943 (1.4%) | 16/1082 (1.5%) | 176/2943 (6%) |
| Gastrectomy | 12/3116 (0.4%) | 1/1485 (0.1%) | 13/3116 (0.4%) | ||||
| - | OR 0.17 (0.1–4.9) | RR 5.7 (1.5–21.9) | RR 10.2 (5.9–17.1) | ||||
| Gu L, 2019 [62] | - | ND | ND | ND | ND | OR 4.94 (3.04–8.03) | OR 8.64 (5.00–14.95) |
| Li H, 2020 [63] | - | WMD −140 (−254 to −34) | −5.41 (−5.93 to −4.89) | OR 0.39 (0.28–0.55) | OR 9.24 (5.94–14.36) | ND | ND |
| Liu Q, 2020 [64] | - | MD −128 (−204 to −52) | −7.13 (−7.98 to −6.28) | OR 0.47 (0.34–0.63) | OR 5.42 (2.91–10.11) | OR 6.59 (1.96–22.1) | OR 10.84 (6.43–18.26) |
| Xu X, 2022 a [65] | - | ND | ND | OR 0.49 (0.34–0.72) | ND | OR 9.09 (2.17–50) | OR 8.33 (4–20) |
| Author, Year | Type of Resection | Overall Survival | Disease-Specific Survival | Disease-Free Survival |
|---|---|---|---|---|
| Abdelfatah MM, 2019 [61] | ESD | 2914/3034 (96%) | 2437/2451 (99.4%) | 1415/1476 (95.9%) |
| Gastrectomy | 3088/3203 (96%) | 1962/1977 (99.2%) | 1816/1844 (98.5%) | |
| - | OR 0.96 (0.74–1.25) | OR 0.7 (0.16–2.9) | OR 1.86 (0.57–6.0) | |
| Gu L, 2019 [62] | ESD | 2238/2324 (96.3%) | 5/1425 (99.7%) | 1241/1376 (90.2%) |
| Gastrectomy | 2563/2662 (96.3%) | 17/1841 (99.1%) | 1261/1298 (97.2%) | |
| - | RR 0.90 (0.68–1.19) | RR 0.40 (0.15–1.03) | RR 3.40 (2.39–4.84) | |
| Li H, 2020 [63] | - | HR 0.51 (0.26–1.00) | ND | ND |
| Liu Q, 2020 [64] | - | HR 0.92 (0.71–1.19) | HR 0.73 (0.36–1.49) | HR 4.58 (2.79–7.52) |
| Huh CW, 2021 a [67] | - | OR 2.29 (0.98–5.36) | ND | ND |
| Xu X, 2022 b [65] | - | HR 1.22 (0.66–2.25) | ND | HR 3.29 (1.60–6.76) |
| Yang HJ, 2022 a [68] | ESD | 383/400 (95.8%) | 396/400 (99.0%) | 362/400 (90.5%) |
| Gastrectomy | 492/508 (96.9%) | 506/508 (99.6%) | 491/508 (96.7%) | |
| - | RR 1.18 (0.60–2.32) | RR 2.49 (0.47–37.93) | RR 2.49 (1.42–4.35) |
6. Management after Resection
- Very-low-risk resections (LNM risk < 0.5–1%), i.e., when a differentiated mucosal (pT1a) lesion, without lymphovascular invasion, and independent of size if there are no ulceration findings or ≤30 mm in size if ulcerated, is resected en bloc and with negative margins;
- Low-risk resections (LNM risk <3%), i.e., when a poorly differentiated pT1a lesion ≤ 20 mm in size or a differentiated pT1b lesion (submucosal invasion ≤ 500 µm) ≤30 mm in size, that present neither ulceration nor lymphovascular invasion, is resected en bloc with negative margins.
7. Future Perspectives
8. Conclusions
- Prediction of and decrease in adverse events: The identification of patients at higher risk of adverse outcomes is important in order to provide patients with more comprehensive information and implement preventive strategies such as defect closure or defect shielding.
- Better patient selection: Up to 20% of endoscopically resected lesions still do not meet curative criteria, and it is desirable to improve pre-resection endoscopic assessments to avoid unnecessary procedures conducted on patients who would not benefit from them and to better allocate scarce resources. In this regard, AI will probably have a clear role in assisting endoscopists in treatment allocation.
- The optimization of the management of patients with non-curative resection: The stratification of the risk of LNM, with individualized predictions, should be pursued; this can be achieved through the refinement of existing scoring systems (eCura) and possibly by incorporating additional variables (and possibly molecular features that can help predict this undesirable outcome of LNM). Less invasive alternatives to gastrectomy with lymphadenectomy among patients with non-curative resections should also be pursued, but more studies are needed to clarify the potential role of LLND and SLNB.
