Intracranial Germinoma—Association between Delayed Diagnosis, Altered Clinical Manifestations, and Prognosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keene, D.; Johnston, D.; Strother, D.; Fryer, C.; Carret, A.S.; Crooks, B.; Eisenstat, D.; Moghrabi, A.; Wilson, B.; Brossard, J.; et al. Epidemiological Survey of Central Nervous System Germ Cell Tumors in Canadian Children. J. Neurooncol. 2006, 82, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, J.W.; Looijenga, L.H.J. Testicular Germ-Cell Tumours in a Broader Perspective. Nat. Rev. Cancer 2005, 5, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Poynter, J.N.; Fonstad, R.; Tolar, J.; Spector, L.G.; Ross, J.A. Incidence of Intracranial Germ Cell Tumors by Race in the United States, 1992–2010. J. Neurooncol. 2014, 120, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Bartels, U.; Nishikawa, R.; Fangusaro, J.; Matsutani, M.; Nicholson, J.C. Consensus on the Management of Intracranial Germ-Cell Tumours. Lancet Oncol. 2015, 16, e470–e477. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, J.W.; Stoop, H.; Honecker, F.; Looijenga, L.H.J. Why Human Extragonadal Germ Cell Tumours Occur in the Midline of the Body: Old Concepts, New Perspectives. Int. J. Androl. 2007, 30, 256–264. [Google Scholar] [CrossRef]
- Mollgard, K.; Jespersen, A.; Lutterodt, M.C.; Yding Andersen, C.; Hoyer, P.E.; Byskov, A.G. Human Primordial Germ Cells Migrate along Nerve Fibers and Schwann Cells from the Dorsal Hind Gut Mesentery to the Gonadal Ridge. Mol. Hum. Reprod. 2010, 16, 621–631. [Google Scholar] [CrossRef]
- Sato, K.; Takeuchi, H.; Kubota, T. Pathology of Intracranial Germ Cell Tumors. Prog. Neurol. Surg. 2009, 23, 59–75. [Google Scholar]
- Frappaz, D.; Pedone, C.; Thiesse, P.; Szathmari, A.; Conter, C.F.; Mottolese, C.; Carrie, C. Visual Complaints in Intracranial Germinomas. Pediatr. Blood Cancer 2017, 64, e26543. [Google Scholar] [CrossRef]
- Frappaz, D.; Dhall, G.; Murray, M.J.; Goldman, S.; Faure Conter, C.; Allen, J.; Kortmann, R.D.; Haas-Kogen, D.; Morana, G.; Finlay, J.; et al. EANO, SNO and Euracan Consensus Review on the Current Management and Future Development of Intracranial Germ Cell Tumors in Adolescents and Young Adults. Neuro-Oncol. 2021, 24, 516–527. [Google Scholar] [CrossRef]
