Beyond CTLA-4 and PD-1 Inhibition: Novel Immune Checkpoint Molecules for Melanoma Treatment
Abstract
Simple Summary
Abstract
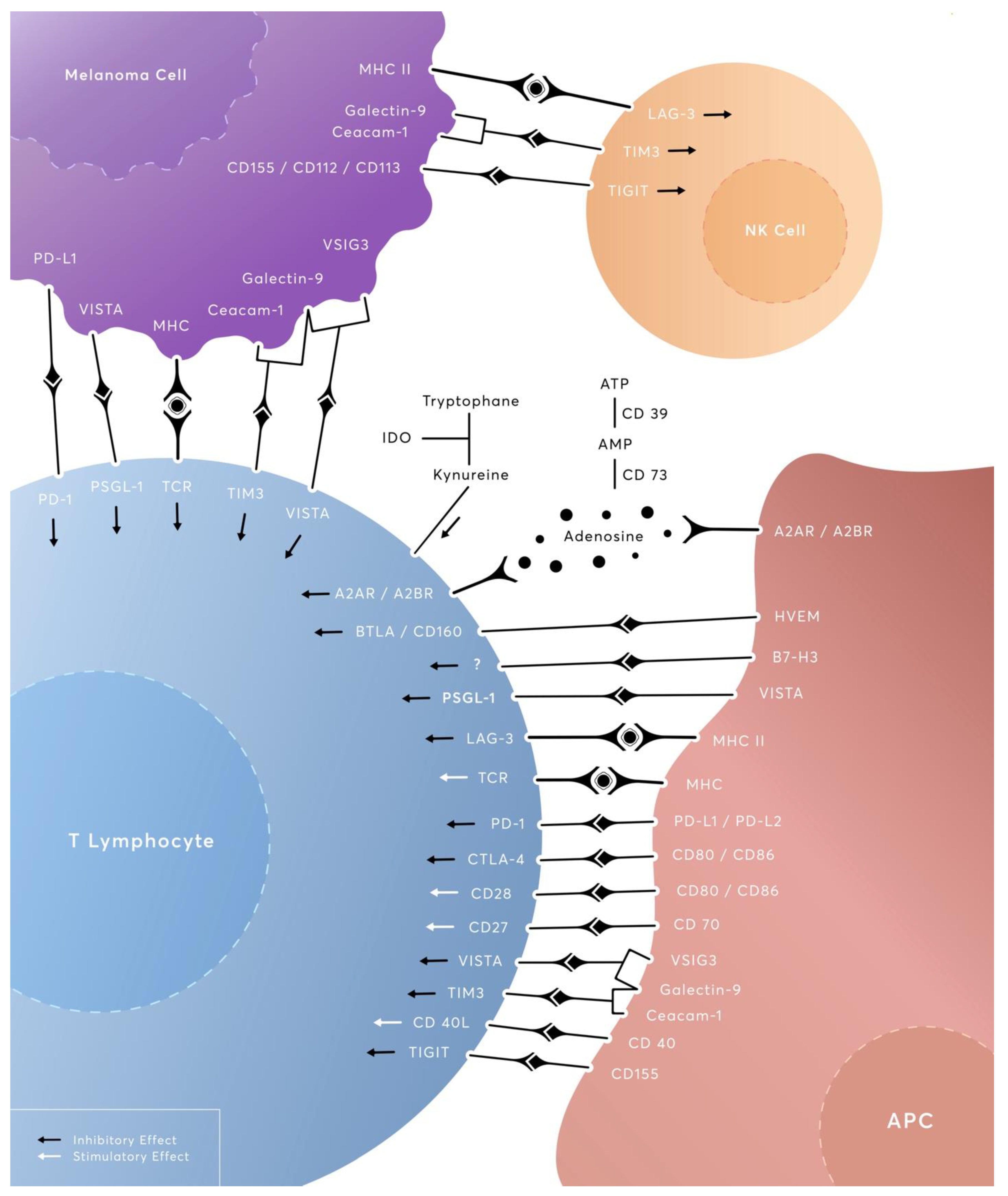
1. Introduction
2. LAG-3 (Lymphocyte Activation Gene-3, CD223)
3. TIGIT (T-Cell Immunoglobulin and ITIM Domain)
4. TIM-3 (T-Cell Immunoglobulin- and Mucin-Domain-Containing Molecule-3)
5. VISTA (V-Domain Immunoglobulin Suppressor of T-Cell Activation)
6. IDO1 (Indoleamine 2,3-Dioxygenase 1), IDO2 (Indoleamine 2,3-Dioxygenase 2), TDO (Tryptophan 2,3-Dioxygenase)
7. CD27/CD70
8. CD39 (Ectonucleoside Triphosphate Diphosphohydrolase-1, E-NTPDase1) and CD73 (Ecto-5′-Nucleotidase, Ecto5′NTase)
9. HVEM/BTLA/CD160
10. B7-H3 (B7 Homolog 3 Protein, CD276)
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OS | overall survival |
| PD-1 | programmed cell death protein-1 |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| LAG-3 | lymphocyte activation gene-3 |
| TIGIT | T-cell immunoglobulin and ITIM domain |
| TIM-3 | T-cell immunoglobulin- and mucin-domain-containing molecule-3 |
| VISTA | V-domain Immunoglobulin Suppressor of T-cell Activation |
| IDO1 | indoleamine 2, 3-dioxygenase 1 |
| IDO2 | indoleamine 2, 3-dioxygenase 2 |
| TDO | tryptophan 2, 3-dioxygenase |
| B7-H3 | B7 homolog 3 protein |
| HVEM | herpesvirus entry mediator |
| BTLA | B- and T-lymphocyte attenuator |
| MHC | major histocompatibility complex |
| TCR | T-cell receptor |
| TME | tumor microenvironment |
| HLA | human leukocyte antigen |
| DCR | disease control rate |
| PFS | progress-free survival |
| TIL | tumor-infiltrating leukocytes |
| IFN-γ | interferon gamma |
| TMB | tumor mutational burden |
| mAb | monoclonal antibody |
| TRAE | treatment-related adverse event |
| RFS | relapse-free survival |
| ORR | objective response rate |
| mDOR | median duration of response |
| DLT | dose-limiting toxicity |
| NK cells | natural killer cells |
| CAECAM1 | carcinoembryonic antigen-related cell adhesion molecule 1 |
| HMGB1 | high-mobility-group box 1 |
| ITIM | immunoreceptor tyrosine-based inhibitory motif |
| ADCC | antibody-dependent cellular cytotoxicity |
| MDSC | myeloid-derived suppressor cells |
| CR | complete response |
| APC | antigen-presenting cells |
| CTL | cytotoxic lymphocyte |
| DC | dendritic cell |
| GPI | glycosylphosphatidylinositol |
| miRNA | microRNA |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J. Clin. Oncol. 2021, 39, 9506. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schumacher, T.N. T Cell Dysfunction in Cancer. Cancer Cell 2018, 33, 547–562. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Chen, H.; Zhao, J.; Li, K.; Sun, J.; Zhou, M. Pan-cancer landscape of T-cell exhaustion heterogeneity within the tumor microenvironment revealed a progressive roadmap of hierarchical dysfunction associated with prognosis and therapeutic efficacy. EBioMedicine 2022, 83, 104207. [Google Scholar] [CrossRef]
- Zehn, D.; Thimme, R.; Lugli, E.; de Almeida, G.P.; Oxenius, A. ‘Stem-like’ precursors are the fount to sustain persistent CD8(+) T cell responses. Nat. Immunol. 2022, 23, 836–847. [Google Scholar] [CrossRef]
- Tsui, C.; Kretschmer, L.; Rapelius, S.; Gabriel, S.S.; Chisanga, D.; Knopper, K.; Utzschneider, D.T.; Nussing, S.; Liao, Y.; Mason, T.; et al. MYB orchestrates T cell exhaustion and response to checkpoint inhibition. Nature 2022, 609, 354–360. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef]
- Ziogas, D.C.; Theocharopoulos, C.; Koutouratsas, T.; Haanen, J.; Gogas, H. Mechanisms of resistance to immune checkpoint inhibitors in melanoma: What we have to overcome? Cancer Treat. Rev. 2022, 113, 102499. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Macon-Lemaitre, L.; Triebel, F. The negative regulatory function of the lymphocyte-activation gene-3 co-receptor (CD223) on human T cells. Immunology 2005, 115, 170–178. [Google Scholar] [CrossRef]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernandez-Rubio, L.; Morente, P.; Fernandez-Hinojal, G.; Echaide, M.; Garnica, M.; et al. Understanding LAG-3 Signaling. Int. J. Mol. Sci. 2021, 22, 5282. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Xu, K.; Liu, X.; He, Y. Update on lymphocyte-activation gene 3 (LAG-3) in cancers: From biological properties to clinical applications. Chin. Med. J. 2022, 135, 1203–1212. [Google Scholar] [CrossRef]
- Guy, C.; Mitrea, D.M.; Chou, P.C.; Temirov, J.; Vignali, K.M.; Liu, X.; Zhang, H.; Kriwacki, R.; Bruchez, M.P.; Watkins, S.C.; et al. LAG3 associates with TCR-CD3 complexes and suppresses signaling by driving co-receptor-Lck dissociation. Nat. Immunol. 2022, 23, 757–767. [Google Scholar] [CrossRef]
- Maruhashi, T.; Okazaki, I.M.; Sugiura, D.; Takahashi, S.; Maeda, T.K.; Shimizu, K.; Okazaki, T. LAG-3 inhibits the activation of CD4(+) T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat. Immunol. 2018, 19, 1415–1426. [Google Scholar] [CrossRef]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.M.; Shimizu, K.; Maeda, T.K.; Ikubo, J.; Yoshikawa, H.; Maenaka, K.; Ishimaru, N.; Kosako, H.; et al. Binding of LAG-3 to stable peptide-MHC class II limits T cell function and suppresses autoimmunity and anti-cancer immunity. Immunity 2022, 55, 912–924 e918. [Google Scholar] [CrossRef]
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015, 3, 412–423. [Google Scholar] [CrossRef]
- Shi, A.P.; Tang, X.Y.; Xiong, Y.L.; Zheng, K.F.; Liu, Y.J.; Shi, X.G.; Lv, Y.; Jiang, T.; Ma, N.; Zhao, J.B. Immune Checkpoint LAG3 and Its Ligand FGL1 in Cancer. Front. Immunol. 2021, 12, 785091. [Google Scholar] [CrossRef]
- Xu, F.; Liu, J.; Liu, D.; Liu, B.; Wang, M.; Hu, Z.; Du, X.; Tang, L.; He, F. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 2014, 74, 3418–3428. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, A.; Qiu, C.; Wang, W.; Yang, Y.; Qiu, C.; Liu, A.; Zhu, L.; Yuan, S.; Hu, H.; et al. The upregulation of LAG-3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV-infected subjects. J. Immunol. 2015, 194, 3873–3882. [Google Scholar] [CrossRef]
- Huang, C.T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in regulatory T cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef]