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; IARC Publications; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- World Health Organization. Cancer Tomorrow—International Agency for Research on Cancer; World Health Organization: Lyon, France, 2020. [Google Scholar]
- Weir, H.K.; Thompson, T.D.; Stewart, S.L.; White, M.C. Cancer Incidence Projections in the United States Between 2015 and 2050. Prev. Chronic Dis. 2021, 18, E59. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Piazuelo, M.B. The Gastric Precancerous Cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, C.; Kato, K.; Miyashiro, I.; Nishida, H.; Takaku, R.; Terasawa, T.; Yoshikawa, T.; Honjo, S.; Inoue, K.; Nakayama, T.; et al. Update Version of the Japanese Guidelines for Gastric Cancer Screening. Jpn. J. Clin. Oncol. 2018, 48, 673–683. [Google Scholar] [CrossRef]
- Park, H.A.; Nam, S.Y.; Lee, S.K.; Kim, S.G.; Shim, K.N.; Park, S.M.; Lee, S.Y.; Han, H.S.; Shin, Y.M.; Kim, K.M.; et al. The Korean Guideline for Gastric Cancer Screening. J. Korean Med. Assoc. 2015, 58, 373–384. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma—2nd English Edition. Gastric Cancer 1998, 1, 10–24. [Google Scholar] [CrossRef]
- Yanzhang, W.; Guanghua, L.; Zhihao, Z.; Zhixiong, W.; Zhao, W. The Risk of Lymph Node Metastasis in Gastric Cancer Conforming to Indications of Endoscopic Resection and Pylorus-Preserving Gastrectomy: A Single-Center Retrospective Study. BMC Cancer 2021, 21, 1280. [Google Scholar] [CrossRef]
- Park, Y.M.; Cho, E.; Kang, H.Y.; Kim, J.M. The Effectiveness and Safety of Endoscopic Submucosal Dissection Compared with Endoscopic Mucosal Resection for Early Gastric Cancer: A Systematic Review and Metaanalysis. Surg. Endosc. 2011, 25, 2666–2677. [Google Scholar] [CrossRef]
- Suzuki, H.; Takizawa, K.; Hirasawa, T.; Takeuchi, Y.; Ishido, K.; Hoteya, S.; Yano, T.; Tanaka, S.; Endo, M.; Nakagawa, M.; et al. Short-Term Outcomes of Multicenter Prospective Cohort Study of Gastric Endoscopic Resection: ‘Real-World Evidence’ in Japan. Dig. Endosc. 2019, 31, 30–39. [Google Scholar] [CrossRef]
- Kim, S.G.; Park, C.M.; Lee, N.R.; Kim, J.; Lyu, D.H.; Park, S.H.; Choi, I.J.; Lee, W.S.; Park, S.J.; Kim, J.J.; et al. Long-Term Clinical Outcomes of Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer: A Prospective Multicenter Cohort Study. Gut Liver 2018, 12, 402–410. [Google Scholar] [CrossRef]
- Endoscopic Classification Review Group. Update on the Paris Classification of Superficial Neoplastic Lesions in the Digestive Tract. Endoscopy 2005, 37, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F. Gastrointestinal Epithelial Neoplasia: Vienna Revisited. Gut 2002, 51, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Libânio, D.; Castro, R.; Ferreira, A.; Barreiro, P.; Boal Carvalho, P.; Capela, T.; Pimentel-Nunes, P.; Santos, C.; Dinis-Ribeiro, M. Reliability of Paris Classification for Superficial Neoplastic Gastric Lesions Improves with Training and Narrow Band Imaging. Endosc. Int. Open 2019, 07, E633–E640. [Google Scholar] [CrossRef] [PubMed]
- Gotoda, T.; Yanagisawa, A.; Sasako, M.; Ono, H.; Nakanishi, Y.; Shimoda, T.; Kato, Y. Incidence of Lymph Node Metastasis from Early Gastric Cancer: Estimation with a Large Number of Cases at Two Large Centers. Gastric Cancer 2000, 3, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Tsuruta, O.; Tateishi, H.; Arima, N.; Takeda, J.; Toyonaga, A.; Sata, M. Extended Indication Criteria for Endoscopic Mucosal Resection of Early Gastric Cancer with Special Reference to Lymph Node Metastasis Examination by Multivariate Analysis. Kurume Med. J. 2004, 51, 9–14. [Google Scholar] [CrossRef]
- Hirasawa, T.; Gotoda, T.; Miyata, S.; Kato, Y.; Shimoda, T.; Taniguchi, H.; Fujisaki, J.; Sano, T.; Yamaguchi, T. Incidence of Lymph Node Metastasis and the Feasibility of Endoscopic Resection for Undifferentiated-Type Early Gastric Cancer. Gastric Cancer 2009, 12, 148–152. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.; Wang, Y. Analysis of Lymph Node Metastasis in Early Gastric Cancer: A Single Institutional Experience from China. World J. Surg. Oncol. 2020, 18, 57. [Google Scholar] [CrossRef]
- Hasuike, N.; Ono, H.; Boku, N.; Mizusawa, J.; Takizawa, K.; Fukuda, H.; Oda, I.; Doyama, H.; Kaneko, K.; Hori, S.; et al. A Non-Randomized Confirmatory Trial of an Expanded Indication for Endoscopic Submucosal Dissection for Intestinal-Type Gastric Cancer (CT1a): The Japan Clinical Oncology Group Study (JCOG0607). Gastric Cancer 2017, 21, 114–123. [Google Scholar] [CrossRef]