- Liang, L.; Korogi, Y.; Sugahara, T.; Ikushima, I.; Shigematsu, Y.; Okuda, T.; Takahashi, M.; Kochi, M.; Ushio, Y. MRI of Intracranial Germ-Cell Tumours. Neuroradiology 2002, 44, 382–388. [Google Scholar] [CrossRef]
- Iizuka, H.; Nojima, T.; Kadoya, S. Germinoma of the optic nerve: Case report. Brain Tumor Pathol. 1996, 13, 95–98. [Google Scholar]
- Ostreni, I.; Gurevitz, M.; Morvillo, G. Radiographic Findings of an Intracranial Germinoma in a 42-Year-Old Male. Cureus 2022, 14, e27535. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Y.; Chiu, C.-F.; Jung, S.-M.; Wong, A.M.-C.; Wu, C.-T.; Lo, F.-S. Neurological and Endocrinological Manifestations of 49 Children with Intracranial Pure Germinoma at Initial Diagnosis in Taiwan. Pediatr. Neonatol. 2021, 62, 106–112. [Google Scholar] [CrossRef]
- Crawford, J.R.; Santi, M.R.; Vezina, G.; Myseros, J.S.; Keating, R.F.; LaFond, D.A.; Rood, B.R.; MacDonald, T.J.; Packer, R.J. CNS Germ Cell Tumor (CNSGCT) of Childhood: Presentation and Delayed Diagnosis. Neurology 2007, 68, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Horan, G.; Lowis, S.; Nicholson, J.C. Highlights from the Third International Central Nervous System Germ Cell Tumour Symposium: Laying the Foundations for Future Consensus. eCancer 2013, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.J.; Shibui, S.; Kayama, T.; Miyaoka, E.; Narita, Y.; Murakami, M.; Matsuda, A.; Matsuda, T.; Sobue, T.; Palis, B.E.; et al. Primary CNS Germ Cell Tumors in Japan and the United States: An Analysis of 4 Tumor Registries. Neuro-Oncol. 2012, 14, 1194–1200. [Google Scholar] [CrossRef]
- Gittleman, H.; Cioffi, G.; Vecchione-Koval, T.; Ostrom, Q.T.; Kruchko, C.; Osorio, D.S.; Finlay, J.L.; Barnholtz-Sloan, J.S. Descriptive Epidemiology of Germ Cell Tumors of the Central Nervous System Diagnosed in the United States from 2006 to 2015. J. Neurooncol. 2019, 143, 251–260. [Google Scholar] [CrossRef]
- Lee, J.H.; Eom, K.-Y.; Phi, J.H.; Park, C.-K.; Kim, S.K.; Cho, B.-K.; Kim, T.M.; Heo, D.S.; Hong, K.T.; Choi, J.Y.; et al. Long-Term Outcomes and Sequelae Analysis of Intracranial Germinoma: Need to Reduce the Extended-Field Radiotherapy Volume and Dose to Minimize Late Sequelae. Cancer Res. Treat. 2021, 53, 983–990. [Google Scholar] [CrossRef]
