Alpha-Fetoprotein Response after First Transarterial Chemoembolization (TACE) and Complete Pathologic Response in Patients with Hepatocellular Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
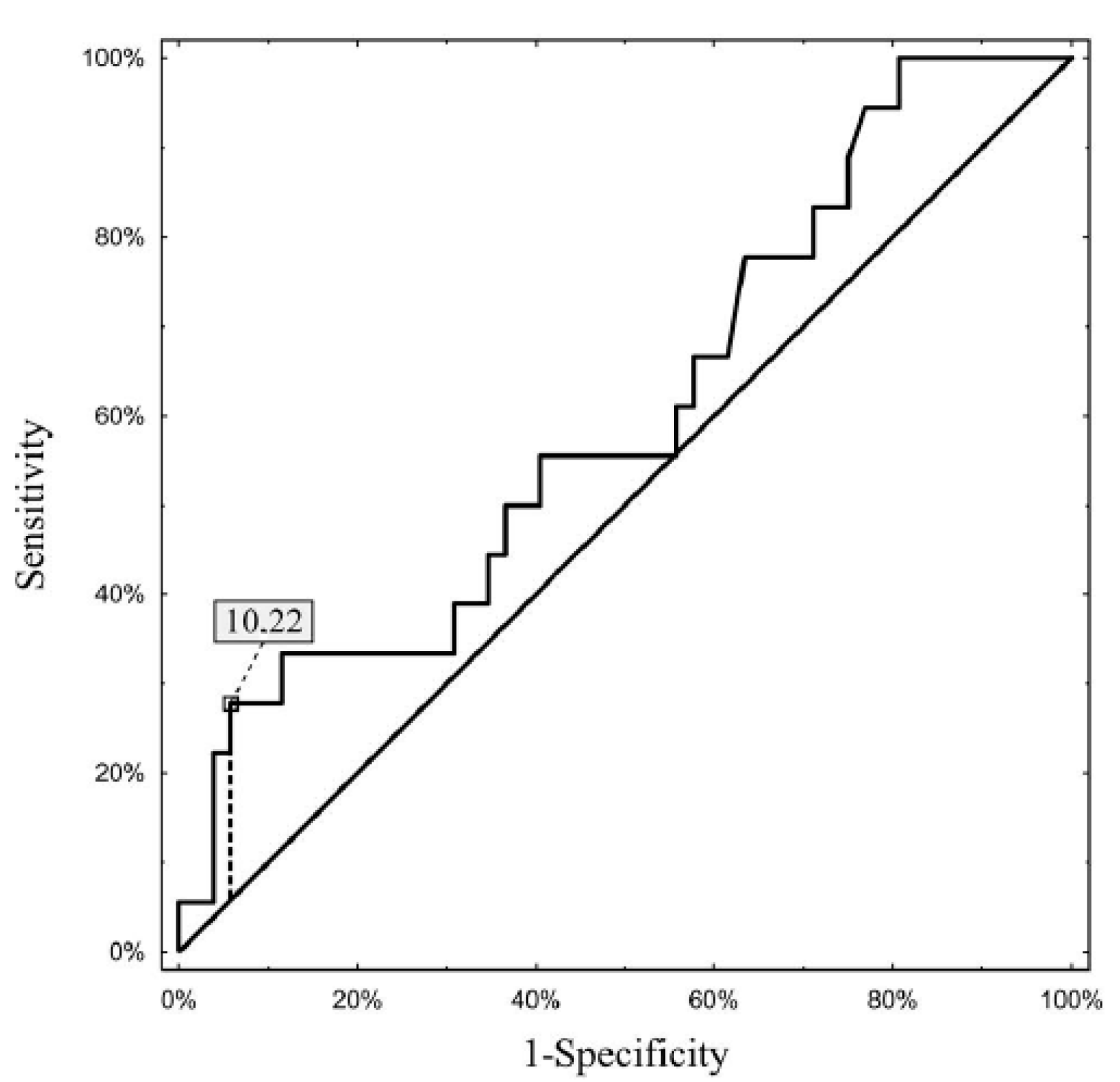
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venook, A.P.; Papandreou, C.; Furuse, J.; de Guevara, L.L. The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist 2010, 15 (Suppl. 4), 5–13. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, L.Y.; Cruz-Ramón, V.; Chinchilla-López, P.; Torres, H.A.; LoConte, N.K.; Rice, J.P.; Foxhall, L.E.; Sturgis, E.M.; Merrill, J.K.; Bailey, H.H.; et al. Global epidemiology, prevention, and management of hepatocellular carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 262–279. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Vitale, A.; Iesari, S.; Finkenstedt, A.; Mennini, G.; Spoletini, G.; Hoppe-Lotichius, M.; Vennarecci, G.; Manzia, T.M.; Nicolini, D.; et al. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology 2017, 66, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.D.; Yang, T.; Mazzaferro, V.; De Carlis, L.; Zhou, J.; Roayaie, S.; Shen, F.; Sposito, C.; Cescon, M.; Di Sandro, S.; et al. Liver transplantation and hepatic resection can achieve cure for hepatocellular carcinoma. Ann. Surg. 2018, 268, 868–875. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Costentin, C.E.; Bababekov, Y.J.; Zhu, A.X.; Yeh, H. Is it time to reconsider the Milan Criteria for selecting patients with hepatocellular carcinoma for deceased-donor liver transplantation? Hepatology 2019, 69, 1324–1336. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology 2012, 143, 986–994.e3. [Google Scholar] [CrossRef]
- Grąt, M.; Wronka, K.M.; Stypułkowski, J.; Bik, E.; Krasnodębski, M.; Masior, Ł.; Lewandowski, Z.; Grąt, K.; Patkowski, W.; Krawczyk, M. The Warsaw Proposal for the Use of Extended Selection Criteria in Liver Transplantation for Hepatocellular Cancer. Ann. Surg. Oncol. 2017, 24, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Halazun, K.J.; Tabrizian, P.; Najjar, M.; Florman, S.; Schwartz, M.; Michelassi, F.; Samstein, B.; Brown, R.S., Jr.; Emond, J.C.; Busuttil, R.W.; et al. Is it Time to Abandon the Milan Criteria? Results of a Bicoastal US Collaboration to Redefine Hepatocellular Carcinoma Liver Transplantation Selection Policies. Ann. Surg. 2018, 268, 690–699. [Google Scholar] [CrossRef]
