Risk Factors for Palbociclib-Induced Early Developing Neutropenia in Patients with Hormone Receptor-Positive Metastatic Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients and Study Design
2.2. Outcome Measurements
2.3. Statistical Analysis
2.4. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glass, A.G.; Lacey, J.V., Jr.; Carreon, J.D.; Hoover, R.N. Breast cancer incidence, 1980–2006: Combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J. Natl. Cancer Inst. 2007, 99, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Maximov, P.Y.; Lee, T.M.; Jordan, V.C. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr. Clin. Pharmacol. 2013, 8, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Schiff, R. Mechanisms of endocrine resistance in breast cancer. Annu. Rev. Med. 2011, 62, 233–247. [Google Scholar] [CrossRef]
- Chlebowski, R.T. Changing concepts of hormone receptor-positive advanced breast cancer therapy. Clin. Breast Cancer 2013, 13, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Dong, P.; Gassler, N.; Taheri, M.; Baniahmad, A.; Dilmaghani, N.A. A review on the role of cyclin dependent kinases in cancers. Cancer Cell Int. 2022, 22, 325. [Google Scholar] [CrossRef]
- Finn, R.S.; Dering, J.; Conklin, D.; Kalous, O.; Cohen, D.J.; Desai, A.J.; Ginther, C.; Atefi, M.; Chen, I.; Fowst, C.; et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009, 11, R77. [Google Scholar] [CrossRef]
- Shah, M.; Nunes, M.R.; Stearns, V. CDK4/6 Inhibitors: Game Changers in the Management of Hormone Receptor-Positive Advanced Breast Cancer? Oncology 2018, 32, 216–222. [Google Scholar]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Turner, N.C.; Ro, J.; Andre, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Mukai, H.; Park, I.H.; Masuda, N.; Shimizu, C.; Kim, S.B.; Im, Y.H.; Ohtani, S.; Huang Bartlett, C.; Lu, D.R.; et al. Palbociclib Plus Letrozole as First-Line Therapy in Postmenopausal Asian Women with Metastatic Breast Cancer: Results From the Phase III, Randomized PALOMA-2 Study. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dieras, V.; Rugo, H.S.; Schnell, P.; Gelmon, K.; Cristofanilli, M.; Loi, S.; Colleoni, M.; Lu, D.R.; Mori, A.; Gauthier, E.; et al. Long-term Pooled Safety Analysis of Palbociclib in Combination with Endocrine Therapy for HR+/HER2- Advanced Breast Cancer. J. Natl. Cancer Inst. 2019, 111, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Thill, M.; Schmidt, M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther. Adv. Med. Oncol. 2018, 10, 1758835918793326. [Google Scholar] [CrossRef]
