T-Cell Engagers in Solid Cancers—Current Landscape and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
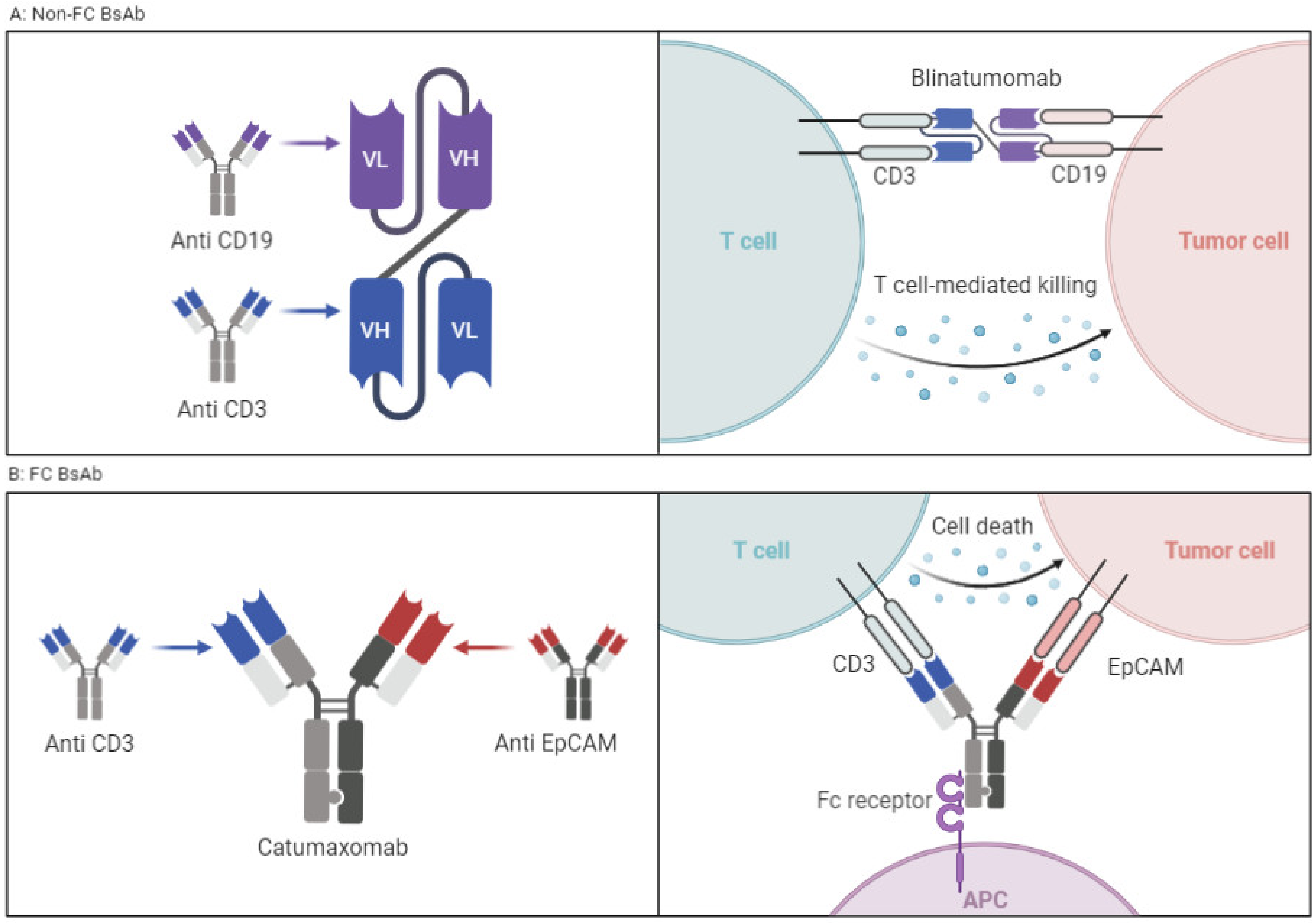
2. Structural Mechanism of T-Cell Engagers
2.1. T-Cell Engagers without FC Fragment
2.2. T-Cell Engagers with FC Fragment
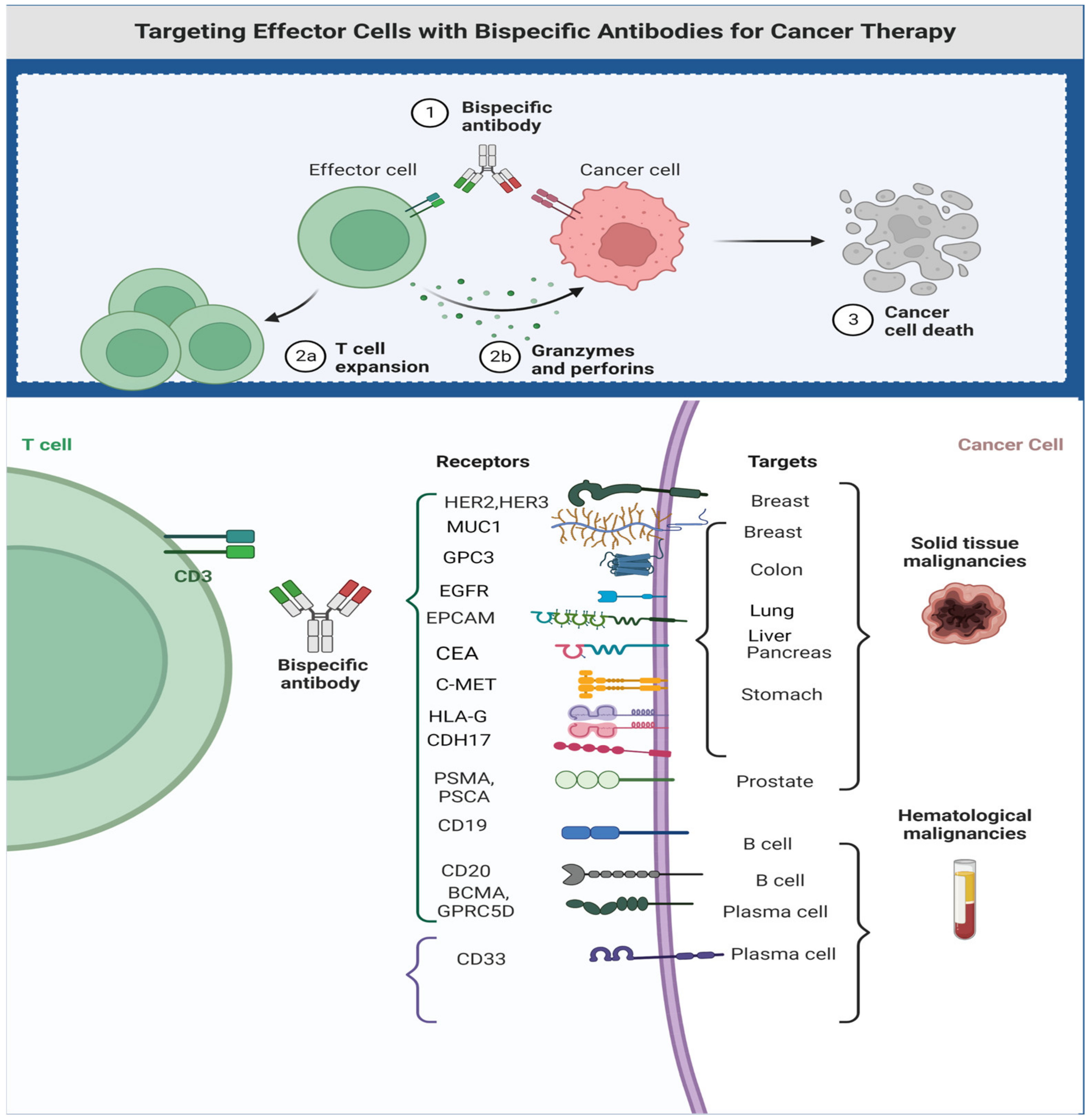
3. T-Cell Engagers in Solid Tumors
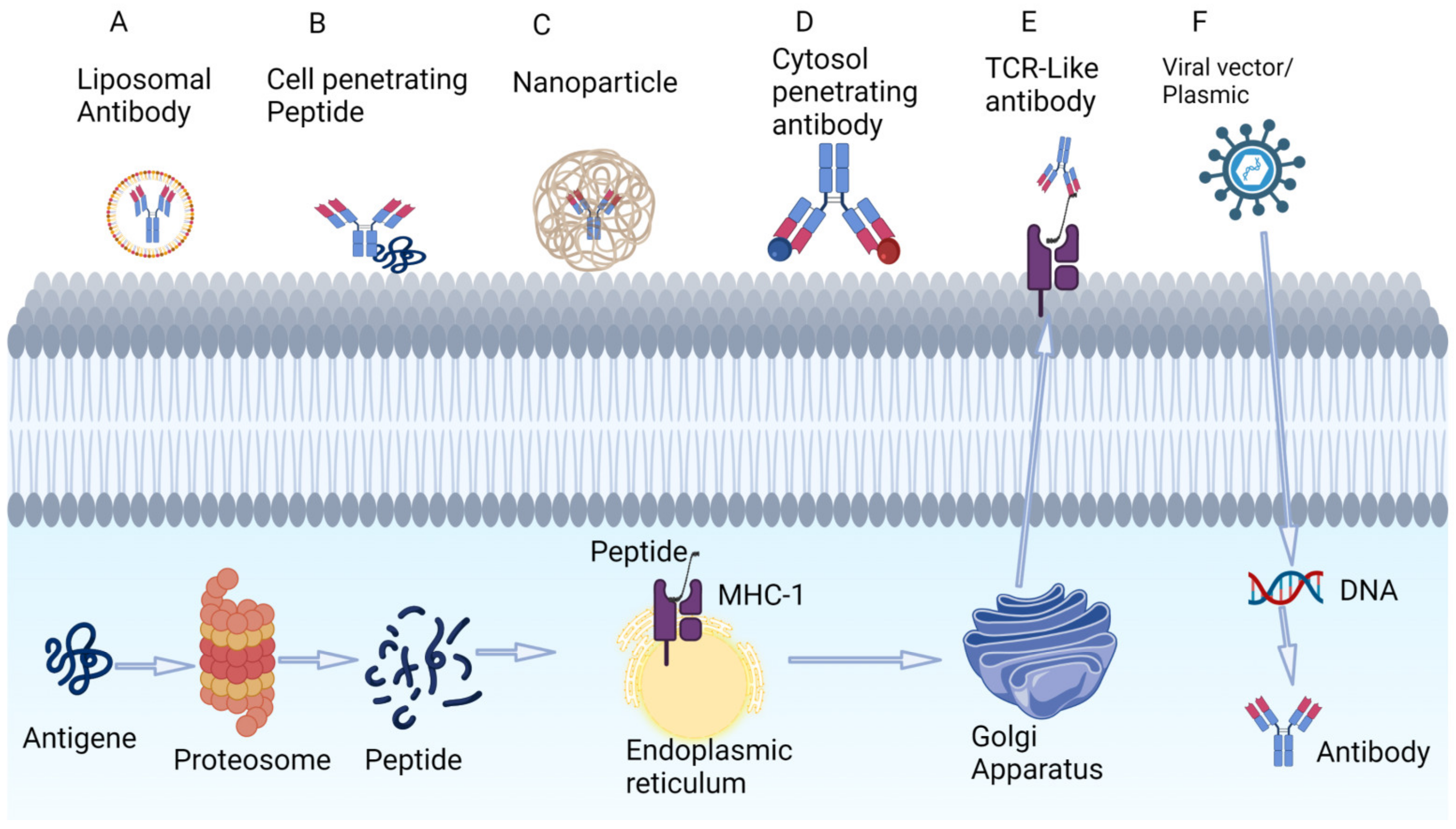
3.1. Targeting Tumor-Associated Peptides Presented by MHC
3.2. Intracellular Delivery of Antibodies
3.3. Successful Examples of T-Cell Engagers in Solid Tumors
- GP100
- EPCAM
- PSMA
- CD33 (MDSC-targeting)
3.4. Toxicity Profile and Management of Toxicity of T-Cell Engagers in Solid Tumors
- Cytokine release syndrome
- Neurotoxicity
- TAA-specific toxicities
- T-cell engagers undergoing clinical evaluation in solid tumors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oostindie, S.C.; Lazar, G.A.; Schuurman, J.; Parren, P. Avidity in antibody effector functions and biotherapeutic drug design. Nat. Rev. Drug. Discov. 2022, 21, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Robbins, P.F.; Rosenberg, S.A. ‘Final common pathway’ of human cancer immunotherapy: Targeting random somatic mutations. Nat. Immunol. 2017, 18, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Torka, P.; Barth, M.; Ferdman, R.; Hernandez-Ilizaliturri, F.J. Mechanisms of Resistance to Monoclonal Antibodies (mAbs) in Lymphoid Malignancies. Curr. Hematol. Malig. Rep. 2019, 14, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, T.M.; Limper, C.B.; Richards, K.L. Past, Present, and Future of Rituximab-The World’s First Oncology Monoclonal Antibody Therapy. Front. Oncol. 2018, 8, 163. [Google Scholar] [CrossRef]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616. [Google Scholar] [CrossRef]
- Abanades, B.; Georges, G.; Bujotzek, A.; Deane, C.M. ABlooper: Fast accurate antibody CDR loop structure prediction with accuracy estimation. Bioinformatics 2022, 38, 1877–1880. [Google Scholar] [CrossRef]
- Pantazes, R.J.; Maranas, C.D. OptCDR: A general computational method for the design of antibody complementarity determining regions for targeted epitope binding. Protein Eng. Des. Sel. 2010, 23, 849–858. [Google Scholar] [CrossRef]
