Vitamin D and Calcium Supplement Use and High-Risk Breast Cancer: A Case–Control Study among BRCA1 and BRCA2 Mutation Carriers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
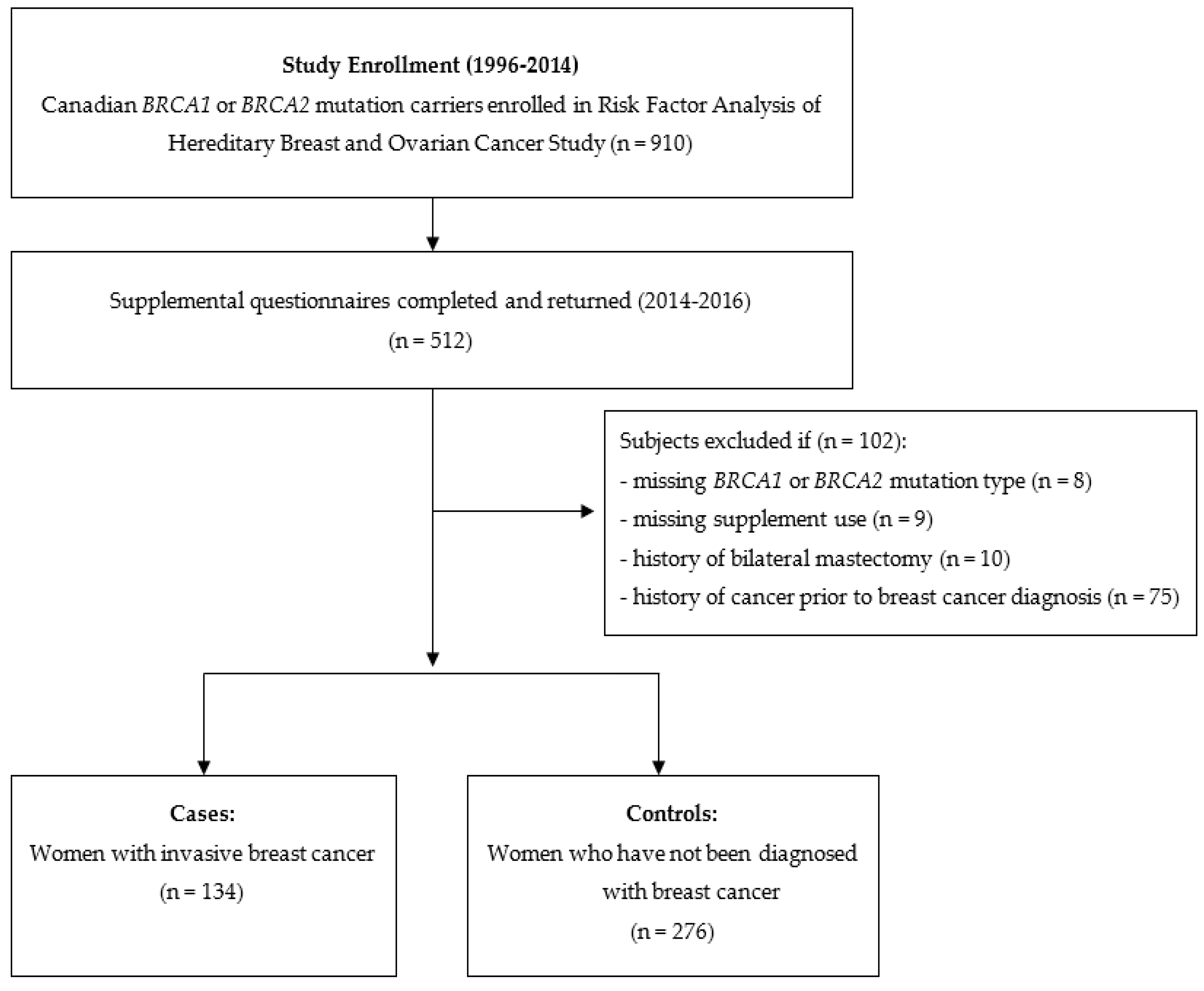
2.1. Study Population
2.2. Data Collection
2.3. Assessment of Supplement Use
2.4. Assessment of Case and Control Status
2.5. Subject Selection
2.6. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Vitamin D Supplement Use and Breast Cancer
3.3. Calcium Supplement Use and Breast Cancer
3.4. Joint Effects of Vitamin D and Calcium and Breast Cancer
3.5. Quantitative Bias Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadian Cancer Society. Breast Cancer Statistics. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/breast/statistics/?region=on (accessed on 22 July 2021).
