Nationwide Trends and the Influence of Age and Gender in the In-Patient Care of Patients with Hepatocellular Carcinoma in Germany between 2010 and 2020
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Patient Cohort and Variables
2.3. Comorbidity
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics between 2010 and 2019
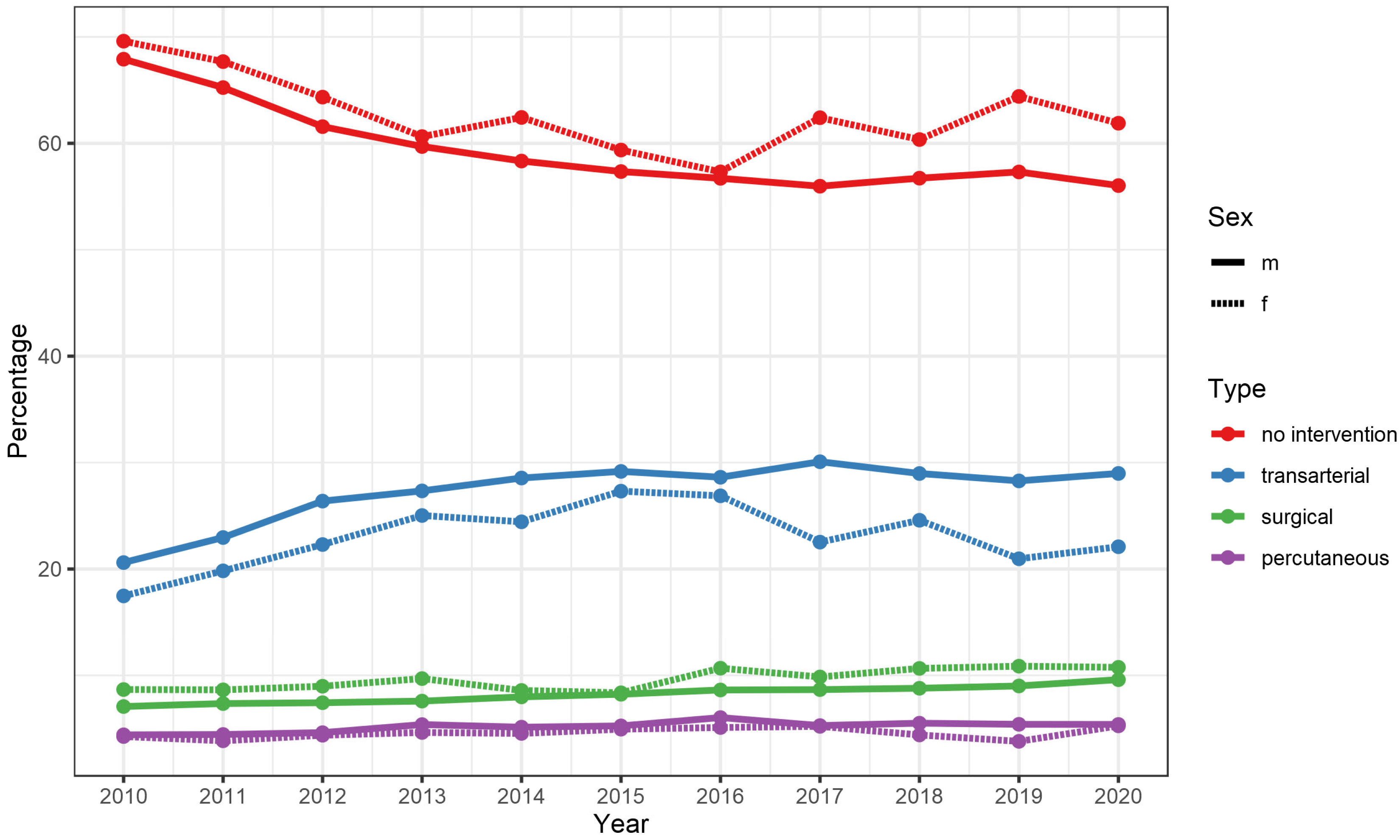
3.2. Trends between 2010 and 2019
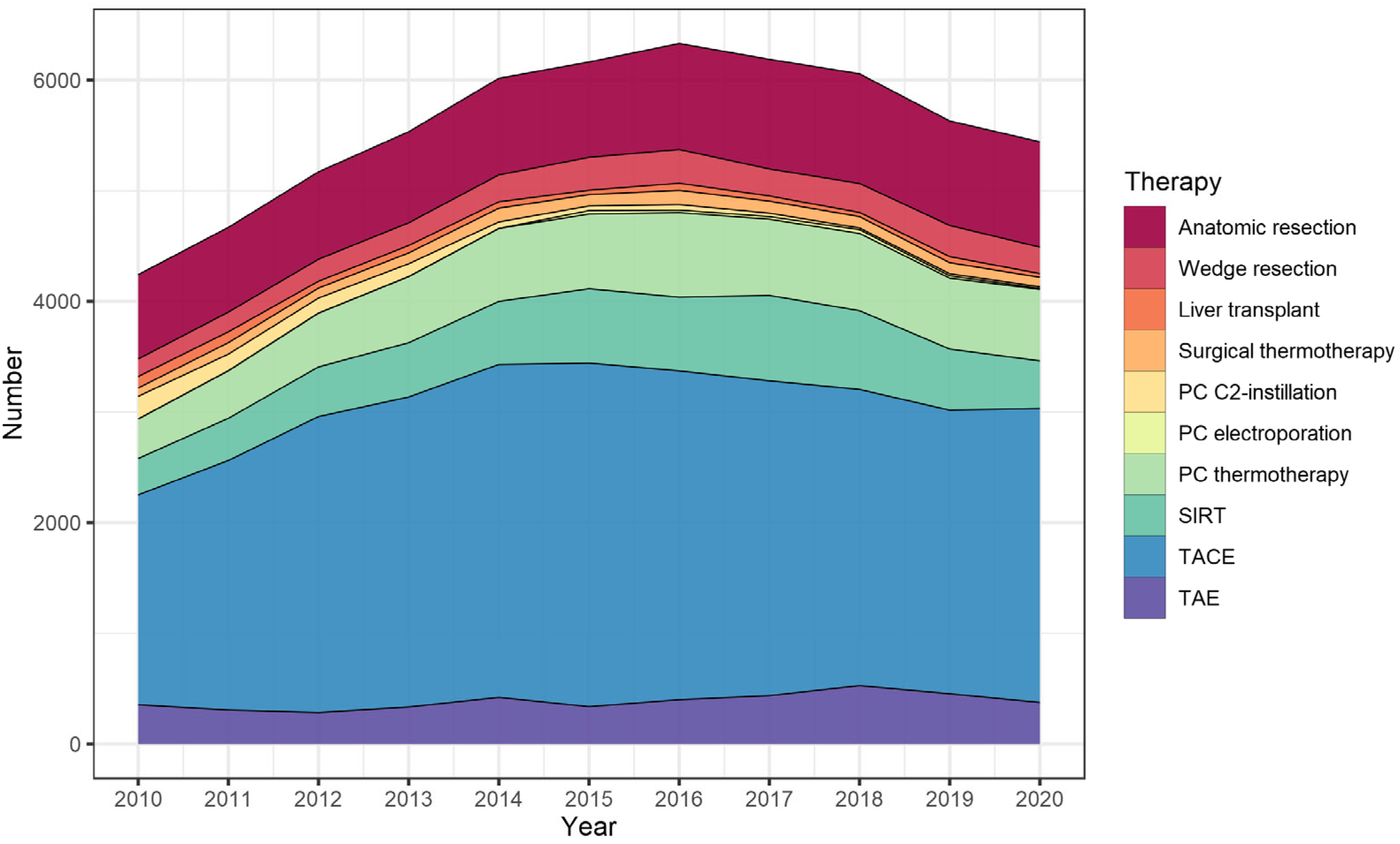
3.3. Liver-Directed Interventions
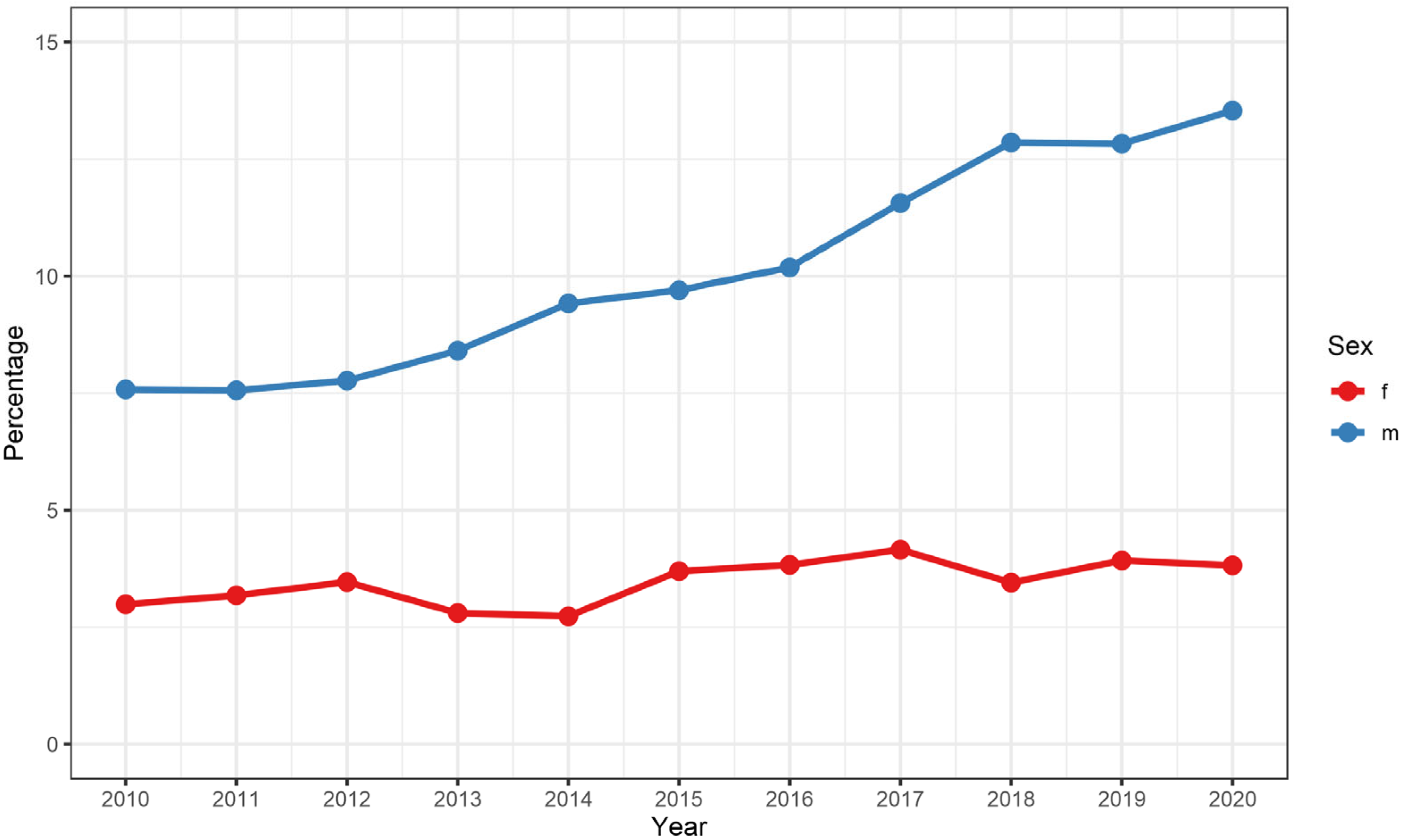
3.4. Gender-Specific and Age-Specific Differences
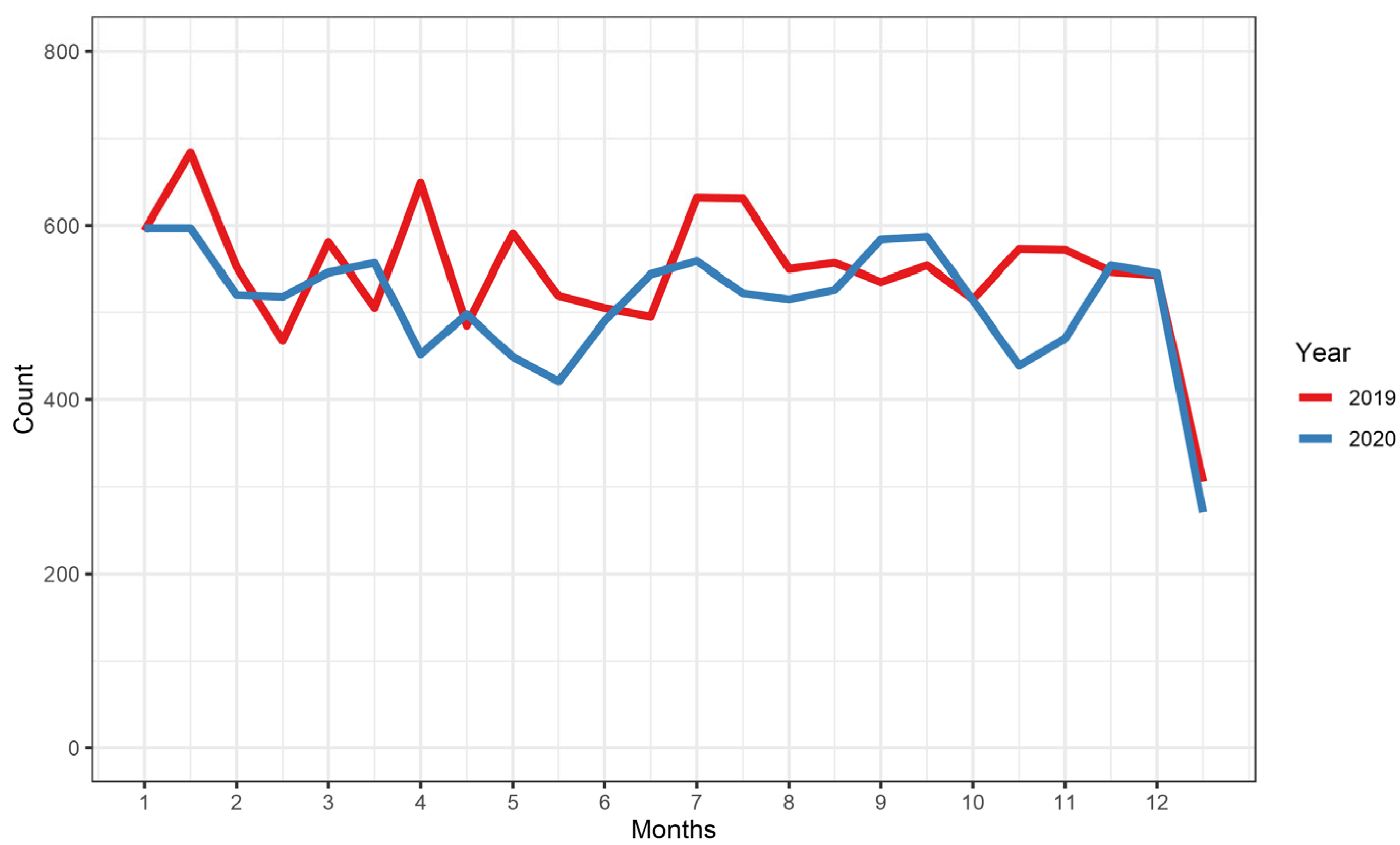
3.5. Effect of the First Year of the COVID-19 Pandemic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global Epidemiology of Alcohol-Associated Cirrhosis and HCC: Trends, Projections and Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2022, 20, 37–49. [Google Scholar] [CrossRef]
