Impact of Oocyte Extract Supplement on Quality of Life after Hepatectomy for Liver Tumours: A Prospective, Multicentre, Double-Blind Randomized Clinical Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Endpoint
2.3. Definitions
2.4. Eligibility Criteria
2.5. QoL Assessment
2.6. Randomization and Masking
2.7. Surgery
2.8. Data Collection
2.9. Intervention
2.10. Statistical Analysis
3. Results
3.1. Patients
3.2. Surgical Outcomes
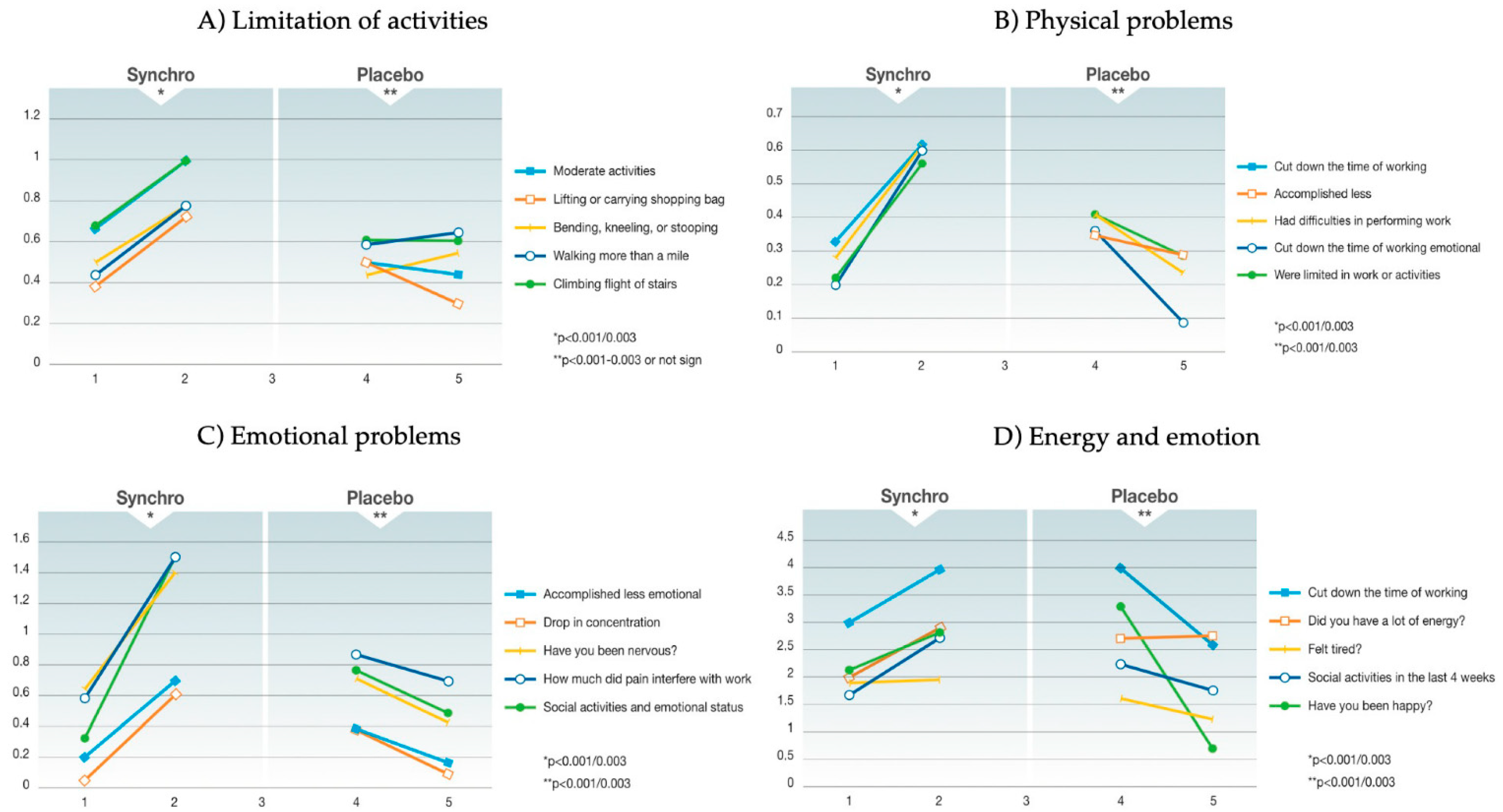
3.3. QoL Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torzilli, G.; Belghiti, J.; Kokudo, N.; Takayama, T.; Capussotti, L.; Nuzzo, G.; Vauthey, J.N.; Choti, M.A.; De Santibanes, E.; Donadon, M.; et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: Is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East-West study group. Ann. Surg. 2013, 257, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, N.; Cillo, U.; Cucchetti, A.; Donadon, M.; Pinna, A.D.; Torzilli, G.; Kokudo, N. Surgery and Hepatocellular Carcinoma. Liver Cancer 2016, 6, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; Fondevila, C.; Donadon, M.; Gringeri, E.; Mocchegiani, F.; Schlitt, H.J.; Ijzermans, J.N.M.; Vivarelli, M.; Zieniewicz, K.; Olde Damink, S.W.M.; et al. Surgery for cholangiocarcinoma. Liver Int. 2019, 39, 143–155. [Google Scholar] [CrossRef]
- Ganz, P.A.; Moinpour, C.M.; Cella, D.F.; Fetting, J.H. Quality-of-life assessment in cancer clinical trials: A status report. J. Natl. Cancer Inst. 1992, 84, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Nayfield, S.G.; Ganz, P.A.; Moinpour, C.M.; Cella, D.F.; Hailey, B.J. Report from a National Cancer Institute (USA) workshop on quality-of-life assessment in cancer clinical trials. Qual. Life Res. 1992, 1, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.; Baum, A.; Carr, B. Quality of life in patients diagnosed with primary hepatocellular carcinoma: Hepatic arterial infusion of Cisplatin versus 90-Yttrium microspheres (Therasphere®). Psychooncology 2004, 13, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Shun, S.C.; Chiou, J.F.; Lai, Y.H.; Yu, P.J.; Wei, L.L.; Tsai, J.T.; Kao, C.Y.; Hsiao, Y.L. Changes in quality of life and its related factors in liver cancer patients receiving stereo- tactic radiation therapy. Support. Care Cancer 2008, 16, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Shun, S.-C.; Chen, C.-H.; Sheu, J.-C.; Liang, J.-D.; Yang, J.-C.; Lai, Y.-H. Quality of life and its associated factors in patients with hepatocellular carcinoma receiving one course o transarterial chemoembolization treatment: A longitudinal study. Oncologist 2012, 17, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Buiting, H.M.; Olthuis, G. Importance of Quality-of-Life Measurement Throughout the Disease Course. JAMA Netw. Open 2020, 3, e200388. [Google Scholar] [CrossRef]