- Guest, J.D.; Vreven, T.; Zhou, J.; Moal, I.; Jeliazkov, J.R.; Gray, J.J.; Weng, Z.; Pierce, B.G. An expanded benchmark for antibody-antigen docking and affinity prediction reveals insights into antibody recognition determinants. Structure 2021, 29, 606–621.e5. [Google Scholar] [CrossRef]
- Ambrosetti, F.; Jiménez-García, B.; Roel-Touris, J.; Bonvin, A.M.J.J. Modeling Antibody-Antigen Complexes by Information-Driven Docking. Structure 2019, 28, 119–129. [Google Scholar] [CrossRef]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef]
- Dai, B.; Bailey-Kellogg, C. Protein Interaction Interface Region Prediction by Geometric Deep Learning. Bioinformatics 2021, 37, 2580–2588. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, D.; Tsumoto, K. Antibody Affinity Maturation by Computational Design. Methods Mol. Biol. 2018, 1827, 15–34. [Google Scholar] [PubMed]
- Garrido, F. HLA class-I expression and cancer immunotherapy. In MHC Class-I Loss and Cancer Immune Escape; Springer: Berlin/Heidelberg, Germany, 2019; pp. 79–90. [Google Scholar]
- Dovedi, S.; Mazor, Y.; Elder, M.; Hasani, S.; Wang, B.; Mosely, S.; Jones, D.; Hansen, A.; Yang, C.; Wu, Y.; et al. Abstract 2776: MEDI5752: A novel bispecific antibody that preferentially targets CTLA-4 on PD-1 expressing T-cells. Cancer Res. 2018, 78, 2776. [Google Scholar] [CrossRef]
- Halim, L.; Das, K.K.; Larcombe-Young, D.; Ajina, A.; Candelli, A.; Benjamin, R.; Dillon, R.; Davies, D.M.; Maher, J. Engineering of an Avidity-Optimized CD19-Specific Parallel Chimeric Antigen Receptor That Delivers Dual CD28 and 4-1BB Co-Stimulation. Front. Immunol. 2022, 13, 836549. [Google Scholar] [CrossRef]
- Marei, H.E.; Cenciarelli, C.; Hasan, A. Potential of antibody-drug conjugates (ADCs) for cancer therapy. Cancer Cell. Int. 2022, 22, 255. [Google Scholar] [CrossRef]
- de Goeij, B.E.; Vink, T.; Ten Napel, H.; Breij, E.C.; Satijn, D.; Wubbolts, R.; Miao, D.; Parren, P.W. Efficient Payload Delivery by a Bispecific Antibody-Drug Conjugate Targeting HER2 and CD63. Mol. Cancer Ther. 2016, 15, 2688–2697. [Google Scholar] [CrossRef]
- Andreev, J.; Thambi, N.; Perez Bay, A.E.; Delfino, F.; Martin, J.; Kelly, M.P.; Kirshner, J.R.; Rafique, A.; Kunz, A.; Nittoli, T.; et al. Bispecific Antibodies and Antibody-Drug Conjugates (ADCs) Bridging HER2 and Prolactin Receptor Improve Efficacy of HER2 ADCs. Mol. Cancer Ther. 2017, 16, 681–693. [Google Scholar] [CrossRef]