- Ramani, A.; Tapper, E.B.; Griffin, C.; Shankar, N.; Parikh, N.D.; Asrani, S.K. Hepatocellular Carcinoma-Related Mortality in the USA, 1999–2018. Dig. Dis. Sci. 2022, 67, 4100–4111. [Google Scholar] [CrossRef]
- Huber, T.C.; Bochnakova, T.; Koethe, Y.; Park, B.; Farsad, K. Percutaneous Therapies for Hepatocellular Carcinoma: Evolution of Liver Directed Therapies. J. Hepatocell Carcinoma 2021, 8, 1181–1193. [Google Scholar] [CrossRef]
- O’Leary, C.; Mahler, M.; Soulen, M.C. Liver-Directed Therapy for Hepatocellular Carcinoma. Chin. Clin. Oncol. 2021, 10, 8. [Google Scholar] [CrossRef]
- Viveiros, P.; Riaz, A.; Lewandowski, R.J.; Mahalingam, D. Current State of Liver-Directed Therapies and Combinatory Approaches with Systemic Therapy in Hepatocellular Carcinoma (HCC). Cancers 2019, 11, 1085. [Google Scholar] [CrossRef]
- Kis, B.; El-Haddad, G.; Sheth, R.A.; Parikh, N.S.; Ganguli, S.; Shyn, P.B.; Choi, J.; Brown, K.T. Liver-Directed Therapies for Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 107327481772924. [Google Scholar] [CrossRef]