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J. BRCA Mutations and Breast Cancer Prevention. Cancers 2018, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Use of Nutritional Supplements. Available online: https://www150.statcan.gc.ca/n1/pub/82-625-x/2017001/article/14831-eng.htm (accessed on 22 July 2021).
- Hossain, S.; Beydoun, M.A.; Beydoun, H.A.; Chen, X.; Zonderman, A.B.; Wood, R.J. Vitamin D and breast cancer: A systematic review and meta-analysis of observational studies. Clin. Nutr. ESPEN 2019, 30, 170–184. [Google Scholar] [CrossRef]
- Anderson, L.N.; Cotterchio, M.; Vieth, R.; Knight, J.A. Vitamin D and calcium intakes and breast cancer risk in pre- and postmenopausal women. Am. J. Clin. Nutr. 2010, 91, 1699–1707. [Google Scholar] [CrossRef]
- Visvanathan, K.; Mondul, A.M.; Zeleniuch-Jacquotte, A.; Wang, M.; Gail, M.H.; Yaun, S.S.; Weinstein, S.J.; McCullough, M.L.; Eliassen, A.H.; Cook, N.R.; et al. Circulating vitamin D and breast cancer risk: An international pooling project of 17 cohorts. Eur. J. Epidemiol. 2023, 38, 11–29. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Zhang, J.; Huang, X.; Thabane, L.; Li, G. Effect of vitamin D supplementation on risk of breast cancer: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2021, 8, 655727. [Google Scholar] [CrossRef]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Dietary supplement use among adults: United States, 2017–2018. NCHS Data Brief 2021, 399, 1–8. [Google Scholar]
- Grotsky, D.A.; Gonzalez-Suarez, I.; Novell, A.; Neumann, M.A.; Yaddanapudi, S.C.; Croke, M.; Martinez-Alonso, M.; Redwood, A.B.; Ortega-Martinez, S.; Feng, Z.; et al. BRCA1 loss activates cathepsin L–mediated degradation of 53BP1 in breast cancer cells. J. Cell Biol. 2013, 200, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Lubinski, J.; Lynch, H.T.; Tung, N.; Armel, S.; Senter, L.; Singer, C.F.; Fruscio, R.; Couch, F.; Weitzel, J.N.; et al. Oophorectomy and risk of contralateral breast cancer among BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 175, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Zhang, C.X.W.; Demsky, R.; Armel, S.; Kim, Y.I.; Narod, S.A.; Kotsopoulos, J. Folic acid supplement use and breast cancer risk in BRCA1 and BRCA2 mutation carriers: A case–control study. Breast Cancer Res. Treat. 2019, 174, 741–748. [Google Scholar] [CrossRef]
- Satia-Abouta, J.; Patterson, R.E.; King, I.B.; Stratton, K.L.; Shattuck, A.L.; Kristal, A.R.; Potter, J.D.; Thornquist, M.D.; White, E. Reliability and validity of self-report of vitamin and mineral supplement use in the vitamins and lifestyle study. Am. J. Epidemiol. 2003, 157, 944–954. [Google Scholar] [CrossRef]