- Camisaschi, C.; Casati, C.; Rini, F.; Perego, M.; De Filippo, A.; Triebel, F.; Parmiani, G.; Belli, F.; Rivoltini, L.; Castelli, C. LAG-3 expression defines a subset of CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. J. Immunol. 2010, 184, 6545–6551. [Google Scholar] [CrossRef]
- Durham, N.M.; Nirschl, C.J.; Jackson, C.M.; Elias, J.; Kochel, C.M.; Anders, R.A.; Drake, C.G. Lymphocyte Activation Gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo. PLoS ONE 2014, 9, e109080. [Google Scholar] [CrossRef] [PubMed]
- Camisaschi, C.; De Filippo, A.; Beretta, V.; Vergani, B.; Villa, A.; Vergani, E.; Santinami, M.; Cabras, A.D.; Arienti, F.; Triebel, F.; et al. Alternative activation of human plasmacytoid DCs in vitro and in melanoma lesions: Involvement of LAG-3. J. Investig. Dermatol. 2014, 134, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Hemon, P.; Jean-Louis, F.; Ramgolam, K.; Brignone, C.; Viguier, M.; Bachelez, H.; Triebel, F.; Charron, D.; Aoudjit, F.; Al-Daccak, R.; et al. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J. Immunol. 2011, 186, 5173–5183. [Google Scholar] [CrossRef]
- Souri, Z.; Wierenga, A.P.A.; Kroes, W.G.M.; van der Velden, P.A.; Verdijk, R.M.; Eikmans, M.; Luyten, G.P.M.; Jager, M.J. LAG3 and Its Ligands Show Increased Expression in High-Risk Uveal Melanoma. Cancers 2021, 13, 4445. [Google Scholar] [CrossRef]
- Machiraju, D.; Wiecken, M.; Lang, N.; Hulsmeyer, I.; Roth, J.; Schank, T.E.; Eurich, R.; Halama, N.; Enk, A.; Hassel, J.C. Soluble immune checkpoints and T-cell subsets in blood as biomarkers for resistance to immunotherapy in melanoma patients. Oncoimmunology 2021, 10, 1926762. [Google Scholar] [CrossRef]
- Johnson, D.B.; Nixon, M.J.; Wang, Y.; Wang, D.Y.; Castellanos, E.; Estrada, M.V.; Ericsson-Gonzalez, P.I.; Cote, C.H.; Salgado, R.; Sanchez, V.; et al. Tumor-specific MHC-II expression drives a unique pattern of resistance to immunotherapy via LAG-3/FCRL6 engagement. JCI Insight 2018, 3, e120360. [Google Scholar] [CrossRef]
- Gestermann, N.; Saugy, D.; Martignier, C.; Tille, L.; Fuertes Marraco, S.A.; Zettl, M.; Tirapu, I.; Speiser, D.E.; Verdeil, G. LAG-3 and PD-1+LAG-3 inhibition promote anti-tumor immune responses in human autologous melanoma/T cell co-cultures. Oncoimmunology 2020, 9, 1736792. [Google Scholar] [CrossRef]
- Shen, R.; Postow, M.A.; Adamow, M.; Arora, A.; Hannum, M.; Maher, C.; Wong, P.; Curran, M.A.; Hollmann, T.J.; Jia, L.; et al. LAG-3 expression on peripheral blood cells identifies patients with poorer outcomes after immune checkpoint blockade. Sci. Transl. Med. 2021, 13, eabf5107. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernandez-Rubio, L.; Bocanegra, A.; Echaide, M.; Garnica, M.; Ramos, P.; Fernandez-Hinojal, G.; Vera, R.; et al. Clinical landscape of LAG-3-targeted therapy. Immunooncol. Technol. 2022, 14, 100079. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutierrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Amaria, R.N.; Postow, M.; Burton, E.M.; Tezlaff, M.T.; Ross, M.I.; Torres-Cabala, C.; Glitza, I.C.; Duan, F.; Milton, D.R.; Busam, K.; et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature 2022, 611, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Bono, P.; Bhatia, S.; Melero, I.; Nyakas, M.S.; Svane, I.M.; Larkin, J.; Gomez-Roca, C.; Schadendorf, D.; Dummer, R.; et al. Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma who progressed during prior anti–PD-1/PD-L1 therapy (mel prior IO) in all-comer and biomarker-enriched populations. Ann. Oncol. 2017, 28, v611–v612. [Google Scholar] [CrossRef]
- Bai, X.; Li, M.; Pu, X.; Cheng, Y.; Chen, J.; Jiang, Y.; Chen, X.; Liu, J.; Fan, L.; Guo, J.; et al. Anti-LAG-3 antibody LBL-007 in combination with toripalimab in patients with unresectable or metastatic melanoma: A phase Ⅰ, open-label, multicenter, dose escalation/expansion study. J. Clin. Oncol. 2022, 40, 9538. [Google Scholar] [CrossRef]
- Hamid, O.; Lewis, K.; Weise, A.; McKean, M.; Papadopoulos, K.; Crown, J.P.; Thomas, S.; Kaczmar, J.; Lakhani, N.; Kim, T.M.; et al. 150P Phase I study of fianlimab: A human lymphocyte activation gene-3 (LAG-3) monoclonal antibody, in combination with cemiplimab in advanced melanoma (mel)—Subgroup analysis. Immuno-Oncol. Technol. 2022, 16, 100262. [Google Scholar] [CrossRef]
- Robert, C.; Schadendorf, D.; Long, G.V.; Ascierto, P.; Intagliata, S.; Meier, F.; van der Veldt, A.A.M.; Ribas, A.; Weber, J.S.; Stenson, L.; et al. 1084P PLATForM: Descriptive analysis from a randomised, phase II study of novel spartalizumab combinations in previously treated unresectable/metastatic melanoma. Ann. Oncol. 2021, 32, S898. [Google Scholar] [CrossRef]
- Schoffski, P.; Tan, D.S.W.; Martin, M.; Ochoa-de-Olza, M.; Sarantopoulos, J.; Carvajal, R.D.; Kyi, C.; Esaki, T.; Prawira, A.; Akerley, W.; et al. Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) +/- anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J. Immunother. Cancer 2022, 10, e003776. [Google Scholar] [CrossRef]
- Legat, A.; Maby-El Hajjami, H.; Baumgaertner, P.; Cagnon, L.; Abed Maillard, S.; Geldhof, C.; Iancu, E.M.; Lebon, L.; Guillaume, P.; Dojcinovic, D.; et al. Vaccination with LAG-3Ig (IMP321) and Peptides Induces Specific CD4 and CD8 T-Cell Responses in Metastatic Melanoma Patients--Report of a Phase I/IIa Clinical Trial. Clin. Cancer Res. 2016, 22, 1330–1340. [Google Scholar] [CrossRef]
- Atkinson, V.; Khattak, A.; Haydon, A.; Eastgate, M.; Roy, A.; Prithviraj, P.; Mueller, C.; Brignone, C.; Triebel, F. Eftilagimod alpha, a soluble lymphocyte activation gene-3 (LAG-3) protein plus pembrolizumab in patients with metastatic melanoma. J. Immunother. Cancer 2020, 8, e001681. [Google Scholar] [CrossRef]
- Natoli, M.; Hatje, K.; Gulati, P.; Junker, F.; Herzig, P.; Jiang, Z.; Davydov, I.I.; Germann, M.; Trub, M.; Marbach, D.; et al. Deciphering molecular and cellular ex vivo responses to bispecific antibodies PD1-TIM3 and PD1-LAG3 in human tumors. J. Immunother. Cancer 2022, 10, e005548. [Google Scholar] [CrossRef]