- Toro, A.; Pulvirenti, E.; Palermo, F.; Di Carlo, I. Health-related quality of life in patients with hepatocellular carcinoma after hepatic resection, transcatheter arterial chemoembolization, radiofrequency ablation or no treatment. Surg. Oncol. 2012, 21, e23–e30. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Fan, S.T.; Yu, W.C.; Lam, B.K.-Y.; Chan, F.Y.-S.; Wong, J. A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch. Surg. 2001, 136, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Mise, Y.; Satou, S.; Ishizawa, T.; Kaneko, J.; Aoki, T.; Hasegawa, K.; Sugawara, Y.; Makuuchi, M.; Kokudo, N. Impact of surgery on quality of life in patients with hepatocellular carcinoma. World J. Surg. 2014, 38, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Biava, P.M.; Fiorito, A.; Negro, C.; Mariani, M. Effects of treatment with embryonic and uterine tissue homogenates on Lewis lung carcinoma development. Cancer Lett. 1988, 41, 265–270. [Google Scholar]
- Biava, P.M.; Bonsignorio, D.; Hoxha, M. Mother– embryo cross-talk: The anti-cancer substances produced by mother and embryo during cell differentiation. A review of experimental data. J. Tumor Marker Oncol. 2002, 17, 55–58. [Google Scholar]
- Biava, P.M.; Bonsignorio, D.; Hoxha, M. Cell proliferation curves of different human tumor lines after in vitro treatment with zebrafish embryonic extracts. J. Tumor Marker Oncol. 2001, 16, 195–201. [Google Scholar]
- Biava, P.M.; Bonsignorio, D.; Hoxha, M. Life protecting factor (LPF): An anticancer low molecular weight fraction isolated from pregnant uterine mucosa during embryo organogenesis. J. Tumor Marker Oncol. 2000, 15, 223–233. [Google Scholar]
- Proietti, S.; Cucina, A.; Pensotti, A.; Biava, P.M.; Minini, M.; Monti, N.; Catizone, A.; Ricci, G.; Leonetti, E.; Harrath, A.H.; et al. Active Fraction from Embryo Fish Extracts Induces Reversion of the Malignant Invasive Phenotype in Breast Cancer through Down-regulation of TCTP and Modulation of E-cadherin/β-catenin Pathway. Int. J. Mol. Sci. 2019, 20, 2151. [Google Scholar] [CrossRef]
- Susini, L.; Besse, S.; Duflaut, D.; Lespagnol, A.; Beekman, C.; Fiucci, G.; Atkinson, A.R.; Busso, D.; Poussin, P.; Marine, J.C.; et al. TCTP protects from apoptotic cell death by antagonizing bax function. Cell Death Differ. 2008, 15, 1211–1220. [Google Scholar] [CrossRef]
- Amson, R.; Pece, S.; Lespagnol, A.; Vyas, R.; Mazzarol, G.; Tosoni, D.; Colaluca, I.; Viale, G.; Rodrigues-Ferreira, S.; Wynendaele, J.; et al. Reciprocal repression between P53 and TCTP. Nat. Med. 2011, 18, 91–99. [Google Scholar] [CrossRef]