- Makary, M.S.; Ramsell, S.; Miller, E.; Beal, E.W.; Dowell, J.D. Hepatocellular Carcinoma Locoregional Therapies: Outcomes and Future Horizons. World J. Gastroenterol. 2021, 27, 7462. [Google Scholar] [CrossRef]
- Covey, A.M.; Hussain, S.M. Liver-Directed Therapy for Hepatocellular Carcinoma: An Overview of Techniques, Outcomes, and Posttreatment Imaging Findings. Am. J. Roentgenol. 2017, 209, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, L.; Bargellini, I.; Cioni, R. Loco-Regional Treatment of HCC: Current Status. Clin. Radiol. 2017, 72, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Sangiovanni, A.; Colombo, M. Treatment of Hepatocellular Carcinoma: Beyond International Guidelines. Liver Int. 2016, 36, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Martinelli, E.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; et al. Updated Treatment Recommendations for Hepatocellular Carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, H.; Wang, Z.; Cong, W.; Wang, J.; Zeng, M.; Zhou, W.; Bie, P.; Liu, L.; Wen, T.; et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer 2020, 9, 682–720. [Google Scholar] [CrossRef]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International Trends in Hepatocellular Carcinoma Incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef]
- Chen, C.L.; Kuo, M.J.; Ming-Fang Yen, A.; Yang, W.S.; Kao, J.H.; Chen, P.J.; Chen, H.H. Gender Difference in the Association Between Metabolic Factors and Hepatocellular Carcinoma. JNCI Cancer Spectr. 2020, 4, pkaa036. [Google Scholar] [CrossRef]
- Robert Koch Institute. RKI—Cancer in Germany 2017/2018. 2017. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2021/krebs_in_deutschland_2021.pdf?__blob=publicationFile (accessed on 14 January 2023).