- Spring, L.M.; Zangardi, M.L.; Moy, B.; Bardia, A. Clinical Management of Potential Toxicities and Drug Interactions Related to Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: Practical Considerations and Recommendations. Oncologist 2017, 22, 1039–1048. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Ettl, J.; Schmidt, M.; Bondarenko, I.M.; Lang, I.; Pinter, T.; Boer, K.; Patel, R.; Randolph, S.; et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: Expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res. 2016, 18, 67. [Google Scholar] [CrossRef]
- Dieras, V.; Harbeck, N.; Joy, A.A.; Gelmon, K.; Ettl, J.; Verma, S.; Lu, D.R.; Gauthier, E.; Schnell, P.; Mori, A.; et al. Palbociclib with Letrozole in Postmenopausal Women with ER+/HER2- Advanced Breast Cancer: Hematologic Safety Analysis of the Randomized PALOMA-2 Trial. Oncologist 2019, 24, 1514–1525. [Google Scholar] [CrossRef]
- Ettl, J.; Im, S.A.; Ro, J.; Masuda, N.; Colleoni, M.; Schnell, P.; Bananis, E.; Lu, D.R.; Cristofanilli, M.; Rugo, H.S.; et al. Hematologic adverse events following palbociclib dose reduction in patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Pooled analysis from randomized phase 2 and 3 studies. Breast Cancer Res. 2020, 22, 27. [Google Scholar] [CrossRef]
- Vazquez, L.; Coussirou, J.; Arvers, P.; Rossi, J.; Arnaud, A. Palbociclib Associated with Endocrine Therapy in Patients with Metastatic Breast Cancer: Predictive Factors of Severe Early Hematological Toxicity. J. Cancer Sci. Ther. 2019, 11, 290–294. [Google Scholar]
- Bruno, R.; Vivier, N.; Vergniol, J.C.; De Phillips, S.L.; Montay, G.; Sheiner, L.B. A population pharmacokinetic model for docetaxel (Taxotere): Model building and validation. J. Pharm. Biopharm. 1996, 24, 153–172. [Google Scholar] [CrossRef]