- Takizawa, K.; Ono, H.; Hasuike, N.; Takashima, A.; Minashi, K.; Boku, N.; Kushima, R.; Katayama, H.; Ogawa, G.; Fukuda, H.; et al. A Nonrandomized, Single-Arm Confirmatory Trial of Expanded Endoscopic Submucosal Dissection Indication for Undifferentiated Early Gastric Cancer: Japan Clinical Oncology Group Study (JCOG1009/1010). Gastric Cancer 2021, 24, 479–491. [Google Scholar] [CrossRef]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Uedo, N.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; et al. Guidelines for Endoscopic Submucosal Dissection and Endoscopic Mucosal Resection for Early Gastric Cancer (Second Edition). Dig. Endosc. 2021, 33, 4–20. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Bastiaansen, B.A.J.; Bhandari, P.; Bisschops, R.; Bourke, M.J.; Esposito, G.; Lemmers, A.; Maselli, R.; Messmann, H.; et al. Endoscopic Submucosal Dissection for Superficial Gastrointestinal Lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2022. Endoscopy 2022, 54, 591–622. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th Edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Figueirôa, G.; Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Libânio, D. Gastric Endoscopic Submucosal Dissection: A Systematic Review and Meta-Analysis on Risk Factors for Poor Short-Term Outcomes. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Park, J.C.; Song, I.J.; Kim, Y.J.; Joh, D.H.; Hahn, K.Y.; Lee, Y.K.; Kim, H.Y.; Chung, H.; Shin, S.K.; et al. Prediction Model for Non-Curative Resection of Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer. Gastrointest. Endosc. 2016, 85, 976–983. [Google Scholar] [CrossRef]
- Nam, H.S.; Choi, C.W.; Kim, S.J.; Kang, D.H.; Kim, H.W.; Park, S.B.; Ryu, D.G.; Choi, J.S. Preprocedural Prediction of Non-Curative Endoscopic Submucosal Dissection for Early Gastric Cancer. PLoS ONE 2018, 13, e0206179. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Fujisaki, J.; Yamamoto, N.; Ishizuka, N.; Omae, M.; Ishiyama, A.; Yoshio, T.; Hirasawa, T.; Yamamoto, Y.; Nagahama, M.; et al. Undifferentiated-Type Component Mixed with Differentiated-Type Early Gastric Cancer Is a Significant Risk Factor for Endoscopic Non-Curative Resection. Dig. Endosc. 2018, 30, 624–632. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y.; Dang, Y.; Huang, Q.; Wang, J.; Zhang, W.; Zhang, Y.; Zhang, G. Predictive Factors and Long-Term Outcomes of Early Gastric Carcinomas in Patients with Non-Curative Resection by Endoscopic Submucosal Dissection. Cancer Manag. Res. 2020, 12, 8037–8046. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhu, S.; Zhang, S.; Sun, X. Risk Factors and Prediction Model for Non-Curative Resection of Early Gastric Cancer With Endoscopic Resection and the Evaluation. Front. Med. 2021, 8, 637875. [Google Scholar] [CrossRef]
- Kadota, T.; Hasuike, N.; Ono, H.; Boku, N.; Mizusawa, J.; Oda, I.; Oyama, T.; Horiuchi, Y.; Hirasawa, K.; Yoshio, T.; et al. Clinical Factors Associated with Noncurative Endoscopic Submucosal Dissection for the Expanded Indication of Intestinal-type Early Gastric Cancer: Post Hoc Analysis of a Multi-institutional, Single-arm, Confirmatory Trial (JCOG0607). Dig. Endosc. 2022, 35, 494–502. [Google Scholar] [CrossRef]
- Yang, P.; Zheng, X.-D.; Wang, J.-M.; Geng, W.-B.; Wang, X. Undifferentiated-Predominant Mixed-Type Early Gastric Cancer Is More Aggressive than Pure Undifferentiated Type: A Systematic Review and Meta-Analysis. BMJ Open 2022, 12, e054473. [Google Scholar] [CrossRef]
- Du, M.Z.; Gan, W.J.; Yu, J.; Liu, W.; Zhan, S.H.; Huang, S.; Huang, R.P.; Chuan Guo, L.; Huang, Q. Risk Factors of Lymph Node Metastasis in 734 Early Gastric Carcinoma Radical Resections in a Chinese Population: Nodes Metastasis in Early Gastric Cancer. J. Dig. Dis. 2018, 19, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Milhomem, L.M.; Milhomem-Cardoso, D.M.; da Mota, O.M.; Mota, E.D.; Kagan, A.; Filho, J.B.S. Risk of Lymph Node Metastasis in Early Gastric Cancer and Indications for Endoscopic Resection: Is It Worth Applying the East Rules to the West? Surg. Endosc. 2021, 35, 4380–4388. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Kim, D.H.; Han, W.H.; Eom, B.W.; Kim, Y.I.; Yoon, H.M.; Lee, J.Y.; Kim, C.G.; Kook, M.-C.; Choi, I.J.; et al. Risk Factors for Lymph Node Metastasis in Early Gastric Cancer without Lymphatic Invasion after Endoscopic Submucosal Dissection. Eur. J. Surg. Oncol. 2021, 47, 3059–3063. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Oda, I.; Shimazu, T.; Kinjo, T.; Tada, K.; Sakamoto, T.; Kusano, C.; Gotoda, T. Depth-Predicting Score for Differentiated Early Gastric Cancer. Gastric Cancer 2011, 14, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Nakayoshi, T.; Tajiri, H.; Matsuda, K.; Kaise, M.; Ikegami, M.; Sasaki, H. Magnifying Endoscopy Combined with Narrow Band Imaging System for Early Gastric Cancer: Correlation of Vascular Pattern with Histo-pathology (Including Video). Endoscopy 2004, 36, 1080–1084. [Google Scholar] [CrossRef]