- Livraghi, T.; Meloni, F.; Frosi, A.; Lazzaroni, S.; Bizzarri, T.M.; Frati, L.; Biava, P.M. Treatment with stem cell differentiation stage factors of intermediate-advanced hepatocellular carcinoma: An open randomized clinical trial. Oncol. Res. 2005, 15, 399–408. [Google Scholar] [CrossRef]
- Livraghi, T.; Ceriani, R.; Palmisano, A.; Pedicini, V.; Pich, M.G.; Tommasini, M.A.; Torzilli, G. Complete response in 5 out of 38 patients with advanced hepatocellular carcinoma treated with stem cell differentiation stage factors: Case reports from a single centre. Curr. Pharm. Biotechnol. 2011, 12, 254–260. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S.; Belghiti, J.; Clavien, P.-A.; Gadzijev, E.; Garden, J.; Lau, W.-Y.; Makuuchi, M.; Strong, R. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000, 2, 333–339. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the international study group of liver surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, E.T.; Feurer, I.D.; Russell, R.T.; Pinson, C.W. Correlation of health-related quality of life after liver transplant with the model for end-stage liver disease score. Arch. Surg. 2009, 144, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Bito, S.; Green, J.; Hsiao, A.; Kurokawa, K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J. Clin. Epidemiol. 1998, 51, 1037–1044. [Google Scholar] [CrossRef]
- Fukuhara, S.; Ware Jr, J.E.; Kosinski, M.; Wada, S.; Gandek, B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J. Clin. Epidemiol. 1998, 51, 045–1053. [Google Scholar] [CrossRef]
- Ware, J.E., Jr. SF-36 Health Survey update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Gandek, B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J. Clin. Epidemiol. 1998, 51, 903–912. [Google Scholar] [CrossRef]
- Kumar, A.; Chakraborty, B.S. Interim analysis: A rational approach of decision making in clinical trial. J. Adv. Pharm. Technol. Res. 2016, 7, 118–122. [Google Scholar] [PubMed]
- Di Maio, M.; Basch, E.; Denis, F.; Fallowfield, L.J.; Ganz, P.A.; Howell, D.; Kowalski, C.; Perrone, F.; Stover, A.M.; Sundaresan, P.; et al. ESMO Guidelines Committee. The role of patient-reported outcom emeasures in the continuum of cancer clinical care: ESMO Clinical Practice Guideline. Ann. Oncol. 2022, 33, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Biava, P.M.; Canaider, S.; Facchin, F.; Bianconi, E.; Ljungberg, L.; Rotilio, D.; Burigana, F.; Ventura, C. Stem Cell Differentiation Stage Factors from Zebrafish Embryo: A Novel Strategy to Modulate the Fate of Normal and Pathological Human (Stem) Cells. Curr. Pharm. Biotechnol. 2015, 16, 782–792. [Google Scholar] [CrossRef]