- Decker, J.A.; Varga-Szemes, A.; Schoepf, U.J.; Emrich, T.; Schwarz, F.; Kroencke, T.J.; Scheurig-Muenkler, C. In-Patient Care Trends in Peripheral Artery Disease in the German Healthcare System over the Past Decade. Eur. Radiol. 2021, 32, 1697–1708. [Google Scholar] [CrossRef]
- Source: Research Data Center (RDC) of the Federal Statistical Office and Statistical Offices of the Federal States, DRG Statistics 2010 to 2020, Own Calculations.
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity Measures for Use with Administrative Data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef] [PubMed]
- van Walraven, C.; Austin, P.C.; Jennings, A.; Quan, H.; Forster, A.J. A Modification of the Elixhauser Comorbidity Measures into a Point System for Hospital Death Using Administrative Data. Med. Care 2009, 47, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.B.; Zhu, A.X. Metabolic Syndrome and Hepatocellular Carcinoma: Two Growing Epidemics with a Potential Link. Cancer 2009, 115, 5651. [Google Scholar] [CrossRef] [PubMed]
- Jinjuvadia, R.; Patel, S.; Liangpunsakul, S. The Association between Metabolic Syndrome and Hepatocellular Carcinoma: Systemic Review and Meta-Analysis. J. Clin. Gastroenterol. 2014, 48, 172–177. [Google Scholar] [CrossRef]
- Kubicka, S.; Rudolph, K.L.; Hanke, M.; Tietze, M.K.; Tillmann, H.L.; Trautwein, C.; Manns, M. Hepatocellular Carcinoma in Germany: A Retrospective Epidemiological Study from a Low-Endemic Area. Liver 2000, 20, 312–318. [Google Scholar] [CrossRef]
- Schönfeld, I.; Kraywinkel, K. Epidemiology of Hepatocellular Carcinoma in Germany. Onkologe 2018, 24, 653–658. [Google Scholar] [CrossRef]
- de Toni, E.N.; Schlesinger-Raab, A.; Fuchs, M.; Schepp, W.; Ehmer, U.; Geisler, F.; Ricke, J.; Paprottka, P.; Friess, H.; Werner, J.; et al. Age Independent Survival Benefit for Patients with Hepatocellular Carcinoma (HCC) without Metastases at Diagnosis: A Population-Based Study. Gut 2020, 69, 168–176. [Google Scholar] [CrossRef]
- Plentz, R.R.; Malek, N.P. Early Detection of Hepatocellular Carcinoma: How to Screen and Follow up Patients with Liver Cirrhosis According to the GERMAN S3 Guideline? Diagnostics 2015, 5, 497–503. [Google Scholar] [CrossRef]
- Couri, T.; Pillai, A. Goals and Targets for Personalized Therapy for HCC. Hepatol. Int. 2019, 13, 125–137. [Google Scholar] [CrossRef]
- Cheng, H.; Sun, G.; Chen, H.; Li, Y.; Han, Z.; Li, Y.; Zhang, P.; Yang, L.; Li, Y. Trends in the Treatment of Advanced Hepatocellular Carcinoma: Immune Checkpoint Blockade Immunotherapy and Related Combination Therapies. Am. J. Cancer Res. 2019, 9, 1536. [Google Scholar] [PubMed]
- Li, C.; Wang, M.d.; Lu, L.; Wu, H.; Yu, J.J.; Zhang, W.G.; Pawlik, T.M.; Zhang, Y.M.; Zhou, Y.H.; Gu, W.M.; et al. Preoperative Transcatheter Arterial Chemoembolization for Surgical Resection of Huge Hepatocellular Carcinoma (≥10 cm): A Multicenter Propensity Matching Analysis. Hepatol. Int. 2019, 13, 736–747. [Google Scholar] [CrossRef]
- Lencioni, R. Loco-Regional Treatment of Hepatocellular Carcinoma. Hepatology 2010, 52, 762–773. [Google Scholar] [CrossRef]