- Sung, E.; Ko, M.; Won, J.Y.; Jo, Y.; Park, E.; Kim, H.; Choi, E.; Jung, U.J.; Jeon, J.; Kim, Y.; et al. LAG-3xPD-L1 bispecific antibody potentiates antitumor responses of T cells through dendritic cell activation. Mol. Ther. 2022, 30, 2800–2816. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ni, H.; Zhang, P.; Guo, X.; Wu, M.; Shen, H.; Wang, J.; Wu, W.; Wu, Z.; Ding, J.; et al. PD-L1/LAG-3 bispecific antibody enhances tumor-specific immunity. Oncoimmunology 2021, 10, 1943180. [Google Scholar] [CrossRef] [PubMed]
- Rohrberg, K.S.; Garralda, E.; Calvo, E.; Moreno Garcia, V.; Guidi, M.; Kraus, D.G.; McIntyre, C.; Kao, H.; Codarri Deak, L.; Michielin, F.; et al. 745P Clinical activity, safety, and PK/PD from the first in human study (NP41300) of RO7247669, a PD1-LAG3 bispecific antibody. Ann. Oncol. 2022, 33, S884–S885. [Google Scholar] [CrossRef]
- Long, G.V.; Amaria, R.; Tarhini, A.; Ascierto, P.A.; Cha, E.; Cirovic, O.; Cotting, D.; Teichgräber, V.; Blank, C.U. 884TiP A phase Ib/II multicenter, randomized, umbrella study evaluating novel treatment combinations in melanoma (MORPHEUS-MELANOMA). Ann. Oncol. 2022, 33, S952–S953. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Li, M.; Hu, D.; Li, C.; Ge, B.; Jin, B.; Fan, Z. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ. 2013, 20, 456–464. [Google Scholar] [CrossRef]
- Li, M.; Xia, P.; Du, Y.; Liu, S.; Huang, G.; Chen, J.; Zhang, H.; Hou, N.; Cheng, X.; Zhou, L.; et al. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. J. Biol. Chem. 2014, 289, 17647–17657. [Google Scholar] [CrossRef]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef]
- Levin, S.D.; Taft, D.W.; Brandt, C.S.; Bucher, C.; Howard, E.D.; Chadwick, E.M.; Johnston, J.; Hammond, A.; Bontadelli, K.; Ardourel, D.; et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur. J. Immunol. 2011, 41, 902–915. [Google Scholar] [CrossRef]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef]
- Boles, K.S.; Vermi, W.; Facchetti, F.; Fuchs, A.; Wilson, T.J.; Diacovo, T.G.; Cella, M.; Colonna, M. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur. J. Immunol. 2009, 39, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Tahara-Hanaoka, S.; Shibuya, K.; Onoda, Y.; Zhang, H.; Yamazaki, S.; Miyamoto, A.; Honda, S.; Lanier, L.L.; Shibuya, A. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112). Int. Immunol. 2004, 16, 533–538. [Google Scholar] [CrossRef]
- Fuchs, A.; Cella, M.; Giurisato, E.; Shaw, A.S.; Colonna, M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155). J. Immunol. 2004, 172, 3994–3998. [Google Scholar] [CrossRef] [PubMed]
- Dougall, W.C.; Kurtulus, S.; Smyth, M.J.; Anderson, A.C. TIGIT and CD96: New checkpoint receptor targets for cancer immunotherapy. Immunol. Rev. 2017, 276, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Masson, D.; Jarry, A.; Baury, B.; Blanchardie, P.; Laboisse, C.; Lustenberger, P.; Denis, M.G. Overexpression of the CD155 gene in human colorectal carcinoma. Gut 2001, 49, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Nakai, R.; Maniwa, Y.; Tanaka, Y.; Nishio, W.; Yoshimura, M.; Okita, Y.; Ohbayashi, C.; Satoh, N.; Ogita, H.; Takai, Y.; et al. Overexpression of Necl-5 correlates with unfavorable prognosis in patients with lung adenocarcinoma. Cancer Sci. 2010, 101, 1326–1330. [Google Scholar] [CrossRef]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Clinical significance of CD155 expression in human pancreatic cancer. Anticancer Res. 2015, 35, 2287–2297. [Google Scholar]
- Bevelacqua, V.; Bevelacqua, Y.; Candido, S.; Skarmoutsou, E.; Amoroso, A.; Guarneri, C.; Strazzanti, A.; Gangemi, P.; Mazzarino, M.C.; D’Amico, F.; et al. Nectin like-5 overexpression correlates with the malignant phenotype in cutaneous melanoma. Oncotarget 2012, 3, 882–892. [Google Scholar] [CrossRef]
- Stalhammar, G.; Seregard, S.; Grossniklaus, H.E. Expression of immune checkpoint receptors Indoleamine 2,3-dioxygenase and T cell Ig and ITIM domain in metastatic versus nonmetastatic choroidal melanoma. Cancer Med. 2019, 8, 2784–2792. [Google Scholar] [CrossRef]
- Wen, J.; Mao, X.; Cheng, Q.; Liu, Z.; Liu, F. A pan-cancer analysis revealing the role of TIGIT in tumor microenvironment. Sci. Rep. 2021, 11, 22502. [Google Scholar] [CrossRef]
- Inozume, T.; Yaguchi, T.; Furuta, J.; Harada, K.; Kawakami, Y.; Shimada, S. Melanoma Cells Control Antimelanoma CTL Responses via Interaction between TIGIT and CD155 in the Effector Phase. J. Investig. Dermatol. 2016, 136, 255–263. [Google Scholar] [CrossRef]
- Josefsson, S.E.; Huse, K.; Kolstad, A.; Beiske, K.; Pende, D.; Steen, C.B.; Inderberg, E.M.; Lingjaerde, O.C.; Ostenstad, B.; Smeland, E.B.; et al. T Cells Expressing Checkpoint Receptor TIGIT Are Enriched in Follicular Lymphoma Tumors and Characterized by Reversible Suppression of T-cell Receptor Signaling. Clin. Cancer Res. 2018, 24, 870–881. [Google Scholar] [CrossRef]
- Joller, N.; Hafler, J.P.; Brynedal, B.; Kassam, N.; Spoerl, S.; Levin, S.D.; Sharpe, A.H.; Kuchroo, V.K. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 2011, 186, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, H.; Li, J.; Hou, X.; Li, L.; Wang, W.; Shi, Y.; Li, D.; Li, L.; Zhao, Z.; et al. Involvement of TIGIT in Natural Killer Cell Exhaustion and Immune Escape in Patients and Mouse Model With Liver Echinococcus multilocularis Infection. Hepatology 2021, 74, 3376–3393. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.M.; Ka, M.; Pagliano, O.; Menna, C.; Ding, Q.; DeBlasio, R.; Sanders, C.; Hou, J.; Li, X.Y.; Ferrone, S.; et al. IL15 Stimulation with TIGIT Blockade Reverses CD155-mediated NK-Cell Dysfunction in Melanoma. Clin. Cancer Res. 2020, 26, 5520–5533. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hou, H.; Wu, S.; Tang, Q.; Liu, W.; Huang, M.; Yin, B.; Huang, J.; Mao, L.; Lu, Y.; et al. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur. J. Immunol. 2015, 45, 2886–2897. [Google Scholar] [CrossRef]
- Joller, N.; Lozano, E.; Burkett, P.R.; Patel, B.; Xiao, S.; Zhu, C.; Xia, J.; Tan, T.G.; Sefik, E.; Yajnik, V.; et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014, 40, 569–581. [Google Scholar] [CrossRef]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.F.; Joller, N.; Tan, D.J.; Teng, M.W.; Smyth, M.J.; Kuchroo, V.K.; Anderson, A.C. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Investig. 2015, 125, 4053–4062. [Google Scholar] [CrossRef]