- Esfandiari, A.; Cassidy, S.; Webster, R.M. Bispecific antibodies in oncology. Nat. Rev. Drug. Discov. 2022, 21, 411–412. [Google Scholar] [CrossRef]
- Schram, A.M.; Goto, K.; Kim, D.-W.; Martin-Romano, P.; Ou, S.-H.I.; O’Kane, G.M.; O’Reilly, E.M.; Umemoto, K.; Duruisseaux, M.; Neuzillet, C.; et al. Efficacy and safety of zenocutuzumab, a HER2 x HER3 bispecific antibody, across advanced NRG1 fusion (NRG1+) cancers. J. Clin. Oncol. 2022, 40, 105. [Google Scholar] [CrossRef]
- Kariolis, M.S.; Wells, R.C.; Getz, J.A.; Kwan, W.; Mahon, C.S.; Tong, R.; Kim, D.J.; Srivastava, A.; Bedard, C.; Henne, K.R.; et al. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci. Transl. Med. 2020, 12, eaay1359. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Zhao, Y.; Kang, X.; Zhao, P.; Fu, X.; Mo, X.; Wang, Y.; Huang, Y. BBB-penetrating codelivery liposomes treat brain metastasis of non-small cell lung cancer with EGFR(T790M) mutation. Theranostics 2020, 10, 6122–6135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Y.; Park, J.; Liu, X.; Hu, Y.; Wang, T.; McFarland, K.; Betenbaugh, M.J. Design and Production of Bispecific Antibodies. Antibodies 2019, 8, 43. [Google Scholar] [CrossRef]
- Wang, L.; Hoseini, S.S.; Xu, H.; Ponomarev, V.; Cheung, N.K. Silencing Fc Domains in T cell-Engaging Bispecific Antibodies Improves T-cell Trafficking and Antitumor Potency. Cancer Immunol. Res. 2019, 7, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Ellerman, D. Bispecific T-cell engagers: Towards understanding variables influencing the in vitro potency and tumor selectivity and their modulation to enhance their efficacy and safety. Methods 2019, 154, 102–117. [Google Scholar] [CrossRef]
- Middelburg, J.; Kemper, K.; Engelberts, P.J.; Labrijn, A.F.; Schuurman, J.; van Hall, T. Overcoming Challenges for CD3-Bispecific Antibody Therapy in Solid Tumors. Cancers 2021, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A. Abstract IAP0301: Bispecific T cell engagers (TCEs) for treatment of solid tumors: Challenges and opportunities. Mol. Cancer Ther. 2021, 20, IAP0301. [Google Scholar] [CrossRef]