- Toso, C.; Meeberg, G.; Hernandez-Alejandro, R.; Dufour, J.F.; Marotta, P.; Majno, P.; Kneteman, N.M. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015, 62, 158–165. [Google Scholar] [CrossRef]
- Kulik, L.; Heimbach, J.K.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Wang, Z.; Murad, M.H.; Mohammed, K. Therapies for Patients with Hepatocellular Carcinoma Awaiting for Liver Transplantation: A Systematic Review and Meta-analysis. Hepatology 2018, 67, 381–400. [Google Scholar] [CrossRef]
- Di Martino, M.; Vitale, A.; Ferraro, D.; Maniscalco, M.; Pisaniello, D.; Arenga, G.; Falaschi, F.; Terrone, A.; Iacomino, A.; Galeota Lanza, A.; et al. Downstaging Therapies for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation: A Systematic Review and Meta-Analysis on Intention-to-Treat Outcomes. Cancers 2022, 14, 5102. [Google Scholar] [CrossRef]
- Mehta, N.; Frenette, C.; Tabrizian, P.; Hoteit, M.; Guy, J.; Parikh, N.; Ghaziani, T.T.; Dhanasekaran, R.; Dodge, J.L.; Natarajan, B.; et al. Downstaging Outcomes for Hepatocellular Carcinoma: Results from the Multicenter Evaluation of Reduction in Tumor Size before Liver Transplantation (MERITS-LT) Consortium. Gastroenterology 2021, 161, 1502–1512. [Google Scholar] [CrossRef]
- Tabrizian, P.; Holzner, M.L.; Mehta, N.; Halazun, K.; Agopian, V.G.; Yao, F.; Busuttil, R.W.; Roberts, J.; Emond, J.C.; Samstein, B.; et al. Ten-Year Outcomes of Liver Transplant and Downstaging for Hepatocellular Carcinoma. JAMA Surg. 2022, 157, 779–788. [Google Scholar] [CrossRef]
- Agopian, V.G.; Morshedi, M.M.; McWilliams, J.; Harlander-Locke, M.P.; Markovic, D.; Zarrinpar, A.; Kaldas, F.M.; Farmer, D.G.; Yersiz, H.; Hiatt, J.R.; et al. Complete Pathologic Response to Pretransplant Locoregional Therapy for Hepatocellular Carcinoma Defines Cancer Cure After Liver Transplantation Analysis of 501 Consecutively Treated Patients. Ann. Surg. 2015, 262, 536–545; discussion 543–545. [Google Scholar] [CrossRef]
- Grąt, M.; Krawczyk, M.; Stypułkowski, J.; Morawski, M.; Krasnodębski, M.; Wasilewicz, M.; Lewandowski, Z.; Grąt, K.; Patkowski, W.; Zieniewicz, K. Prognostic Relevance of a Complete Pathologic Response in Liver Transplantation for Hepatocellular Carcinoma. Ann. Surg. Oncol. 2019, 26, 4556–4565. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Ouyang, Q.; Xia, F.; Fan, G.; Yu, J.; Zhang, C.; Wang, D. Alpha-fetoprotein response following transarterial chemoembolization indicates improved survival for intermediate-stage hepatocellular carcinoma. HPB 2019, 21, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Allaire, M.; Bruix, J.; Korenjak, M.; Manes, S.; Maravic, Z.; Reeves, H.; Salem, R.; Sangro, B.; Sherman, M. What to do about hepatocellular carcinoma: Recommendations for health authorities from the International Liver Cancer Association. JHEP Rep. 2022, 4, 100578. [Google Scholar] [CrossRef] [PubMed]
- Kardashian, A.; Florman, S.S.; Haydel, B.; Ruiz, R.M.; Klintmalm, G.B.; Lee, D.D.; Taner, C.B.; Aucejo, F.; Tevar, A.D.; Humar, A.; et al. Liver Transplantation Outcomes in a U.S. Multicenter Cohort of 789 Patients with Hepatocellular Carcinoma Presenting Beyond Milan Criteria. Hepatology 2020, 72, 2014–2028. [Google Scholar] [CrossRef]
- Mehta, N.; Dodge, J.L.; Grab, J.D.; Yao, F.Y. National Experience on Down-Staging of Hepatocellular Carcinoma Before Liver Transplant: Influence of Tumor Burden, Alpha-Fetoprotein, and Wait Time. Hepatology 2020, 71, 943–954. [Google Scholar] [CrossRef]
- DiNorcia, J.; Florman, S.S.; Haydel, B.; Tabrizian, P.; Ruiz, R.M.; Klintmalm, G.B.; Senguttuvan, S.; Lee, D.D.; Taner, C.B.; Verna, E.C.; et al. Pathologic Response to Pretransplant Locoregional Therapy is Predictive of Patient Outcome After Liver Transplantation for Hepatocellular Carcinoma. Analysis from the US Multicenter HCC Transplant Consortium. Ann. Surg. 2020, 271, 616–624. [Google Scholar] [CrossRef]
- Agopian, V.G.; Harlander-Locke, M.P.; Ruiz, R.M.; Klintmalm, G.B.; Senguttuvan, S.; Florman, S.S.; Haydel, B.; Hoteit, M.; Levine, M.H.; Lee, D.D.; et al. Impact of Pretransplant Bridging Locoregional Therapy for Patients with Hepatocellular Carcinoma Within Milan Criteria Undergoing Liver Transplantation. Analysis of 3601 Patients from the US Multicenter HCC Transplant Consortium. Ann. Surg. 2017, 266, 525–535. [Google Scholar] [CrossRef]
- Lai, Q.; Vitale, A.; Iesari, S.; Finkenstedt, A.; Mennini, G.; Onali, S.; Hoppe-Lotichius, M.; Manzia, T.M.; Nicolini, D.; Avolio, A.W.; et al. The Intention-to-Treat Effect of Bridging Treatments in the Setting of Milan Criteria–In Patients Waiting for Liver Transplantation. Liver Transpl. 2019, 25, 1023–1033. [Google Scholar] [CrossRef]
- Mishra, G.; Dev, A.; Paul, E.; Cheung, W.; Koukounaras, J.; Jhamb, A.; Marginson, B.; Lim, B.G.; Simkin, P.; Borsaru, A.; et al. Prognostic role of alpha-fetoprotein in patients with hepatocellular carcinoma treated with repeat transarterial chemoembolisation. BMC Cancer 2020, 20, 483. [Google Scholar] [CrossRef]