- Lash, T.L.; Fox, M.P.; Fink, A.K. Applying Quantitative Bias Analysis to Epidemiologic Data, 1st ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- VanderWeele, T.J.; Ding, P. Sensitivity analysis in observational research: Introducing the E-value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, N.; Gómez-Acebo, I.; Palazuelos, C.; Llorca, J.; Dierssen-Sotos, T. Vitamin D exposure and risk of breast cancer: A meta-analysis. Sci. Rep. 2018, 8, 9039. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Sandler, D.P.; Taylor, J.A.; Weinberg, C.R. Serum vitamin D and risk of breast cancer within five years. Environ. Health Perspect. 2017, 125, 077004. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Vitamin D supplements and cancer incidence and mortality: A meta-analysis. Br. J. Cancer 2014, 111, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Bristow, S.M.; Bolland, M.J.; MacLennan, G.S.; Avenell, A.; Grey, A.; Gamble, G.D.; Reid, I.R. Calcium supplements and cancer risk: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1384–1393. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Gamble, G.D.; Reid, I.R. Calcium and vitamin D supplements and health outcomes: A reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am. J. Clin. Nutr. 2011, 94, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Muñoz, A. An update on vitamin d signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Colston, K.W.; Hansen, C.M. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr. Relat. Cancer 2002, 9, 45–59. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Its role in cancer prevention and treatment. Prog. Biophys. Mol. Biol. 2006, 92, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J.; Pawlowska, E.; Chojnacki, J.; Szczepanska, J.; Fila, M.; Chojnacki, C. Vitamin D in triple-negative and BRCA1-deficient breast cancer—Implications for pathogenesis and therapy. Int. J. Mol. Sci. 2020, 21, 3670. [Google Scholar] [CrossRef]
- Heublein, S.; Mayr, D.; Meindl, A.; Kircher, A.; Jeschke, U.; Ditsch, N. Vitamin D receptor, Retinoid X receptor and peroxisome proliferator-activated receptor γ are overexpressed in BRCA1 mutated breast cancer and predict prognosis. J. Exp. Clin. Cancer Res. 2017, 36, 57. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Wang, B.; Picon-Ruiz, M.; Buchwald, P.; Ince, T.A. Vitamin D and androgen receptor-targeted therapy for triple-negative breast cancer. Breast Cancer Res. Treat. 2016, 157, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Peterlik, M.; Grant, W.; Cross, H. Calcium, Vitamin D and Cancer. Anticancer Res. 2009, 29, 3687–3698. [Google Scholar] [PubMed]
| Characteristic | Cases | Controls | p a |
|---|---|---|---|
| (n = 134) | (n = 276) | ||
| Age at breast cancer diagnosis (year) | 41.5 ± 8.3 b | ||
| Age (year) c | 41.5 ± 8.3 | 42.7 ± 12.2 | 0.24 |
| Mutation (%) | 0.15 | ||
| BRCA1 | 61.9 | 54.3 | |
| BRCA2 | 38.1 | 45.7 | |
| BMI (kg/m2) | 26.4 ± 5.7 | 24.9 ± 4.7 | 0.01 |
| Age at menarche (year) | 12.4 ± 1.4 | 12.6 ± 1.5 | 0.13 |
| Menopausal status (%) | 0.01 | ||
| Premenopausal | 78.1 | 65.6 | |
| Postmenopausal | 21.9 | 34.4 | |