- Lin, S.J.; Rocha, S.S.; Kwant, K.; Dayao, M.R.; Ng, T.M.; Banzon, R.R.; Thothathri, S.; Aaron, W.; Callihan, E.; Hemmati, G.; et al. Abstract 933: ProTriTAC is a modular and robust T cell engager prodrug platform with therapeutic index expansion observed across multiple tumor targets. Cancer Res. 2021, 81, 933. [Google Scholar] [CrossRef]
- Weidle, U.H.; Maisel, D.; Klostermann, S.; Schiller, C.; Weiss, E.H. Intracellular proteins displayed on the surface of tumor cells as targets for therapeutic intervention with antibody-related agents. Cancer Genom. Proteom. 2011, 8, 49–63. [Google Scholar]
- Thura, M.; Al-Aidaroos, A.Q.O.; Yong, W.P.; Kono, K.; Gupta, A.; Lin, Y.B.; Mimura, K.; Thiery, J.P.; Goh, B.C.; Tan, P.; et al. PRL3-zumab, a first-in-class humanized antibody for cancer therapy. JCI Insight 2016, 1, e87607. [Google Scholar] [CrossRef]
- Hsiue, E.H.; Wright, K.M.; Douglass, J.; Hwang, M.S.; Mog, B.J.; Pearlman, A.H.; Paul, S.; DiNapoli, S.R.; Konig, M.F.; Wang, Q.; et al. Targeting a neoantigen derived from a common TP53 mutation. Science 2021, 371, eabc8697. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Van Tine, B.A.; Biswas, S.; McAlpine, C.; Johnson, M.L.; Olszanski, A.J.; Clarke, J.M.; Araujo, D.; Blumenschein, G.R., Jr.; Kebriaei, P.; et al. Autologous T cell therapy for MAGE-A4(+) solid cancers in HLA-A*02(+) patients: A phase 1 trial. Nat. Med. 2023, 29, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-M.; Kim, J.-S.; Park, S.-W.; Jun, S.-Y.; Kweon, H.-J.; Choi, D.-K.; Lee, D.; Cho, Y.B.; Kim, Y.-S. Direct targeting of oncogenic RAS mutants with a tumor-specific cytosol-penetrating antibody inhibits RAS mutant–driven tumor growth. Sci. Adv. 2020, 6, eaay2174. [Google Scholar] [CrossRef] [PubMed]
- Sosman, J.A.; Carrillo, C.; Urba, W.J.; Flaherty, L.; Atkins, M.B.; Clark, J.I.; Dutcher, J.; Margolin, K.A.; Mier, J.; Gollob, J.; et al. Three phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma. J. Clin. Oncol. 2008, 26, 2292–2298. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Nathan, P.; Sacco, J.J.; Orloff, M.; Hernandez-Aya, L.F.; Yang, J.; Luke, J.J.; Butler, M.O.; Stanhope, S.; Collins, L.; et al. Phase I Study of Safety, Tolerability, and Efficacy of Tebentafusp Using a Step-Up Dosing Regimen and Expansion in Patients With Metastatic Uveal Melanoma. J. Clin. Oncol. 2022, 40, 1939–1948. [Google Scholar] [CrossRef]