- D’Anselmi, F.; Valerio, M.; Cucina, A.; Galli, L.; Proietti, S.; Dinicola, S.; Pasqualato, A.; Manetti, C.; Ricci, G.; Giuliani, A.; et al. Metabolism and cell shape in cancer: A fractal analysis. Int. J. Biochem. Cell Biol. 2011, 43, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Cucina, A.; Pensotti, A.; Fuso, A.; Marchese, C.; Nicolini, A.; Bizzarri, M. Tumor reversion and embryo morphogenetic factors. Semin. Cancer Biol. 2022, 79, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Cucina, A.; Catizone, A.; Ricci, G.; Pensotti, A.; Bizzarri, M. Zebrafish embryo extracts enhance 5-FU anti-cancer effects upon breast cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3235–3245. [Google Scholar] [CrossRef]
- Proietti, S.; Cucina, A.; Giuliani, A.; Verna, R.; Palombi, E.; Biava, P.M.; Pensotti, A. Fish protein extract enhances clinical response to salvage chemotherapy in colon cancer patients. Organisms. J. Biol. Sci. 2018, 2, 81–90. [Google Scholar]
- Anselmi, F.; Cucina, A.; Biava, P.M.; Proietti, S.; Coluccia, P.; Frati, L.; Bizzarri, M. Zebrafish stem cell differentiation stage factors suppress Bcl-xL release and enhance 5-Fu-mediated apoptosis in colon cancer cells. Curr Pharm Biotechnol. 2011, 12, 261–267. [Google Scholar] [CrossRef]
- Dong, K.; Zhao, Q.; Xue, Y.; Wei, Y.; Zhang, Y.; Yang, Y. TCTP participates in hepatic metabolism by regulating gene expression involved in insulin resistance. Gene 2021, 768, 145263. [Google Scholar] [CrossRef]
- Zhu, W.L.; Cheng, H.X.; Han, N.; Liu, D.L.; Zhu, W.X.; Fan, B.L.; Duan, F.L. Messenger RNA expression of translationally controlled tumor protein (TCTP) in liver regeneration and cancer. Anticancer Res. 2008, 28, 1575–1580. [Google Scholar]
- Kondo, Y.; Yoshida, H.; Tateishi, R.; Shiina, S.; Mine, N.; Yamashiki, N.; Sato, S.; Kato, N.; Kanai, F.; Yanase, M.; et al. Health-related quality of life of chronic liver disease patients with and without hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2007, 22, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.Y.; Eiser, C.; Ho, M.C. Health-related quality of life in patients with hepatocellular carcinoma: A systematic review. Clin. Gastroenterol. Hepatol. 2010, 8, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, D.; Smith, A.B.; Hamilton-Burke, W.; Prasad, K.R.; Toogood, G.J.; Velikova, G.; Lodge, J.P.A. Quality of life after liver resection for hepatobiliary malignancies. Br. J. Surg. 2008, 95, 845–854. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Full Series | Treatment Group | Placebo Group | p-Value |
|---|---|---|---|---|
| Patient number | 66 | 33 | 33 | - |
| Age | ||||
| Median; range | 70; 27–85 | 68; 27–74 | 69; 61–85 | 0.9144 |
