Incidental Findings in Pediatric Patients: How to Manage Liver Incidentaloma in Pediatric Patients
Abstract
Simple Summary
Abstract
1. Introduction and Definition
2. Definition
2.1. Prevalence, Epidemiology
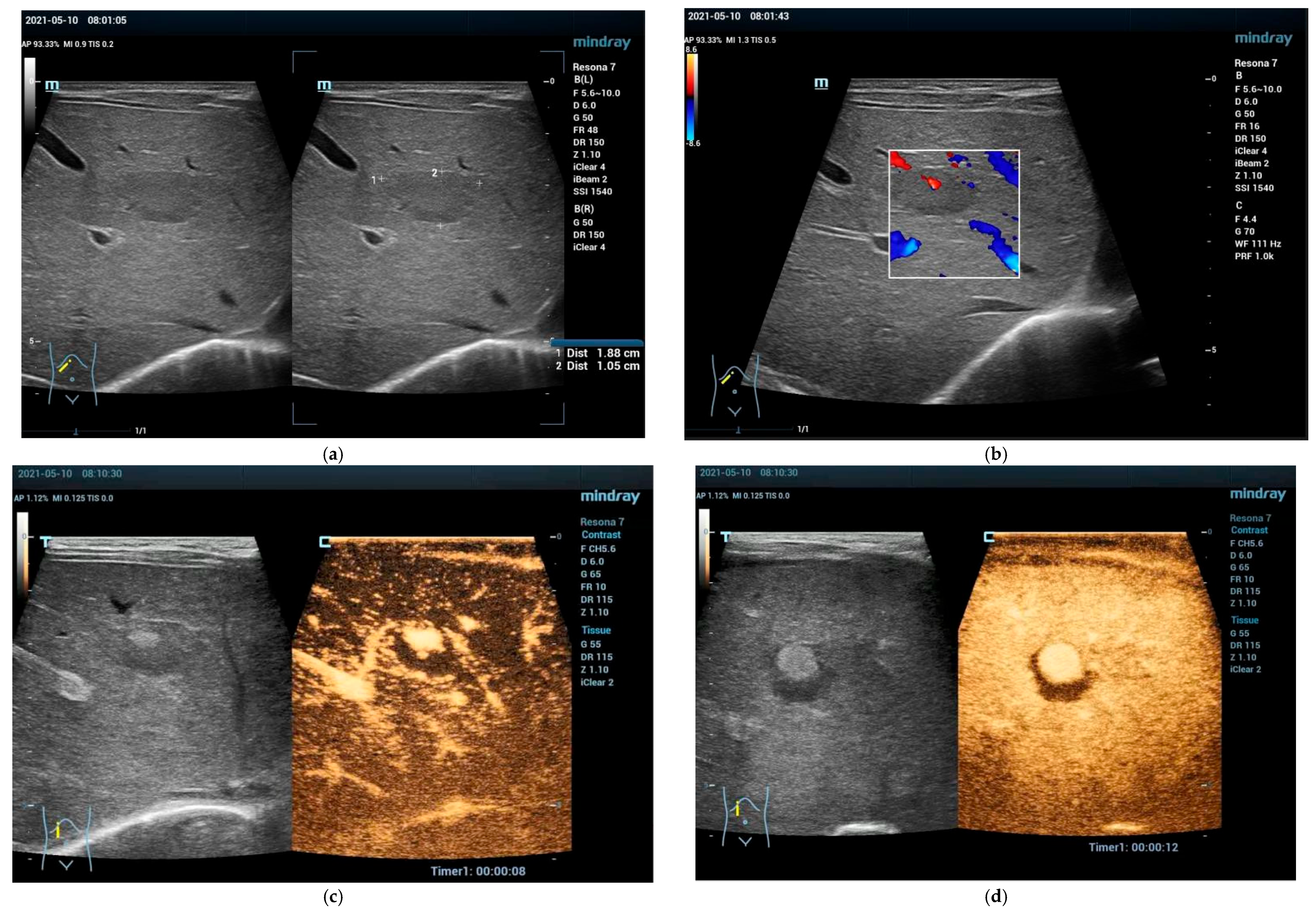
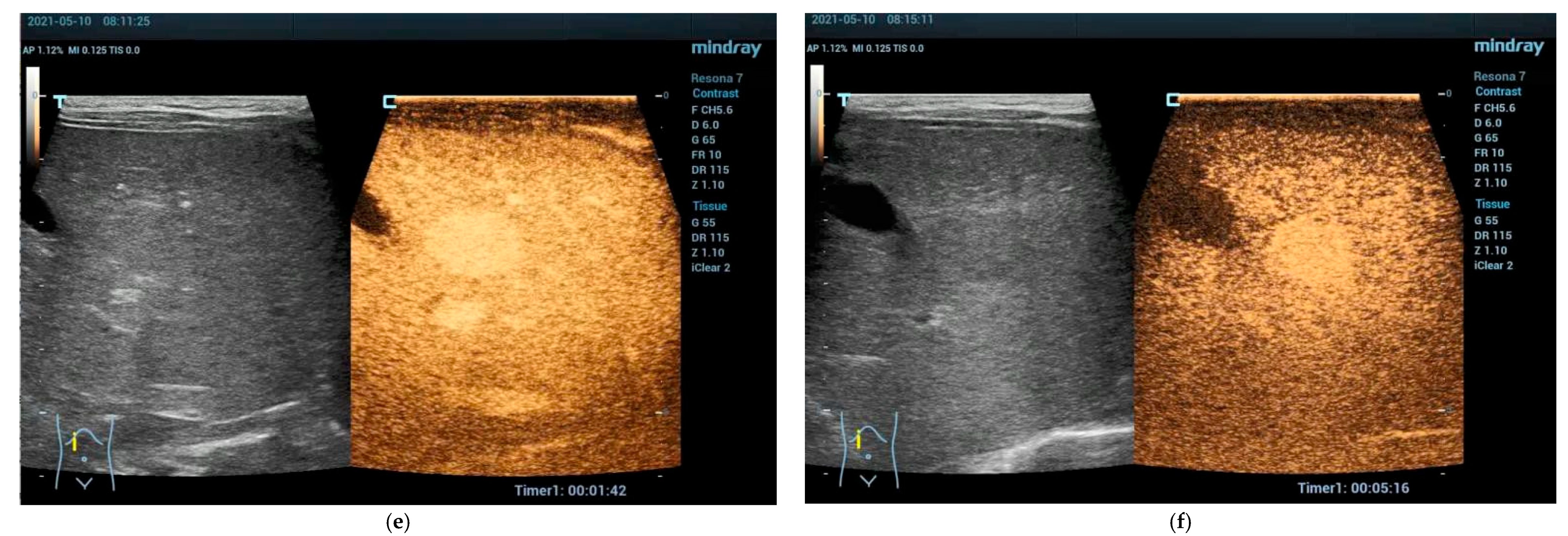
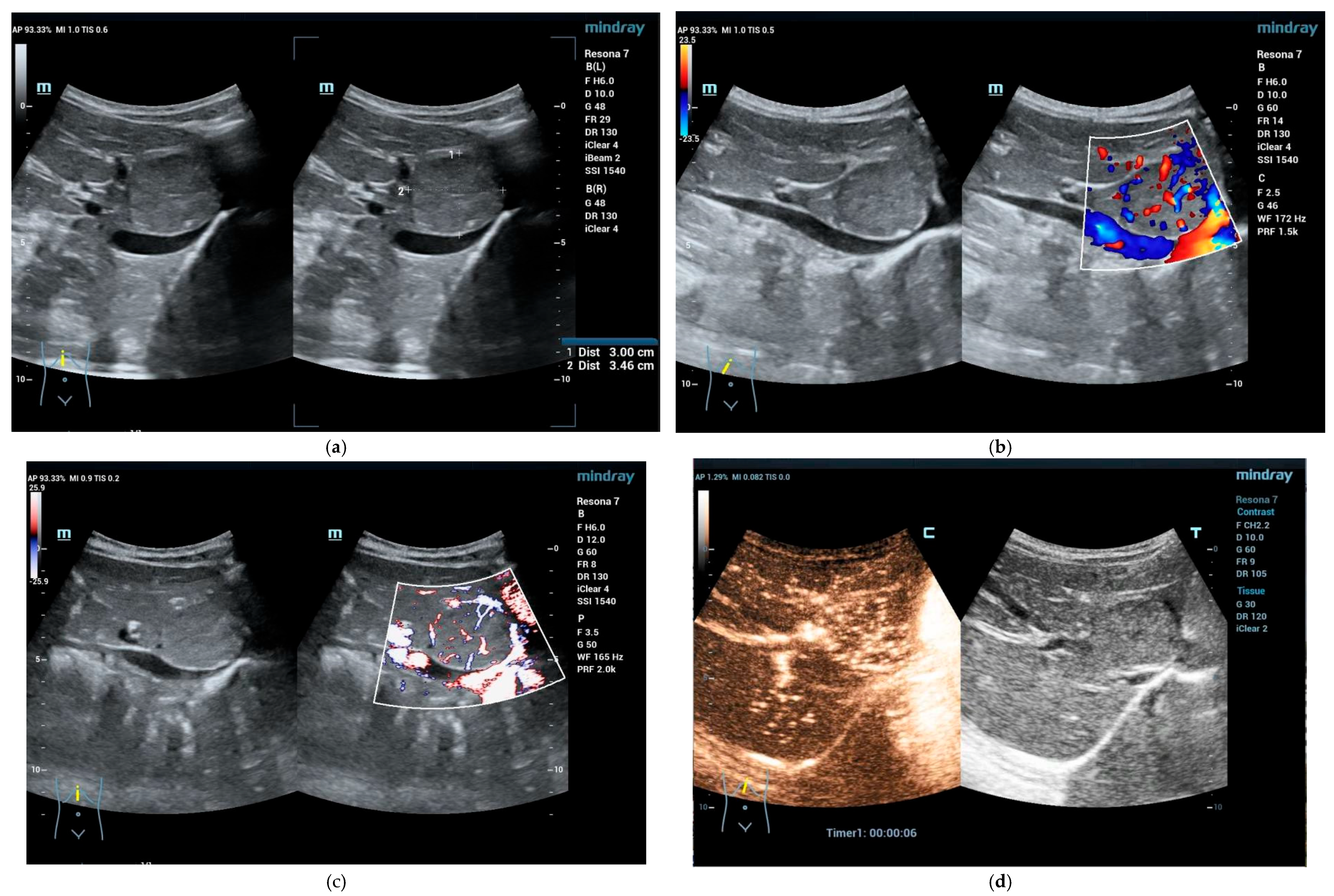
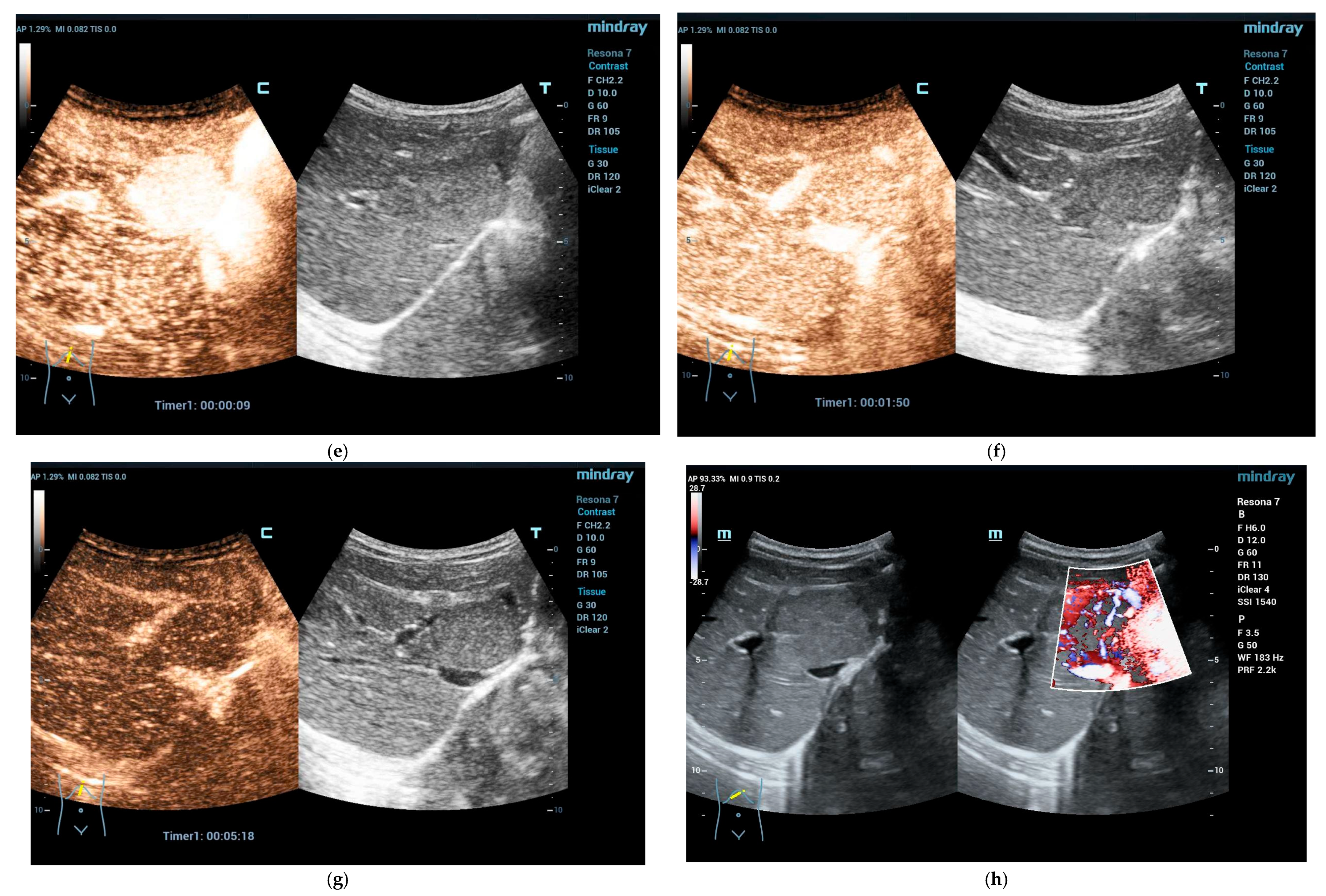
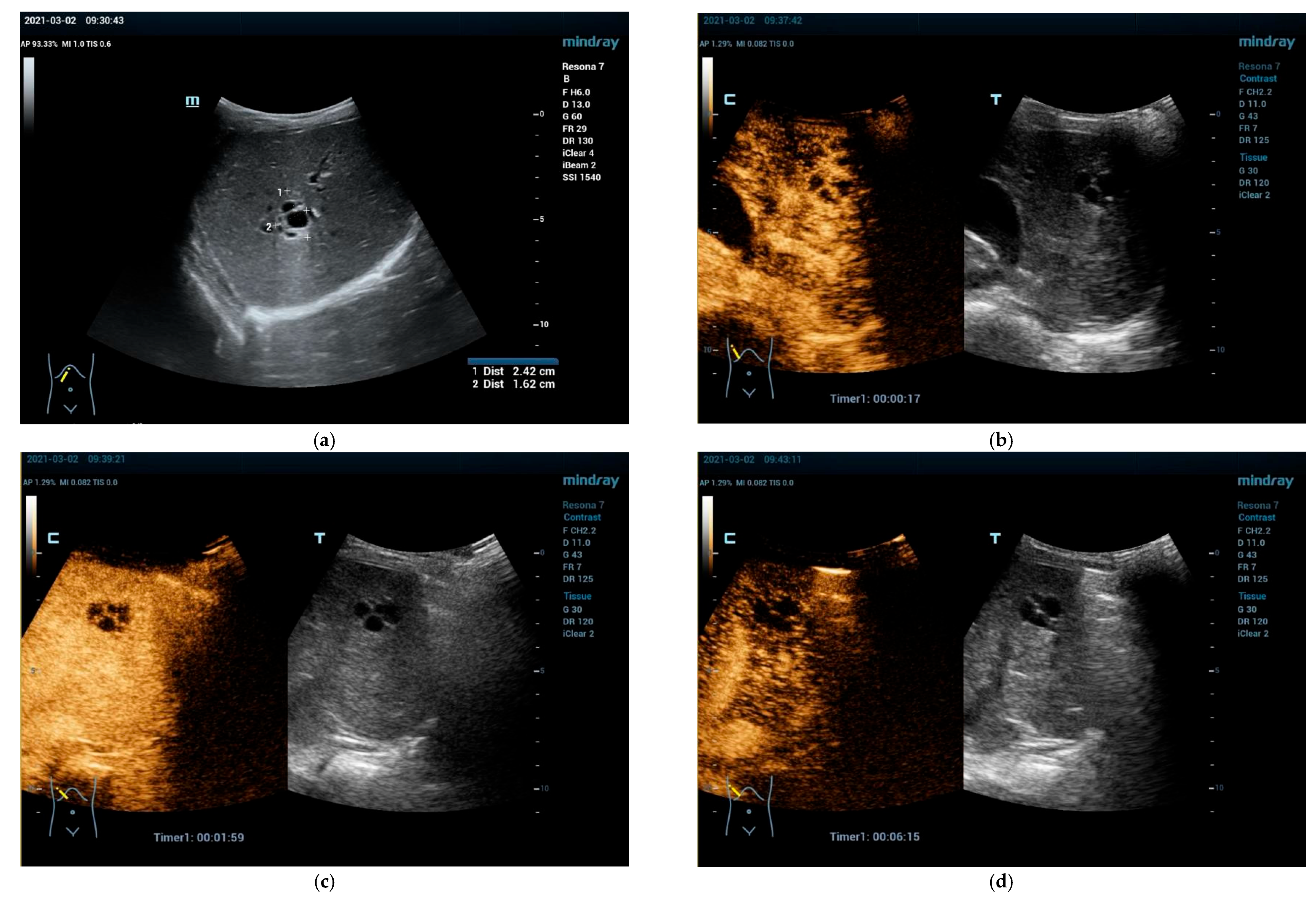
2.2. Clinical Scenarios and the Role of Ultrasound and CEUS
3. Situs Inversus, Size and Shape Variations
3.1. Hepatomegaly and Variants of Shape and Echogenicity
3.2. Inhomogeneous Liver Parenchyma
3.3. Elastography
Diagnostic Work-Up and Follow-Up Strategy
3.4. Liver Cysts
Diagnostic Work-Up and Follow-Up Strategy
4. Solid Focal Liver Lesions: Introduction
4.1. Diagnostic Work-Up and Follow-Up Strategy
4.2. Liver Calcification
Diagnostic Work-Up and Follow-Up Strategy
4.3. Vascular Malformations, Vascular Tumors and Hemangioma
4.3.1. Hepatic Hemangioma
4.3.2. Congenital Hepatic Hemangioma
4.3.3. Infantile Hepatic Hemangioma
4.4. Vascular Malformations Other than Hemangioma
Focal Nodular Hyperplasia (FNH)
4.5. Hepatocellular Adenoma (HCA)
4.6. Inflammatory Hepatocellular Adenoma
4.7. HNF-1-Inactivated Hepatocellular Adenoma
4.8. β-Catenin-Activated Hepatocellular Adenoma
4.9. Von Meyenburg Complex
4.10. Mesenchymal Hamartoma of the Liver
4.11. Cholangiocellular and Bile Duct Adenoma
5. Focal Fatty Infiltration and Focal Fatty Sparing (Focal Fatty Lesions, FFL)
5.1. Focal Fatty Sparing (FFS)
5.2. Focal Fatty Infiltration (FFI)
5.3. Inflammatory Focal Liver Lesions
6. Malignant Focal Liver Lesions
6.1. Hepatoblastoma
6.2. Hepatocellular Carcinoma
6.3. Primary Sarcoma of the Liver
6.4. Primary Hepatic Lymphoma
6.5. Hepatic Metastases
7. What Is the Impact of an Incidental Finding in Pediatric Ultrasound?
7.1. Psychological and Social Burden
7.2. Benefits and Economic Consequences (Costs)
8. Strategy
8.1. Iso- and Hyperechoic FLL
8.2. Isoechoic FLL
8.3. Hypoechoic FLL
9. Inconclusive CT/MRI Findings
10. Conclusions
11. Summary Statements
- An incidental finding (IF) during an imaging examination is the unintended and unexpected discovery of an abnormality that may be clinically significant (or not).