- Middleton, M.R.; McAlpine, C.; Woodcock, V.K.; Corrie, P.; Infante, J.R.; Steven, N.M.; Evans, T.R.J.; Anthoney, A.; Shoushtari, A.N.; Hamid, O.; et al. Tebentafusp, A TCR/Anti-CD3 Bispecific Fusion Protein Targeting gp100, Potently Activated Antitumor Immune Responses in Patients with Metastatic Melanoma. Clin. Cancer Res. 2020, 26, 5869–5878. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Linke, R.; Klein, A.; Seimetz, D. Catumaxomab: Clinical development and future directions. mAbs 2010, 2, 129–136. [Google Scholar] [CrossRef]
- Borlak, J.; Länger, F.; Spanel, R.; Schöndorfer, G.; Dittrich, C. Immune-mediated liver injury of the cancer therapeutic antibody catumaxomab targeting EpCAM, CD3 and Fcγ receptors. Oncotarget 2016, 7, 28059–28074. [Google Scholar] [CrossRef]
- Hummel, H.-D.; Kufer, P.; Grüllich, C.; Deschler-Baier, B.; Chatterjee, M.; Goebeler, M.-E.; Miller, K.; Santis, M.D.; Loidl, W.C.; Buck, A.; et al. Phase 1 study of pasotuxizumab (BAY 2010112), a PSMA-targeting Bispecific T cell Engager (BiTE) immunotherapy for metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2019, 37, 5034. [Google Scholar] [CrossRef]
- Tran, B.; Horvath, L.G.; Dorff, T.B.; Greil, R.; Machiels, J.-P.H.; Roncolato, F.T.; Autio, K.A.; Rettig, M.B.; Fizazi, K.; Lolkema, M.P.; et al. Phase I study of AMG 160, a half-life extended bispecific T-cell engager (HLE BiTE) immune therapy targeting prostate-specific membrane antigen (PSMA), in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, TPS5590. [Google Scholar] [CrossRef]
- ESMO Virtual Congress 2020: Novel Immunotherapy for Prostate Cancer—AMG 160—PSMA-Targeted, Bispecific T-Cell Engager (BiTE®) Immune Therapy for Metastatic Castration-Resistant Prostate Cancer—Invited Discussan. Available online: https://www.urotoday.com/conference-highlights/esmo-2020/prostate-cancer/124635-esmo-virtual-congress-2020-novel-immunotherapy-for-prostate-cancer-amg-160-psma-targeted-bispecific-t-cell-engager-bite-immune-therapy-for-metastatic-castration-resistant-prostate-cancer-invited-discussant.html (accessed on 14 May 2023).