- Lee, J.S.; Chon, Y.E.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H.; Kang, W.; Choi, M.S.; Gwak, G.Y.; et al. Prognostic Value of Alpha-Fetoprotein in Patients Who Achieve a Complete Response to Transarterial Chemoembolization for Hepatocellular Carcinoma. Yonsei Med. J. 2021, 62, 12–20. [Google Scholar] [CrossRef]
- Golfieri, R.; Cappelli, A.; Cucchetti, A.; Piscaglia, F.; Carpenzano, M.; Peri, E.; Ravaioli, M.; D’Errico-Grigioni, A.; Pinna, A.D.; Bolondi, L. Efficacy of Selective Transarterial Chemoembolization in Inducing Tumor Necrosis in Small (<5 cm) Hepatocellular Carcinomas. Hepatology 2011, 53, 1580–1589. [Google Scholar]
- Terzi, E.; Golfieri, R.; Piscaglia, F.; Galassi, M.; Dazzi, A.; Leoni, S.; Giampalma, E.; Renzulli, M.; Bolondi, L. Response rate and clinical outcome of HCC after first and repeated cTACE performed “on demand”. J. Hepatol. 2012, 57, 1258–1267. [Google Scholar] [CrossRef]
- Gabr, A.; Kulik, L.; Mouli, S.; Riaz, A.; Ali, R.; Desai, K.; Mora, R.A.; Ganger, D.; Maddur, H.; Flamm, S.; et al. Liver Transplantation Following Yttrium-90 Radioembolization: 15-year Experience in 207-Patient Cohort. Hepatology 2021, 73, 998–1010. [Google Scholar] [CrossRef]
- Takayasu, K.; Arii, S.; Ikai, I.; Omata, M.; Okita, K.; Ichida, T.; Matsuyama, Y.; Nakanuma, Y.; Kojiro, M.; Makuuchi, M.; et al. Prospective Cohort Study of Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma in 8510 Patients. Gastroenterology 2006, 131, 461–469. [Google Scholar] [CrossRef]
- Hyun, M.H.; Lee, Y.S.; Kim, J.H.; Lee, C.U.; Jung, Y.K.; Seo, Y.S.; Yim, H.J.; Yeon, J.E.; Byun, K.S. Hepatic Resection Compared to Chemoembolization in Intermediate to Advanced-Stage Hepatocellular Carcinoma: A Meta-Analysis of High-Quality Studies. Hepatology 2018, 68, 977–993. [Google Scholar] [CrossRef]
- Yang, B.; Zheng, B.; Yang, M.; Zeng, Z.; Yang, F.; Pu, J.; Li, C.; Liao, Z. Liver resection versus transarterial chemoembolization for the initial treatment of Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Hepatol. Int. 2018, 12, 417–428. [Google Scholar] [CrossRef]
- Guo, C.; Zou, X.; Hong, Z.; Sun, J.; Xiao, W.; Sun, K.; Li, X.; Shen, Y.; Liang, T.; Bai, X. Preoperative transarterial chemoembolization for barcelona clinic liver cancer stage A/B hepatocellular carcinoma beyond the milan criteria: A propensity score matching analysis. HPB 2021, 23, 1427–1438. [Google Scholar] [CrossRef]

| Median (Range) or n (%) | |