- The term IF can be used for any focal changes of the liver echogenicity and architecture that is causing no symptoms to the patient.
- Liver incidentalomas are frequently detected.
- Ultrasound is the primary and first-line imaging modality for the evaluation of liver pathology in children, including liver incidentalomas.
- Liver elastography should be applied in all patients with liver pathology to determine underlying risk constellation.
- Conventional B-mode ultrasound is accurate for the diagnosis of simple hepatic cysts, typical hemangiomas and hydatid cysts. As a result, such lesions could be followed up with US.
- Contrast-enhanced ultrasound (CEUS) is appropriate to precisely characterize solitary and multiple solid and cystic FLL with excellent accuracy in differentiating benign from malignant lesions.
- CEUS has an excellent safety profile.
- MRI is probably superior for the documentation of multiple lesions prior to surgery.
- Once characterization with CT/MRI has been initially performed, long-term follow-up can be undertaken with US/CEUS if necessary.
- Benign hepatic tumors are commonly observed in adults, but more rarely reported in children.
- The pretest probability—including presentation of the patient, past medical and family history with patient’s background risk of malignant and inflammatory liver disease (“all clinical information available”), laboratory data and liver stiffness using elastography—and the lesion’s characteristics including size and echogenicity are taken into account for appropriate management, final diagnosis and follow-up of these patients.
- According to the factors mentioned, patients can be subcategorized into no, low and high risk for clinical and imaging features.
- The psychological, healthcare and economic impact of every imaging modality using a multiparametric imaging approach should be taken into account.
- In some patients, imaging will be inconclusive, and some form of tissue diagnosis will be required, either imaging-guided biopsy or surgical resection.
- In doubtful cases, a multi-disciplinary team (MDT) dedicated to liver diseases should be consulted.