- Rogers, S.; Mosleh-Shirazi, M.; Saran, F. Radiotherapy of Localised Intracranial Germinoma: Time to Sever Historical Ties? Lancet Oncol. 2005, 6, 509–519. [Google Scholar] [CrossRef]
- Hardenbergh, P.H.; Golden, J.; Billet, A.; Scott, R.M.; Shrieve, D.C.; Silver, B.; Loeffler, J.S.; Tarbell, N.J. Intracranial Germinoma: The Case for Lower Dose Radiation Therapy. Int. J. Radiat. Oncol. 1997, 39, 419–426. [Google Scholar] [CrossRef]
- da Silva, N.S.; Cappellano, A.M.; Diez, B.; Cavalheiro, S.; Gardner, S.; Wisoff, J.; Kellie, S.; Parker, R.; Garvin, J.; Finlay, J. Primary Chemotherapy for Intracranial Germ Cell Tumors: Results of the Third International CNS Germ Cell Tumor Study. Pediatr. Blood Cancer 2010, 54, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Calaminus, G.; Kortmann, R.; Worch, J.; Nicholson, J.C.; Alapetite, C.; Garre, M.L.; Patte, C.; Ricardi, U.; Saran, F.; Frappaz, D. SIOP CNS GCT 96: Final Report of Outcome of a Prospective, Multinational Nonrandomized Trial for Children and Adults with Intracranial Germinoma, Comparing Craniospinal Irradiation Alone with Chemotherapy Followed by Focal Primary Site Irradiation for Patients with Localized Disease. Neuro-Oncol. 2013, 15, 788–796. [Google Scholar]
- Kang, Y.-M.; Lee, Y.-Y.; Lin, S.-C.; Chang, F.-C.; Hsu, S.P.C.; Lin, C.-F.; Liang, M.-L.; Chen, H.-H.; Wong, T.-T.; Lan, K.-L.; et al. Bifocal Lesions Have a Poorer Treatment Outcome than a Single Lesion in Adult Patients with Intracranial Germinoma. PLoS ONE 2022, 17, e0264641. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, D.R.; Alden, T.; DiPatri, A.; Xi, G.; Goldman, S.; Tomita, T. Pediatric Suprasellar Germ Cell Tumors: A Clinical and Radiographic Review of Solitary vs. Bifocal Tumors and Its Therapeutic Implications. Cancers 2020, 12, 2621. [Google Scholar] [CrossRef] [PubMed]
- Paximadis, P.; Hallock, A.; Bhambhani, K.; Chu, R.; Sood, S.; Wang, Z.; Konski, A. Patterns of Failure in Patients With Primary Intracranial Germinoma Treated With Neoadjuvant Chemotherapy and Radiotherapy. Pediatr. Neurol. 2012, 47, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Hou, X.; Yan, J.; Sun, S.; Miao, Z.; Liu, Z.; Wang, W.; Shen, J.; Shen, J.; Hu, K.; et al. Treatment Outcomes of Intracranial Germinoma: A Retrospective Analysis of 170 Patients from a Single Institution. J. Cancer Res. Clin. Oncol. 