- Leenhardt, F.; Fiteni, F.; Gauthier, L.; Alexandre, M.; Guiu, S.; Firmin, N.; Pouderoux, S.; Viala, M.; Lossaint, G.; Gautier, C.; et al. Pharmacokinetic Variability Drives Palbociclib-Induced Neutropenia in Metastatic Breast Cancer Patients: Drug-Drug Interactions Are the Usual Suspects. Pharmaceutics 2022, 14, 841. [Google Scholar] [CrossRef] [PubMed]
- Roncato, R.; Gerratana, L.; Palmero, L.; Gagno, S.; Poetto, A.S.; Peruzzi, E.; Zanchetta, M.; Posocco, B.; De Mattia, E.; Canil, G.; et al. An Integrated Pharmacological Counselling Approach to Guide Decision-Making in the Treatment with CDK4/6 Inhibitors for Metastatic Breast Cancer. Front. Pharmacol. 2022, 13, 897951. [Google Scholar] [CrossRef] [PubMed]
- Westerdijk, K.; Desar, I.M.E.; Steeghs, N.; van der Graaf, W.T.A.; van Erp, N.P.; Dutch, P.; Oncology, G. Imatinib, sunitinib and pazopanib: From flat-fixed dosing towards a pharmacokinetically guided personalized dose. Br. J. Clin. Pharmacol. 2020, 86, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Morita, S.Y.; Terada, T. Dose Individualization of Oral Multi-Kinase Inhibitors for the Implementation of Therapeutic Drug Monitoring. Biol. Pharm. Bull. 2022, 45, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; Druker, B.J.; Guilhot, F.; O’Brien, S.G.; Riviere, G.J.; Krahnke, T.; Gathmann, I.; Wang, Y.; Group, I.S. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: A subanalysis of the IRIS study. Blood 2008, 111, 4022–4028. [Google Scholar] [CrossRef]
- Iwata, H.; Im, S.A.; Masuda, N.; Im, Y.H.; Inoue, K.; Rai, Y.; Nakamura, R.; Kim, J.H.; Hoffman, J.T.; Zhang, K.; et al. PALOMA-3: Phase III Trial of Fulvestrant with or Without Palbociclib in Premenopausal and Postmenopausal Women with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer That Progressed on Prior Endocrine Therapy-Safety and Efficacy in Asian Patients. J. Glob. Oncol. 2017, 3, 289–303. [Google Scholar] [CrossRef]
- Kimura, M.; Usami, E.; Yoshimura, T. Association between severe neutropenia and progression-free survival in patients with advanced or recurrent breast cancer treated with palbociclib. Pharmazie 2020, 75, 662–665. [Google Scholar] [CrossRef]
- Lavery, L.; DiSogra, K.; Lea, J.; Trufan, S.J.; Symanowski, J.T.; Roberts, A.; Moore, D.C.; Heeke, A.; Pal, S. Risk factors associated with palbociclib-induced neutropenia in patients with metastatic breast cancer. Support. Care Cancer 2022, 30, 9803–9809. [Google Scholar] [CrossRef]