Author Contributions
Funding
Conflicts of Interest
References
- Jenssen, C.; Lorentzen, T.; Dietrich, C.F.; Lee, J.Y.; Chaubal, N.; Choi, B.I.; Rosenberg, J.; Gutt, C.; Nolsøe, C.P. Incidental Findings of Gallbladder and Bile Ducts-Management Strategies: General Aspects, Gallbladder Polyps and Gallbladder Wall Thickening-A World Federation of Ultrasound in Medicine and Biology (WFUMB) Position Paper. Ultrasound. Med. Biol. 2022, 48, 2355–2378. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Fraser, A.G.; Dong, Y.; Guth, S.; Hari, R.; Hoffmann, B.; Prosch, H.; Walter, R.; Abramowicz, J.S.; Nolsoe, C.P.; et al. Managing Incidental Findings Reported by Medical, Sonography and Other Students Performing Educational Ultrasound Examinations. Ultrasound Med. Biol. 2022, 48, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Petousis, S.; Chatzakis, C.; Westerway, S.C.; Abramowicz, J.S.; Dinas, K.; Dong, Y.; Dietrich, C.F.; Sotiriadis, A. World Federation for Ultrasound in Medicine Review Paper: Incidental Findings during Obstetrical Ultrasound. Ultrasound Med. Biol. 2022, 48, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Lewicki, A.; Freeman, S.; Jedrzejczyk, M.; Dobruch, J.; Dong, Y.; Bertolotto, M.; Dietrich, C.F. Incidental Findings and How to Manage Them: Testis- A WFUMB Position Paper. Ultrasound Med. Biol. 2021, 47, 2787–2802. [Google Scholar] [CrossRef]
- Bialek, E.J.; Lim, A.; Dong, Y.; Fodor, D.; Gritzmann, N.; Dietrich, C.F. WFUMB position paper. Incidental findings of the salivary glands. Med. Ultrason. 2021, 23, 329–338. [Google Scholar] [CrossRef]
- Trenker, C.; Gorg, C.; Freeman, S.; Jenssen, C.; Dong, Y.; Caraiani, C.; Ioanitescu, E.S.; Dietrich, C.F. WFUMB Position Paper-Incidental Findings, How to Manage: Spleen. Ultrasound Med. Biol. 2021, 47, 2017–2032. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Westerway, S.; Nolsoe, C.; Kim, S.; Jenssen, C. Commentary on the World Federation for Ultrasound in Medicine and Biology Project “Incidental Findings”. Ultrasound Med. Biol. 2020, 46, 1815–1820. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Correas, J.M.; Dong, Y.; Nolsoe, C.; Westerway, S.C.; Jenssen, C. WFUMB position paper on the management incidental findings: Adrenal incidentaloma. Ultrasonography 2020, 39, 11–21. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Riemer-Hommel, P. Challenges for the German Health Care System. Z Gastroenterol. 2012, 50, 557–572. [Google Scholar] [CrossRef]
- Scharitzer, M.; Tamandl, D.; Ba-Ssalamah, A. Incidental findings of liver, biliary system, pancreas and spleen in asymptomatic patients: Assessment and management recommendations. Radiologe 2017, 57, 270–278. [Google Scholar] [CrossRef]
- Boutros, C.; Katz, S.C.; Espat, N.J. Management of an incidental liver mass. Surg. Clin. North Am. 2010, 90, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Algarni, A.A.; Alshuhri, A.H.; Alonazi, M.M.; Mourad, M.M.; Bramhall, S.R. Focal liver lesions found incidentally. World J. Hepatol. 2016, 8, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, L.; Cui, X.W.; Tannapfel, A.; Franke, D.; Stenzel, M.; Kosiak, W.; Schreiber-Dietrich, D.; Jungert, J.; Chang, J.M.; Dietrich, C.F. Benign liver tumors in pediatric patients—Review with emphasis on imaging features. World J. Gastroenterol. 2015, 21, 8541–8561. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, L.; Caraiani, C.; Radzina, M.; Jedrzejczyk, M.; Schreiber-Dietrich, D.; Dietrich, C.F. Vascular phases in imaging and their role in focal liver lesions assessment. Clin. Hemorheol. Microcirc. 2015, 62, 299–326. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Nolsoe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Augustiniene, R.; Batko, T.; Cantisani, V.; Cekuolis, A.; Deganello, A.; Dong, Y.; Franke, D.; Harkanyi, Z.; Humphries, P.D.; et al. European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB): An Update on the Pediatric CEUS Registry on Behalf of the “EFSUMB Pediatric CEUS Registry Working Group”. Ultraschall Med. 2021, 42, 270–277. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Deganello, A.; Dietrich, C.F.; Duran, C.; Franke, D.; Harkanyi, Z.; Kosiak, W.; Miele, V.; Ntoulia, A.; et al. Role of Contrast-Enhanced Ultrasound (CEUS) in Paediatric Practice: An EFSUMB Position Statement. Ultraschall Med. 2017, 38, 33–43. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Deganello, A.; Dietrich, C.F.; Duran, C.; Franke, D.; Harkanyi, Z.; Kosiak, W.; Miele, V.; Ntoulia, A.; et al. Authors’ Reply to Letter: Role of Contrast-Enhanced Ultrasound (CEUS) in Paediatric Practice: An EFSUMB Position Statement. Ultraschall Med. 2017, 38, 447–448. [Google Scholar] [CrossRef]
- Fang, C.; Anupindi, S.A.; Back, S.J.; Franke, D.; Green, T.G.; Harkanyi, Z.; Jungert, J.; Kwon, J.K.; Paltiel, H.J.; Squires, J.H.; et al. Contrast-enhanced ultrasound of benign and malignant liver lesions in children. Pediatr. Radiol. 2021, 51, 2181–2197. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Brabrand, K.; Cantisani, V.; Correas, J.M.; Cui, X.W.; D’Onofrio, M.; Essig, M.; Freeman, S.; Gilja, O.H.; Gritzmann, N.; et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part II. Diagnostic Ultrasound-Guided Interventional Procedures (Long Version). Ultraschall Med. 2015, 36, E15–E35. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Brabrand, K.; Cantisani, V.; Correas, J.M.; Cui, X.W.; D’Onofrio, M.; Essig, M.; Freeman, S.; Gilja, O.H.; Gritzmann, N.; et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part II. Diagnostic Ultrasound-Guided Interventional Procedures (Short Version). Ultraschall Med. 2015, 36, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Lorentzen, T.; Sidhu, P.S.; Jenssen, C.; Gilja, O.H.; Piscaglia, F.; Efsumb. An Introduction to the EFSUMB Guidelines on Interventional Ultrasound (INVUS). Ultraschall Med. 2015, 36, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Lorentzen, T.; Appelbaum, L.; Buscarini, E.; Cantisani, V.; Correas, J.M.; Cui, X.W.; D’Onofrio, M.; Gilja, O.H.; Hocke, M.; et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part III—Abdominal Treatment Procedures (Short Version). Ultraschall Med. 2016, 37, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Lorentzen, T.; Appelbaum, L.; Buscarini, E.; Cantisani, V.; Correas, J.M.; Cui, X.W.; D’Onofrio, M.; Gilja, O.H.; Hocke, M.; et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part III—Abdominal Treatment Procedures (Long Version). Ultraschall Med. 2016, 37, E1–E32. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Kratzer, W.; Strobe, D.; Danse, E.; Fessl, R.; Bunk, A.; Vossas, U.; Hauenstein, K.; Koch, W.; Blank, W.; et al. Assessment of metastatic liver disease in patients with primary extrahepatic tumors by contrast-enhanced sonography versus CT and MRI. World J. Gastroenterol. 2006, 12, 1699–1705. [Google Scholar] [CrossRef]
- Wu, M.; Li, L.; Wang, J.; Zhang, Y.; Guo, Q.; Li, X.; Zhang, X. Contrast-enhanced US for characterization of focal liver lesions: A comprehensive meta-analysis. Eur. Radiol. 2018, 28, 2077–2088. [Google Scholar] [CrossRef]
- Strobel, D.; Seitz, K.; Blank, W.; Schuler, A.; Dietrich, C.; von Herbay, A.; Friedrich-Rust, M.; Kunze, G.; Becker, D.; Will, U.; et al. Contrast-enhanced ultrasound for the characterization of focal liver lesions—Diagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall Med. 2008, 29, 499–505. [Google Scholar] [CrossRef]
- Seitz, K.; Greis, C.; Schuler, A.; Bernatik, T.; Blank, W.; Dietrich, C.F.; Strobel, D. Frequency of tumor entities among liver tumors of unclear etiology initially detected by sonography in the noncirrhotic or cirrhotic livers of 1349 patients. Results of the DEGUM multicenter study. Ultraschall Med. 2011, 32, 598–603. [Google Scholar] [CrossRef]
- Strobel, D.; Bernatik, T.; Blank, W.; Schuler, A.; Greis, C.; Dietrich, C.F.; Seitz, K. Diagnostic accuracy of CEUS in the differential diagnosis of small (</=20 mm) and subcentimetric (</=10 mm) focal liver lesions in comparison with histology. Results of the DEGUM multicenter trial. Ultraschall Med. 2011, 32, 593–597. [Google Scholar] [CrossRef]
- Bernatik, T.; Seitz, K.; Blank, W.; Schuler, A.; Dietrich, C.F.; Strobel, D. Unclear focal liver lesions in contrast-enhanced ultrasonography--lessons to be learned from the DEGUM multicenter study for the characterization of liver tumors. Ultraschall Med. 2010, 31, 577–581. [Google Scholar] [CrossRef]
- Seitz, K.; Strobel, D.; Bernatik, T.; Blank, W.; Friedrich-Rust, M.; Herbay, A.; Dietrich, C.F.; Strunk, H.; Kratzer, W.; Schuler, A. Contrast-Enhanced Ultrasound (CEUS) for the characterization of focal liver lesions—Prospective comparison in clinical practice: CEUS vs. CT (DEGUM multicenter trial). Parts of this manuscript were presented at the Ultrasound Dreilandertreffen 2008, Davos. Ultraschall Med. 2009, 30, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Strobel, D.; Seitz, K.; Blank, W.; Schuler, A.; Dietrich, C.F.; von Herbay, A.; Friedrich-Rust, M.; Bernatik, T. Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1,349 liver lesions in contrast-enhanced ultrasound (CEUS). Ultraschall Med. 2009, 30, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Ungermann, L.; Elias, P.; Zizka, J.; Ryska, P.; Klzo, L. Focal nodular hyperplasia: Spoke-wheel arterial pattern and other signs on dynamic contrast-enhanced ultrasonography. Eur. J. Radiol. 2007, 63, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, M.H.; Yin, S.S.; Yan, K.; Fan, Z.H.; Yang, W.; Dai, Y.; Huo, L.; Li, J.Y. The role of contrast-enhanced sonography of focal liver lesions before percutaneous biopsy. AJR Am. J. Roentgenol. 2006, 187, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Trillaud, H.; Bruel, J.M.; Valette, P.J.; Vilgrain, V.; Schmutz, G.; Oyen, R.; Jakubowski, W.; Danes, J.; Valek, V.; Greis, C. Characterization of focal liver lesions with SonoVue-enhanced sonography: International multicenter-study in comparison to CT and MRI. World J. Gastroenterol. 2009, 15, 3748–3756. [Google Scholar] [CrossRef]
- Sporea, I.; Badea, R.; Martie, A.; Dumitru, E.; Ioanitescu, S.; Sirli, R.; Socaciu, M.; Popescu, A.; Danila, M.; Voiculescu, M. Contrast Enhanced Ultrasound for the evaluation of focal liver lesions in daily practice. A multicentre study. Med. Ultrason. 2012, 14, 95–100. [Google Scholar]
- Soye, J.A.; Mullan, C.P.; Porter, S.; Beattie, H.; Barltrop, A.H.; Nelson, W.M. The use of contrast-enhanced ultrasound in the characterisation of focal liver lesions. Ulst. Med. J. 2007, 76, 22–25. [Google Scholar]
- Wang, Z.L.; Tang, J.; Weskott, H.P.; Li, J.L.; Wang, W.; Luo, Y.K.; An, L.C.; Xu, J.H. Undetermined focal liver lesions on gray-scale ultrasound in patients with fatty liver: Characterization with contrast-enhanced ultrasound. J. Gastroenterol. Hepatol. 2008, 23, 1511–1519. [Google Scholar] [CrossRef]
- Kim, T.K.; Jang, H.J.; Burns, P.N.; Murphy-Lavallee, J.; Wilson, S.R. Focal nodular hyperplasia and hepatic adenoma: Differentiation with low-mechanical-index contrast-enhanced sonography. AJR Am. J. Roentgenol. 2008, 190, 58–66. [Google Scholar] [CrossRef]
- Pei, X.Q.; Liu, L.Z.; Liu, M.; Zheng, W.; Han, F.; Li, A.H.; Cai, M.Y. Contrast-enhanced ultrasonography of hepatocellular carcinoma: Correlation between quantitative parameters and histological grading. Br. J. Radiol. 2012, 85, e740–e747. [Google Scholar] [CrossRef]