- Yokoyama, A.; Inoue, H.; Minami, H.; Wada, Y.; Sato, Y.; Satodate, H.; Hamatani, S.; Kudo, S. Novel Nar-row-Band Imaging Magnifying Endoscopic Classification for Early Gastric Cancer. Dig. Liver Dis. 2010, 42, 704–708. [Google Scholar] [CrossRef]
- Tanaka, K.; Toyoda, H.; Kadowaki, S.; Kosaka, R.; Shiraishi, T.; Imoto, I.; Shiku, H.; Adachi, Y. Features of Early Gastric Cancer and Gastric Adenoma by Enhanced-Magnification Endoscopy. J. Gastroenterol. 2006, 41, 332–338. [Google Scholar] [CrossRef]
- Ok, K.-S.; Kim, G.H.; Park, D.Y.; Lee, H.J.; Jeon, H.K.; Baek, D.H.; Lee, B.E.; Song, G.A. Magnifying En-doscopy with Narrow Band Imaging of Early Gastric Cancer: Correlation with Histopathology and Mucin Phenotype. Gut Liver 2016, 10, 532–541. [Google Scholar] [CrossRef]
- Kanesaka, T.; Uedo, N.; Doyama, H.; Yoshida, N.; Nagahama, T.; Ohtsu, K.; Uchita, K.; Kojima, K.; Ueo, T.; Takahashi, H.; et al. Diagnosis of Histological Type of Early Gastric Cancer by Magnifying Narrow-band Imaging: A Multicenter Prospective Study. DEN Open 2021, 2, e61. [Google Scholar] [CrossRef]
- Inoue, H.; Takeshita, K.; Hori, H.; Muraoka, Y.; Yoneshima, H.; Endo, M. Endoscopic Mucosal Resection with a Cap-Fitted Panendoscope for Esophagus, Stomach, and Colon Mucosal Lesions. Gastrointest. Endosc. 1993, 39, 58–62. [Google Scholar] [CrossRef]
- Ono, H.; Kondo, H.; Gotoda, T.; Shirao, K.; Yamaguchi, H.; Saito, D.; Hosokawa, K.; Shimoda, T.; Yoshida, S. Endoscopic Mucosal Resection for Treatment of Early Gastric Cancer. Gut 2001, 48, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Gotoda, T.; Kondo, H.; Ono, H.; Saito, Y.; Yamaguchi, H.; Saito, D.; Yokota, T. A New Endoscopic Mucosal Resection Procedure Using an Insulation-Tipped Electrosurgical Knife for Rectal Flat Lesions: Report of Two Cases. Gastrointest. Endosc. 1999, 50, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Zhou, X.; Hu, M.; Pan, J. Endoscopic Submucosal Dissection versus Endoscopic Mucosal Resection for Patients with Early Gastric Cancer: A Meta-Analysis. BMJ Open 2019, 9, e025803. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Chen, S.; Zhang, Y.; Qiu, F. A Meta-Analysis of Endoscopic Submucosal Dissection and EMR for Early Gastric Cancer. Gastrointest. Endosc. 2012, 76, 763–770. [Google Scholar] [CrossRef]
- Facciorusso, A.; Antonino, M.; Di Maso, M.; Muscatiello, N. Endoscopic Submucosal Dissection vs Endoscopic Mucosal Resection for Early Gastric Cancer: A Meta-Analysis. World J. Gastrointest. Endosc. 2014, 6, 555. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C. Long-Term Clinical Efficacy and Perioperative Safety of Endoscopic Submucosal Dissection versus Endoscopic Mucosal Resection for Early Gastric Cancer: An Updated Meta-Analysis. BioMed Res. Int. 2018, 2018, 3152346. [Google Scholar] [CrossRef]
- Tanabe, S.; Ishido, K.; Matsumoto, T.; Kosaka, T.; Oda, I.; Suzuki, H.; Fujisaki, J.; Ono, H.; Kawata, N.; Oyama, T.; et al. Long-Term Outcomes of Endoscopic Submucosal Dissection for Early Gastric Cancer: A Multicenter Collaborative Study. Gastric Cancer 2017, 20, 45–52. [Google Scholar] [CrossRef]
- Peng, L.J.; Tian, S.N.; Lu, L.; Chen, H.; Ouyang, Y.Y.; Wu, Y.J. Outcome of Endoscopic Submucosal Dissection for Early Gastric Cancer of Conventional and Expanded Indications: Systematic Review and Meta-Analysis. J. Dig. Dis. 2015, 16, 67–74. [Google Scholar] [CrossRef]
- Suzuki, H.; Ono, H.; Hirasawa, T.; Takeuchi, Y.; Ishido, K.; Hoteya, S.; Yano, T.; Tanaka, S.; Toya, Y.; Nakagawa, M.; et al. Long-Term Survival After Endoscopic Resection For Gastric Cancer: Real-World Evidence From a Multicenter Prospective Cohort. Clin. Gastroenterol. Hepatol. 2022, 21, 307–318.e2. [Google Scholar] [CrossRef]
- Shichijo, S.; Uedo, N.; Kanesaka, T.; Ohta, T.; Nakagawa, K.; Shimamoto, Y.; Ohmori, M.; Arao, M.; Iwatsubo, T.; Suzuki, S.; et al. Long-term Outcomes after Endoscopic Submucosal Dissection for Differentiated-type Early Gastric Cancer That Fulfilled Expanded Indication Criteria: A Prospective Cohort Study. J. Gastroenterol. Hepatol. 2020, 36, 664–670. [Google Scholar] [CrossRef]