- Tolcher, A.W.; Gordon, M.; Mahoney, K.M.; Seto, A.; Zavodovskaya, M.; Hsueh, C.H.; Zhai, S.; Tarnowski, T.; Jürgensmeier, J.M.; Stinson, S.; et al. Phase 1 first-in-human study of dalutrafusp alfa, an anti-CD73-TGF-β-trap bifunctional antibody, in patients with advanced solid tumors. J. Immunother. Cancer 2023, 11, e005267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Chen, X.; Dalton, R.; Calescibetta, A.; So, T.; Gilvary, D.; Ward, G.; Smith, V.; Eckard, S.; Fox, J.A.; et al. Immunodepletion of MDSC by AMV564, a novel bivalent, bispecific CD33/CD3 T cell engager, ex vivo in MDS and melanoma. Mol. Ther. 2022, 30, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Mettu, N.B.; Starodub, A.; Piha-Paul, S.A.A.; Abdul-Karim, R.M.; Tinoco, G.; Shafique, M.R.; Smith, V.; Baccei, C.; Chun, P.Y. Results of a phase 1 dose-escalation study of AMV564, a novel T-cell engager, alone and in combination with pembrolizumab in patients with relapsed/refractory solid tumors. J. Clin. Oncol. 2021, 39, 2555. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, H.; Cao, J.; Zhang, C.; Liu, H.; Huang, H.; Cheng, H.; Qiao, J.; Wang, Y.; Wang, Y.; et al. Characteristics and Risk Factors of Cytokine Release Syndrome in Chimeric Antigen Receptor T Cell Treatment. Front. Immunol. 2021, 12, 611366. [Google Scholar] [CrossRef]
- Weddell, J. Mechanistically modeling peripheral cytokine dynamics following bispecific dosing in solid tumors. CPT Pharmacomet. Syst. Pharmacol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Levy, M.Y.; Dalovisio, A.P.; Bahlis, N.J.; Solh, M.; Sebag, M.; Jakubowiak, A.; Jethava, Y.S.; Costello, C.L.; Chu, M.P.; et al. Preliminary Safety, Efficacy, Pharmacokinetics, and Pharmacodynamics of Subcutaneously (SC) Administered PF-06863135, a B-Cell Maturation Antigen (BCMA)-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 8–9. [Google Scholar] [CrossRef]
- Selvaggio, G.; Parolo, S.; Bora, P.; Leonardelli, L.; Harrold, J.; Mehta, K.; Rock, D.A.; Marchetti, L. Computational Analysis of Cytokine Release Following Bispecific T-Cell Engager Therapy: Applications of a Logic-Based Model. Front. Oncol. 2022, 12, 818641. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Teachey, D.T. Spotlight on Tocilizumab in the Treatment of CAR-T-Cell-Induced Cytokine Release Syndrome: Clinical Evidence to Date. Ther. Clin. Risk Manag. 2020, 16, 705–714. [Google Scholar] [PubMed]
- Gust, J.; Hay, K.A.; Hanafi, L.A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, O.O.; Bouabdallah, K.; Muñoz, J.; De Guibert, S.; Vose, J.M.; Bartlett, N.L.; Lin, Y.; Deol, A.; McSweeney, P.A.; Goy, A.H.; et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br. J. Haematol. 2021, 194, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Lum, L.G.; Al-Kadhimi, Z.; Deol, A.; Kondadasula, V.; Schalk, D.; Tomashewski, E.; Steele, P.; Fields, K.; Giroux, M.; Liu, Q.; et al. Phase II clinical trial using anti-CD3 × anti-HER2 bispecific antibody armed activated T cells (HER2 BATs) consolidation therapy for HER2 negative (0-2+) metastatic breast cancer. J. Immunother. Cancer 2021, 9, e002194. [Google Scholar] [CrossRef] [PubMed]
- Sternjak, A.; Lee, F.; Thomas, O.; Balazs, M.; Wahl, J.; Lorenczewski, G.; Ullrich, I.; Muenz, M.; Rattel, B.; Bailis, J.M.; et al. Preclinical Assessment of AMG 596, a Bispecific T-cell Engager (BiTE) Immunotherapy Targeting the Tumor-specific Antigen EGFRvIII. Mol. Cancer Ther. 2021, 20, 925–933. [Google Scholar] [CrossRef]