| Sex | ||||
| M | 51 (77.2) | 23 (69.9) | 26 (78.7) | |
| F | 15 (22.8) | 10 (29.1) | 7 (21.3%) | 0.7131 |
| Aetiology | ||||
| Hepatitis C virus | 24 (36.3) | 14 (42.4) | 10 (30.3) | |
| Hepatitis B virus | 7 (10.6) | 3 (9.2) | 4 (12.1) | |
| Alcohol | 9 (13.6) | 5 (15.1) | 4 (12.1) | |
| Negative | 26 (39.5) | 11 (33.3) | 15 (45.5) | 0.6739 |
| Underlying liver | ||||
| Chronic hepatitis or cirrhosis | 22 (33.3) | 9 (27.3) | 13 (39.3) | |
| Normal | 44 (66.7) | 24 (72.7) | 20 (60.7) | 0.2962 |
| Pathology | ||||
| HCC | 55 (83.3%) | 26 (78.7) | 29 (87.8) | |
| iCCA | 11 (16.7%) | 7 (21.2) | 4 (12.2) | 0.3217 |
| Alpha Fetoprotein | ||||
| Median; range | 8; 1–82 | 7; 1–45 | 8; 3–82 | 0.9471 |
| Ca19-9 | ||||
| Median; range | 1.2; 0.8–31 | 2.2; 4–31 | 2.6; 0.8–22 | 0.8719 |
| Platelet count | ||||
| Median; range | 187; 97–282 | 154; 97–127 | 131; 111–282 | 0.7194 |
| CPT score A | 66 (100) | 33 (100) | 33 (100) | - |
| MELD | ||||
| Median; range | 7; 6–17 | 6; 6–11 | 7; 6–17 | 0.9181 |
| Tumour size (cm) | ||||
| Median; range | 4; 1–11 | 4; 1–7 | 3.5; 1–11 | 0.8740 |
| Tumour number | ||||
| Median; range | 1; 1–3 | 1; 1–3 | 1; 1–2 | 0.8981 |
| Vascular Invasion | ||||
| Micro | 35 (53) | 25 (75.7) | 8 (24.2) | |
| Macro | 9 (13.6) | 4 (12.1) | 5 (15.1) | 0.0711 |
| Grading | ||||
| 1–2 | 28 (42.4) | 11 (45.4) | 21 (63.6) | |
| 3–4 | 35 (53.1) | 19 (72.7) | 12 (36.3) | |
| Unknown | 3 (4.5) | 3 (9) | - | 0.0321 |
| Characteristic | Full Series | Treatment Group | Placebo Group | p-Value |
|---|---|---|---|---|
| Extent of hepatectomy | ||||
| Major (>3 segments) | 11 (16.6) | 5 (15.1) | 6 (18.1) | |
| Minor | 55 (83.4) | 28 (84.9) | 27 (81.9) | 0.7411 |
| Approach | ||||
| Open surgery | 56 (84.8) | 25 (75.7) | 31 (94) | |
| Laparoscopic surgery | 10 (15.2) | 8 (24.3) | 2 (6) | 0.0394 |
| Length of operations (minutes) | ||||
| Median; range | 314; 96–654 | 213; 96–234 | 296; 108–654 | 0.9341 |
| Length of Pringle maneuver | ||||
| Median; range | 27; 0–145 | 22; 0–81 | 18; 0–145 | 0.6714 |
| Blood loss (mL) | ||||
| Median; range | 200; 0–1400 | 180; 0–340 | 250; 0–1400 | 0.8713 |
| Red packed cell transfusion | 11 (16.6) | 5 (15.1) | 6 (18.1) | - |
| Postoperative complications | ||||
| Overall | 14 (21.2) | 9 (27.2) | 6 (18.1) | |
| Clavien–Dindo 1–2 | 14 (21.2) | - | - | |
| Clavien–Dindo 3–4 | - | - | - | - |
| Length of stay (day) | ||||
| Median; range | 7; 4–12 | 7; 5–12 | 6; 4–9 | 0.5618 |
| 90-day mortality | - | - | - | - |
| Item | Treatment Group (N = 33) | Placebo Group (N = 33) | ||||
|---|---|---|---|---|---|---|
| Baseline | At 6 Months | p-Value | Baseline | At 6 Months | p-Value | |