- Peng, Z.; Wei, M.; Chen, S.; Lin, M.; Jiang, C.; Mei, J.; Li, B.; Wang, Y.; Li, J.; Xie, X.; et al. Combined Transcatheter Arterial Chemoembolization and Radiofrequency Ablation versus Hepatectomy for Recurrent Hepatocellular Carcinoma after Initial Surgery: A Propensity Score Matching Study. Eur. Radiol. 2018, 28, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.W.; Hur, Y.H.; Shin, S.S.; Heo, S.H.; Cho, S.B.; Kang, Y.J.; Lim, H.S.; Seon, H.J.; Jeong, Y.Y. Combined Therapy of Transcatheter Arterial Chemoembolization and Radiofrequency Ablation versus Surgical Resection for Single 2–3 Cm Hepatocellular Carcinoma: A Propensity-Score Matching Analysis. J. Vasc. Interv. Radiol. 2017, 28, 1240–1247.e3. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Mu, L.W.; Wu, C.; Wu, X.Q.; Xie, Q.K.; Li, X.S.; Lyu, N.; Li, S.L.; Deng, H.J.; Jiang, Z.B.; et al. Comparison of Combined Transcatheter Arterial Chemoembolization and CT-Guided Radiofrequency Ablation with Surgical Resection in Patients with Hepatocellular Carcinoma within the Up-to-Seven Criteria: A Multicenter Case-Matched Study. J. Cancer 2017, 8, 3506. [Google Scholar] [CrossRef]
- Kishore, S.A.; Bajwa, R.; Madoff, D.C. Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers 2020, 12, 791. [Google Scholar] [CrossRef]
- Ladenheim, M.R.; Kim, N.G.; Nguyen, P.; Le, A.; Stefanick, M.L.; Garcia, G.; Nguyen, M.H. Sex Differences in Disease Presentation, Treatment and Clinical Outcomes of Patients with Hepatocellular Carcinoma: A Single-Centre Cohort Study. BMJ Open Gastroenterol. 2016, 3, e000107. [Google Scholar] [CrossRef]
- Rich, N.E.; Murphy, C.C.; Yopp, A.C.; Tiro, J.; Marrero, J.A.; Singal, A.G. Sex Disparities in Presentation and Prognosis of 1110 Patients with Hepatocellular Carcinoma. Aliment. Pharm. 2020, 52, 701–709. [Google Scholar] [CrossRef]
- Sobotka, L.; Hinton, A.; Conteh, L. Women Receive More Inpatient Resections and Ablations for Hepatocellular Carcinoma than Men. World J. Hepatol. 2017, 9, 1346. [Google Scholar] [CrossRef]
- Yang, D.; Hanna, D.L.; Usher, J.; LoCoco, J.; Chaudhari, P.; Lenz, H.J.; Setiawan, V.W.; El-Khoueiry, A. Impact of Sex on the Survival of Patients with Hepatocellular Carcinoma: A Surveillance, Epidemiology, and End Results Analysis. Cancer 2014, 120, 3707–3716. [Google Scholar] [CrossRef] [PubMed]
- Cauble, S.; Abbas, A.; Balart, L.; Bazzano, L.; Medvedev, S.; Shores, N. United States Women Receive More Curative Treatment for Hepatocellular Carcinoma than Men. Dig. Dis. Sci. 2013, 58, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, J.; Chang, Z. Trends and Age-Period-Cohort Effects on the Prevalence, Incidence and Mortality of Hepatocellular Carcinoma from 2008 to 2017 in Tianjin, China. Int. J. Environ. Res. Public Health 2021, 18, 6034. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and Surveillance for Hepatocellular Carcinoma: New Trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Cho, H.A.; Jun, C.H.; Kim, H.J.; Cho, S.B.; Choi, S.K. A Review of Hepatocellular Carcinoma in Elderly Patients Focused on Management and Outcomes. Vivo 2019, 33, 1411–1420. [Google Scholar] [CrossRef]
- Park, S.; Rim, C.H.; Jung, Y.K.; Yoon, W.S. Therapeutic Decision Making in Hepatocellular Carcinoma According to Age and Child-Pugh Class: A Nationwide Cohort Analysis in South Korea. CA. J. Gastroenterol. Hepatol. 2021, 2021, 6640121. [Google Scholar] [CrossRef]
- Rho, S.Y.; Lee, H.W.; Kim, D.Y.; Kim, K.S. Current Status of Therapeutic Choice and Feasibility for Patients with Hepatocellular Carcinoma Aged ≥ 70 Years: A Nationwide Cancer Registry Analysis. J. Hepatocell Carcinoma 2021, 8, 321–332. [Google Scholar] [CrossRef]