- Iwata, H.; Umeyama, Y.; Liu, Y.; Zhang, Z.; Schnell, P.; Mori, Y.; Fletcher, O.; Marshall, J.C.; Johnson, J.G.; Wood, L.S.; et al. Evaluation of the Association of Polymorphisms with Palbociclib-Induced Neutropenia: Pharmacogenetic Analysis of PALOMA-2/-3. Oncologist 2021, 26, e1143–e1155. [Google Scholar] [CrossRef]
- Kanbayashi, Y.; Sakaguchi, K.; Ishikawa, T.; Takayama, K.; Taguchi, T. Predictors for development of palbociclib-induced neutropenia in breast cancer patients as determined by ordered logistic regression analysis. Sci. Rep. 2021, 11, 20055. [Google Scholar] [CrossRef]
- Bellet, M.; Ahmad, F.; Villanueva, R.; Valdivia, C.; Palomino-Doza, J.; Ruiz, A.; Gonzalez, X.; Adrover, E.; Azaro, A.; Valls-Margarit, M.; et al. Palbociclib and ribociclib in breast cancer: Consensus workshop on the management of concomitant medication. Ther. Adv. Med. Oncol. 2019, 11, 1758835919833867. [Google Scholar] [CrossRef] [PubMed]
- Del Re, M.; Omarini, C.; Diodati, L.; Palleschi, M.; Meattini, I.; Crucitta, S.; Lorenzini, G.; Isca, C.; Fontana, A.; Livi, L.; et al. Drug-drug interactions between palbociclib and proton pump inhibitors may significantly affect clinical outcome of metastatic breast cancer patients. ESMO Open 2021, 6, 100231. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Miura, M.; Niioka, T.; Sawada, K. Influence of H2-receptor antagonists and proton pump inhibitors on dasatinib pharmacokinetics in Japanese leukemia patients. Cancer Chemother. Pharm. 2012, 69, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Klamerus, K.J.; Yuhas, L.M.; Pawlak, S.; Plotka, A.; O’Gorman, M.; Kirkovsky, L.; Kosa, M.; Wang, D. Impact of Acid-Reducing Agents on the Pharmacokinetics of Palbociclib, a Weak Base with pH-Dependent Solubility, with Different Food Intake Conditions. Clin. Pharmacol. Drug Dev. 2017, 6, 614–626. [Google Scholar] [CrossRef] [PubMed]
- El Badri, S.; Tahir, B.; Balachandran, K.; Bezecny, P.; Britton, F.; Davies, M.; Desouza, K.; Dixon, S.; Hills, D.; Moe, M.; et al. Palbociclib in combination with aromatase inhibitors in patients >/= 75 years with oestrogen receptor-positive, human epidermal growth factor receptor 2 negative advanced breast cancer: A real-world multicentre UK study. Breast 2021, 60, 199–205. [Google Scholar] [CrossRef]