- Chao, J.; Buxó, E.; Cervantes, A.; Dayyani, F.; Lima, C.M.S.P.R.; Greil, R.; Laarhoven, H.W.M.V.; Lorenzen, S.; Heinemann, V.; Kischel, R.; et al. Trial in progress: A phase I study of AMG 199, a half-life extended bispecific T-cell engager (HLE BiTE) immune therapy, targeting MUC17 in patients with gastric and gastroesophageal junction (G/GEJ) cancer. J. Clin. Oncol. 2020, 38, TPS4649. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Yu, H.; Tang, Y. Efficacy and safety of MUC1 targeted CIK cells for the treatment of advanced liver cancer. J. Clin. Oncol. 2021, 39, e16278. [Google Scholar] [CrossRef]
- Wermke, M.; Felip, E.; Gambardella, V.; Kuboki, Y.; Morgensztern, D.; Oum’Hamed, Z.; Geng, J.; Studeny, M.; Owonikoko, T.K. A phase I, open-label, dose-escalation trial of BI 764532, a DLL3/CD3 bispecific antibody, in patients (pts) with small cell lung carcinoma (SCLC) or other neuroendocrine neoplasms expressing DLL3. J. Clin. Oncol. 2021, 39, TPS8588. [Google Scholar] [CrossRef]
- Danila, D.C.; Waterhouse, D.M.; Appleman, L.J.; Pook, D.W.; Matsubara, N.; Dorff, T.B.; Lee, J.-L.; Armstrong, A.J.; Kim, M.; Horvath, L.; et al. A phase 1 study of AMG 509 in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2022, 40, TPS5101. [Google Scholar] [CrossRef]
- Heiss, M.M.; Murawa, P.; Koralewski, P.; Kutarska, E.; Kolesnik, O.O.; Ivanchenko, V.V.; Dudnichenko, A.S.; Aleknaviciene, B.; Razbadauskas, A.; Gore, M.; et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int. J. Cancer 2010, 127, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Chao, J.; Buxò, E.; van Laarhoven, H.; Lima, C.; Lorenzen, S.; Dayyani, F.; Heinemann, V.; Greil, R.; Stienen, S. 1496TiP Phase I study evaluating safety and tolerability of AMG 910, a half-life extended bispecific T cell engager targeting claudin-18.2 (CLDN18. 2) in gastric and gastroesophageal junction (G/GEJ) adenocarcinoma. Ann. Oncol. 2020, 31, S928–S929. [Google Scholar] [CrossRef]
- de Souza, J.E.; Galante, P.A.; de Almeida, R.V.; da Cunha, J.P.; Ohara, D.T.; Ohno-Machado, L.; Old, L.J.; de Souza, S.J. SurfaceomeDB: A cancer-orientated database for genes encoding cell surface proteins. Cancer Immun. 2012, 12, 15. [Google Scholar] [PubMed]
- Malekzadeh, P.; Pasetto, A.; Robbins, P.F.; Parkhurst, M.R.; Paria, B.C.; Jia, L.; Gartner, J.J.; Hill, V.; Yu, Z.; Restifo, N.P.; et al. Neoantigen screening identifies broad TP53 mutant immunogenicity in patients with epithelial cancers. J. Clin. Investig. 2019, 129, 1109–1114. [Google Scholar] [CrossRef]
- Cattaruzza, F.; Nazeer, A.; Lange, Z.; Hammond, M.; Koski, C.; Henkensiefken, A.; Derynck, M.K.; Irving, B.; Schellenberger, V. Abstract 3376: HER2-XPAT and EGFR-XPAT: Pro-drug T-cell engagers (TCEs) engineered to address on-target, off-tumor toxicity with potent efficacy in vitro and in vivo and large safety margins in NHP. Cancer Res. 2020, 80, 3376. [Google Scholar] [CrossRef]

| Target Antigen | Specific Toxicity |
|---|---|
| EPCAM | Immune-mediated hepatotoxicity |
| HER2 | Hypotension, hypertension, tachycardia |
| PSMA | Hepatotoxicity |
| DLL3 | Pneumonitis |
| Myeloid-derived suppressor cells (MDSC) | Anemia, hypotension, pruritis |
| EGFRvIII | Dermatologic toxicities, SJS, TEN |
| CEA | Hepatotoxicity |
| Target Population | Phase of Study/References | Number of Patients | Results | Clinical Trial Number | |
|---|---|---|---|---|---|
| CD3/HER2 | Advanced HER2-positive breast cancer | Phase 2 [57] | 32 patients, 8 patients had stable disease. | The median OS was 13.1, 15.2 and 12.3 months for the entire group, HER2-HR+ and TNBC patients, respectively. Plan for phase 3. | NCT03272334 |
| EGFRvIII/CD3 (AMG596), (CX-904) EGFR/CD3. | EGFRvIII-positive GBM or malignant glioma | Phase 1/1b [58] | Total 14 patients. | 1 partial response, 2 stable disease. | NCT03296696 |