- Akbulut, S.; Garzali, I.U.; Hargura, A.S.; Aloun, A.; Yilmaz, S. Screening, Surveillance, and Management of Hepatocellular Carcinoma During the COVID-19 Pandemic: A Narrative Review. J. Gastrointest. Cancer 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Amaddeo, G.; Brustia, R.; Allaire, M.; Lequoy, M.; Hollande, C.; Regnault, H.; Blaise, L.; Ganne-Carrié, N.; Séror, O.; Larrey, E.; et al. Impact of COVID-19 on the Management of Hepatocellular Carcinoma in a High-Prevalence Area. JHEP Rep. 2021, 3, 100199. [Google Scholar] [CrossRef]
- Scheurig-Muenkler, C.; Schwarz, F.; Kroencke, T.J.; Decker, J.A. Impact of the COVID-19 Pandemic on In-Patient Treatment of Peripheral Artery Disease in Germany during the First Pandemic Wave. J. Clin. Med. 2022, 11, 2008. [Google Scholar] [CrossRef]
- Adwan, H.; Vogl, T.J.; Balaban, Ü.; Nour-Eldin, N.E.A. Percutaneous Thermal Ablation Therapy of Hepatocellular Carcinoma (HCC): Microwave Ablation (MWA) versus Laser-Induced Thermotherapy (LITT). Diagnostics 2022, 12, 564. [Google Scholar] [CrossRef]
- Cha, D.I.; Kang, T.W.; Song, K.D.; Lee, M.W.; Rhim, H.; Lim, H.K.; Sinn, D.H.; Kim, K. Radiofrequency Ablation for Subcardiac Hepatocellular Carcinoma: Therapeutic Outcomes and Risk Factors for Technical Failure. Eur. Radiol. 2019, 29, 2706–2715. [Google Scholar] [CrossRef]
- Yang, J.D.; Kim, W.R.; Park, K.W.; Chaiteerakij, R.; Kim, B.; Sanderson, S.O.; Larson, J.J.; Pedersen, R.A.; Therneau, T.M.; Gores, G.J.; et al. Model to Estimate Survival in Ambulatory Patients with Hepatocellular Carcinoma. Hepatology 2012, 56, 614–621. [Google Scholar] [CrossRef]
- Kim, B.H.; Park, J.W.; Nam, B.H.; Kwak, H.W.; Kim, W.R. Validation of a Model to Estimate Survival in Ambulatory Patients with Hepatocellular Carcinoma: A Single-Centre Cohort Study. Liver Int. 2014, 34, e317–e323. [Google Scholar] [CrossRef]
- Facciorusso, A.; Di Maso, M.; Muscatiello, N. Microwave Ablation Versus Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials. Cancers 2020, 12, 3796. [Google Scholar] [CrossRef]
| 2010 | 2016 | 2019 | Absolute Change | Relative Change | |
|---|---|---|---|---|---|
| 2010–2019 | 2010–2019 | ||||
| Total number | 12,707 | 13,966 | 13,143 | +436 (+3.4%) | |
| Age, years | 67.6 ± 10.3 | 69.2 ± 10.1 | 69.6 ± 10.7 | +2.0 (+3.0%) | |
| Male gender | 10,332 (81.3%) | 11,204 (80.2%) | 10,424 (79.3%) | −92.0 (−2.0%) | −2.5% |
| In-hospital stay, days | 7.2 ± 9.3 | 6.5 ± 8.7 | 6.1 ± 8.2 | −1.1 (−15.0%) | |
| Reimbursement per case, EUR | 4396.6 ± 9391.0 | 5133.7 ± 9291.0 | 5341.2 ± 9610.9 | +944.6 (+21.5%) | |
| In-hospital death | 862 (6.8%) | 796 (5.7%) | 685 (5.2%) | −204.0 (−1.6%) | −23.2% |
| Van Walraven score | 14.3 ± 8.0 | 14.7 ± 8.5 | 14.6 ± 8.6 | +0.3 (+1.9%) | |
| Elixhauser score | 3.6 ± 1.7 | 3.9 ± 1.9 | 3.9 ± 1.9 | +0.3 (+7.8%) | |
| Type of therapy: | |||||
| none | 8679 (68.3%) | 7938 (56.8%) | 7724 (58.8%) | −955 (−9.5%) | −11.0% |
| endovascular | 2534 (19.9%) | 3940 (28.2%) | 3513 (26.7%) | +979 (+6.8%) | +38.6% |
| surgical | 932 (7.3%) | 1254 (9.0%) | 1226 (9.3%) | +294 (+2.0%) | +31.5% |
| percutaneous | 533 (4.2%) | 762 (5.4%) | 636 (4.8%) | +103 (+0.6%) | +19.3% |
| combined | 29 (0.2%) | 72 (0.5%) | 44 (0.3%) | +15 (+0.1%) | +51.7% |
| Endovascular | Surgical | Percutaneous | Combined | No Treatment | |
|---|---|---|---|---|---|
| Number | 35,446 | 11,199 | 6369 | 513 | 81,186 |
| Age, years | 69.0 ± 9.5 | 68.2 ± 10.6 | 69.0 ± 9.6 | 68.3 ± 9.5 | 68.9 ± 10.7 |
| Male gender | 29,293 (82.6%) | 8690 (77.6%) | 5229 (82.1%) | 416 (81.1%) | 64,540 (79.5%) |
| In-hospital stay, days | 3.9 ± 4.5 | 17.6 ± 14.2 | 4.4 ± 5.3 | 9.5 ± 11.2 | 6.4 ± 7.9 |