- Fourcade, J.; Sun, Z.; Chauvin, J.M.; Ka, M.; Davar, D.; Pagliano, O.; Wang, H.; Saada, S.; Menna, C.; Amin, R.; et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight 2018, 3, e121157. [Google Scholar] [CrossRef]
- Simon, S.; Voillet, V.; Vignard, V.; Wu, Z.; Dabrowski, C.; Jouand, N.; Beauvais, T.; Khammari, A.; Braudeau, C.; Josien, R.; et al. PD-1 and TIGIT coexpression identifies a circulating CD8 T cell subset predictive of response to anti-PD-1 therapy. J. Immunother. Cancer 2020, 8, e001631. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Lee, Y.J.; Choi, M.E.; Yun, K.A.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E. Expression of lymphocyte-activating gene 3 and T-cell immunoreceptor with immunoglobulin and ITIM domains in cutaneous melanoma and their correlation with programmed cell death 1 expression in tumor-infiltrating lymphocytes. J. Am. Acad. Dermatol. 2019, 81, 219–227. [Google Scholar] [CrossRef]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Zhu, C.; Kondo, Y.; Anderson, A.C.; Gandhi, A.; Russell, A.; Dougan, S.K.; Petersen, B.S.; Melum, E.; Pertel, T.; et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015, 517, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Casasnovas, J.M.; Umetsu, D.T.; DeKruyff, R.H. TIM genes: A family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 2010, 235, 172–189. [Google Scholar] [CrossRef]
- Joller, N.; Kuchroo, V.K. Tim-3, Lag-3, and TIGIT. Curr. Top. Microbiol. Immunol. 2017, 410, 127–156. [Google Scholar] [CrossRef]
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Yao, J.; Li, H.; Yan, M.; Chang, W.C.; Hsu, J.M.; Cha, J.H.; et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef]
- da Silva, I.P.; Gallois, A.; Jimenez-Baranda, S.; Khan, S.; Anderson, A.C.; Kuchroo, V.K.; Osman, I.; Bhardwaj, N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol. Res. 2014, 2, 410–422. [Google Scholar] [CrossRef]
- Huang, L.; Xu, Y.; Fang, J.; Liu, W.; Chen, J.; Liu, Z.; Xu, Q. Targeting STAT3 Abrogates Tim-3 Upregulation of Adaptive Resistance to PD-1 Blockade on Regulatory T Cells of Melanoma. Front. Immunol. 2021, 12, 654749. [Google Scholar] [CrossRef]
- Chiba, S.; Baghdadi, M.; Akiba, H.; Yoshiyama, H.; Kinoshita, I.; Dosaka-Akita, H.; Fujioka, Y.; Ohba, Y.; Gorman, J.V.; Colgan, J.D.; et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 2012, 13, 832–842. [Google Scholar] [CrossRef]
- Ito, M.; Mimura, K.; Nakajima, S.; Saito, K.; Min, A.K.T.; Okayama, H.; Saito, M.; Momma, T.; Saze, Z.; Ohtsuka, M.; et al. Immune escape mechanism behind resistance to anti-PD-1 therapy in gastrointestinal tract metastasis in malignant melanoma patients with multiple metastases. Cancer Immunol. Immunother. 2022, 71, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cai, P.; Wang, N.; Zhang, Q.; Chen, F.; Shi, L.; Zhang, Y.; Wang, L.; Hu, L. Combined blockade of Tim-3 and MEK inhibitor enhances the efficacy against melanoma. Biochem. Biophys. Res. Commun. 2017, 484, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Tallerico, R.; Cristiani, C.M.; Staaf, E.; Garofalo, C.; Sottile, R.; Capone, M.; Pico de Coana, Y.; Madonna, G.; Palella, E.; Wolodarski, M.; et al. IL-15, TIM-3 and NK cells subsets predict responsiveness to anti-CTLA-4 treatment in melanoma patients. Oncoimmunology 2017, 6, e1261242. [Google Scholar] [CrossRef] [PubMed]
- Graves, M.; CelliMarchett, G.; van Zyl, B.; Tang, D.; Vilain, R.E.; van der Westhuizen, A.; Bowden, N.A. Monitoring Patient Response to Pembrolizumab With Peripheral Blood Exhaustion Marker Profiles. Front. Med. 2019, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Khunger, A.; Sarikonda, G.; Tsau, J.; Pahuja, A.; Alfonso, Z.; Gao, J.; Laing, C.; Vaupel, C.; Dakappagari, N.; Tarhini, A.A. Multimarker scores of Th1 and Th2 immune cellular profiles in peripheral blood predict response and immune related toxicity with CTLA4 blockade and IFNalpha in melanoma. Transl. Oncol. 2021, 14, 101014. [Google Scholar] [CrossRef]
- Conway, J.W.; Rawson, R.V.; Lo, S.; Ahmed, T.; Vergara, I.A.; Gide, T.N.; Attrill, G.H.; Carlino, M.S.; Saw, R.P.M.; Thompson, J.F.; et al. Unveiling the tumor immune microenvironment of organ-specific melanoma metastatic sites. J. Immunother. Cancer 2022, 10, e004884. [Google Scholar] [CrossRef]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef]
- Falchook, G.S.; Ribas, A.; Davar, D.; Eroglu, Z.; Wang, J.S.; Luke, J.J.; Hamilton, E.P.; Di Pace, B.; Wang, T.; Ghosh, S.; et al. Phase 1 trial of TIM-3 inhibitor cobolimab monotherapy and in combination with PD-1 inhibitors nivolumab or dostarlimab (AMBER). J. Clin. Oncol. 2022, 40, 2504. [Google Scholar] [CrossRef]
- Schwartz, S.; Patel, N.; Longmire, T.; Jayaraman, P.; Jiang, X.; Lu, H.; Baker, L.; Velez, J.; Ramesh, R.; Wavreille, A.S.; et al. Characterization of sabatolimab, a novel immunotherapy with immuno-myeloid activity directed against TIM-3 receptor. Immunother. Adv. 2022, 2, ltac019. [Google Scholar] [CrossRef]
- Gutierrez, M.E.; Tang, S.C.; Powderly, J.D.; Balmanoukian, A.S.; Janik, J.; Hoyle, P.; Wei, W.; Gong, X.; Hamid, O. 730MO First-in-human phase I study of INCAGN02390, a TIM-3 monoclonal antibody antagonist in patients with advanced malignancies. Ann. Oncol. 2022, 33, S876–S877. [Google Scholar] [CrossRef]
- ElTanbouly, M.A.; Zhao, Y.; Nowak, E.; Li, J.; Schaafsma, E.; Le Mercier, I.; Ceeraz, S.; Lines, J.L.; Peng, C.; Carriere, C.; et al. VISTA is a checkpoint regulator for naive T cell quiescence and peripheral tolerance. Science 2020, 367, eaay0524. [Google Scholar] [CrossRef] [PubMed]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014, 74, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Maddineni, S.; Mathews, I.I.; Andres Parra Sperberg, R.; Huang, P.S.; Cochran, J.R. Structure and Functional Binding Epitope of V-domain Ig Suppressor of T Cell Activation. Cell Rep. 2019, 28, 2509–2516.e5. [Google Scholar] [CrossRef]
- Xu, W.; Dong, J.; Zheng, Y.; Zhou, J.; Yuan, Y.; Ta, H.M.; Miller, H.E.; Olson, M.; Rajasekaran, K.; Ernstoff, M.S.; et al. Immune-Checkpoint Protein VISTA Regulates Antitumor Immunity by Controlling Myeloid Cell-Mediated Inflammation and Immunosuppression. Cancer Immunol. Res. 2019, 7, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011, 208, 577–592. [Google Scholar] [CrossRef]