- Kong, W.T.; Wang, W.P.; Huang, B.J.; Ding, H.; Mao, F.; Si, Q. Contrast-enhanced ultrasound in combination with color Doppler ultrasound can improve the diagnostic performance of focal nodular hyperplasia and hepatocellular adenoma. Ultrasound Med. Biol. 2015, 41, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-T.; Ji, Z.-B.; Wang, W.-P.; Cai, H.; Huang, B.-J.; Ding, H. Evaluation of Liver Metastases Using Contrast-Enhanced Ultrasound: Enhancement Patterns and Influencing Factors. Gut Liver 2016, 10, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Schuessler, G.; Trojan, J.; Fellbaum, C.; Ignee, A. Differentiation of focal nodular hyperplasia and hepatocellular adenoma by contrast-enhanced ultrasound. Br. J. Radiol. 2005, 78, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Mertens, J.C.; Braden, B.; Schuessler, G.; Ott, M.; Ignee, A. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology 2007, 45, 1139–1145. [Google Scholar] [CrossRef]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver—update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med. Biol. 2013, 39, 187–210. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.A.; et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2018, 4, E2–E15. [Google Scholar] [CrossRef]
- Caraiani, C.; Yi, D.; Petresc, B.; Dietrich, C. Indications for abdominal imaging: When and what to choose? J. Ultrason. 2020, 20, e43–e54. [Google Scholar] [CrossRef]
- Caraiani, C.; Petresc, B.; Dong, Y.; Dietrich, C.F. Contraindications and adverse effects in abdominal imaging. Med. Ultrason. 2019, 21, 456–463. [Google Scholar] [CrossRef]
- Caraiani, C.; Dong, Y.; Rudd, A.G.; Dietrich, C.F. Reasons for inadequate or incomplete imaging techniques. Med. Ultrason. 2018, 20, 498–507. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Sharma, M.; Gibson, R.N.; Schreiber-Dietrich, D.; Jenssen, C. Fortuitously discovered liver lesions. World J. Gastroenterol. 2013, 19, 3173–3188. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Jenssen, C. Focal liver lesion, incidental finding. Dtsch Med. Wochenschr. 2012, 137, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S. Multiparametric Ultrasound (MPUS) Imaging: Terminology Describing the Many Aspects of Ultrasonography. Ultraschall Med. 2015, 36, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Gore, R.M.; Pickhardt, P.J.; Mortele, K.J.; Fishman, E.K.; Horowitz, J.M.; Fimmel, C.J.; Talamonti, M.S.; Berland, L.L.; Pandharipande, P.V. Management of Incidental Liver Lesions on CT: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2017, 14, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, L.; Tana, C.; Braden, B.; Caraiani, C.; Sparchez, Z.; Cui, X.W.; Baum, U.; Dietrich, C.F. Advantages and Limitations of Focal Liver Lesions Assessment with Ultrasound Contrast Agents: Comments to the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Guidelines. Med. Princ Pract. 2016, 25, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Averkiou, M.A.; Correas, J.M.; Lassau, N.; Leen, E.; Piscaglia, F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012, 33, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Claudon, M.; Cosgrove, D.; Albrecht, T.; Bolondi, L.; Bosio, M.; Calliada, F.; Correas, J.M.; Darge, K.; Dietrich, C.; D’Onofrio, M.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)—Update 2008. Ultraschall Med. 2008, 29, 28–44. [Google Scholar] [CrossRef]
- Piscaglia, F.; Nolsoe, C.; Dietrich, C.F.; Cosgrove, D.O.; Gilja, O.H.; Bachmann, N.M.; Albrecht, T.; Barozzi, L.; Bertolotto, M.; Catalano, O.; et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med. 2012, 33, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Ignee, A.; Greis, C.; Cui, X.W.; Schreiber-Dietrich, D.G.; Hocke, M. Artifacts and pitfalls in contrast-enhanced ultrasound of the liver. Ultraschall Med. 2014, 35, 108–125, quiz 126-107. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Ignee, A.; Hocke, M.; Schreiber-Dietrich, D.; Greis, C. Pitfalls and artefacts using contrast enhanced ultrasound. Z Gastroenterol. 2011, 49, 350–356. [Google Scholar] [CrossRef]
- Fetzer, D.; Dietrich, C.F. CEUS technique: Artifacts and pitfalls. In Speciality Imaging. Fundamentals of CEUS; Lyshchik, A., Dietrich, C.F., Sidhu, P.S., Wilson, S., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 42–49. [Google Scholar]
- Schmidt, C.O.; Sierocinski, E.; Hegenscheid, K.; Baumeister, S.E.; Grabe, H.J.; Volzke, H. Impact of whole-body MRI in a general population study. Eur. J. Epidemiol. 2016, 31, 31–39. [Google Scholar] [CrossRef]
- Li, X.; Fu, Z.; Zhong, J.; Cao, K.; Chen, X.; Ding, N.; Liu, L.; Zhai, J.; Qu, Z. Coexistence of situs inversus totalis and hepatocellular carcinoma: A series of nine cases and a literature review. J. Interv. Med. 2022, 5, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Eitler, K.; Bibok, A.; Telkes, G. Situs Inversus Totalis: A Clinical Review. Int. J. Gen. Med. 2022, 15, 2437–2449. [Google Scholar] [CrossRef] [PubMed]
- Sienz, M.; Ignee, A.; Dietrich, C.F. Reference values in abdominal ultrasound—Liver and liver vessels. Z Gastroenterol. 2010, 48, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Serra, C.; Jedrejczyk, M. Ultrasound of the liver. In EFSUMB Course Book on Ultrasound; Dietrich, C.F., Ed.; EFSUMB: London, UK, 2012; pp. 31–90. [Google Scholar]
- Sienz, M.; Ignee, A.; Dietrich, C.F. Reference values in abdominal ultrasound—Biliopancreatic system and spleen. Z Gastroenterol. 2011, 49, 845–870. [Google Scholar] [CrossRef]
- Ferraioli, G.; Barr, R.G.; Farrokh, A.; Radzina, M.; Cui, X.W.; Dong, Y.; Rocher, L.; Cantisani, V.; Polito, E.; D’Onofrio, M.; et al. How to perform shear wave elastography. Part II. Med. Ultrason. 2022, 24, 196–210. [Google Scholar] [CrossRef]
- Ferraioli, G.; Barr, R.G.; Farrokh, A.; Radzina, M.; Cui, X.W.; Dong, Y.; Rocher, L.; Cantisani, V.; Polito, E.; D’Onofrio, M.; et al. How to perform shear wave elastography. Part I. Med. Ultrason. 2022, 24, 95–106. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Shi, L.; Wei, Q.; Dong, Y.; Cui, X.W.; Lowe, A.; Worni, M.; Ferraioli, G. What does liver elastography measure? Technical aspects and methodology. Minerva Gastroenterol. 2021, 67, 129–140. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Ferraioli, G.; Sirli, R.; Popescu, A.; Sporea, I.; Pienar, C.; Kunze, C.; Taut, H.; Schrading, S.; Bota, S.; et al. General advice in ultrasound based elastography of pediatric patients. Med. Ultrason. 2019, 21, 315–326. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef]
- Dong, Y.; Sirli, R.; Ferraioli, G.; Sporea, I.; Chiorean, L.; Cui, X.; Fan, M.; Wang, W.P.; Gilja, O.H.; Sidhu, P.S.; et al. Shear wave elastography of the liver—Review on normal values. Z Gastroenterol. 2017, 55, 153–166. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Short Version). Ultraschall Med. 2017, 38, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017, 38, e16–e47. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Berzigotti, A.; Barr, R.G.; Choi, B.I.; Cui, X.W.; Dong, Y.; Gilja, O.H.; Lee, J.Y.; Lee, D.H.; Moriyasu, F.; et al. Quantification of Liver Fat Content with Ultrasound: A WFUMB Position Paper. Ultrasound Med. Biol. 2021, 47, 2803–2820. [Google Scholar] [CrossRef] [PubMed]
- Anupindi, S.A.; Biko, D.M.; Ntoulia, A.; Poznick, L.; Morgan, T.A.; Darge, K.; Back, S.J. Contrast-enhanced US Assessment of Focal Liver Lesions in Children. RadioGraphics 2017, 37, 1632–1647. [Google Scholar] [CrossRef] [PubMed]
- Faust, D.; Fellbaum, C.; Zeuzem, S.; Dietrich, C.F. Nodular regenerative hyperplasia of the liver: A rare differential diagnosis of cholestasis with response to ursodeoxycholic acid. Z Gastroenterol. 2003, 41, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Sirli, R.; Ferraioli, G.; Popescu, A.; Sporea, I.; Pienar, C.; Kunze, C.; Taut, H.; Schrading, S.; Bota, S.; et al. Current knowledge in ultrasound-based liver elastography of pediatric patients. Appl. Sci. 2018, 8, 944. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef]
- Ferraioli, G.; Filice, C.; Castera, L.; Choi, B.I.; Sporea, I.; Wilson, S.R.; Cosgrove, D.; Dietrich, C.F.; Amy, D.; Bamber, J.C.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: Liver. Ultrasound Med. Biol. 2015, 41, 1161–1179. [Google Scholar] [CrossRef]
- Berzigotti, A.; Ferraioli, G.; Bota, S.; Gilja, O.H.; Dietrich, C.F. Novel ultrasound-based methods to assess liver disease: The game has just begun. Dig. Liver. Dis. 2018, 50, 107–112. [Google Scholar] [CrossRef]
- Cui, X.W.; Pirri, C.; Ignee, A.; De Molo, C.; Hirche, T.O.; Schreiber-Dietrich, D.G.; Dietrich, C.F. Measurement of shear wave velocity using acoustic radiation force impulse imaging is not hampered by previous use of ultrasound contrast agents. Z Gastroenterol. 2014, 52, 649–653. [Google Scholar] [CrossRef]
- Venkatesh, S.K.; Chandan, V.; Roberts, L.R. Liver masses: A clinical, radiologic, and pathologic perspective. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2014, 12, 1414–1429. [Google Scholar] [CrossRef] [PubMed]
- Mavilia, M.G.; Pakala, T.; Molina, M.; Wu, G.Y. Differentiating Cystic Liver Lesions: A Review of Imaging Modalities, Diagnosis and Management. J. Clin. Transl. Hepatol. 2018, 6, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Schreiber-Dietrich, D.G.; Leuschner, I.; Tannapfel, A.; Franke, D.; Stenzel, M.; Juengert, J.; Dietrich, C.F. Primary liver tumours in childhood. Z Gastroenterol. 2015, 53, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Obaro, A.E.; Ryan, S.M. Benign liver lesions: Grey-scale and contrast-enhanced ultrasound appearances. Ultrasound 2015, 23, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, W.P.; Mao, F.; Fan, M.; Ignee, A.; Serra, C.; Sparchez, Z.; Sporea, I.; Braden, B.; Dietrich, C.F. Contrast enhanced ultrasound features of hepatic cystadenoma and hepatic cystadenocarcinoma. Scand. J. Gastroenterol. 2017, 52, 365–372. [Google Scholar] [CrossRef]
- Fergusson, J. Investigation and management of hepatic incidentalomas. J. Gastroenterol. Hepatol. 2012, 27, 1772–1782. [Google Scholar] [CrossRef]
- Brunetti, E.; Tamarozzi, F.; Macpherson, C.; Filice, C.; Piontek, M.S.; Kabaalioglu, A.; Dong, Y.; Atkinson, N.; Richter, J.; Schreiber-Dietrich, D.; et al. Ultrasound and Cystic Echinococcosis. Ultrasound Int. Open 2018, 4, E70–E78. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Douira-Khomsi, W.; Gharbi, H.; Sharma, M.; Cui, X.W.; Sparchez, Z.; Richter, J.; Kabaalioglu, A.; Atkinson, N.S.S.; Schreiber-Dietrich, D.; et al. Cystic and alveolar echinococcosis of the hepatobiliary tract—The role of new imaging techniques for improved diagnosis. Med. Ultrason. 2020, 22, 75–84. [Google Scholar] [CrossRef]
- Schwarze, V.; Mueller-Peltzer, K.; Negrao de Figueiredo, G.; Lindner, F.; Rubenthaler, J.; Clevert, D.A. The use of contrast-enhanced ultrasound (CEUS) for the diagnostic evaluation of hepatic echinococcosis. Clin. Hemorheol. Microcirc. 2018, 70, 449–455. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Douira-Khomsi, W.; Gharbi, H.; Sharma, M.; Cui, X.W.; Sparchez, Z.; Richter, J.; Kabaalioglu, A.; Atkinson, N.S.; Schreiber-Dietrich, D.; et al. Cystic echinococcosis, review and illustration of non-hepatic manifestations. Med. Ultrason. 2020, 22, 319–324. [Google Scholar] [CrossRef]
- European Association for the Study of the, L. EASL Clinical Practice Guidelines on the management of benign liver tumours. J. Hepatol. 2016, 65, 386–398. [Google Scholar] [CrossRef]
- Anomalies ISftSoV. 2018 Classification. Available online: Issva.org/classification (accessed on 28 October 2020).