- Libânio, D.; Costa, M.N.; Pimentel-Nunes, P.; Dinis-Ribeiro, M. Risk Factors for Bleeding after Gastric Endoscopic Submucosal Dissection: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2016, 84, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Uedo, N.; Takeuchi, Y.; Yamada, T.; Ishihara, R.; Ogiyama, H.; Yamamoto, S.; Kato, M.; Tatsumi, K.; Masuda, E.; Tamai, C.; et al. Effect of a Proton Pump Inhibitor or an H2-Receptor Antagonist on Prevention of Bleeding From Ulcer After Endoscopic Submucosal Dissection of Early Gastric Cancer: A Prospective Randomized Controlled Trial. Am. J. Gastroenterol. 2007, 102, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, Q.; Liu, Z.; Wu, K.; Fan, D. Proton Pump Inhibitors versus Histamine-2-Receptor Antagonists for the Management of Iatrogenic Gastric Ulcer after Endoscopic Mucosal Resection or Endoscopic Submucosal Dissection: A Meta-Analysis of Randomized Trials. Digestion 2011, 84, 315–320. [Google Scholar] [CrossRef]
- Nishizawa, T.; Suzuki, H.; Akimoto, T.; Maehata, T.; Morizane, T.; Kanai, T.; Yahagi, N. Effects of Preoperative Proton Pump Inhibitor Administration on Bleeding after Gastric Endoscopic Submucosal Dissection: A Systematic Review and Meta-analysis. United Eur. Gastroenterol. J. 2016, 4, 5–10. [Google Scholar] [CrossRef]
- Takizawa, K.; Oda, I.; Gotoda, T.; Yokoi, C.; Matsuda, T.; Saito, Y.; Saito, D.; Ono, H. Routine Coagulation of Visible Vessels May Prevent Delayed Bleeding after Endoscopic Submucosal Dissection—An Analysis of Risk Factors. Endoscopy 2008, 40, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X.; Wang, D.; Liu, X.; Chen, J.; Song, J.; Bai, T.; Hou, X. Endoscopic Delivery of Polymers Reduces Delayed Bleeding after Gastric Endoscopic Submucosal Dissection: A Systematic Review and Meta-Analysis. Polymers 2022, 14, 2387. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Luo, H.; Duan, H. Risk Factors for Perforation of Gastric Endoscopic Submucosal Dissection: A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1481–1488. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, Z.; Liang, J.; Guo, S.; Huang, D. Endoscopic Submucosal Dissection for Early Gastric Cancer in Elderly vs. Non-Elderly Patients: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 11, 5767. [Google Scholar] [CrossRef]
- Abdelfatah, M.M.; Barakat, M.; Ahmad, D.; Ibrahim, M.; Ahmed, Y.; Kurdi, Y.; Grimm, I.S.; Othman, M.O. Long-term Outcomes of Endoscopic Submucosal Dissection versus Surgery in Early Gastric Cancer: A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2019, 31, 418–424. [Google Scholar] [CrossRef]
- Gu, L.; Khadaroo, P.A.; Chen, L.; Li, X.; Zhu, H.; Zhong, X.; Pan, J.; Chen, M. Comparison of Long-Term Outcomes of Endoscopic Submucosal Dissection and Surgery for Early Gastric Cancer: A Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2019, 23, 1493–1501. [Google Scholar] [CrossRef]
- Li, H.; Feng, L.-Q.; Bian, Y.-Y.; Yang, L.-L.; Liu, D.-X.; Huo, Z.-B.; Zeng, L. Comparison of Endoscopic Submucosal Dissection with Surgical Gastrectomy for Early Gastric Cancer: An Updated Meta-Analysis. World J. Gastrointest. Oncol. 2019, 11, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ding, L.; Qiu, X.; Meng, F. Updated Evaluation of Endoscopic Submucosal Dissection versus Surgery for Early Gastric Cancer: A Systematic Review and Meta-Analysis. Int. J. Surg. 2019, 73, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, G.; Gao, N.; Zheng, Z. Long-Term Outcomes and Clinical Safety of Expanded Indication Early Gastric Cancer Treated with Endoscopic Submucosal Dissection versus Surgical Resection: A Meta-Analysis. BMJ Open 2022, 12, e055406. [Google Scholar] [CrossRef]
- Nakamura, R.; Omori, T.; Mayanagi, S.; Irino, T.; Wada, N.; Kawakubo, H.; Kameyama, K.; Kitagawa, Y. Risk of Lymph Node Metastasis in Undifferentiated-Type Mucosal Gastric Carcinoma. World J. Surg. Oncol. 2019, 17, 32. [Google Scholar] [CrossRef]
- Huh, C.-W.; Ma, D.W.; Kim, B.-W.; Kim, J.S.; Lee, S.J. Endoscopic Submucosal Dissection versus Surgery for Undifferentiated-Type Early Gastric Cancer: A Systematic Review and Meta-Analysis. Clin. Endosc. 2021, 54, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Kim, J.-H.; Kim, N.W.; Choi, I.J. Comparison of Long-Term Outcomes of Endoscopic Submucosal Dissection and Surgery for Undifferentiated-Type Early Gastric Cancer Meeting the Expanded Criteria: A Systematic Review and Meta-Analysis. Surg. Endosc. 2022, 36, 3686–3697. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, Y.-W.; Choi, I.J.; Cho, J.Y.; Kim, J.H.; Kwon, J.-W.; Lee, J.Y.; Lee, N.R.; Seol, S.-Y. Cost Comparison between Surgical Treatments and Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer in Korea. Gut Liver 2015, 9, 174–180. [Google Scholar] [CrossRef]