| General health | 2.76 ± 0.56 | 2.94 ± 0.66 | 0.1530 | 3.33 ± 1.02 | 2.89 ± 0.91 | 0.0281 |
| General health, compared to one year ago | 3.00 ± 0.94 | 3.94 ± 1.09 | <0.001 | 4.06 ± 0.98 | 2.61 ± 1.11 | <0.001 |
| Limitation of activities | ||||||
| Vigorous activities | 1.22 ± 0.62 | 1.44 ± 0.65 | 0.0930 | 0.94 ± 0.77 | 0.75 ± 0.86 | 0.2570 |
| Moderate activities | 0.67 ± 0.77 | 1.00 ± 0.77 | 0.0384 | 0.5 ± 0.79 | 0.44 ± 0.78 | 0.7089 |
| Lifting or carrying shopping bag | 0.39 ± 0.57 | 0.72 ± 0.61 | 0.0074 | 0.5 ± 0.71 | 0.39 ± 0.78 | 0.4718 |
| Climbing flights of stairs | 0.68 ± 0.49 | 1.00 ± 0.69 | 0.0082 | 0.61 ± 0.78 | 0.61 ± 0.85 | 1 |
| Climbing one flight of stairs | 0.22 ± 0.59 | 0.33 ± 0.43 | 0.2992 | 0.33 ± 0.59 | 0.33 ± 0.69 | 1 |
| Bending, kneeling, or stooping | 0.5 ± 0.73 | 0.78 ± 0.62 | 0.0457 | 0.44 ± 0.7 | 0.5 ± 0.79 | 0.6946 |
| Walking more than a mile | 0.44 ± 0.81 | 0.78 ± 0.62 | 0.0231 | 0.59 ± 0.71 | 0.65 ± 0.86 | 0.7102 |
| Walking several blocks | 0.35 ± 0.61 | 0.35 ± 0.49 | 1 | 0.5 ± 0.62 | 0.33 ± 0.69 | 0.2073 |
| Walking one block | 0.18 ± 0.39 | 0.18 ± 0.39 | 1 | 0.28 ± 0.57 | 0.17 ± 0.51 | 0.3216 |
| Bathing or dressing yourself | 0.06 ± 0.24 | 0.00 ±0.00 | 1 | 0.17 ± 0.38 | 0.11 ± 0.47 | 0.4933 |
| Physical problems | ||||||
| Cut down on the time of working | 0.33 ± 0.5 | 0.61 ± 0.49 | 0.0064 | 0.41 ± 0.51 | 0.24 ± 0.44 | 0.0836 |
| Less accomplishment | 0.22 ± 0.51 | 0.56 ± 0.43 | 0.0006 | 0.35 ± 0.49 | 0.29 ± 0.47 | 0.5419 |
| Limitation in work or activities | 0.22 ± 0.51 | 0.56 ± 0.43 | 0.0006 | 0.41 ± 0.51 | 0.29 ± 0.47 | 0.2336 |
| Difficulties in performing work | 0.28 ± 0.5 | 0.61 ± 0.46 | 0.0011 | 0.41 ± 0.51 | 0.24 ± 0.44 | 0.2336 |
| Emotional problems | ||||||
| Cut down on the time of working | 0.20 ± 0.52 | 0.60 ± 0.42 | <0.001 | 0.36 ± 0.5 | 0.09 ± 0.3 | 0.0018 |
| Less accomplishment | 0.22 ± 0.46 | 0.71 ± 0.43 | <0.001 | 0.41 ± 0.51 | 0.18 ± 0.39 | 0.0148 |
| Drop in concentration | 0.06 ± 0.5 | 0.61 ± 0.24 | <0.001 | 0.39 ± 0.5 | 0.11 ± 0.32 | 0.0015 |
| Social activities and emotional status | 0.33 ± 0.79 | 1.51 ± 0.69 | <0.001 | 0.78 ± 1.06 | 0.5 ± 0.92 | 0.1702 |
| Pain in the last 4 weeks | 1.41 ± 1.33 | 1.12 ± 1.27 | 0.2774 | 0.72 ± 1.02 | 0.44 ± 1.04 | 0.1862 |
| How much pain interfered with work | 0.65 ± 0.87 | 1.41 ± 0.93 | <0.001 | 0.56 ± 0.86 | 0.39 ± 0.98 | 0.3687 |
| Energy and emotion in the last 4 weeks | ||||||
| Full of pep? | 2.38 ± 1.31 | 2.75 ± 1 | 0.1232 | 3.17 ± 1.49 | 2.28 ± 1.38 | 0.0031 |
| Have you been nervous? | 0.59 ± 1.1 | 1.5 ± 0.48 | <0.001 | 0.88 ± 0.93 | 0.71 ± 1.26 | 0.4539 |
| Felt down? | 0.88 ± 1.02 | 0.69 ± 1.01 | 0.3615 | 0.59 ± 0.87 | 0.53 ± 0.87 | 0.7362 |