2018, 145, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Morii, K.; Okazaki, H.; Tamura, T.; Tanaka, R. Neurohypophyseal Germinoma Traced from Its Earliest Stage via Magnetic Resonance Imaging: Case Report. Surg. Neurol. 2001, 56, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Cormenzana Carpio, M.; Nehme Álvarez, D.; Hernández Marqúes, C.; Pérez Martínez, A.; Lassaletta Atienza, A.; Madero López, L. Intracranial Germ Cell Tumours: A 21-Year Review. An. Pediatría (Engl. Ed.) 2017, 86, 20–27. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, L.; Gao, B. Intracranial Germinoma: Clinical and MRI Findings in 56 Patients. Childs Nerv. Syst. 2010, 26, 1773–1777. [Google Scholar] [CrossRef]
- Hadjikoutis, S.; Hughes, T. Germinoma with Synchronous Involvement of the Pineal Gland and the Suprasellar Region: A Treatable Cause of Visual Failure in a Young Adult. Eye 2004, 18, 525–526. [Google Scholar] [CrossRef]
- Hill, P.; Lepre, F.; Allison, R. CNS Germinomas: Curable Tumours in Two Adolescents. J. Paediatr. Child Health 1994, 30, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Sklar, C.A.; Grumbach, M.M.; Kaplan, S.L.; Conte, F.A. Hormonal and Metabolic Abnormalities Associated with Central Nervous System Germinoma in Children and Adolescents and the Effect of Therapy: Report of 10 Patients. J. Clin. Endocr. Metab. 1981, 52, 9–16. [Google Scholar] [CrossRef] [PubMed]



| All Patients n = 35 | Early Diagnosis n = 18 | Delayed Diagnosis n = 17 | p-Value | |
|---|---|---|---|---|
| Sex | 0.228 | |||
| Female | 7 (20.0%) | 2 (11.1%) | 5 (29.4%) | |
| Male | 28 (80.0%) | 16 (88.9%) | 12 (70.6%) | |
| Age (years) | 0.879 * | |||
| Median (IQR) | 16 (13–22) | 17.5 (13–21) | 16 (13–22) | |
| Mean (min; max) | 17.84 (5–27) | 17.97 (5–26) | 17.68 (7–27) | |
| Age group | 0.388 † | |||
| Children (<18 years old) | 19 (54.3%) | 8 (44.4%) | 11 (64.7%) | |
| Young adults (>18 years old) | 16 (45.7%) | 10 (55.6%) | 6 (35.3%) | |
| Tumour location | ||||
| Pineal gland | 11 (31.4%) | 8 (44.4%) | 3 (17.6%) | 0.179 † |
| Bifocal | 10 (28.6%) | 4 (22.2%) | 6 (35.3%) | 0.471 |
| Suprasellar | 8 (22.9%) | 2 (11.1%) | 6 (35.3%) | 0.121 |
| Disseminated | 3 (8.6%) | 2 (11.1%) | 1 (5.9%) | 1.000 |
| Basal ganglia | 3 (8.6%) | 2 (11.1%) | 1 (5.9%) | 1.000 |
| Maximum tumour dimension | 0.040 ‡ | |||