- Kunimoto, K.; Yamamoto, Y.; Jinnin, M. ISSVA Classification of Vascular Anomalies and Molecular Biology. Int. J. Mol. Sci. 2022, 23, 2358. [Google Scholar] [CrossRef] [PubMed]
- Kollipara, R.; Dinneen, L.; Rentas, K.E.; Saettele, M.R.; Patel, S.A.; Rivard, D.C.; Lowe, L.H. Current classification and terminology of pediatric vascular anomalies. AJR Am. J. Roentgenol. 2013, 201, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Biecker, E.; Fischer, H.P.; Strunk, H.; Sauerbruch, T. Benign hepatic tumours. Z Gastroenterol. 2003, 41, 191–200. [Google Scholar] [CrossRef]
- Vilgrain, V.; Uzan, F.; Brancatelli, G.; Federle, M.P.; Zappa, M.; Menu, Y. Prevalence of hepatic hemangioma in patients with focal nodular hyperplasia: MR imaging analysis. Radiology 2003, 229, 75–79. [Google Scholar] [CrossRef]
- Mathieu, D.; Zafrani, E.S.; Anglade, M.C.; Dhumeaux, D. Association of focal nodular hyperplasia and hepatic hemangioma. Gastroenterology 1989, 97, 154–157. [Google Scholar] [CrossRef]
- Gnarra, M.; Behr, G.; Kitajewski, A.; Wu, J.K.; Anupindi, S.A.; Shawber, C.J.; Zavras, N.; Schizas, D.; Salakos, C.; Economopoulos, K.P. History of the infantile hepatic hemangioma: From imaging to generating a differential diagnosis. World J. Clin. Pediatr. 2016, 5, 273–280. [Google Scholar] [CrossRef]
- Merrow, A.C.; Gupta, A.; Patel, M.N.; Adams, D.M. 2014 Revised Classification of Vascular Lesions from the International Society for the Study of Vascular Anomalies: Radiologic-Pathologic Update. Radiographics 2016, 36, 1494–1516. [Google Scholar] [CrossRef]
- Iacobas, I.; Phung, T.L.; Adams, D.M.; Trenor, C.C., 3rd; Blei, F.; Fishman, D.S.; Hammill, A.; Masand, P.M.; Fishman, S.J. Guidance Document for Hepatic Hemangioma (Infantile and Congenital) Evaluation and Monitoring. J. Pediatr. 2018, 203, 294–300 e292. [Google Scholar] [CrossRef]
- Kulungowski, A.M.; Alomari, A.I.; Chawla, A.; Christison-Lagay, E.R.; Fishman, S.J. Lessons from a liver hemangioma registry: Subtype classification. J. Pediatr. Surg 2012, 47, 165–170. [Google Scholar] [CrossRef]
- Gorincour, G.; Kokta, V.; Rypens, F.; Garel, L.; Powell, J.; Dubois, J. Imaging characteristics of two subtypes of congenital hemangiomas: Rapidly involuting congenital hemangiomas and non-involuting congenital hemangiomas. Pediatr Radiol. 2005, 35, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Kassarjian, A.; Zurakowski, D.; Dubois, J.; Paltiel, H.J.; Fishman, S.J.; Burrows, P.E. Infantile hepatic hemangiomas: Clinical and imaging findings and their correlation with therapy. AJR Am. J. Roentgenol. 2004, 182, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, B.; Mulliken, J.B.; Enjolras, O.; Boon, L.M.; Wassef, M.; Josset, P.; Burrows, P.E.; Perez-Atayde, A.R.; Kozakewich, H.P. Rapidly involuting congenital hemangioma: Clinical and histopathologic features. Pediatr. Dev. Pathol. 2003, 6, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pan, F.S.; Wang, W.; Zhang, X.E.; Li, X.J.; Hong, Y.; Zhou, L.Y.; Xie, X.Y.; Lyu, M.D. The value of clinical and ultrasound features for the diagnosis of infantile hepatic hemangioma: Comparison with contrast-enhanced CT/MRI. Clin. Imaging 2018, 51, 311–317. [Google Scholar] [CrossRef]
- Piorkowska, M.A.; Dezman, R.; Sellars, M.E.; Deganello, A.; Sidhu, P.S. Characterization of a hepatic haemangioma with contrast-enhanced ultrasound in an infant. Ultrasound 2018, 26, 178–181. [Google Scholar] [CrossRef]
- Restrepo, R.; Palani, R.; Cervantes, L.F.; Duarte, A.M.; Amjad, I.; Altman, N.R. Hemangiomas revisited: The useful, the unusual and the new. Part 1: Overview and clinical and imaging characteristics. Pediatr. Radiol. 2011, 41, 895–904. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.P.; Lim, A.; Lee, W.J.; Clevert, D.A.; Höpfner, M.; Tannapfel, A.; Dietrich, C.F. Ultrasound findings in peliosis hepatis. ULTRASONOGRAPHY 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.P.; Cantisani, V.; D’Onofrio, M.; Ignee, A.; Mulazzani, L.; Saftoiu, A.; Sparchez, Z.; Sporea, I.; Dietrich, C.F. Contrast-enhanced ultrasound of histologically proven hepatic epithelioid hemangioendothelioma. World J. Gastroenterol. 2016, 22, 4741–4749. [Google Scholar] [CrossRef]
- Klinger, C.; Stuckmann, G.; Dietrich, C.F.; Berzigotti, A.; Horger, M.S.; Debove, I.; Gilot, B.J.; Pauluschke-Frohlich, J.; Hoffmann, T.; Sipos, B.; et al. Contrast-enhanced imaging in hepatic epithelioid hemangioendothelioma: Retrospective study of 10 patients. Z Gastroenterol. 2019, 57, 753–766. [Google Scholar] [CrossRef]
- Trojan, J.; Hammerstingl, R.; Engels, K.; Schneider, A.R.; Zeuzem, S.; Dietrich, C.F. Contrast-enhanced ultrasound in the diagnosis of malignant mesenchymal liver tumors. J. Clin. Ultrasound 2010, 38, 227–231. [Google Scholar] [CrossRef]
- El-Ali, A.M.; McCormick, A.; Thakrar, D.; Yilmaz, S.; Malek, M.M.; Squires, J.H. Contrast-Enhanced Ultrasound of Congenital and Infantile Hemangiomas: Preliminary Results from a Case Series. AJR Am. J. Roentgenol. 2020, 214, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Schooler, G.R.; Hull, N.C.; Lee, E.Y. Hepatobiliary MRI Contrast Agents: Pattern Recognition Approach to Pediatric Focal Hepatic Lesions. AJR Am. J. Roentgenol. 2020, 214, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Pugmire, B.S.; Towbin, A.J. Magnetic resonance imaging of primary pediatric liver tumors. Pediatr. Radiol. 2016, 46, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.E.; Drolet, B.A. Classification of Vascular Anomalies: An Update. Semin. Interv. Radiol. 2017, 34, 225–232. [Google Scholar] [CrossRef]
- Fetzer, D.T.; Kono, Y.; Rodgers, S.K. Using Contrast-Enhanced Ultrasound to Characterize Focal Liver Lesions. Clin. Liver Dis. 2021, 17, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A.; Salisbury, S.; Martin, R.; Towbin, A.J. Incidence and etiology of new liver lesions in pediatric patients previously treated for malignancy. AJR Am. J. Roentgenol. 2012, 199, 186–191. [Google Scholar] [CrossRef]
- Hussain, S.M.; Terkivatan, T.; Zondervan, P.E.; Lanjouw, E.; de Rave, S.; Ijzermans, J.N.; de Man, R.A. Focal nodular hyperplasia: Findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. Radiographics 2004, 24, 3–17; discussion 18–19. [Google Scholar] [CrossRef]
- Bioulac-Sage, P.; Cubel, G.; Balabaud, C.; Zucman-Rossi, J. Revisiting the pathology of resected benign hepatocellular nodules using new immunohistochemical markers. Semin. Liver Dis. 2011, 31, 91–103. [Google Scholar] [CrossRef]
- Fang, C.; Bernardo, S.; Sellars, M.E.; Deganello, A.; Sidhu, P.S. Contrast-enhanced ultrasound in the diagnosis of pediatric focal nodular hyperplasia and hepatic adenoma: Interobserver reliability. Pediatr. Radiol. 2019, 49, 82–90. [Google Scholar] [CrossRef]
- Dietrich, C.F. Liver tumor characterization--comments and illustrations regarding guidelines. Ultraschall Med. 2012, 33 (Suppl. S1), S22–S30. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Cui, X.W.; Schreiber-Dietrich, D.G.; Ignee, A. EFSUMB guidelines 2011: Comments and illustrations. Ultraschall Med. 2012, 33 (Suppl. S1), S11–S21. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.M.; Cube, R.; Lewis, R.B.; Conran, R.M. Pediatric Liver Masses: Radiologic-Pathologic Correlation Part 1. Benign Tumors. RadioGraphics 2010, 30, 801–826. [Google Scholar] [CrossRef]
- Frohlich, E.; Muller, R.; Cui, X.W.; Schreiber-Dietrich, D.; Dietrich, C.F. Dynamic contrast-enhanced ultrasound for quantification of tissue perfusion. J. Ultrasound Med. 2015, 34, 179–196. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Dong, Y.; Froehlich, E.; Hocke, M. Dynamic contrast-enhanced endoscopic ultrasound: A quantification method. Endosc. Ultrasound 2017, 6, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.W.; Ignee, A.; Jedrzejczyk, M.; Dietrich, C.F. Dynamic Vascular Pattern (DVP), a quantification tool for contrast enhanced ultrasound. Z Gastroenterol. 2013, 51, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F. Comments and illustrations regarding the guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS)--update 2008. Ultraschall Med. 2008, 29 (Suppl. S4), S188–S202. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Ignee, A.; Trojan, J.; Fellbaum, C.; Schuessler, G. Improved characterisation of histologically proven liver tumours by contrast enhanced ultrasonography during the portal venous and specific late phase of SHU 508A. Gut 2004, 53, 401–405. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Greis, C. How to perform contrast enhanced ultrasound. Dtsch Med. Wochenschr. 2016, 141, 1019–1024. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Flejou, J.F.; Terris, B.; Belghiti, J.; Degott, C. Focal nodular hyperplasia of the liver: A comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am. J. Surg. Pathol. 1999, 23, 1441–1454. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Maddalena, M.E.; Cui, X.W.; Schreiber-Dietrich, D.; Ignee, A. Liver tumor characterization--review of the literature. Ultraschall Med. 2012, 33 (Suppl. S1), S3–S10. [Google Scholar] [CrossRef]