| Tumor expression EGFR | Early phase 1 | Plan for 100 patients | Not published | NCT05387265 | |
| Tyrosinase Related Protein 1 (TYRP1) (RO7293583) | Melanoma | Phase 1 | 20 patients | Not published | NCT04551352 |
| MUC17/CD3 | Advanced gastric, GE junction, CRC and pancreatic (AMG199) | Phase 1 [59] | Total 64 patients. | 13 had PR, 17 SD. CRS > grade 3 occurred in 2% | NCT04117958 |
| Advanced liver cancer | Phase 2 [60] | 11 Patients | Median PFS 4 months, median OS 13.2 months; 5 discontinued treatments due to severe side effects | NCT03146637 | |
| DLL3/CD3 | Small cell lung cancer | Phase 1 [61] | Confirmed partial responses in 20% of patients and duration of response of 8.7 months | Phase 2 ongoing | NCT05060016 |
| CEA (MEDI-565) | Advanced GI cancers | Phase 1 | Total 39 patients, | 11 patients have stable disease as best response. | NCT01284231 |
| PSMA | Prostate cancer | Phase ½ | LAVA-1207 | Not published | NCT05369000 |
| Phase 1 [62] | AMG 509 | Not published | NCT04221542 | ||
| Phase 1 | BAY 2010112 | 47 patients, 12 patients had >50% decrease in PSA | NCT01723475 | ||
| EpCAM | Advanced solid tumors | Solitomab: Phase 1 Catumoximab: Phase 2 [63] | Catumoximab and Solitomab: for malignant ascites both associated with sever toxicities precluding development of Solitomab. | Solitomab DLT in phase-limiting escalation. Catumoximab, withdrawn from market due to toxicities. | NCT00635596 NCT00836654 |
| Myeloid-derived suppressor cells (MDSC) | Advanced solid tumors. with and without pembrolizumab | Phase 1 | 20 patients in monotherapy arm. 10 in combination arm. | Not fully published, study mentioned One CR. Study is going to phase 2. | NCT04128423 |
| CLDN18.2 (AMG 910) | Gastric and gastroesophageal junction (G/GEJ) adenocarcinoma | Phase 1 [64] | Plan recruitment 34 patients | Not finished | (NCT04260191) |
| HLA-G | Advanced solid tumors | Phase 1 | Actively recruiting | Not finished | NCT04991740 |
| T-Cell Engagers | CAR-T | |
|---|---|---|
| Basic Structure | Bispecific antibody that binds TAA and CD3 on T-cells | Engineered T-cell that express engineered scFV fused to linker and activation domain |
| Source of T-cells | Endogenous T-cell activation | Requires ex vivo expansion of engineered T-cells |
| Availability | Outpatient | Inpatient and only at high-volume medical centers |
| Drug properties | Off the shelf | Must be engineered (2–4 weeks) |
| Dosing | Requires multiple doses, sometimes requires pump | One dose, sometimes multiple dosing if HLA is eliminated |
| Toxicity | Less CRS | Higher CRS and neurotoxicity |
| Lymphodepletion prior to treatment | Not required | Required |
| Operational Cost | High (USD 90,000) per course | Very high (USD 450,000 to 750,000) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanshal, M.; Caimi, P.F.; Adjei, A.A.; Ma, W.W. T-Cell Engagers in Solid Cancers—Current Landscape and Future Directions. Cancers 2023, 15, 2824. https://doi.org/10.3390/cancers15102824
Shanshal M, Caimi PF, Adjei AA, Ma WW. T-Cell Engagers in Solid Cancers—Current Landscape and Future Directions. Cancers. 2023; 15(10):2824. https://doi.org/10.3390/cancers15102824
Chicago/Turabian StyleShanshal, Mohamed, Paolo F. Caimi, Alex A. Adjei, and Wen Wee Ma. 2023. "T-Cell Engagers in Solid Cancers—Current Landscape and Future Directions" Cancers 15, no. 10: 2824. https://doi.org/10.3390/cancers15102824
APA StyleShanshal, M., Caimi, P. F., Adjei, A. A., & Ma, W. W. (2023). T-Cell Engagers in Solid Cancers—Current Landscape and Future Directions. Cancers, 15(10), 2824. https://doi.org/10.3390/cancers15102824