| Median (IQR) | 2.7 cm (2.0–3.8) | 3.1 cm (2.5–4.0) | 2.35 cm (1.9–3.2) | |
| Mean (min; max) | 2.85 cm (1.1; 4.5) | 3.16 cm (2.0; 4.5) | 2.46 cm (1.1; 3.9) | |
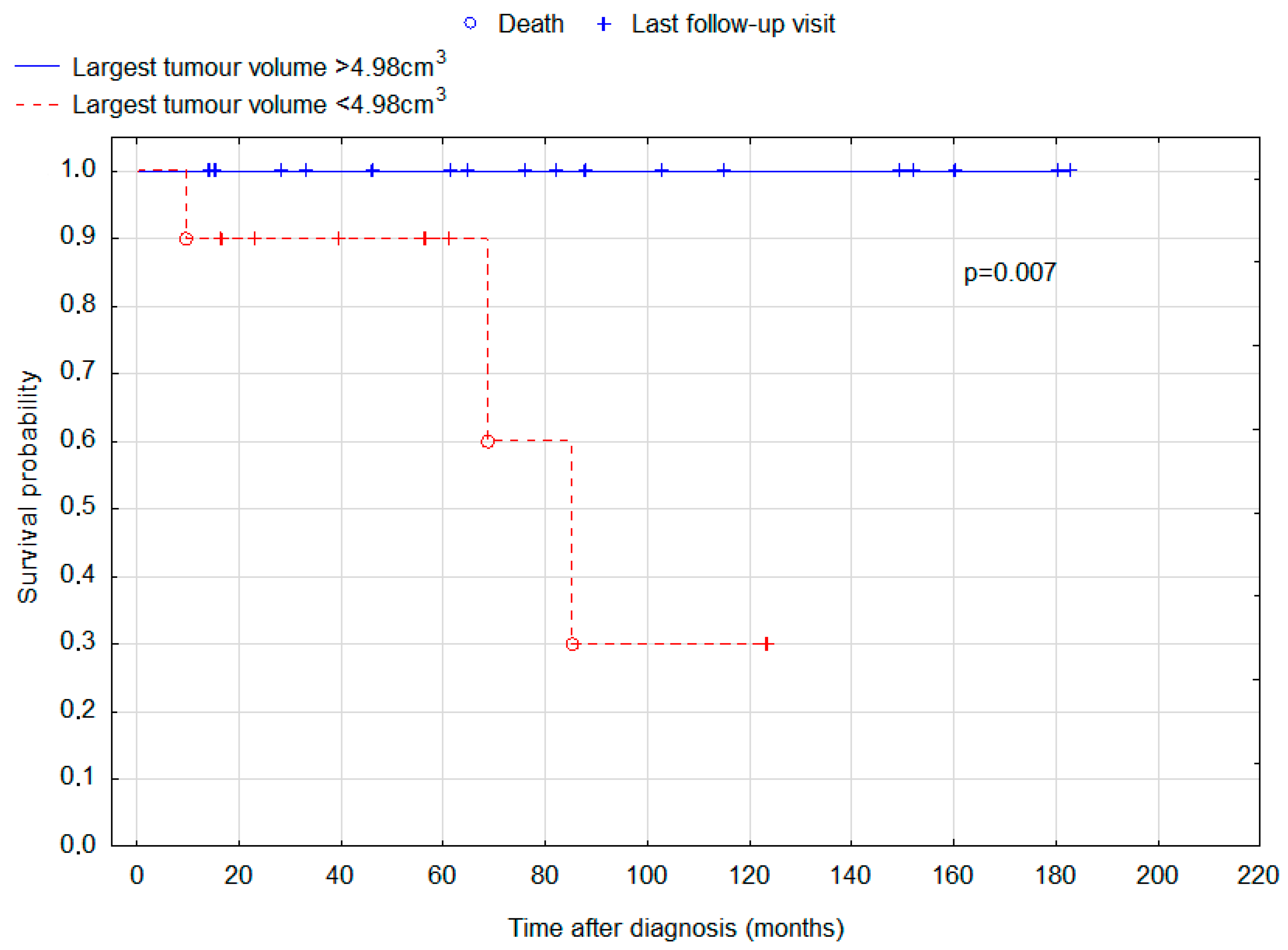
| Largest tumour volume | 0.051 § | |||
| Median (IQR) | 6.37 cm3 (1.99–13.09) | 8.93 cm3 (5.85–13.18) | 4.03 cm3 (1.34–10.97) | |
| Mean (min; max) | 8.64 cm3 (0.29; 24.88) | 10.36 cm3 (17.70; 24.88) | 6.49 cm3 (0.29; 20.89) | |
| Total tumour volume | 0.174 * | |||
| Median (IQR) | 7.70 cm3 (2.96–13.09) | 8.93 cm3 (5.94–14.26) | 4.11 cm3 (1.60–10.97) | |
| Mean (min; max) | 8.89 cm3 (0.29; 24.88) | 10.48 cm3 (1.77; 24.88) | 6.90 cm3 (0.29; 20.89) | |
| Hydrocephalus | 20 (57.1%) | 11 (61.1%) | 9 (52.9%) | 0.884 † |
| Ventriculostomy | 10 (28.6%) | 5 (27.8%) | 5 (29.4%) | 0.402 |
| Ventriculoperitoneal shunts | 7 (20.0%) | 5 (27.8%) | 2 (11.8%) | 1.000 |
| Comorbidities | ||||
| Hypertension | 2 (5.7%) | 0 | 2 (11.8%) | 0.229 |
| Epilepsy | 1 (2.9%) | 1 (5.6%) | 0 | 1.000 |
| Type 1 diabetes | 1 (2.9%) | 0 | 1 (5.9%) | 0.486 |
| Treatment | ||||
| Radiotherapy | 35 (100%) | 18 (100%) | 17 (100%) | 1.000 |
| Chemotherapy | 26 (74.3%) | 13 (72.2%) | 13 (76.5%) | 1.000 |
| Incomplete excision | 9 (25.7%) | 4 (22.2%) | 5 (29.4%) | 0.711 |
| Complete excision | 2 (5.7%) | 1 (5.6%) | 1 (5.9%) | 1.000 |
| All Patients n = 35 | Early Diagnosis n = 18 | Delayed Diagnosis n = 17 | p-Value | |
|---|---|---|---|---|
| Initial manifestation | 0.282 ‖ | |||
| Neurological | 16 (45.7%) | 10 (55.6%) | 6 (35.3%) | 0.388 † |
| Endocrinological | 5 (14.3%) | 1 (5.6%) | 4 (23.5%) | 0.177 |
| Mixed | 14 (40.0%) | 7 (38.9%) | 7 (41.2%) | 0.836 † |
| Neurological symptoms | ||||
| Headache | 21 (60%) | 14 (77.8%) | 7 (41.2%) | 0.062 † |
| Nausea/vomiting | 10 (28.6%) | 5 (27.8%) | 5 (29.4%) | 1.000 |
| Vertigo and balance disorders | 8 (22.9%) | 4 (22.2%) | 4 (23.5%) | 1.000 |
| Drowsiness | 5 (14.3%) | 2 (11.1%) | 3 (17.6%) | 0.658 |
| Memory defects | 2 (5.7%) | 1 (5.6%) | 1 (5.9%) | 1.000 |
| Hand tremor | 1 (2.9%) | 0 | 1 (5.9%) | 0.486 |
| Ocular symptoms | 24 (68.6%) | 13 (72.2%) | 11 (64.7%) | 0.909 † |
| Diplopia | 11 (31.4%) | 7 (38.9%) | 4 (23.5%) | 0.539 † |
| Blurred vision | 5 (14.3%) | 2 (11.1%) | 3 (17.6%) | 0.658 |
| Nystagmus | 5 (14.3%) | 2 (11.1%) | 3 (17.6%) | 0.658 † |