| Oral contraceptive use | 0.42 | ||
| Never | 15.6 | 19.0 | |
| Ever | 84.4 | 81.0 | |
| Parity (%) d | 0.0004 | ||
| 0 | 17.9 | 32.6 | |
| 1–2 | 61.9 | 41.7 | |
| ≥3 | 20.2 | 25.7 | |
| Prophylactic bilateral oophorectomy (%) | 6.8 | 12.0 | 0.11 |
| Alcohol consumption (%) | 0.16 | ||
| Never | 25.4 | 19.3 | |
| Ever | 74.6 | 80.7 | |
| Smoking status (%) | 0.07 | ||
| Never | 55.2 | 64.5 | |
| Ever | 44.8 | 35.5 | |
| Multivitamin supplement use (%) | 0.12 | ||
| Never | 76.9 | 69.2 | |
| Ever | 23.1 | 30.8 | |
| Prenatal supplement use (%) e | 0.16 | ||
| Never | 54.1 | 45.7 | |
| Ever | 45.9 | 54.3 | |
| Vitamin D | |||
| Vitamin D-specific supplement use (%) | 7.7 | 15.0 | 0.05 |
| Vitamin D-containing supplement use (%) | 60.2 | 68.5 | 0.11 |
| Total average daily vitamin D supplement use (mcg/day) | 2.5 ± 5.0 | 4.6 ± 8.4 | 0.01 |
| Calcium | |||
| Calcium-specific supplement use (%) | 3.4 | 8.7 | 0.06 |
| Calcium-containing supplement use (%) | 60.3 | 66.5 | 0.25 |
| Total average daily calcium supplement use (mg/day) | 36.9 ± 75.9 | 65.6 ± 126.6 | 0.02 |
| Cases/Controls | OR (95% CI) Basic Model a | p | OR (95% CI) Multivariable Model b | p | |
|---|---|---|---|---|---|
| Any vitamin D-containing supplement use c | |||||
| All Participants | |||||
| Never | 47/79 | Ref. | Ref. | Ref. | Ref. |
| Ever | 71/172 | 0.70 (0.44, 1.10) | 0.12 | 0.54 (0.31, 0.91) | 0.02 |
| BRCA1 | |||||
| Never | 30/41 | Ref. | Ref. | Ref. | Ref. |
| Ever | 41/94 | 0.60 (0.33, 1.10) | 0.10 | 0.40 (0.20, 0.81) | 0.01 |
| BRCA2 | |||||
| Never | 17/38 | Ref. | Ref. | Ref. | Ref. |
| Ever | 30/78 | 0.86 (0.42, 1.75) | 0.67 | 0.79 (0.35, 1.78) | 0.57 |
| Total average daily vitamin D supplement use (mcg/day) c | |||||
| All Participants | |||||
| None | 46/79 | Ref. | Ref. | Ref. | Ref. |
| 1.07 ≤ 7.50 | 26/44 | 1.03 (0.56, 1.90) | 0.91 | 0.72 (0.35, 1.46) | 0.36 |
| >7.50 | 22/75 | 0.51 (0.28, 0.92) | 0.03 | 0.44 (0.22, 0.89) | 0.02 |
| p-trend per mcg increase in vitamin D | 0.03 | 0.04 | |||
| BRCA1 | |||||
| None | 29/41 | Ref. | Ref. | Ref. | Ref. |
| 1.07 ≤ 7.50 | 17/20 | 1.19 (0.53, 2.65) | 0.68 | 0.74 (0.30, 1.86) | 0.53 |
| >7.50 | 11/42 | 0.37 (0.16, 0.84) | 0.02 | 0.28 (0.11, 0.71) | 0.007 |
| p-trend per mcg increase in vitamin D | 0.03 | 0.01 | |||
| BRCA2 | |||||
| None | 17/38 | Ref. | Ref. | Ref. | Ref. |
| 1.07 ≤ 7.50 | 9/24 | 0.87 (0.33, 2.27) | 0.77 | 0.66 (0.22, 1.97) | 0.46 |
| >7.50 | 11/33 | 0.75 (0.31, 1.84) | 0.53 | 0.82 (0.29, 2.27) | 0.70 |
| p-trend per mcg increase in vitamin D | 0.41 | 0.63 |
| Cases/Controls | OR (95% CI) Basic Model a | p | OR (95% CI) Multivariable Model b | p | |
|---|---|---|---|---|---|
| Any calcium-containing supplement use c | |||||
| All Participants | |||||
| Never | 46/86 | Ref. | Ref. | Ref. | Ref. |
| Ever | 70/171 | 0.77 (0.49, 1.22) | 0.27 | 0.61 (0.36, 1.05) | 0.07 |
| BRCA1 | |||||
| Never | 32/44 | Ref. | Ref. | Ref. | Ref. |