- Wang, J.; Wu, G.; Manick, B.; Hernandez, V.; Renelt, M.; Erickson, C.; Guan, J.; Singh, R.; Rollins, S.; Solorz, A.; et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology 2019, 156, 74–85. [Google Scholar] [CrossRef]
- Yasinska, I.M.; Meyer, N.H.; Schlichtner, S.; Hussain, R.; Siligardi, G.; Casely-Hayford, M.; Fiedler, W.; Wellbrock, J.; Desmet, C.; Calzolai, L.; et al. Ligand-Receptor Interactions of Galectin-9 and VISTA Suppress Human T Lymphocyte Cytotoxic Activity. Front. Immunol. 2020, 11, 580557. [Google Scholar] [CrossRef]
- Fu, J.; Li, S.; Ma, H.; Yang, J.; Pagnotti, G.M.; Weiss, S.J.; Mapara, M.Y.; Lentzsch, S. Checkpoint Inhibitor PD-1H/VISTA Mediates MMP-13 Induced Osteoclast Activation and Multiple Myeloma Bone Disease. Blood 2020, 136, 15–16. [Google Scholar] [CrossRef]
- Yoon, K.W.; Byun, S.; Kwon, E.; Hwang, S.Y.; Chu, K.; Hiraki, M.; Jo, S.H.; Weins, A.; Hakroush, S.; Cebulla, A.; et al. Control of signaling-mediated clearance of apoptotic cells by the tumor suppressor p53. Science 2015, 349, 1261669. [Google Scholar] [CrossRef]
- Flies, D.B.; Wang, S.; Xu, H.; Chen, L. Cutting edge: A monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J. Immunol. 2011, 187, 1537–1541. [Google Scholar] [CrossRef]
- Bharaj, P.; Chahar, H.S.; Alozie, O.K.; Rodarte, L.; Bansal, A.; Goepfert, P.A.; Dwivedi, A.; Manjunath, N.; Shankar, P. Characterization of programmed death-1 homologue-1 (PD-1H) expression and function in normal and HIV infected individuals. PLoS ONE 2014, 9, e109103. [Google Scholar] [CrossRef] [PubMed]
- ElTanbouly, M.A.; Schaafsma, E.; Noelle, R.J.; Lines, J.L. VISTA: Coming of age as a multi-lineage immune checkpoint. Clin. Exp. Immunol. 2020, 200, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Eltanbouly, M.; Smits, N.; Sarde, A.; Noelle, R.; Mabaera, R. VISTA Is a Novel Regulator of Macrophage Biology. Blood 2019, 134, 2320. [Google Scholar] [CrossRef]
- ElTanbouly, M.A.; Schaafsma, E.; Smits, N.C.; Shah, P.; Cheng, C.; Burns, C.; Blazar, B.R.; Noelle, R.J.; Mabaera, R. VISTA Re-programs Macrophage Biology Through the Combined Regulation of Tolerance and Anti-inflammatory Pathways. Front. Immunol. 2020, 11, 580187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Le Mercier, I.; Putra, J.; Chen, W.; Liu, J.; Schenk, A.D.; Nowak, E.C.; Suriawinata, A.A.; Li, J.; Noelle, R.J. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc. Natl. Acad. Sci. USA 2014, 111, 14846–14851. [Google Scholar] [CrossRef]
- Kang, C.W.; Dutta, A.; Chang, L.Y.; Mahalingam, J.; Lin, Y.C.; Chiang, J.M.; Hsu, C.Y.; Huang, C.T.; Su, W.T.; Chu, Y.Y.; et al. Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer. Sci. Rep. 2015, 5, 15659. [Google Scholar] [CrossRef]
- Le Mercier, I.; Chen, W.; Lines, J.L.; Day, M.; Li, J.; Sergent, P.; Noelle, R.J.; Wang, L. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014, 74, 1933–1944. [Google Scholar] [CrossRef]
- Johnston, R.J.; Su, L.J.; Pinckney, J.; Critton, D.; Boyer, E.; Krishnakumar, A.; Corbett, M.; Rankin, A.L.; Dibella, R.; Campbell, L.; et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature 2019, 574, 565–570. [Google Scholar] [CrossRef]
- Tinoco, R.; Carrette, F.; Barraza, M.L.; Otero, D.C.; Magana, J.; Bosenberg, M.W.; Swain, S.L.; Bradley, L.M. PSGL-1 Is an Immune Checkpoint Regulator that Promotes T Cell Exhaustion. Immunity 2016, 44, 1470. [Google Scholar] [CrossRef]
- Kuklinski, L.F.; Yan, S.; Li, Z.; Fisher, J.L.; Cheng, C.; Noelle, R.J.; Angeles, C.V.; Turk, M.J.; Ernstoff, M.S. VISTA expression on tumor-infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease-specific survival. Cancer Immunol. Immunother. 2018, 67, 1113–1121. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, Y.J.; Yun, K.A.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E.; Lee, W.J. The prognostic significance of VISTA and CD33-positive myeloid cells in cutaneous melanoma and their relationship with PD-1 expression. Sci. Rep. 2020, 10, 14372. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.R.; Knecht, M.; Mollaee, M.; Zhong, Z.; Erkes, D.A.; McCue, P.A.; Chervoneva, I.; Berger, A.C.; Lo, J.A.; Fisher, D.E.; et al. FOXD3 Regulates VISTA Expression in Melanoma. Cell Rep. 2020, 30, 510–524.e6. [Google Scholar] [CrossRef] [PubMed]
- Kakavand, H.; Jackett, L.A.; Menzies, A.M.; Gide, T.N.; Carlino, M.S.; Saw, R.P.M.; Thompson, J.F.; Wilmott, J.S.; Long, G.V.; Scolyer, R.A. Negative immune checkpoint regulation by VISTA: A mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod. Pathol. 2017, 30, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ward, J.F.; Pettaway, C.A.; Shi, L.Z.; Subudhi, S.K.; Vence, L.M.; Zhao, H.; Chen, J.; Chen, H.; Efstathiou, E.; et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017, 23, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Powderly, J.; Patel, M.R.; Lee, J.J.; Brody, J.; Meric-Bernstam, F.; Hamilton, E.; Ponce Aix, S.; Garcia-Corbacho, J.; Bang, Y.J.; Ahn, M.J.; et al. CA-170, a first in class oral small molecule dual inhibitor of immune checkpoints PD-L1 and VISTA, demonstrates tumor growth inhibition in pre-clinical models and promotes T cell activation in Phase 1 study. Ann. Oncol. 2017, 28, v405–v406. [Google Scholar] [CrossRef]
- Sasikumar, P.G.; Sudarshan, N.S.; Adurthi, S.; Ramachandra, R.K.; Samiulla, D.S.; Lakshminarasimhan, A.; Ramanathan, A.; Chandrasekhar, T.; Dhudashiya, A.A.; Talapati, S.R.; et al. PD-1 derived CA-170 is an oral immune checkpoint inhibitor that exhibits preclinical anti-tumor efficacy. Commun. Biol. 2021, 4, 699. [Google Scholar] [CrossRef]
- Radhakrishnan, V.; Banavali, S.; Gupta, S.; Kumar, A.; Deshmukh, C.D.; Nag, S.; Beniwal, S.K.; Gopichand, M.; Naik, R.; Lakshmaiah, K.C.; et al. Excellent CBR and prolonged PFS in non-squamous NSCLC with oral CA-170, an inhibitor of VISTA and PD-L1. Ann. Oncol. 2019, 30, v494. [Google Scholar] [CrossRef]