| Felt calm and peaceful? | 2.69 ± 1.01 | 3 ± 1.26 | 0.1742 | 3.47 ± 1.5 | 3.24 ± 1.71 | 0.4853 |
| Did you have a lot of energy? | 2 ± 1.26 | 2.88 ± 1.02 | 0.0003 | 2.71 ± 1.71 | 2.65 ± 1.46 | 0.8542 |
| Felt downhearted? | 1.24 ± 0.75 | 1.06 ± 1.25 | 0.3945 | 0.94 ± 0.9 | 0.59 ± 0.8 | 0.0469 |
| Did you feel worn out? | 0.81 ± 1.05 | 0.63 ± 1.09 | 0.4120 | 0.59 ± 0.71 | 0.76 ± 0.9 | 0.3069 |
| Have you been happy? | 2.13 ± 1.19 | 2.8 ± 1.21 | 0.0041 | 3.29 ± 1.31 | 0.71 ± 1.16 | 0.0239 |
| Felt tired? | 1.88 ± 0.78 | 1.94 ± 0.97 | <0.001 | 1.65 ± 1.27 | 1.24 ± 1.2 | 0.1074 |
| Social activities in the last 4 weeks | 1.65 ± 1.11 | 2.71 ± 0.69 | <0.001 | 2.24 ± 0.9 | 1.76 ± 1.03 | 0.0169 |
| General health | ||||||
| I seem to get sick easier than others | 2.24 ± 1.37 | 2.54 ± 1.2 | 0.4359 | 2.25 ± 1.39 | 1.75 ± 0.93 | 0.0411 |
| I am as healthy as anybody | 2.94 ± 1.34 | 3 ± 1.46 | 0.8343 | 3 ± 1.1 | 2.94 ± 1.44 | 0.8191 |
| I expect my health to get worse | 2.59 ± 1.33 | 2.47 ± 1.28 | 0.6535 | 2.81 ± 1.42 | 2.19 ± 0.98 | 0.0145 |
| My health is excellent | 2.81 ± 1.38 | 3.19 ± 1.33 | 0.1728 | 3.56 ± 1.31 | 2.88 ± 1.09 | 0.0069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donadon, M.; Palmisano, A.; Bizzarri, M.; Ceriani, R.; Veneroni, L.; Donati, G.; Tassinari, D.; Viola, M.G.; Tamburini, E.; Torzilli, G. Impact of Oocyte Extract Supplement on Quality of Life after Hepatectomy for Liver Tumours: A Prospective, Multicentre, Double-Blind Randomized Clinical Trial. Cancers 2023, 15, 2809. https://doi.org/10.3390/cancers15102809
Donadon M, Palmisano A, Bizzarri M, Ceriani R, Veneroni L, Donati G, Tassinari D, Viola MG, Tamburini E, Torzilli G. Impact of Oocyte Extract Supplement on Quality of Life after Hepatectomy for Liver Tumours: A Prospective, Multicentre, Double-Blind Randomized Clinical Trial. Cancers. 2023; 15(10):2809. https://doi.org/10.3390/cancers15102809
Chicago/Turabian StyleDonadon, Matteo, Angela Palmisano, Mariano Bizzarri, Roberto Ceriani, Luigi Veneroni, Gabriele Donati, Davide Tassinari, Massimo Giuseppe Viola, Emiliano Tamburini, and Guido Torzilli. 2023. "Impact of Oocyte Extract Supplement on Quality of Life after Hepatectomy for Liver Tumours: A Prospective, Multicentre, Double-Blind Randomized Clinical Trial" Cancers 15, no. 10: 2809. https://doi.org/10.3390/cancers15102809
APA StyleDonadon, M., Palmisano, A., Bizzarri, M., Ceriani, R., Veneroni, L., Donati, G., Tassinari, D., Viola, M. G., Tamburini, E., & Torzilli, G. (2023). Impact of Oocyte Extract Supplement on Quality of Life after Hepatectomy for Liver Tumours: A Prospective, Multicentre, Double-Blind Randomized Clinical Trial. Cancers, 15(10), 2809. https://doi.org/10.3390/cancers15102809