|---|---|
| Recipient age (years) | 58 (27–70) |
| Recipient sex (male) | 79 (78.2%) |
| BMI | 27.4 (25.4–33.8) |
| HBV (n = 100) | 42 (42%) |
| HCV (n = 99) | 59 (59.6%) |
| MELD | 9 (6–22) |
| AFP before first TACE < 100 (ng/mL) | 69 (68.3%) |
| Previous liver resection (n = 88) | 8 (9.1%) |
| Number of tumors | 1.0 (1–10) |
| Size of the largest tumor (mm) | 35 (7–140) |
| Number of TACEs | 2 (1–6) |
| Microvascular invasion (n = 84) | 25 (29.8%) |
| Grading G3 (n = 66) | 8 (12.1%) |
| Milan criteria (n = 84) | 52 (61.9%) |
| Up-to-7 criteria (n = 84) | 61 (72.6%) |
| Complete pathologic response (whole cohort) | 20 (19.8%) |
| Complete pathologic response AFP ≤ 100 (ng/mL, n = 69) | 18 (26.1%) |
| Complete pathologic response AFP > 100 (ng/mL, n = 32) | 2 (6.3%) |
| Data are presented as medians (range) or n (%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masior, Ł.; Krasnodębski, M.; Kuncewicz, M.; Karaban, K.; Jaszczyszyn, I.; Kruk, E.; Małecka-Giełdowska, M.; Korzeniowski, K.; Figiel, W.; Krawczyk, M.; et al. Alpha-Fetoprotein Response after First Transarterial Chemoembolization (TACE) and Complete Pathologic Response in Patients with Hepatocellular Cancer. Cancers 2023, 15, 3962. https://doi.org/10.3390/cancers15153962
Masior Ł, Krasnodębski M, Kuncewicz M, Karaban K, Jaszczyszyn I, Kruk E, Małecka-Giełdowska M, Korzeniowski K, Figiel W, Krawczyk M, et al. Alpha-Fetoprotein Response after First Transarterial Chemoembolization (TACE) and Complete Pathologic Response in Patients with Hepatocellular Cancer. Cancers. 2023; 15(15):3962. https://doi.org/10.3390/cancers15153962
Chicago/Turabian StyleMasior, Łukasz, Maciej Krasnodębski, Mikołaj Kuncewicz, Kacper Karaban, Igor Jaszczyszyn, Emilia Kruk, Milena Małecka-Giełdowska, Krzysztof Korzeniowski, Wojciech Figiel, Marek Krawczyk, and et al. 2023. "Alpha-Fetoprotein Response after First Transarterial Chemoembolization (TACE) and Complete Pathologic Response in Patients with Hepatocellular Cancer" Cancers 15, no. 15: 3962. https://doi.org/10.3390/cancers15153962
APA StyleMasior, Ł., Krasnodębski, M., Kuncewicz, M., Karaban, K., Jaszczyszyn, I., Kruk, E., Małecka-Giełdowska, M., Korzeniowski, K., Figiel, W., Krawczyk, M., Wróblewski, T., & Grąt, M. (2023). Alpha-Fetoprotein Response after First Transarterial Chemoembolization (TACE) and Complete Pathologic Response in Patients with Hepatocellular Cancer. Cancers, 15(15), 3962. https://doi.org/10.3390/cancers15153962