- Harbeck, N.; Bartlett, M.; Spurden, D.; Hooper, B.; Zhan, L.; Rosta, E.; Cameron, C.; Mitra, D.; Zhou, A. CDK4/6 inhibitors in HR+/HER2− advanced/metastatic breast cancer: A systematic literature review of real-world evidence studies. Future Oncol. 2021, 17, 2107–2122. [Google Scholar] [CrossRef]
- McAndrew, N.P.; Dickson, M.A.; Clark, A.S.; Troxel, A.B.; O’Hara, M.H.; Colameco, C.; Gallager, M.; Gramlich, K.; Zafman, K.; Vaughn, D.; et al. Early treatment-related neutropenia predicts response to palbociclib. Br. J. Cancer 2020, 123, 912–918. [Google Scholar] [CrossRef]
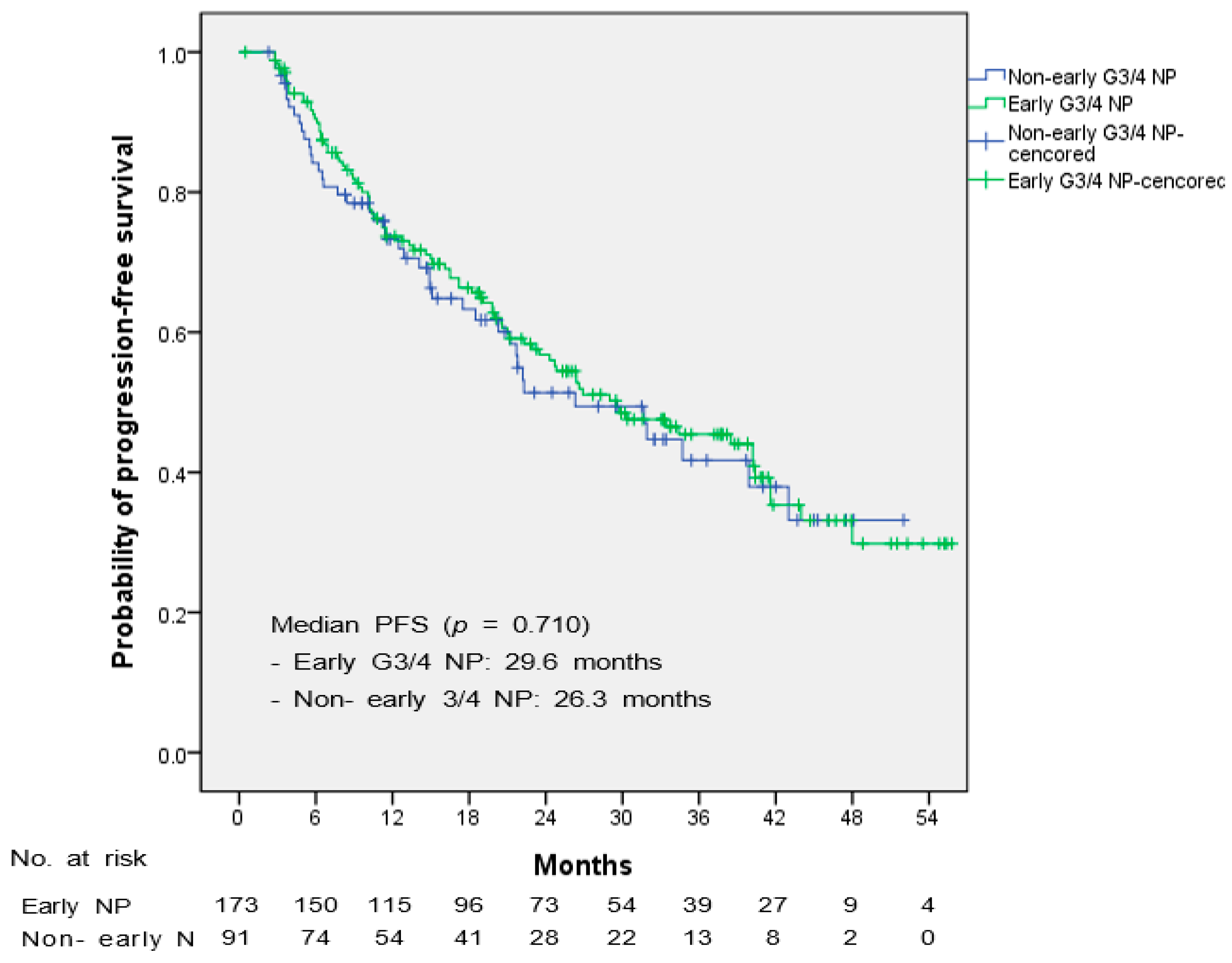
| Characteristic | Total | Early G3/4 NP | Non-Early G3/4 NP | p |
|---|---|---|---|---|
| (n = 264) | (n = 173) | (n = 91) | ||
| Age, years—median (range) | ||||
| 55 (29–90) | 55 (29–90) | 56 (35–90) | 0.751 | |
| Weight (kg)—n (%) | ||||
| <58 | 130 (49.2) | 92 (53.2) | 38 (41.8) | 0.078 |
| ≥58 | 134 (50.8) | 81 (46.8) | 53 (58.2) | |
| BSA (m2)—n (%) | ||||
| <1.58 | 119 (45.1) | 86 (49.9) | 33 (36.3) | 0.037 |
| ≥1.58 | 145 (54.9) | 87 (50.3) | 58 (63.7) | |
| BMI (kg/m2)—n (%) | ||||
| <23 | 105 (39.8) | 77 (45.8) | 28 (30.8) | 0.030 |
| ≥23 | 159 (60.2) | 96 (55.5) | 64 (69.2) | |
| ECOG PS—n (%) | 0.382 | |||
| 0 | 98 (37.3) | 64 (37.2) | 34 (37.4) | |
| 1 | 98 (37.3) | 69 (40.1) | 29 (31.9) | |
| ≥2 | 20 (7.6) | 11 (6.4) | 9 (9.9) | |
| Unknown | 47 (17.9) | 28 (16.3) | 19 (20.9) | |
| Disease status—n (%) | 0.896 | |||
| De novo | 68 (25.8) | 45 (26.0) | 23 (25.3) | |
| Recurrence | 196 (74.2) | 128 (74.0) | 68 (74.7) | |
| Metastatic disease site—n (%) | 0.367 | |||
| Viceral | 155 (58.7) | 105 (60.7) | 50 (54.9) | |
| Nonviceral | 109 (41.3) | 68 (39.3) | 41 (45.1) | |
| Bone | 154 (58.3) | 94 (54.3) | 60 (65.9) | 0.069 |
| Bone only | 53 (20.1) | 32 (18.5) | 21 (23.1) | 0.377 |
| Metastatic disease site—n (%) | 0.211 | |||
| 1 | 119 (45.1) | 80 (46.2) | 39 (42.9) | |
| 2 | 83 (31.4) | 58 (33.5) | 25 (27.5) | |
| ≥3 | 62 (23.5) | 35 (20.2) | 27 (29.7) | |
| Menopausal status—n (%) | 0.739 | |||