- Dietrich, C.F. Contrast-Enhanced Ultrasound of Benign Focal Liver Lesions. Ultraschall Med. 2019, 40, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Couchy, G.; Balabaud, C.; Morcrette, G.; Caruso, S.; Blanc, J.F.; Bacq, Y.; Calderaro, J.; Paradis, V.; Ramos, J.; et al. Molecular Classification of Hepatocellular Adenoma Associates With Risk Factors, Bleeding, and Malignant Transformation. Gastroenterology 2017, 152, 880–894 e886. [Google Scholar] [CrossRef] [PubMed]
- Seitz, K.; Strobel, D. A Milestone: Approval of CEUS for Diagnostic Liver Imaging in Adults and Children in the USA. Ultraschall Med. 2016, 37, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Tannapfel, A.; Jang, H.J.; Kim, T.K.; Burns, P.N.; Dong, Y. Ultrasound Imaging of Hepatocellular Adenoma Using the New Histology Classification. Ultrasound Med. Biol. 2019, 45, 1–10. [Google Scholar] [CrossRef]
- Bioulac-Sage, P.; Laumonier, H.; Couchy, G.; Le Bail, B.; Sa Cunha, A.; Rullier, A.; Laurent, C.; Blanc, J.F.; Cubel, G.; Trillaud, H.; et al. Hepatocellular adenoma management and phenotypic classification: The Bordeaux experience. Hepatology 2009, 50, 481–489. [Google Scholar] [CrossRef]
- Bioulac-Sage, P.; Rebouissou, S.; Thomas, C.; Blanc, J.F.; Saric, J.; Sa Cunha, A.; Rullier, A.; Cubel, G.; Couchy, G.; Imbeaud, S.; et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 2007, 46, 740–748. [Google Scholar] [CrossRef]
- Zucman-Rossi, J.; Jeannot, E.; Nhieu, J.T.; Scoazec, J.Y.; Guettier, C.; Rebouissou, S.; Bacq, Y.; Leteurtre, E.; Paradis, V.; Michalak, S.; et al. Genotype-phenotype correlation in hepatocellular adenoma: New classification and relationship with HCC. Hepatology 2006, 43, 515–524. [Google Scholar] [CrossRef]
- Ros, P.R.; Goodman, Z.D. Genetics and imaging of hepatocellular adenomas: 2011 update. Invited commentary. Radiographics 2011, 31, 1543–1545; discussion 1545. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.P.; Lee, W.J.; Meloni, M.F.; Clevert, D.A.; Chammas, M.C.; Tannapfel, A.; Forgione, A.; Piscaglia, F.; Dietrich, C.F. Contrast-Enhanced Ultrasound Features of Histopathologically Proven Hepatocellular Carcinoma in the Non-cirrhotic Liver: A Multicenter Study. Ultrasound Med. Biol. 2022, 48, 1797–1805. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.P.; Lee, W.J.; Meloni, M.F.; Clevert, D.A.; Chammas, M.C.; Tannapfel, A.; Forgione, A.; Piscaglia, F.; Dietrich, C.F. Hepatocellular carcinoma in the non-cirrhotic liver. Clin. Hemorheol. Microcirc. 2022, 80, 423–436. [Google Scholar] [CrossRef]
- Nault, J.C.; Paradis, V.; Cherqui, D.; Vilgrain, V.; Zucman-Rossi, J. Molecular classification of hepatocellular adenoma in clinical practice. J. Hepatol. 2017, 67, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, N.; Matsui, O.; Kitao, A.; Kozaka, K.; Kobayashi, S.; Sasaki, M.; Yoshida, K.; Inoue, D.; Minami, T.; Gabata, T. Benign Hepatocellular Nodules: Hepatobiliary Phase of Gadoxetic Acid-enhanced MR Imaging Based on Molecular Background. Radiographics 2016, 36, 2010–2027. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Dong, Y.; Zhang, W.; Han, H.; Mao, F.; Zhang, Q.; Zheng, Z.; He, W.; Wang, W.P. Analysis of contrast-enhanced ultrasound features of hepatocellular adenoma according to different pathological molecular classifications. Clin. Hemorheol. Microcirc. 2020, 76, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Laumonier, H.; Cailliez, H.; Balabaud, C.; Possenti, L.; Zucman-Rossi, J.; Bioulac-Sage, P.; Trillaud, H. Role of contrast-enhanced sonography in differentiation of subtypes of hepatocellular adenoma: Correlation with MRI findings. AJR Am. J. Roentgenol. 2012, 199, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Manichon, A.F.; Bancel, B.; Durieux-Millon, M.; Ducerf, C.; Mabrut, J.Y.; Lepogam, M.A.; Rode, A. Hepatocellular adenoma: Evaluation with contrast-enhanced ultrasound and MRI and correlation with pathologic and phenotypic classification in 26 lesions. HPB Surg. 2012, 2012, 418745. [Google Scholar] [CrossRef] [PubMed]
- Pech, L.; Favelier, S.; Falcoz, M.T.; Loffroy, R.; Krause, D.; Cercueil, J.P. Imaging of Von Meyenburg complexes. Diagn. Interv. Imaging 2016, 97, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ros, P.R.; Goodman, Z.D.; Ishak, K.G.; Dachman, A.H.; Olmsted, W.W.; Hartman, D.S.; Lichtenstein, J.E. Mesenchymal hamartoma of the liver: Radiologic-pathologic correlation. Radiology 1986, 158, 619–624. [Google Scholar] [CrossRef]
- Shi, Q.S.; Xing, L.X.; Jin, L.F.; Wang, H.; Lv, X.H.; Du, L.F. Imaging findings of bile duct hamartomas: A case report and literature review. Int. J. Clin. Exp. Med. 2015, 8, 13145–13153. [Google Scholar]
- Zheng, R.Q.; Zhang, B.; Kudo, M.; Onda, H.; Inoue, T. Imaging findings of biliary hamartomas. World J. Gastroenterol. 2005, 11, 6354–6359. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, W.S.; Cheon, J.E.; Yoon, H.K.; Kang, G.H.; Kim, I.O.; Yeon, K.M. Radiological spectrum of hepatic mesenchymal hamartoma in children. Korean J. Radiol. 2007, 8, 498–505. [Google Scholar] [CrossRef]
- Lung, P.F.; Jaffer, O.S.; Akbar, N.; Sidhu, P.S.; Ryan, S.M. Appearances of von meyenburg complex on cross sectional imaging. J. Clin. Imaging Sci. 2013, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, J.; Loddenkemper, C.; Albrecht, T. Assessment of a biliary hamartoma with contrast-enhanced sonography using two different contrast agents. Ultraschall Med. 2009, 30, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Ignee, A.; Piscaglia, F.; Ott, M.; Salvatore, V.; Dietrich, C.F. A benign tumour of the liver mimicking malignant liver disease--cholangiocellular adenoma. Scand. J. Gastroenterol. 2009, 44, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.; Sahoo, B.; Choudhury, A.K.; Sofi, N.Y.; Kumar, R.; Bhadoria, A.S. Childhood obesity: Causes and consequences. J Fam. Med. Prim. Care 2015, 4, 187–192. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Wehrmann, T.; Zeuzem, S.; Braden, B.; Caspary, W.F.; Lembcke, B. Analysis of hepatic echo patterns in chronic hepatitis C. Ultraschall Med. 1999, 20, 9–14. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Schall, H.; Kirchner, J.; Seifert, H.; Herrmann, G.; Caspary, W.F.; Lembcke, B. Sonographic detection of focal changes in the liver hilus in patients receiving corticosteroid therapy. Z Gastroenterol. 1997, 35, 1051–1057. [Google Scholar]
- Hirche, T.O.; Ignee, A.; Hirche, H.; Schneider, A.; Dietrich, C.F. Evaluation of hepatic steatosis by ultrasound in patients with chronic hepatitis C virus infection. Liver Int. 2007, 27, 748–757. [Google Scholar] [CrossRef]
- Rafailidis, V.; Fang, C.; Leenknegt, B.; Ballal, K.; Deganello, A.; Sellars, M.E.; Yusuf, G.T.; Huang, D.Y.; Sidhu, P.S. Contrast-Enhanced Ultrasound (CEUS) quantification assessment of focal fatty variations in liver parenchyma: Challenging the traditional qualitative paradigm of uniform enhancement with adjacent parenchyma. J. Ultrasound Med. 2020. In press. [Google Scholar]
- Dietrich, C.F.; Shi, L.; Lowe, A.; Dong, Y.; Potthoff, A.; Sparchez, Z.; Teufel, A.; Guth, S.; Koch, J.; Barr, R.G.; et al. Conventional ultrasound for diagnosis of hepatic steatosis is better than believed. Z Gastroenterol. 2021, 60, 1235–1248. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Lee, W.J. Focal liver lesions. In WFUMB Course Book; Nürnberg, D., Chammas, M.C., Gilja, O.H., Sporea, I., Sirli, R., Abramowicz, J., Westerway, S.C., Condous, G., et al., Eds.; WFUMB: Laurel, MD, USA, 2021; pp. 1–16. Available online: www.wfumb.org (accessed on 30 November 2022).