| Ever | 41/95 | 0.60 (0.33, 1.08) | 0.09 | 0.41 (0.21, 0.81) | 0.01 |
| BRCA2 | |||||
| Never | 14/42 | Ref. | Ref. | Ref. | Ref. |
| Ever | 29/76 | 1.15 (0.55, 2.41) | 0.72 | 1.13 (0.49, 2.63) | 0.77 |
| Total average daily calcium supplement use (mg/day) c | |||||
| All Participants | |||||
| None | 45/86 | Ref. | Ref. | Ref. | Ref. |
| 15.63 ≤ 105.88 | 24/45 | 1.02 (0.55, 1.90) | 0.94 | 0.71 (0.35, 1.44) | 0.34 |
| >105.88 | 24/76 | 0.61 (0.34, 1.09) | 0.09 | 0.56 (0.28, 1.09) | 0.09 |
| p-trend per mg increase in calcium | 0.05 | 0.04 | |||
| BRCA1 | |||||
| None | 31/44 | Ref. | Ref. | Ref. | Ref. |
| 15.63 ≤ 105.88 | 14/24 | 0.81 (0.36, 1.81) | 0.60 | 0.50 (0.20, 1.25) | 0.14 |
| >105.88 | 14/39 | 0.50 (0.23, 1.08) | 0.08 | 0.38 (0.16, 0.91) | 0.03 |
| p-trend per mg increase in calcium | 0.08 | 0.05 | |||
| BRCA2 | |||||
| None | 14/42 | Ref. | Ref. | Ref. | Ref. |
| 15.63 ≤ 105.88 | 10/21 | 1.47 (0.56, 3.86) | 0.44 | 1.17 (0.39, 3.48) | 0.78 |
| >105.88 | 10/37 | 0.80 (0.32, 2.03) | 0.64 | 1.00 (0.35, 2.84) | 0.99 |
| p-trend per mg increase in calcium | 0.31 | 0.54 |
| Supplement Use | Cases/Controls | OR (95% CI) Basic Model a | p | OR (95% CI) Multivariable Model b | p |
|---|---|---|---|---|---|
| Never vitamin D user/never calcium user | 43/76 | Ref. | Ref. | Ref. | Ref. |
| Ever calcium use only | 1/3 | 0.57 (0.06, 5.80) | 0.64 | 0.80 (0.07, 9.24) | 0.86 |
| Ever vitamin D use only | 1/7 | 0.24 (0.03, 2.04) | 0.19 | 0.28 (0.03, 2.60) | 0.26 |
| Ever vitamin D use/ever calcium use | 69/164 | 0.75 (0.47, 1.20) | 0.24 | 0.60 (0.34, 1.03) | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guyonnet, E.; Kim, S.J.; Pullella, K.; Zhang, C.X.W.; McCuaig, J.M.; Armel, S.; Narod, S.A.; Kotsopoulos, J. Vitamin D and Calcium Supplement Use and High-Risk Breast Cancer: A Case–Control Study among BRCA1 and BRCA2 Mutation Carriers. Cancers 2023, 15, 2790. https://doi.org/10.3390/cancers15102790
Guyonnet E, Kim SJ, Pullella K, Zhang CXW, McCuaig JM, Armel S, Narod SA, Kotsopoulos J. Vitamin D and Calcium Supplement Use and High-Risk Breast Cancer: A Case–Control Study among BRCA1 and BRCA2 Mutation Carriers. Cancers. 2023; 15(10):2790. https://doi.org/10.3390/cancers15102790
Chicago/Turabian StyleGuyonnet, Emma, Shana J. Kim, Katherine Pullella, Cindy X. W. Zhang, Jeanna M. McCuaig, Susan Armel, Steven A. Narod, and Joanne Kotsopoulos. 2023. "Vitamin D and Calcium Supplement Use and High-Risk Breast Cancer: A Case–Control Study among BRCA1 and BRCA2 Mutation Carriers" Cancers 15, no. 10: 2790. https://doi.org/10.3390/cancers15102790
APA StyleGuyonnet, E., Kim, S. J., Pullella, K., Zhang, C. X. W., McCuaig, J. M., Armel, S., Narod, S. A., & Kotsopoulos, J. (2023). Vitamin D and Calcium Supplement Use and High-Risk Breast Cancer: A Case–Control Study among BRCA1 and BRCA2 Mutation Carriers. Cancers, 15(10), 2790. https://doi.org/10.3390/cancers15102790