| EOM (extraocular muscle) impairment | 3 (8.6%) | 0 | 3 (17.6%) | 0.229 |
| Anisocoria | 3 (8.6%) | 2 (11.1%) | 1 (5.9%) | 1.000 |
| Visual field defect | 3 (8.6%) | 2 (11.1%) | 1 (5.9%) | 1.000 |
| Motor impairment | ||||
| Facial asymmetry | 4 (11.4%) | 4 (22.2%) | 0 | 0.104 |
| Hemiparesis | 2 (5.7%) | 1 (5.6%) | 1 (5.9%) | 1.000 |
| Endocrinological symptoms | ||||
| Diabetes insipidus | 15 (42.9%) | 6 (33.3%) | 9 (52.9%) | 0.407 † |
| Combined pituitary Hormone deficiency | 13 (37.1%) | 3 (16.7%) | 10 (58.8%) | 0.015 † |
| Thyroid axis dysfunction | 12 (34.3%) | 4 (22.2%) | 8 (47.1%) | 0.234 † |
| Adrenal axis dysfunction | 10 (28.6%) | 3 (16.7%) | 7 (41.2%) | 0.218 |
| Gonadal axis dysfunction | 7 (20%) | 2 (11.1%) | 5 (29.4%) | 0.228 |
| Hyperprolactinemia | 4 (11.4%) | 1 (5.6%) | 3 (17.6%) | 0.338 |
| Growth deficiency | 3 (8.6%) | 0 | 3 (17.6%) | 0.104 |
| Change of behaviour | 6 (17.1%) | 0 | 6 (35.3%) | 0.008 |
| β-HCG elevation | 10/32 (31.3%) | 7/16 (43.8%) | 3/16 (18.8%) | 0.253 † |
| AFP elevation | 1/28 (3.7%) | 1/16 (6.3%) | 0/12 (0%) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska, I.; Goławski, M.; Nowicka, E.; Drosik-Rutowicz, K.; Trybus, A.; Tarnawski, R.; Miszczyk, M. Intracranial Germinoma—Association between Delayed Diagnosis, Altered Clinical Manifestations, and Prognosis. Cancers 2023, 15, 2789. https://doi.org/10.3390/cancers15102789
Jabłońska I, Goławski M, Nowicka E, Drosik-Rutowicz K, Trybus A, Tarnawski R, Miszczyk M. Intracranial Germinoma—Association between Delayed Diagnosis, Altered Clinical Manifestations, and Prognosis. Cancers. 2023; 15(10):2789. https://doi.org/10.3390/cancers15102789
Chicago/Turabian StyleJabłońska, Iwona, Marcin Goławski, Elżbieta Nowicka, Katarzyna Drosik-Rutowicz, Anna Trybus, Rafał Tarnawski, and Marcin Miszczyk. 2023. "Intracranial Germinoma—Association between Delayed Diagnosis, Altered Clinical Manifestations, and Prognosis" Cancers 15, no. 10: 2789. https://doi.org/10.3390/cancers15102789
APA StyleJabłońska, I., Goławski, M., Nowicka, E., Drosik-Rutowicz, K., Trybus, A., Tarnawski, R., & Miszczyk, M. (2023). Intracranial Germinoma—Association between Delayed Diagnosis, Altered Clinical Manifestations, and Prognosis. Cancers, 15(10), 2789. https://doi.org/10.3390/cancers15102789