- Shin, D.W.; Hwang, H.Y.; Jeon, S.W. Comparison of Endoscopic Submucosal Dissection and Surgery for Differentiated Type Early Gastric Cancer within the Expanded Criteria. Clin. Endosc. 2017, 50, 170–178. [Google Scholar] [CrossRef]
- Qian, M.; Sheng, Y.; Wu, M.; Wang, S.; Zhang, K. Comparison between Endoscopic Submucosal Dissection and Surgery in Patients with Early Gastric Cancer. Cancers 2022, 14, 3603. [Google Scholar] [CrossRef]
- Song, W.; Qiao, X.; Gao, X. A Comparison of Endoscopic Submucosal Dissection (ESD) and Radical Surgery for Early Gastric Cancer: A Retrospective Study. World J. Surg. Oncol. 2015, 13, 309. [Google Scholar] [CrossRef]
- Libânio, D.; Braga, V.; Ferraz, S.; Castro, R.; Lage, J.; Pita, I.; Ribeiro, C.; Abreu De Sousa, J.; Dinis-Ribeiro, M.; Pimentel-Nunes, P. Prospective Comparative Study of Endoscopic Submucosal Dissection and Gastrectomy for Early Neoplastic Lesions Including Patients’ Perspectives. Endoscopy 2019, 51, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Kim, Y.A.; Kim, C.G.; Ryu, K.W.; Kim, Y.-W.; Sim, J.A.; Yun, Y.H.; Choi, I.J. Serial Intermediate-Term Quality of Life Comparison after Endoscopic Submucosal Dissection versus Surgery in Early Gastric Cancer Patients. Surg. Endosc. 2018, 32, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Libânio, D.; Ortigão, R.; Pimentel-Nunes, P.; Dinis-Ribeiro, M. Improving the Diagnosis and Treatment of Early Gastric Cancer in the West. GE Port J. Gastroenterol. 2022, 29, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Libânio, D.; Pimentel-Nunes, P.; Afonso, L.P.; Henrique, R.; Dinis-Ribeiro, M. Long-Term Outcomes of Gastric Endoscopic Submucosal Dissection: Focus on Metachronous and Non-Curative Resection Management. GE Port. J. Gastroenterol. 2016, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kawata, N.; Kakushima, N.; Takizawa, K.; Tanaka, M.; Makuuchi, R.; Tokunaga, M.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Sugino, T.; et al. Risk Factors for Lymph Node Metastasis and Long-Term Outcomes of Patients with Early Gastric Cancer after Non-Curative Endoscopic Submucosal Dissection. Surg. Endosc. 2016, 31, 1607–1616. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: “ECura System”. Am. J. Gastroenterol. 2017, 112, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Ortigão, R.; Figueirôa, G.; Frazzoni, L.; Pimentel-Nunes, P.; Hassan, C.; Dinis-Ribeiro, M.; Fuccio, L.; Libânio, D. Risk Factors for Gastric Metachronous Lesions after Endoscopic or Surgical Resection: A Systematic Review and Meta-Analysis. Endoscopy 2022, 54, 892–901. [Google Scholar] [CrossRef]
- Hahn, K.Y.; Park, J.C.; Kim, E.H.; Shin, S.; Park, C.H.; Chung, H.; Shin, S.K.; Lee, S.K.; Lee, Y.C. Incidence and Impact of Scheduled Endoscopic Surveillance on Recurrence after Curative Endoscopic Resection for Early Gastric Cancer. Gastrointest. Endosc. 2016, 84, 628–638.e1. [Google Scholar] [CrossRef]
- Fan, F.; Wang, Z.; Li, B.; Zhang, H. Effects of Eradicating Helicobacter Pylori on Metachronous Gastric Cancer Prevention: A Systematic Review and Meta-analysis. J. Eval. Clin. Pract. 2020, 26, 308–315. [Google Scholar] [CrossRef]
- Bang, C.S.; Baik, G.H.; Shin, I.S.; Kim, J.B.; Suk, K.T.; Yoon, J.H.; Kim, Y.S.; Kim, D.J. Helicobacter Pylori Eradication for Prevention of Metachronous Recurrence after Endoscopic Resection of Early Gastric Cancer. J. Korean Med. Sci. 2015, 30, 749–756. [Google Scholar] [CrossRef]
- Xiao, S.; Li, S.; Zhou, L.; Jiang, W.; Liu, J. Helicobacter Pylori Status and Risks of Metachronous Recurrence after Endoscopic Resection of Early Gastric Cancer: A Systematic Review and Meta-Analysis. J. Gastroenterol. 2019, 54, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.B.; Park, J.M.; Lim, C.-H.; Cho, Y.K.; Choi, M.-G. Effect of Helicobacter Pylori Eradication on Metachronous Gastric Cancer after Endoscopic Resection of Gastric Tumors: A Meta-Analysis. Helicobacter 2014, 19, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Y.; Aslam, A.; Mihali, A.B.; Shabbir Rawala, M.; Dirweesh, A.; Khan, S.; Adler, D.G.; Siddiqui, A. Effectiveness of Helicobacter Pylori Eradication in Preventing Metachronous Gastric Cancer and Preneoplastic Lesions. A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, J.; Mei, D.; Luo, R.; Lu, H.; Xu, H.; Huang, B. Does Helicobacter Pylori Eradication Reduce the Incidence of Metachronous Gastric Cancer After Curative Endoscopic Resection of Early Gastric Cancer. J. Clin. Gastroenterol. 2020, 54, 235–241. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of Epithelial Precancerous Conditions and Lesions in the Stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) Guideline Update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [PubMed]