| Reimbursement/case, EUR | 3974.5 ± 2519.7 | 17,219.3 ± 15,530.1 | 3683.5 ± 3731.3 | 7279.6 ± 10,694.8 | 3573.4 ± 7590.5 |
| In-hospital death | 196 (0.6%) | 857 (7.7%) | 34 (0.5%) | 9 (1.8%) | 6781 (8.4%) |
| Van Walraven score | 12.4 ± 7.6 | 16.3 ± 8.7 | 13.3 ± 7.2 | 15.3 ± 7.2 | 15.2 ± 8.6 |
| Elixhauser score | 3.4 ± 1.7 | 4.6 ± 2.0 | 3.6 ± 1.7 | 3.8 ± 1.8 | 3.8 ± 1.8 |
| Males | Females | |
|---|---|---|
| (n = 108,179, 80.3%) | (n = 26,534, 19.7%) | |
| Age, years | 68.9 ± 9.8 | 68.9 ± 12.1 |
| In-hospital stay, days | 6.4 ± 7.1 | 7.4 ± 8.3 |
| Reimbursement per case, EUR | 4769.3 ± 6311.6 | 5088.7 ± 7956.6 |
| In-hospital death | 6214 (5.7%) | 1653 (6.2%) |
| Van Walraven score | 14.5 ± 8.3 | 13.9 ± 8.0 |
| Elixhauser score | 3.8 ± 1.8 | 3.6 ± 1.8 |
| Type of therapy: | ||
| none | 64,464 (59.6%) | 16,621 (62.6%) |
| endovascular | 29,389 (27.2%) | 6167 (23.2%) |
| surgical | 8748 (8.1%) | 2522 (9.5%) |
| percutaneous | 5578 (5.2%) | 1224 (4.6%) |
| <80 Years | ≥80 Years | |
|---|---|---|
| (n = 116,863, 86.7%) | (n = 17,850, 13.3%) | |
| Number | 116,863 | 17,850 |
| Age, years | 66.7 ± 9.3 | 82.9 ± 2.8 |
| Male gender | 94,949 (81.2%) | 13,230 (74.1%) |
| In-hospital stay, days | 6.5 ± 8.7 | 6.8 ± 7.6 |
| Reimbursement cost per case, EUR | 4934.9 ± 8821.8 | 4203.1 ± 5373.8 |
| In-hospital death | 6515 (5.6%) | 1362 (7.6%) |
| Van Walraven score | 14.5 ± 8.3 | 14.2 ± 9.0 |
| Elixhauser score | 3.8 ± 1.8 | 3.9 ± 1.9 |
| Type of therapy: | ||
| none | 64,464 (59.6%) | 16,621 (62.6%) |
| endovascular | 29,389 (27.2%) | 6167 (23.2%) |
| surgical | 8748 (8.1%) | 2522 (9.5%) |
| percutaneous | 5578 (5.2%) | 1224 (4.6%) |
| Any Intervention | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Odds Ratio | p Value | Odds Ratio | p Value | |
| Age | ||||
| <80 years (ref) | 1.00 | 1.00 | ||
| ≥80 years | 0.80 [0.78, 0.83] | <0.001 | 0.81 [0.79, 0.84] | <0.001 |
| Gender | ||||
| Male (ref) | 1.00 | 1.00 | ||
| Female | 0.88 [0.86, 0.90] | <0.001 | 0.89 [0.86, 0.91] | <0.001 |
| vWs (continuously) | 0.97 [0.97, 0.97] | <0.001 | 0.98 [0.98, 0.98] | <0.001 |
| In-hospital death | ||||
| No (ref) | 1.00 | 1.00 | ||
| Yes | 0.23 [0.21, 0.24] | <0.001 | 0.27 [0.25, 0.28] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decker, J.A.; Scheurig-Muenkler, C.; Luitjens, J.H.; Kroencke, T. Nationwide Trends and the Influence of Age and Gender in the In-Patient Care of Patients with Hepatocellular Carcinoma in Germany between 2010 and 2020. Cancers 2023, 15, 2792. https://doi.org/10.3390/cancers15102792
Decker JA, Scheurig-Muenkler C, Luitjens JH, Kroencke T. Nationwide Trends and the Influence of Age and Gender in the In-Patient Care of Patients with Hepatocellular Carcinoma in Germany between 2010 and 2020. Cancers. 2023; 15(10):2792. https://doi.org/10.3390/cancers15102792
Chicago/Turabian StyleDecker, Josua A., Christian Scheurig-Muenkler, Jan H. Luitjens, and Thomas Kroencke. 2023. "Nationwide Trends and the Influence of Age and Gender in the In-Patient Care of Patients with Hepatocellular Carcinoma in Germany between 2010 and 2020" Cancers 15, no. 10: 2792. https://doi.org/10.3390/cancers15102792
APA StyleDecker, J. A., Scheurig-Muenkler, C., Luitjens, J. H., & Kroencke, T. (2023). Nationwide Trends and the Influence of Age and Gender in the In-Patient Care of Patients with Hepatocellular Carcinoma in Germany between 2010 and 2020. Cancers, 15(10), 2792. https://doi.org/10.3390/cancers15102792