| Premenopausal | 7 (2.7) | 5 (2.9) | 2 (2.2) | |
| Postmenopausal | 257 (97.3) | 168 (97.1) | 89 (97.8) | |
| BSO in a Month—n (%) | 0.818 | |||
| Yes | 76 (28.2) | 49 (28.3) | 27 (29.7) | |
| No | 188 (71.1) | 124 (71.7) | 64 (70.3) | |
| Treatment line—n (%) | 0.939 | |||
| 1 | 203 (76.9) | 134 (77.5) | 69 (75.8) | |
| 2 | 24 (9.1) | 15 (8.7) | 9 (9.9) | |
| ≥3 | 37 (14.0) | 24 (13.9) | 13 (35.1) | |
| Prior Chemotherapy for metastatic disease—n (%) | 0.807 | |||
| Yes | 33 (12.5) | 21 (12.1) | 12 (13.2) | |
| No | 231 (87.5) | 152 (87.9) | 79 (86.8) | |
| Prior Endocrine therapy for metastatic disease—n (%) | 0.499 | |||
| Yes | 52 (19.7) | 32 (18.5) | 20 (22.0) | |
| No | 212 (80.3) | 141 (81.5) | 71 (78.0) | |
| Prior radiotherapy—n (%) | 0.686 | |||
| Yes | 41 (15.5) | 28 (16.2) | 13 (14.3) | |
| No | 223 (84.5) | 145 (83.8) | 78 (85.7) | |
| Bone RT | 31 (11.7) | 24 (13.9) | 7 (7.7) | 0.138 |
| Bone RT in a year | 21 (8.0) | 17 (9.8) | 4 (4.4) | 0.121 |
| Baseline laboratory test—median (IQR) | ||||
| ANC (cell/mm3) | 3401 (2468–4567) | 3072 (2273–5081) | 4356 (3130–5304) | <0.001 |
| WBC (×103/mm3) | 6.04 (4.76–7.42) | 5.44 (4.26–6.65) | 6.87 (5.99–8.67) | <0.001 |
| Hb (g/dL) | 12.5 (11.5–13.5) | 12.5 (11.6–13.5) | 12.8 (11.8–14.7) | 0.033 |
| PLT (×103/mm3) | 236 (191–293) | 227 (187–285) | 258 (221–315) | 0.001 |
| Total bilirubin(mg/dL) | 0.5 (0.4–0.6) | 0.5 (0.4–0.7) | 0.5 (0.4–0.6) | 0.509 |
| AST (IU/L) | 23 (20–32.5) | 24 (20–33) | 22 (18–31) | 0.822 |
| ALT (IU/L) | 18 (13–29) | 19 (13–31) | 17 (13–26) | 0.879 |
| Comorbidities—n (%) | ||||
| Hypertension | 86 (32.6) | 58 (33.5) | 28 (30.8) | 0.65 |
| DM | 50 (18.9) | 28 (16.2) | 22 (24.2) | 0.115 |
| Dyslipidemia | 49 (18.6) | 32 (18.5) | 17 (18.7) | 0.971 |
| Concomitant medication—n (%) | ||||
| Denosumab | 76 (28.8) | 51 (29.5) | 25 (27.5) | 0.732 |
| CaD | 169 (64.0) | 110 (63.6) | 59 (64.8) | 0.84 |
| CYP3A4 inhibitor | 47 (17.8) | 31 (17.9) | 16 (17.6) | 0.946 |
| ARB | 55 (20.8) | 39 (22.5) | 16 (17.6) | 0.346 |
| Statin | 63 (23.9) | 41 (23.7) | 22 (24.2) | 0.931 |
| H2 blocker | 13 (4.9) | 8 (4.6) | 5 (5.5) | 0.756 |
| PPI | 16 (6.1) | 10 (5.8) | 6 (6.6) | 0.792 |
| Antacid | 18 (6.8) | 13 (7.5) | 5 (5.5) | 0.536 |
| Combination—n (%) | 0.582 | |||