- Thakkar, D.; Paliwal, S.; Dharmadhikari, B.; Guan, S.; Liu, L.; Kar, S.; Tulsian, N.K.; Gruber, J.J.; DiMascio, L.; Paszkiewicz, K.H.; et al. Rationally targeted anti-VISTA antibody that blockades the C-C’ loop region can reverse VISTA immune suppression and remodel the immune microenvironment to potently inhibit tumor growth in an Fc independent manner. J. Immunother. Cancer 2022, 10, e003382. [Google Scholar] [CrossRef]
- Mehta, N.; Maddineni, S.; Kelly, R.L.; Lee, R.B.; Hunter, S.A.; Silberstein, J.L.; Parra Sperberg, R.A.; Miller, C.L.; Rabe, A.; Labanieh, L.; et al. An engineered antibody binds a distinct epitope and is a potent inhibitor of murine and human VISTA. Sci. Rep. 2020, 10, 15171. [Google Scholar] [CrossRef]
- Alanazi, F.E.; As Sobeai, H.M.; Alhazzani, K.; Al-Dhfyan, A.; Alshammari, M.A.; Alotaibi, M.; Al-Hosaini, K.; Korashy, H.M.; Alhoshani, A. Metformin attenuates V-domain Ig suppressor of T-cell activation through the aryl hydrocarbon receptor pathway in Melanoma: In Vivo and In Vitro Studies. Saudi Pharm. J. 2022, 30, 138–149. [Google Scholar] [CrossRef]
- Zhai, L.; Ladomersky, E.; Bell, A.; Dussold, C.; Cardoza, K.; Qian, J.; Lauing, K.L.; Wainwright, D.A. Quantification of IDO1 enzyme activity in normal and malignant tissues. Methods Enzymol. 2019, 629, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Meireson, A.; Devos, M.; Brochez, L. IDO Expression in Cancer: Different Compartment, Different Functionality? Front. Immunol. 2020, 11, 531491. [Google Scholar] [CrossRef] [PubMed]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolusic, E.; Frederick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Salmi, S.; Lin, A.; Hirschovits-Gerz, B.; Valkonen, M.; Aaltonen, N.; Sironen, R.; Siiskonen, H.; Pasonen-Seppanen, S. The role of FoxP3+ regulatory T cells and IDO+ immune and tumor cells in malignant melanoma—An immunohistochemical study. BMC Cancer 2021, 21, 641. [Google Scholar] [CrossRef]
- Lee, G.K.; Park, H.J.; Macleod, M.; Chandler, P.; Munn, D.H.; Mellor, A.L. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 2002, 107, 452–460. [Google Scholar] [CrossRef]
- Qin, R.; Zhao, C.; Wang, C.J.; Xu, W.; Zhao, J.Y.; Lin, Y.; Yuan, Y.Y.; Lin, P.C.; Li, Y.; Zhao, S.; et al. Tryptophan potentiates CD8(+) T cells against cancer cells by TRIP12 tryptophanylation and surface PD-1 downregulation. J. Immunother. Cancer 2021, 9, e002840. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Liu, K.; Bizargity, P.; Hancock, W.W.; Visner, G.A. Reduced cytotoxic function of effector CD8+ T cells is responsible for indoleamine 2,3-dioxygenase-dependent immune suppression. J. Immunol. 2009, 183, 1022–1031. [Google Scholar] [CrossRef]
- Greene, L.I.; Bruno, T.C.; Christenson, J.L.; D’Alessandro, A.; Culp-Hill, R.; Torkko, K.; Borges, V.F.; Slansky, J.E.; Richer, J.K. A Role for Tryptophan-2,3-dioxygenase in CD8 T-cell Suppression and Evidence of Tryptophan Catabolism in Breast Cancer Patient Plasma. Mol. Cancer Res. 2019, 17, 131–139. [Google Scholar] [CrossRef]
- Holmgaard, R.B.; Zamarin, D.; Li, Y.; Gasmi, B.; Munn, D.H.; Allison, J.P.; Merghoub, T.; Wolchok, J.D. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep. 2015, 13, 412–424. [Google Scholar] [CrossRef]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef]
- Li, H.; Bullock, K.; Gurjao, C.; Braun, D.; Shukla, S.A.; Bosse, D.; Lalani, A.A.; Gopal, S.; Jin, C.; Horak, C.; et al. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat. Commun. 2019, 10, 4346. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.J.; Kim, T.M.; et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Beatty, G.L.; O’Dwyer, P.J.; Clark, J.; Shi, J.G.; Bowman, K.J.; Scherle, P.A.; Newton, R.C.; Schaub, R.; Maleski, J.; Leopold, L.; et al. First-in-Human Phase I Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients with Advanced Solid Malignancies. Clin. Cancer Res. 2017, 23, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.J.; Manfredi, M.G.; Zakharia, Y.; Prendergast, G.C. Inhibiting IDO pathways to treat cancer: Lessons from the ECHO-301 trial and beyond. Semin. Immunopathol. 2019, 41, 41–48. [Google Scholar] [CrossRef]
- Prendergast, G.C.; Malachowski, W.P.; DuHadaway, J.B.; Muller, A.J. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res. 2017, 77, 6795–6811. [Google Scholar] [CrossRef]
- Zakharia, Y.; McWilliams, R.R.; Rixe, O.; Drabick, J.; Shaheen, M.F.; Grossmann, K.F.; Kolhe, R.; Pacholczyk, R.; Sadek, R.; Tennant, L.L.; et al. Phase II trial of the IDO pathway inhibitor indoximod plus pembrolizumab for the treatment of patients with advanced melanoma. J. Immunother. Cancer 2021, 9, e002057. [Google Scholar] [CrossRef]
- Kjeldsen, J.W.; Lorentzen, C.L.; Martinenaite, E.; Ellebaek, E.; Donia, M.; Holmstroem, R.B.; Klausen, T.W.; Madsen, C.O.; Ahmed, S.M.; Weis-Banke, S.E.; et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat. Med. 2021, 27, 2212–2223. [Google Scholar] [CrossRef]
- Kapron, B.; Plazinska, A.; Plazinski, W.; Plech, T. Identification of the first-in-class dual inhibitors of human DNA topoisomerase IIalpha and indoleamine-2,3-dioxygenase 1 (IDO 1) with strong anticancer properties. J. Enzym. Inhib. Med. Chem. 2023, 38, 192–202. [Google Scholar] [CrossRef]
- Hu, Z.; Zheng, B.; Xu, J.; Gao, S.; Lu, W. An albumin-bound drug conjugate of paclitaxel and indoleamine-2,3-dioxygenase inhibitor for enhanced cancer chemo-immunotherapy. Nanotechnology 2020, 31, 295101. [Google Scholar] [CrossRef]
- Liu, H.; Gao, H.; Chen, C.; Jia, W.; Xu, D.; Jiang, G. IDO Inhibitor and Gallic Acid Cross-Linked Small Molecule Drug Synergistic Treatment of Melanoma. Front. Oncol. 2022, 12, 904229. [Google Scholar] [CrossRef]
- Denoeud, J.; Moser, M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J. Leukoc. Biol. 2011, 89, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Buchan, S.L.; Rogel, A.; Al-Shamkhani, A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood 2018, 131, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Agematsu, K. Memory B cells and CD27. Histol. Histopathol. 2000, 15, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Flieswasser, T.; Van den Eynde, A.; Van Audenaerde, J.; De Waele, J.; Lardon, F.; Riether, C.; de Haard, H.; Smits, E.; Pauwels, P.; Jacobs, J. The CD70-CD27 axis in oncology: The new kids on the block. J. Exp. Clin. Cancer Res. 2022, 41, 12. [Google Scholar] [CrossRef] [PubMed]