- Kobayashi, M.; Narisawa, R.; Sato, Y.; Takeuchi, M.; Aoyagi, Y. Self-Limiting Risk of Metachronous Gastric Cancers after Endoscopic Resection. Dig. Endosc. 2010, 22, 169–173. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Q.-C.; Xu, M.-D.; Zhang, Z.; Cheng, J.; Zhong, Y.-S.; Zhang, Y.-Q.; Chen, W.-F.; Yao, L.-Q.; Zhou, P.-H.; et al. Application of Convolutional Neural Network in the Diagnosis of the Invasion Depth of Gastric Cancer Based on Conventional Endoscopy. Gastrointest. Endosc. 2019, 89, 806–815.e1. [Google Scholar] [CrossRef]
- Tang, D.; Zhou, J.; Wang, L.; Ni, M.; Chen, M.; Hassan, S.; Luo, R.; Chen, X.; He, X.; Zhang, L.; et al. A Novel Model Based on Deep Convolutional Neural Network Improves Diagnostic Accuracy of Intramucosal Gastric Cancer (With Video). Front. Oncol. 2021, 11, 622827. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, S.; Kim, J.-H.; Keum, J.-S.; Oh, S.-I.; Jo, J.; Chun, J.; Youn, Y.H.; Park, H.; Kwon, I.G.; et al. A Lesion-Based Convolutional Neural Network Improves Endoscopic Detection and Depth Prediction of Early Gastric Cancer. J. Clin. Med. 2019, 8, 1310. [Google Scholar] [CrossRef]
- Nagao, S.; Tsuji, Y.; Sakaguchi, Y.; Takahashi, Y.; Minatsuki, C.; Niimi, K.; Yamashita, H.; Yamamichi, N.; Seto, Y.; Tada, T.; et al. Highly Accurate Artificial Intelligence Systems to Predict the Invasion Depth of Gastric Cancer: Efficacy of Conventional White-Light Imaging, Nonmagnifying Narrow-Band Imaging, and Indigo-Carmine Dye Contrast Imaging. Gastrointest. Endosc. 2020, 92, 866–873.e1. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; He, X.; Zhu, Y.; Jiang, X.; Chen, Y.; Wang, Y.; Huang, L.; Shang, R.; Dong, Z.; et al. Deep Learning System Compared with Expert Endoscopists in Predicting Early Gastric Cancer and Its Invasion Depth and Differentiation Status (with Videos). Gastrointest. Endosc. 2022, 95, 92–104.e3. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Kawahara, Y.; Tanimoto, T.; Ohto, A.; Toda, A.; Aida, T.; Yamasaki, Y.; Gotoda, T.; Ogawa, T.; Abe, M.; et al. Application of Convolutional Neural Networks for Evaluating the Depth of Invasion of Early Gastric Cancer Based on Endoscopic Images. J. Gastroenterol. Hepatol. 2021, 37, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Jiang, X.; Pan, J.; Wen, Y.; Huang, Y.; Weng, S.; Lan, S.; Nie, K.; Zheng, Z.; Ji, S.; et al. Current Evidence and Future Perspective of Accuracy of Artificial Intelligence Application for Early Gastric Cancer Diagnosis With Endoscopy: A Systematic and Meta-Analysis. Front. Med. (Lausanne) 2021, 8, 629080. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, K.; Li, F.; Ma, G.; Ni, Y.; Zhang, W.; Wang, J.; Li, Y. Diagnostic Accuracy of Convolutional Neural Network–Based Endoscopic Image Analysis in Diagnosing Gastric Cancer and Predicting Its Invasion Depth: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2021, 95, 599–609.e7. [Google Scholar] [CrossRef]
- Kim, J.-H.; Oh, S.-I.; Han, S.-Y.; Keum, J.-S.; Kim, K.-N.; Chun, J.-Y.; Youn, Y.-H.; Park, H. An Optimal Artificial Intelligence System for Real-Time Endoscopic Prediction of Invasion Depth in Early Gastric Cancer. Cancers 2022, 14, 6000. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Kubota, N.; Nishikawa, J.; Ogawa, R.; Hamabe, K.; Hashimoto, S.; Ogihara, H.; Hamamoto, Y.; Yanai, H.; Miura, O.; et al. Cooperation between Artificial Intelligence and Endoscopists for Diagnosing Invasion Depth of Early Gastric Cancer. Gastric Cancer 2023, 26, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Mori, T.; Takeuchi, H.; Yoshida, T.; Ohki, A.; Ueki, H.; Yanagida, O.; Masaki, T.; Sugiyama, M.; Atomi, Y. Laparoscopic Lymph Node Dissection after Endoscopic Submucosal Dissection: A Novel and Minimally Invasive Approach to Treating Early-Stage Gastric Cancer. Am. J. Surg. 2005, 190, 496–503. [Google Scholar] [CrossRef]
- Abe, N.; Takeuchi, H.; Ohki, A.; Yanagida, O.; Masaki, T.; Mori, T.; Sugiyama, M. Long-Term Outcomes of Combination of Endoscopic Submucosal Dissection and Laparoscopic Lymph Node Dissection without Gastrectomy for Early Gastric Cancer Patients Who Have a Potential Risk of Lymph Node Metastasis. Gastrointest. Endosc. 2011, 74, 792–797. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Z.-Y.; Chen, J.-Q.; Liu, J.-L. Diagnostic Value of Sentinel Lymph Node Biopsy in Gastric Cancer: A Meta-Analysis. Ann. Surg. Oncol. 2011, 19, 1541–1550. [Google Scholar] [CrossRef]