- Hamer, O.W.; Aguirre, D.A.; Casola, G.; Lavine, J.E.; Woenckhaus, M.; Sirlin, C.B. Fatty liver: Imaging patterns and pitfalls. Radiographics 2006, 26, 1637–1653. [Google Scholar] [CrossRef]
- Piscaglia, F.; Lencioni, R.; Sagrini, E.; Pina, C.D.; Cioni, D.; Vidili, G.; Bolondi, L. Characterization of focal liver lesions with contrast-enhanced ultrasound. Ultrasound Med. Biol. 2010, 36, 531–550. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, M.; Crosara, S.; De Robertis, R.; Canestrini, S.; Mucelli, R.P. Contrast-Enhanced Ultrasound of Focal Liver Lesions. AJR Am. J. Roentgenol. 2015, 205, W56–W66. [Google Scholar] [CrossRef]
- Schuessler, G.; Fellbaum, C.; Fauth, F.; Jacobi, V.; Schmidt-Matthiesen, A.; Ignee, A.; Dietrich, C.F. The infammatory pseudotumour—An unusual liver tumour. Ultraschall Med. 2006, 27, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Schiavone, C.; Ticinesi, A.; Ricci, F.; Giamberardino, M.A.; Cipollone, F.; Silingardi, M.; Meschi, T.; Dietrich, C.F. Ultrasound imaging of abdominal sarcoidosis: State of the art. World J. Clin. Cases 2019, 7, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Dietrich, C.F.; Schiavone, C. Hepatosplenic sarcoidosis: Contrast-enhanced ultrasound findings and implications for clinical practice. Biomed. Res. Int. 2014, 2014, 926203. [Google Scholar] [CrossRef] [PubMed]
- Darbari, A.; Sabin, K.M.; Shapiro, C.N.; Schwarz, K.B. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 2003, 38, 560–566. [Google Scholar] [CrossRef]
- Chung, E.M.; Lattin, G.E., Jr.; Cube, R.; Lewis, R.B.; Marichal-Hernandez, C.; Shawhan, R.; Conran, R.M. From the archives of the AFIP: Pediatric liver masses: Radiologic-pathologic correlation. Part 2. Malignant tumors. Radiographics 2011, 31, 483–507. [Google Scholar] [CrossRef]
- Ferraro, S.; Panzeri, A.; Braga, F.; Panteghini, M. Serum alpha-fetoprotein in pediatric oncology: Not a children’s tale. Clin. Chem. Lab. Med. 2019, 57, 783–797. [Google Scholar] [CrossRef]
- Schooler, G.R.; Squires, J.H.; Alazraki, A.; Chavhan, G.B.; Chernyak, V.; Davis, J.T.; Khanna, G.; Krishnamurthy, R.; Lungren, M.P.; Masand, P.M.; et al. Pediatric Hepatoblastoma, Hepatocellular Carcinoma, and Other Hepatic Neoplasms: Consensus Imaging Recommendations from American College of Radiology Pediatric Liver Reporting and Data System (LI-RADS) Working Group. Radiology 2020, 296, 493–497. [Google Scholar] [CrossRef]
- Khanna, R.; Verma, S.K. Pediatric hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 3980–3999. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Trenker, C.; Fontanilla, T.; Gorg, C.; Hausmann, A.; Klein, S.; Lassau, N.; Miquel, R.; Schreiber-Dietrich, D.; Dong, Y. New Ultrasound Techniques Challenge the Diagnosis of Sinusoidal Obstruction Syndrome. Ultrasound Med. Biol. 2018, 44, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Wiest, I.; Teufel, A.; Ebert, M.P.; Potthoff, A.; Christen, M.; Penkala, N.; Dietrich, C.F. Budd-Chiari syndrome, review and illustration. Z Gastroenterol. 2021, 60, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, T.K.; Markiet, K.; Szurowska, E. Diagnostic Imaging of Hepatocellular Carcinoma—A Pictorial Essay. Curr. Med. Imaging Rev. 2017, 13, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, W.P.; Mao, F.; Zhang, Q.; Yang, D.; Tannapfel, A.; Meloni, M.F.; Neye, H.; Clevert, D.A.; Dietrich, C.F. Imaging Features of Fibrolamellar Hepatocellular Carcinoma with Contrast-Enhanced Ultrasound. Ultraschall Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Teufel, A.; Trojan, J.; Berzigotti, A.; Cui, X.W.; Dietrich, C.F. Contrast enhanced ultrasound in mixed hepatocellular cholangiocarcinoma: Case series and review of the literature. Dig. Liver. Dis. 2018, 50, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Teufel, A.; Wang, W.P.; Dietrich, C.F. Current Opinion about Hepatocellular Carcinoma <10 mm. Digestion 2021, 102, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, T.V.; Terranova, M.C.; Gagliardo, C.; Taibbi, A. CEUS LI-RADS: A pictorial review. Insights Imaging 2020, 11, 9. [Google Scholar] [CrossRef]
- Caraiani, C.; Boca, B.; Bura, V.; Sparchez, Z.; Dong, Y.; Dietrich, C. CT/MRI LI-RADS v2018 vs. CEUS LI-RADS v2017-Can Things Be Put Together? Biology 2021, 10, 412. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Dong, Y.; Kono, Y.; Caraiani, C.; Sirlin, C.B.; Cui, X.W.; Tang, A. LI-RADS ancillary features on contrast-enhanced ultrasonography. Ultrasonography 2020, 39, 221–228. [Google Scholar] [CrossRef]
- Rodgers, S.K.; Fetzer, D.T.; Gabriel, H.; Seow, J.H.; Choi, H.H.; Maturen, K.E.; Wasnik, A.P.; Morgan, T.A.; Dahiya, N.; O’Boyle, M.K.; et al. Role of US LI-RADS in the LI-RADS Algorithm. Radiographics 2019, 39, 690–708. [Google Scholar] [CrossRef]
- Tang, A.; Bashir, M.R.; Corwin, M.T.; Cruite, I.; Dietrich, C.F.; Do, R.K.G.; Ehman, E.C.; Fowler, K.J.; Hussain, H.K.; Jha, R.C.; et al. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology 2018, 286, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Lyshchik, A.; Kono, Y.; Dietrich, C.F.; Jang, H.J.; Kim, T.K.; Piscaglia, F.; Vezeridis, A.; Willmann, J.K.; Wilson, S.R. Contrast-enhanced ultrasound of the liver: Technical and lexicon recommendations from the ACR CEUS LI-RADS working group. Abdom. Radiol. 2018, 43, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Lyshchik, A.; Piscaglia, F.; Cosgrove, D.; Jang, H.J.; Sirlin, C.; Dietrich, C.F.; Kim, T.K.; Willmann, J.K.; Kono, Y. CEUS LI-RADS: Algorithm, implementation, and key differences from CT/MRI. Abdom. Radiol. 2018, 43, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Schellhaas, B.; Bernatik, T.; Bohle, W.; Borowitzka, F.; Chang, J.; Dietrich, C.F.; Dirks, K.; Donoval, R.; Drube, K.; Friedrich-Rust, M.; et al. Contrast-Enhanced Ultrasound Algorithms (CEUS-LIRADS/ESCULAP) for the Noninvasive Diagnosis of Hepatocellular Carcinoma—A Prospective Multicenter DEGUM Study. Ultraschall. Med. 2021, 42, 178–186. [Google Scholar] [CrossRef]
- Strobel, D.; Jung, E.M.; Ziesch, M.; Praktiknjo, M.; Link, A.; Dietrich, C.F.; Klinger, C.; Schultheiss, M.; Jesper, D.; Schellhaas, B. Real-life assessment of standardized contrast-enhanced ultrasound (CEUS) and CEUS algorithms (CEUS LI-RADS(R)/ESCULAP) in hepatic nodules in cirrhotic patients-a prospective multicenter study. Eur. Radiol. 2021, 31, 7614–7625. [Google Scholar] [CrossRef]
- Jacob, J.; Deganello, A.; Sellars, M.E.; Hadzic, N.; Sidhu, P.S. Contrast enhanced ultrasound (CEUS) characterization of grey-scale sonographic indeterminate focal liver lesions in pediatric practice. Ultraschall. Med. 2013, 34, 529–540. [Google Scholar] [CrossRef]
- Khanna, G.; Chavhan, G.B.; Schooler, G.R.; Fraum, T.J.; Alazraki, A.L.; Squires, J.H.; Salter, A.; Podberesky, D.J.; Towbin, A.J. Diagnostic Performance of LI-RADS Version 2018 for Evaluation of Pediatric Hepatocellular Carcinoma. Radiology 2021, 299, 190–199. [Google Scholar] [CrossRef]
- Rees, M.A.; Schooler, G.R.; Chavhan, G.B.; Towbin, A.J.; Alazraki, A.L.; Squires, J.H.; Fraum, T.J.; Zhang, C.; Khanna, G. Imaging Features of Hepatocellular Carcinoma in Children With and Without Underlying Risk Factors. AJR Am. J. Roentgenol. 2022, 219, 647–654. [Google Scholar] [CrossRef]
- Stocker, J.T.; Ishak, K.G. Undifferentiated (embryonal) sarcoma of the liver: Report of 31 cases. Cancer 1978, 42, 336–348. [Google Scholar] [CrossRef]
- Gao, J.; Fei, L.; Li, S.; Cui, K.; Zhang, J.; Yu, F.; Zhang, B. Undifferentiated embryonal sarcoma of the liver in a child: A case report and review of the literature. Oncol. Lett. 2013, 5, 739–742. [Google Scholar] [CrossRef]
- McCarville, B.D.A.; Harkanyi, Z. Contrast enhanced ultrasound: The current state. In Imaging in Pediatric Oncology, 1st ed.; Voss, S.D., McHugh, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 137–155. [Google Scholar]
- Yin, J.; Liu, Z.; Yang, K. Pleomorphic rhabdomyosarcoma of the liver with a hepatic cyst in an adult: Case report and literature review. Medicine 2018, 97, e11335. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, D.J.; Yang, W.T.; Lam, W.W.; Stanley, P. Hepatobiliary rhabdomyosarcoma in children: Diagnostic radiology. Pediatr. Radiol. 1998, 28, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Berger, I.; Shtalrid, M.; Schlanger, H.; Sthoeger, Z.M. Primary hepatic lymphoma: A case report and review of the literature. Age Ageing 2004, 33, 637–640. [Google Scholar] [CrossRef]
- Avlonitis, V.S.; Linos, D. Primary hepatic lymphoma: A review. Eur. J. Surg. 1999, 165, 725–729. [Google Scholar] [PubMed]
- Lu, Q.; Zhang, H.; Wang, W.P.; Jin, Y.J.; Ji, Z.B. Primary non-Hodgkin’s lymphoma of the liver: Sonographic and CT findings. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 75–81. [Google Scholar] [CrossRef]
- Yamashita, Y.; Joshita, S.; Kobayashi, H.; Wakabayashi, S.I.; Sugiura, A.; Yamazaki, T.; Umemura, T. Primary Hepatic Extranodal Marginal Zone Lymphoma of Mucosa-Associated Lymphoid Tissue in a Patient with Chronic Hepatitis B Virus Infection: Case Report and Summary of the Literature. Medicina 2021, 57, 280. [Google Scholar] [CrossRef] [PubMed]
- Foschi, F.G.; Dall’Aglio, A.C.; Marano, G.; Lanzi, A.; Savini, P.; Piscaglia, F.; Serra, C.; Cursaro, C.; Bernardi, M.; Andreone, P.; et al. Role of contrast-enhanced ultrasonography in primary hepatic lymphoma. J. Ultrasound Med. 2010, 29, 1353–1356. [Google Scholar] [CrossRef]
- Kitahata, S.; Hiraoka, A.; Kudo, M.; Murakami, T.; Ochi, M.; Izumoto, H.; Ueki, H.; Kaneto, M.; Aibiki, T.; Okudaira, T.; et al. Abdominal Ultrasound Findings of Tumor-Forming Hepatic Malignant Lymphoma. Dig. Dis. 2017, 35, 498–505. [Google Scholar] [CrossRef]
- Shiozawa, K.; Watanabe, M.; Ikehara, T.; Matsukiyo, Y.; Kikuchi, Y.; Kaneko, H.; Okubo, Y.; Shibuya, K.; Igarashi, Y.; Sumino, Y. A case of contiguous primary hepatic marginal zone B-cell lymphoma and hemangioma ultimately diagnosed using contrast-enhanced ultrasonography. Case Rep. Oncol. 2015, 8, 50–56. [Google Scholar] [CrossRef]
- Yang, X.-W.; Tan, W.-F.; Yu, W.-L.; Shi, S.; Wang, Y.; Zhang, Y.-L.; Zhang, Y.-J.; Wu, M.-C. Diagnosis and surgical treatment of primary hepatic lymphoma. World J. Gastroenterol. 2010, 16, 6016. [Google Scholar]
- Leng-Kit Lei, K.; Hei-Sing Chow, J.; James Johnson, P. Aggressive primary hepatic lymphoma in Chinese patients. Presentation, pathologic features, and outcome. Cancer 1995, 76, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.C.; Kornstein, M.J. Malignant lymphoma imitating hepatitis. Cancer 1993, 71, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, G.; d’Oiron, R.; Amato, A.; Leger-Ravet, M.; Iseni, M.; Smadja, C.; Lemaigre, G.; Franco, D. Primary Lymphoplasmacytic Lymphoma of the Liver Associated with a Serum Monoclonal Peak of IgG κ. Am. J. Gastroenterol. 1995, 90, 137–140. [Google Scholar]