- Kuka, M.; Munitic, I.; Giardino Torchia, M.L.; Ashwell, J.D. CD70 is downregulated by interaction with CD27. J. Immunol. 2013, 191, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.M.; Groothuis, T.A.; Veraar, E.A.; Marsman, M.; Maillette de Buy Wenniger, L.; Janssen, H.; Neefjes, J.; Borst, J. Costimulatory ligand CD70 is delivered to the immunological synapse by shared intracellular trafficking with MHC class II molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 5989–5994. [Google Scholar] [CrossRef]
- Polak, M.E.; Newell, L.; Taraban, V.Y.; Pickard, C.; Healy, E.; Friedmann, P.S.; Al-Shamkhani, A.; Ardern-Jones, M.R. CD70-CD27 interaction augments CD8+ T-cell activation by human epidermal Langerhans cells. J. Investig. Dermatol. 2012, 132, 1636–1644. [Google Scholar] [CrossRef]
- Keller, A.M.; Schildknecht, A.; Xiao, Y.; van den Broek, M.; Borst, J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity 2008, 29, 934–946. [Google Scholar] [CrossRef]
- Keller, A.M.; Xiao, Y.; Peperzak, V.; Naik, S.H.; Borst, J. Costimulatory ligand CD70 allows induction of CD8+ T-cell immunity by immature dendritic cells in a vaccination setting. Blood 2009, 113, 5167–5175. [Google Scholar] [CrossRef]
- Kong, F.; Ye, Q.; Xiong, Y. Comprehensive analysis of prognosis and immune function of CD70-CD27 signaling axis in pan-cancer. Funct. Integr. Genom. 2023, 23, 48. [Google Scholar] [CrossRef]
- Claus, C.; Riether, C.; Schurch, C.; Matter, M.S.; Hilmenyuk, T.; Ochsenbein, A.F. CD27 signaling increases the frequency of regulatory T cells and promotes tumor growth. Cancer Res. 2012, 72, 3664–3676. [Google Scholar] [CrossRef] [PubMed]
- Muth, S.; Klaric, A.; Radsak, M.; Schild, H.; Probst, H.C. CD27 expression on Treg cells limits immune responses against tumors. J. Mol. Med. 2022, 100, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Pich, C.; Sarrabayrouse, G.; Teiti, I.; Mariame, B.; Rochaix, P.; Lamant, L.; Favre, G.; Maisongrosse, V.; Tilkin-Mariame, A.F. Melanoma-expressed CD70 is involved in invasion and metastasis. Br. J. Cancer 2016, 114, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wasiuk, A.; Testa, J.; Weidlick, J.; Sisson, C.; Vitale, L.; Widger, J.; Crocker, A.; Thomas, L.J.; Goldstein, J.; Marsh, H.C.; et al. CD27-Mediated Regulatory T Cell Depletion and Effector T Cell Costimulation Both Contribute to Antitumor Efficacy. J. Immunol. 2017, 199, 4110–4123. [Google Scholar] [CrossRef] [PubMed]
- Arens, R.; Schepers, K.; Nolte, M.A.; van Oosterwijk, M.F.; van Lier, R.A.; Schumacher, T.N.; van Oers, M.H. Tumor rejection induced by CD70-mediated quantitative and qualitative effects on effector CD8+ T cell formation. J. Exp. Med. 2004, 199, 1595–1605. [Google Scholar] [CrossRef]
- Huang, J.; Kerstann, K.W.; Ahmadzadeh, M.; Li, Y.F.; El-Gamil, M.; Rosenberg, S.A.; Robbins, P.F. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: Importance for the therapeutic effectiveness of cell transfer immunotherapy. J. Immunol. 2006, 176, 7726–7735. [Google Scholar] [CrossRef]
- Ahrends, T.; Spanjaard, A.; Pilzecker, B.; Babala, N.; Bovens, A.; Xiao, Y.; Jacobs, H.; Borst, J. CD4(+) T Cell Help Confers a Cytotoxic T Cell Effector Program Including Coinhibitory Receptor Downregulation and Increased Tissue Invasiveness. Immunity 2017, 47, 848–861.e845. [Google Scholar] [CrossRef]
- Roberts, D.J.; Franklin, N.A.; Kingeter, L.M.; Yagita, H.; Tutt, A.L.; Glennie, M.J.; Bullock, T.N. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J. Immunother. 2010, 33, 769–779. [Google Scholar] [CrossRef]
- Ramakrishna, V.; Sundarapandiyan, K.; Zhao, B.; Bylesjo, M.; Marsh, H.C.; Keler, T. Characterization of the human T cell response to in vitro CD27 costimulation with varlilumab. J. Immunother. Cancer 2015, 3, 37. [Google Scholar] [CrossRef]
- Burris, H.A.; Infante, J.R.; Ansell, S.M.; Nemunaitis, J.J.; Weiss, G.R.; Villalobos, V.M.; Sikic, B.I.; Taylor, M.H.; Northfelt, D.W.; Carson, W.E., 3rd; et al. Safety and Activity of Varlilumab, a Novel and First-in-Class Agonist Anti-CD27 Antibody, in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2017, 35, 2028–2036. [Google Scholar] [CrossRef]
- Ahrends, T.; Babala, N.; Xiao, Y.; Yagita, H.; van Eenennaam, H.; Borst, J. CD27 Agonism Plus PD-1 Blockade Recapitulates CD4+ T-cell Help in Therapeutic Anticancer Vaccination. Cancer Res. 2016, 76, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Buchan, S.; Manzo, T.; Flutter, B.; Rogel, A.; Edwards, N.; Zhang, L.; Sivakumaran, S.; Ghorashian, S.; Carpenter, B.; Bennett, C.; et al. OX40- and CD27-mediated costimulation synergizes with anti-PD-L1 blockade by forcing exhausted CD8+ T cells to exit quiescence. J. Immunol. 2015, 194, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Buchan, S.L.; Fallatah, M.; Thirdborough, S.M.; Taraban, V.Y.; Rogel, A.; Thomas, L.J.; Penfold, C.A.; He, L.Z.; Curran, M.A.; Keler, T.; et al. PD-1 Blockade and CD27 Stimulation Activate Distinct Transcriptional Programs That Synergize for CD8(+) T-Cell-Driven Antitumor Immunity. Clin. Cancer Res. 2018, 24, 2383–2394. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, R.E.; Pishvain, M.J.; Callahan, M.K.; Rizvi, N.; Kluger, H.; Yellin, M.; Rawls, T.; Vitale, L.; Halim, A.; Davis, T.; et al. Abstract CT023: Phase I results from the combination of an immune-activating anti-CD27 antibody (varlilumab) in combination with PD-1 blockade (nivolumab): Activation across multiple immune pathways without untoward immune-related adverse events. Cancer Res. 2016, 76, CT023. [Google Scholar] [CrossRef]
- Sanborn, R.E.; Pishvaian, M.J.; Callahan, M.K.; Weise, A.; Sikic, B.I.; Rahma, O.; Cho, D.C.; Rizvi, N.A.; Sznol, M.; Lutzky, J.; et al. Safety, tolerability and efficacy of agonist anti-CD27 antibody (varlilumab) administered in combination with anti-PD-1 (nivolumab) in advanced solid tumors. J. Immunother. Cancer 2022, 10, e005147. [Google Scholar] [CrossRef]
- Riccione, K.A.; He, L.Z.; Fecci, P.E.; Norberg, P.K.; Suryadevara, C.M.; Swartz, A.; Healy, P.; Reap, E.; Keler, T.; Li, Q.J.; et al. CD27 stimulation unveils the efficacy of linked class I/II peptide vaccines in poorly immunogenic tumors by orchestrating a coordinated CD4/CD8 T cell response. Oncoimmunology 2018, 7, e1502904. [Google Scholar] [CrossRef]