| AI | 210 (79.5) | 140 (80.9) | 70 (76.9) | |
| Fulvestrant | 54 (20.5) | 33 (19.1) | 21 (23.1) | |
| Initial dose—n (%) | 0.092 | |||
| 125 mg | 230 (87.1) | 156 (90.2) | 74 (81.3) | |
| 100 mg | 30 (11.4) | 15 (8.7) | 15 (16.5) | |
| 75 mg | 4 (1.5) | 2 (1.2) | 2 (2.2) | |
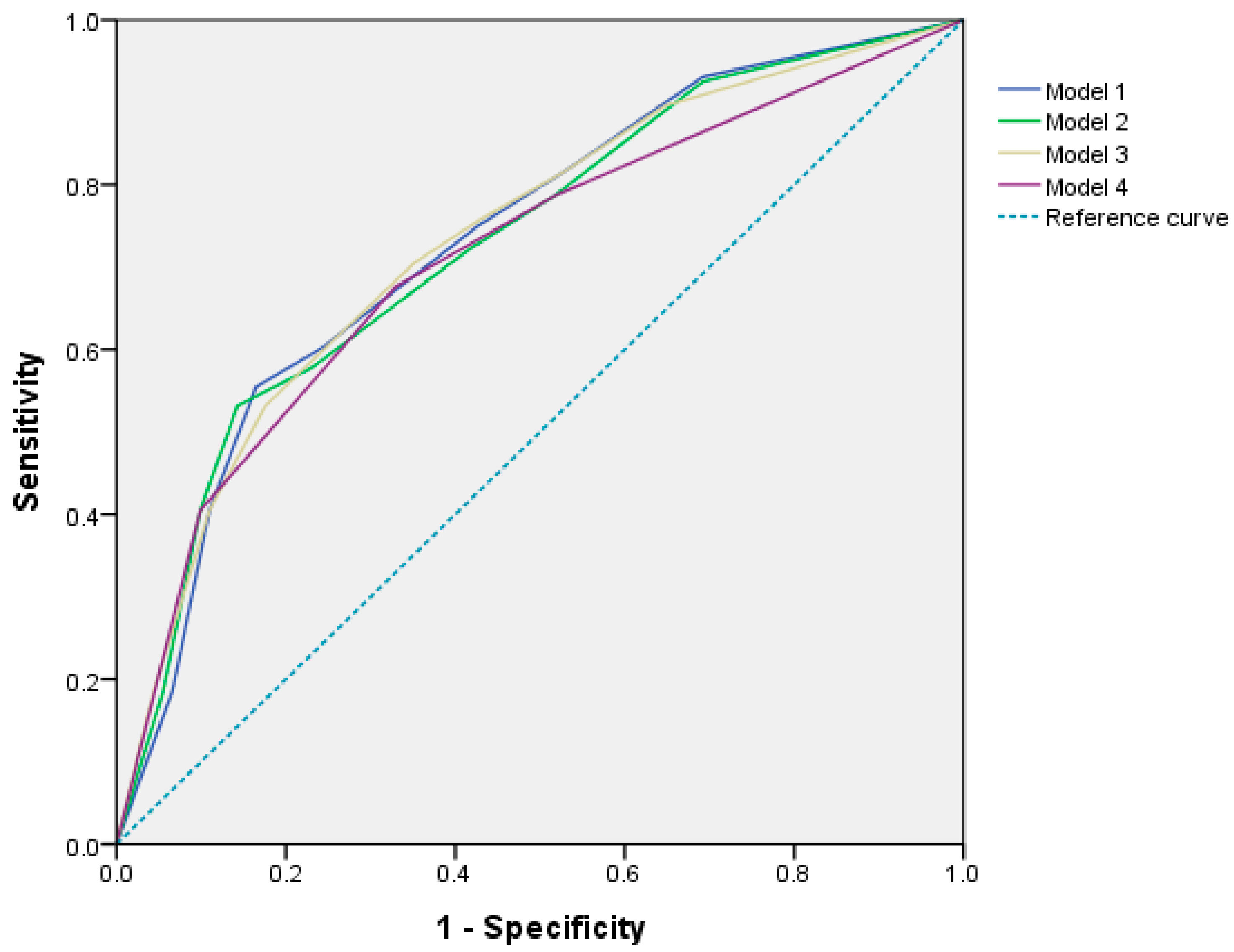
| Predictors | Unadjusted OR | Model Ⅰ | Model Ⅱ | Model Ⅲ | Model Ⅳ |
|---|---|---|---|---|---|
| (95% CIs) | Adjusted OR | Adjusted OR | Adjusted OR | Adjusted OR | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||
| Age < 60 years | 1.021 (0.600–1.739) | ||||
| BSA < 1.58 m2 | 1.737 (1.032–2.925) * | 1.916 (1.087–3.376) * | 2.050 (1.160–3.623) * | ||
| BMI < 23 kg/m2 | 1.805 (1.055–3.087) * | ||||
| ANC < 3700/mm3 | 4.411 (2.569–7.571) *** | 4.110 (2.350–7.189) *** | 3.973 (2.281–6.919) *** | ||
| WBC < 6.30 × 103/mm3 | 4.248 (2.474–7.296) *** | 4.043 (2.295–7.120) *** | 3.732 (2.148–6.483) *** | ||
| PLT < 230 × 103/mm3 | 2.649 (1.538–4.563) *** | 2.194 (1.229–3.915) ** | 2.108 (1.180–3.766) * | 2.083 (1.170–3.711) * | 2.091 (1.180–3.707) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Lee, D.; Seo, I.; Chae, H.; Sim, S.H.; Lee, K.S.; Gwak, H.S. Risk Factors for Palbociclib-Induced Early Developing Neutropenia in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Cancers 2023, 15, 2810. https://doi.org/10.3390/cancers15102810
Lee Y, Lee D, Seo I, Chae H, Sim SH, Lee KS, Gwak HS. Risk Factors for Palbociclib-Induced Early Developing Neutropenia in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Cancers. 2023; 15(10):2810. https://doi.org/10.3390/cancers15102810
Chicago/Turabian StyleLee, Yeonhong, Dayae Lee, Inyoung Seo, Heejung Chae, Sung Hoon Sim, Keun Seok Lee, and Hye Sun Gwak. 2023. "Risk Factors for Palbociclib-Induced Early Developing Neutropenia in Patients with Hormone Receptor-Positive Metastatic Breast Cancer" Cancers 15, no. 10: 2810. https://doi.org/10.3390/cancers15102810
APA StyleLee, Y., Lee, D., Seo, I., Chae, H., Sim, S. H., Lee, K. S., & Gwak, H. S. (2023). Risk Factors for Palbociclib-Induced Early Developing Neutropenia in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Cancers, 15(10), 2810. https://doi.org/10.3390/cancers15102810