- Huang, L.; Wei, T.; Chen, J.; Zhou, D. Feasibility and Diagnostic Performance of Dual-Tracer-Guided Sentinel Lymph Node Biopsy in CT1-2N0M0 Gastric Cancer: A Systematic Review and Meta-Analysis of Diagnostic Studies. World J. Surg. Oncol. 2017, 15, 103. [Google Scholar] [CrossRef]
- Skubleny, D.; Dang, J.T.; Skulsky, S.; Switzer, N.; Tian, C.; Shi, X.; de Gara, C.; Birch, D.W.; Karmali, S. Diagnostic Evaluation of Sentinel Lymph Node Biopsy Using Indocyanine Green and Infrared or Fluorescent Imaging in Gastric Cancer: A Systematic Review and Meta-Analysis. Surg. Endosc. 2018, 32, 2620–2631. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Pan, M.; Deng, Z.; Ji, Y.; Chen, B. How Useful Is Sentinel Lymph Node Biopsy for the Status of Lymph Node Metastasis in CT1N0M0 Gastric Cancer? A Systematic Review and Meta-Analysis. Updates Surg. 2021, 73, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Pan, M.; Chen, B. A Systematic Review and Meta-Analysis of Sentinel Lymph Node Biopsy in Gastric Cancer, an Optimization of Imaging Protocol for Tracer Mapping. World J. Surg. 2021, 45, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Chen, Q.-Y.; Huang, X.-B.; Lin, G.-T.; Liu, Z.-Y.; Chen, J.-Y.; Wang, H.-G.; Weng, K.; Li, P.; Xie, J.-W.; et al. Clinical Implications of Indocyanine Green Fluorescence Imaging-Guided Laparoscopic Lymphadenectomy for Patients with Gastric Cancer: A Cohort Study from Two Randomized, Controlled Trials Using Individual Patient Data. Int. J. Surg. 2021, 94, 106120. [Google Scholar] [CrossRef]
- Park, D.J.; Park, Y.S.; Son, S.Y.; Lee, J.-H.; Lee, H.S.; Park, Y.S.; Lee, K.H.; Kim, Y.H.; Park, K.U.; Lee, W.W.; et al. Long-Term Oncologic Outcomes of Laparoscopic Sentinel Node Navigation Surgery in Early Gastric Cancer: A Single-Center, Single-Arm, Phase II Trial. Ann. Surg. Oncol. 2018, 25, 2357–2365. [Google Scholar] [CrossRef]

| Type of Lesion | European Guidelines | Japanese Guidelines | |
|---|---|---|---|
| Dysplasia, any size | Absolute indication | ||
| Adenocarcinoma | cT1a, well-differentiated, non-ulcerated, any size | Absolute indication | |
| cT1a, well-differentiated, ulcerated, ≤30 mm | Absolute indication | ||
| cT1a, poorly differentiated, non-ulcerated, ≤20 mm | Expanded indication | Absolute indication | |
| Recurrence of an eCura-C1 lesion, staged as cT1a | - | Expanded indication | |
| Author, Year | Type of Resection | Operation Time (in Minutes) | Perforation Rate | Local Recurrence | En Bloc Resection | Complete Resection |
|---|---|---|---|---|---|---|
| Tao M, 2019 [45] | - | SMD 1.12 (0.13–2.10) | OR 2.55 (1.48–4.39) | OR 0.18 (0.09–0.34) | OR 9.00 (6.66–12.17) | OR 8.43 (5.04–14.09) |
| Lian J, 2012 [46] | EMR | ND | 17/1973 (0.9%) | 126/1973 (6.4%) | 1020/1973 (51.7%) | 867/2053 (42.2%) |
| ESD | ND | 62/1438 (4.3%) | 11/1438 (0.8%) | 1328/1437 (92.4%) | 1227/1495 (82.1%) | |
| - | WMD 59.4 (16.8–102.0) | OR 4.67 (2.77–7.87) | OR 0.10 (0.06–0.18) | OR 9.69 (7.74–12.13) | OR 5.66 (2.92–10.96) | |
| Facciorusso A, 2014 [47] | EMR | ND | 17/1973 (0.9%) | 141/2332 (6.0%) | 1020/1973 (51.7%) | 867/2053 (42.2%) |
| ESD | ND | 62/1438 (4.3%) | 12/1859 (0.6%) | 1328/1437 (92.4%) | 1227/1495 (82.1%) | |
| - | SMD 1.73 (0.52–2.95) | OR 4.67 (2.77–7.87) | OR 0.09 (0.05–0.17) | OR 9.69 (7.74–12.13) | OR 5.66 (2.92–10.96) | |
| Zhao Y, 2018 [48] | EMR | - | 26/2134 (1.2%) | 116/2245 (5.2%) | 1422/2551 (55.7%) | 1110/1935 (57.4%) |
| ESD | - | 86/2676 (3.2%) | 4/1932 (0.2%) | 2229/2387 (93.4%) | 1864/2032 (91.7%) | |
| - | MD −49.86 (−71.62 to −28.10) | OR 0.37 (0.24–0.57) | OR 14.94 (7.26–30.74) | OR 0.10 (0.09–0.13) | OR 0.14 (0.12–0.17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, A.C.; Dinis-Ribeiro, M.; Libânio, D. Endoscopic Resection of Early Gastric Cancer and Pre-Malignant Gastric Lesions. Cancers 2023, 15, 3084. https://doi.org/10.3390/cancers15123084
Vasconcelos AC, Dinis-Ribeiro M, Libânio D. Endoscopic Resection of Early Gastric Cancer and Pre-Malignant Gastric Lesions. Cancers. 2023; 15(12):3084. https://doi.org/10.3390/cancers15123084
Chicago/Turabian StyleVasconcelos, Ana Clara, Mário Dinis-Ribeiro, and Diogo Libânio. 2023. "Endoscopic Resection of Early Gastric Cancer and Pre-Malignant Gastric Lesions" Cancers 15, no. 12: 3084. https://doi.org/10.3390/cancers15123084
APA StyleVasconcelos, A. C., Dinis-Ribeiro, M., & Libânio, D. (2023). Endoscopic Resection of Early Gastric Cancer and Pre-Malignant Gastric Lesions. Cancers, 15(12), 3084. https://doi.org/10.3390/cancers15123084