- Ozaki, K.; Ikeno, H.; Koneri, K.; Higuchi, S.; Hosono, N.; Kosaka, N.; Goi, T.; Gabata, T.; Kimura, H. Primary hepatic diffuse large B-cell lymphoma presenting unusual imaging features. Clin. J. Gastroenterol 2020, 13, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Tomasian, A.; Sandrasegaran, K.; Elsayes, K.M.; Shanbhogue, A.; Shaaban, A.; Menias, C.O. Hematologic malignancies of the liver: Spectrum of disease. Radiographics 2015, 35, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Trenker, C.; Kunsch, S.; Michl, P.; Wissniowski, T.T.; Goerg, K.; Goerg, C. Contrast-enhanced ultrasound (CEUS) in hepatic lymphoma: Retrospective evaluation in 38 cases. Ultraschall Med. 2014, 35, 142–148. [Google Scholar] [CrossRef]
- Isaacson, P.; Wright, D.H. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer 1983, 52, 1410–1416. [Google Scholar] [CrossRef]
- Iida, T.; Iwahashi, M.; Nakamura, M.; Nakamori, M.; Yokoyama, S.; Tani, M.; Akamatsu, H.; Nakamine, H.; Yamaue, H. Primary hepatic low-grade B-cell lymphoma of MALT-type associated with Helicobacter pylori infection. Hepatogastroenterology 2007, 54, 1898–1901. [Google Scholar]
- Willenbrock, K.; Kriener, S.; Oeschger, S.; Hansmann, M.L. Nodular lymphoid lesion of the liver with simultaneous focal nodular hyperplasia and hemangioma: Discrimination from primary hepatic MALT-type non-Hodgkin’s lymphoma. Virchows Arch 2006, 448, 223–227. [Google Scholar] [CrossRef]
- Chatelain, D.; Maes, C.; Yzet, T.; Brevet, M.; Bounicaud, D.; Plachot, J.P.; Verhaeghe, P. Primary hepatic lymphoma of MALT-type: A tumor that can simulate a liver metastasis. Ann. Chir. 2006, 131, 121–124. [Google Scholar] [CrossRef]
- Xu, Z.; Pang, C.; Sui, J.; Gao, Z. A case of primary hepatic extranodal marginal zone B-cell mucosa-associated lymphoid tissue (MALT) lymphoma treated by radiofrequency ablation (RFA), and a literature review. J. Int. Med. Res. 2021, 49, 0300060521999539. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, L.; Chen, Y.; Chen, X. Primary hepatic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type: A case report and literature review. Medicine 2017, 96, e6305. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, J.H.; Kim, K.; Kim, M.; Choi, H.J.; Kim, Y.M.; Suh, J.H.; Seo, M.J.; Cha, H.J. Primary hepatic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue. J. Pathol. Transl. Med. 2020, 54, 340–345. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Abramowicz, J.S.; Chammas, M.C.; Chou, Y.H.; Condous, G.; Kim, S.H.; Nolsoe, C.P.; Jenssen, C.; Vinayak, S. World Federation for Ultrasound in Medicine and Biology (WFUMB) Policy Document Development Strategy—Clinical Practice Guidelines, Position Statements and Technological Reviews (on behalf of the WFUMB publication committee and Executive Bureau). Ultrasound Med. Biol. 2021, 47, 2779–2781. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Del Mar, C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: A systematic review. JAMA Intern. Med. 2015, 175, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.C.; Del Mar, C. Clinicians’ Expectations of the Benefits and Harms of Treatments, Screening, and Tests: A Systematic Review. JAMA Intern. Med. 2017, 177, 407–419. [Google Scholar] [CrossRef]
- Booth, T.C.; Jackson, A.; Wardlaw, J.M.; Taylor, S.A.; Waldman, A.D. Incidental findings found in “healthy” volunteers during imaging performed for research: Current legal and ethical implications. Br. J. Radiol. 2010, 83, 456–465. [Google Scholar] [CrossRef]
- Booth, T.C.; Waldman, A.D.; Wardlaw, J.M.; Taylor, S.A.; Jackson, A. Management of incidental findings during imaging research in “healthy” volunteers: Current UK practice. Br. J. Radiol. 2012, 85, 11–21. [Google Scholar] [CrossRef]
- Booth, T.C.; Najim, R.; Petkova, H. Incidental findings discovered during imaging: Implications for general practice. Br. J. Gen. Pract. 2016, 66, 346–347. [Google Scholar] [CrossRef]
- Berland, L.L.; Silverman, S.G.; Gore, R.M.; Mayo-Smith, W.W.; Megibow, A.J.; Yee, J.; Brink, J.A.; Baker, M.E.; Federle, M.P.; Foley, W.D.; et al. Managing incidental findings on abdominal CT: White paper of the ACR incidental findings committee. J. Am. Coll. Radiol. 2010, 7, 754–773. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Ignee, A.; Barreiros, A.P.; Schreiber-Dietrich, D.; Sienz, M.; Bojunga, J.; Braden, B. Contrast-enhanced ultrasound for imaging of adrenal masses. Ultraschall Med. 2010, 31, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Tarique, U.; Tang, B.; Singh, M.; Kulasegaram, K.M.; Ailon, J. Ultrasound Curricula in Undergraduate Medical Education: A Scoping Review. J Ultrasound Med. 2018, 37, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.O.; Hegenscheid, K.; Erdmann, P.; Kohlmann, T.; Langanke, M.; Völzke, H.; Puls, R.; Assel, H.; Biffar, R.; Grabe, H.J. Psychosocial consequences and severity of disclosed incidental findings from whole-body MRI in a general population study. Eur. Radiol. 2013, 23, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.K. Patient explanation guidelines for incidentalomas: Helping patients not to fear the delayed surveillance. AJR Am. J. Roentgenol. 2014, 202, W602. [Google Scholar] [CrossRef]
- Wolf, S.M.; Lawrenz, F.P.; Nelson, C.A.; Kahn, J.P.; Cho, M.K.; Clayton, E.W.; Fletcher, J.G.; Georgieff, M.K.; Hammerschmidt, D.; Hudson, K.; et al. Managing incidental findings in human subjects research: Analysis and recommendations. J. Law Med. Ethics A. J. Am. Soc. Law Med. Ethics 2008, 36, 219–248, 211. [Google Scholar] [CrossRef]
- Kole, J.; Fiester, A. Incidental findings and the need for a revised informed consent process. AJR Am. J. Roentgenol. 2013, 201, 1064–1068. [Google Scholar] [CrossRef]
- Cotter, A.R.; Vuong, K.; Mustelin, L.; Yang, Y.; Rakhmankulova, M.; Barclay, C.J.; Harris, R.P. Do psychological harms result from being labelled with an unexpected diagnosis of abdominal aortic aneurysm or prostate cancer through screening? A systematic review. BMJ Open 2017, 7, e017565. [Google Scholar] [CrossRef]
- Korenstein, D.; Chimonas, S.; Barrow, B.; Keyhani, S.; Troy, A.; Lipitz-Snyderman, A. Development of a Conceptual Map of Negative Consequences for Patients of Overuse of Medical Tests and Treatments. JAMA Intern. Med. 2018, 178, 1401–1407. [Google Scholar] [CrossRef]
- Cawood, T.J.; Hunt, P.J.; O’Shea, D.; Cole, D.; Soule, S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur. J. Endocrinol. 2009, 161, 513–527. [Google Scholar] [CrossRef]
- Chadha, D.S.; Sharma, S.; Sivasankar, R.; Kudva, N.; Sabhiki, G.; Behl, A. Abdominal sonography in the medical evaluation of aviation aspirants. Aviat. Space Environ. Med. 2010, 81, 965–969. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Maurer, M.; Riemer-Hommel, P. Challenges for the German Health Care System–Pharmaceuticals. Endheu 2014, 27, 45–53. [Google Scholar] [CrossRef]
- Ding, A.; Eisenberg, J.D.; Pandharipande, P.V. The economic burden of incidentally detected findings. Radiol. Clin. North Am. 2011, 49, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Orme, N.M.; Fletcher, J.G.; Siddiki, H.A.; Harmsen, W.S.; O’Byrne, M.M.; Port, J.D.; Tremaine, W.J.; Pitot, H.C.; McFarland, E.G.; Robinson, M.E.; et al. Incidental findings in imaging research: Evaluating incidence, benefit, and burden. Arch. Intern. Med. 2010, 170, 1525–1532. [Google Scholar] [CrossRef]
- Yusuf, G.T.; Sellars, M.E.; Deganello, A.; Cosgrove, D.O.; Sidhu, P.S. Retrospective Analysis of the Safety and Cost Implications of Pediatric Contrast-Enhanced Ultrasound at a Single Center. AJR Am. J. Roentgenol. 2017, 208, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Streb, J.W.; Tchelepi, H.; Malhi, H.; Deurdulian, C.; Grant, E.G. Retrospective Analysis of Contrast-enhanced Ultrasonography Effectiveness in Reducing Time to Diagnosis and Imaging-related Expenditures at a Single Large United States County Hospital. Ultrasound Q. 2019, 35, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Westwood, M.; Joore, M.; Grutters, J.; Redekop, K.; Armstrong, N.; Lee, K.; Gloy, V.; Raatz, H.; Misso, K.; Severens, J.; et al. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2013, 17, 1. [Google Scholar] [CrossRef]
- Smajerova, M.; Petrasova, H.; Little, J.; Ovesna, P.; Andrasina, T.; Valek, V.; Nemcova, E.; Miklosova, B. Contrast-enhanced ultrasonography in the evaluation of incidental focal liver lesions: A cost-effectiveness analysis. World J. Gastroenterol. 2016, 22, 8605–8614. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Xue, X.; Gyftopoulos, S.; Kim, D.C.; Nicola, G.N. Downstream Costs Associated With Incidental Pancreatic Cysts Detected at MRI. AJR Am. J. Roentgenol. 2018, 211, 1278–1282. [Google Scholar] [CrossRef]
- Brown, S.D. Professional norms regarding how radiologists handle incidental findings. J. Am. Coll. Radiol. 2013, 10, 253–257. [Google Scholar] [CrossRef]
- Jeon, J.H.; Kim, J.H.; Joo, I.; Lee, S.; Choi, S.Y.; Han, J.K. Transabdominal Ultrasound Detection of Pancreatic Cysts Incidentally Detected at CT, MRI, or Endoscopic Ultrasound. AJR Am. J. Roentgenol. 2018, 210, 518–525. [Google Scholar] [CrossRef]
- Morelli, L.; Guadagni, S.; Borrelli, V.; Pisano, R.; Di Franco, G.; Palmeri, M.; Furbetta, N.; Gambaccini, D.; Marchi, S.; Boraschi, P.; et al. Role of abdominal ultrasound for the surveillance follow-up of pancreatic cystic neoplasms: A cost-effective safe alternative to the routine use of magnetic resonance imaging. World J. Gastroenterol. 2019, 25, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Mork, H.; Ignee, A.; Schuessler, G.; Ott, M.; Dietrich, C.F. Analysis of neuroendocrine tumour metastases in the liver using contrast enhanced ultrasonography. Scand.J.Gastroenterol. 2007, 42, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Venturi, A.; Mancini, M.; Giangregorio, F.; Vidili, G.; Magnolfi, F.; Mirarchi, M.; Fornari, F.; Bolondi, L. Diagnostic features of real-time contrast-enhanced ultrasound in focal nodular hyperplasia of the liver. Ultraschall Med. 2010, 31, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E. Solid focal liver lesions indeterminate by contrast-enhanced CT or MR imaging: The added diagnostic value of contrast-enhanced ultrasound. Abdom. Imaging 2012, 37, 580–590. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cekuolis, A.; Schreiber-Dietrich, D.; Augustinienė, R.; Taut, H.; Squires, J.; Chaves, E.L.; Dong, Y.; Dietrich, C.F. Incidental Findings in Pediatric Patients: How to Manage Liver Incidentaloma in Pediatric Patients. Cancers 2023, 15, 2360. https://doi.org/10.3390/cancers15082360
Cekuolis A, Schreiber-Dietrich D, Augustinienė R, Taut H, Squires J, Chaves EL, Dong Y, Dietrich CF. Incidental Findings in Pediatric Patients: How to Manage Liver Incidentaloma in Pediatric Patients. Cancers. 2023; 15(8):2360. https://doi.org/10.3390/cancers15082360
Chicago/Turabian StyleCekuolis, Andrius, Dagmar Schreiber-Dietrich, Rasa Augustinienė, Heike Taut, Judy Squires, Edda L. Chaves, Yi Dong, and Christoph F. Dietrich. 2023. "Incidental Findings in Pediatric Patients: How to Manage Liver Incidentaloma in Pediatric Patients" Cancers 15, no. 8: 2360. https://doi.org/10.3390/cancers15082360
APA StyleCekuolis, A., Schreiber-Dietrich, D., Augustinienė, R., Taut, H., Squires, J., Chaves, E. L., Dong, Y., & Dietrich, C. F. (2023). Incidental Findings in Pediatric Patients: How to Manage Liver Incidentaloma in Pediatric Patients. Cancers, 15(8), 2360. https://doi.org/10.3390/cancers15082360