- Thiemann, M.; Richards, D.M.; Heinonen, K.; Kluge, M.; Marschall, V.; Merz, C.; Redondo Muller, M.; Schnyder, T.; Sefrin, J.P.; Sykora, J.; et al. A Single-Chain-Based Hexavalent CD27 Agonist Enhances T Cell Activation and Induces Anti-Tumor Immunity. Front. Oncol. 2018, 8, 387. [Google Scholar] [CrossRef]
- Vitale, L.A.; He, L.Z.; Thomas, L.J.; Wasiuk, A.; O’Neill, T.; Widger, J.; Crocker, A.; Mills-Chen, L.; Forsberg, E.; Weidlick, J.; et al. Development of CDX-527: A bispecific antibody combining PD-1 blockade and CD27 costimulation for cancer immunotherapy. Cancer Immunol. Immunother. 2020, 69, 2125–2137. [Google Scholar] [CrossRef]
- Starzer, A.M.; Berghoff, A.S. New emerging targets in cancer immunotherapy: CD27 (TNFRSF7). ESMO Open 2020, 4, e000629. [Google Scholar] [CrossRef]
- Umansky, V.; Shevchenko, I.; Bazhin, A.V.; Utikal, J. Extracellular adenosine metabolism in immune cells in melanoma. Cancer Immunol. Immunother. 2014, 63, 1073–1080. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Hasko, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Chen, X.; Li, L.; Li, Y.; Ping, Y.; Huang, L.; Yue, D.; Zhang, Z.; Wang, F.; et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-beta-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology 2017, 6, e1320011. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Westerman, K.A.; Faigle, M.; Eltzschig, H.K.; Colgan, S.P. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006, 20, 2242–2250. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, S.; Glover, L.; Miller, S.M.; Shannon, J.M.; Guo, X.; Franklin, W.A.; Bridges, J.P.; Schaack, J.B.; Colgan, S.P.; et al. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc. Natl. Acad. Sci. USA 2009, 106, 10684–10689. [Google Scholar] [CrossRef]
- Mastelic-Gavillet, B.; Navarro Rodrigo, B.; Décombaz, L.; Wang, H.; Ercolano, G.; Ahmed, R.; Lozano, L.E.; Ianaro, A.; Derré, L.; Valerio, M.; et al. Adenosine mediates functional and metabolic suppression of peripheral and tumor-infiltrating CD8+ T cells. J. ImmunoTherapy Cancer 2019, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ferris, R.L.; Whiteside, T.L. Chemokine C receptor 7 expression and protection of circulating CD8+ T lymphocytes from apoptosis. Clin. Cancer Res. 2005, 11, 7901–7910. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Y.; Yang, M.; Zhang, Y.; Xie, Q.; Li, Z.; Dong, Z.; Yang, Y.; Deng, B.; Feng, A.; et al. Hypoxia induces T-cell apoptosis by inhibiting chemokine C receptor 7 expression: The role of adenosine receptor A(2). Cell. Mol. Immunol. 2010, 7, 77–82. [Google Scholar] [CrossRef]
- Ohta, A.; Kini, R.; Ohta, A.; Subramanian, M.; Madasu, M.; Sitkovsky, M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012, 3, 190. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Ngiow, S.F.; Gao, Y.; Patch, A.M.; Barkauskas, D.S.; Messaoudene, M.; Lin, G.; Coudert, J.D.; Stannard, K.A.; Zitvogel, L.; et al. A2AR Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment. Cancer Res. 2018, 78, 1003–1016. [Google Scholar] [CrossRef]
- Raskovalova, T.; Huang, X.; Sitkovsky, M.; Zacharia, L.C.; Jackson, E.K.; Gorelik, E. Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. J. Immunol. 2005, 175, 4383–4391. [Google Scholar] [CrossRef]
- Lokshin, A.; Raskovalova, T.; Huang, X.; Zacharia, L.C.; Jackson, E.K.; Gorelik, E. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res. 2006, 66, 7758–7765. [Google Scholar] [CrossRef] [PubMed]
- Ryzhov, S.; Novitskiy, S.V.; Goldstein, A.E.; Biktasova, A.; Blackburn, M.R.; Biaggioni, I.; Dikov, M.M.; Feoktistov, I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J. Immunol. 2011, 187, 6120–6129. [Google Scholar] [CrossRef] [PubMed]
- Challier, J.; Bruniquel, D.; Sewell, A.K.; Laugel, B. Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8(+) T-cell priming capacity. Immunology 2013, 138, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Borodovsky, A.; Barbon, C.M.; Wang, Y.; Ye, M.; Prickett, L.; Chandra, D.; Shaw, J.; Deng, N.; Sachsenmeier, K.; Clarke, J.D.; et al. Small molecule AZD4635 inhibitor of A(2A)R signaling rescues immune cell function including CD103(+) dendritic cells enhancing anti-tumor immunity. J. Immunother. Cancer 2020, 8, e000417. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, L.; Song, Z.; Ren, M.; Yang, Y.; Li, J.; Shen, K.; Li, Y.; Ding, Y.; Yang, Y.; et al. Intratumoral CD73: An immune checkpoint shaping an inhibitory tumor microenvironment and implicating poor prognosis in Chinese melanoma cohorts. Front. Immunol. 2022, 13, 954039. [Google Scholar] [CrossRef]
- Canale, F.P.; Ramello, M.C.; Nunez, N.; Araujo Furlan, C.L.; Bossio, S.N.; Gorosito Serran, M.; Tosello Boari, J.; Del Castillo, A.; Ledesma, M.; Sedlik, C.; et al. CD39 Expression Defines Cell Exhaustion in Tumor-Infiltrating CD8(+) T Cells. Cancer Res. 2018, 78, 115–128. [Google Scholar] [CrossRef]
- Morello, S.; Capone, M.; Sorrentino, C.; Giannarelli, D.; Madonna, G.; Mallardo, D.; Grimaldi, A.M.; Pinto, A.; Ascierto, P.A. Soluble CD73 as biomarker in patients with metastatic melanoma patients treated with nivolumab. J. Transl. Med. 2017, 15, 244. [Google Scholar] [CrossRef]
- Turiello, R.; Capone, M.; Giannarelli, D.; Morretta, E.; Monti, M.C.; Madonna, G.; Mallardo, D.; Festino, L.; Azzaro, R.; Levesque, M.P.; et al. Serum CD73 is a prognostic factor in patients with metastatic melanoma and is associated with response to anti-PD-1 therapy. J. Immunother. Cancer 2020, 8, e001689. [Google Scholar] [CrossRef]
- Capone, M.; Fratangelo, F.; Giannarelli, D.; Sorrentino, C.; Turiello, R.; Zanotta, S.; Galati, D.; Madonna, G.; Tuffanelli, M.; Scarpato, L.; et al. Frequency of circulating CD8+CD73+T cells is associated with survival in nivolumab-treated melanoma patients. J. Transl. Med. 2020, 18, 121. [Google Scholar] [CrossRef]
- Reinhardt, J.; Landsberg, J.; Schmid-Burgk, J.L.; Ramis, B.B.; Bald, T.; Glodde, N.; Lopez-Ramos, D.; Young, A.; Ngiow, S.F.; Nettersheim, D.; et al. MAPK Signaling and Inflammation Link Melanoma Phenotype Switching to Induction of CD73 during Immunotherapy. Cancer Res. 2017, 77, 4697–4709. [Google Scholar] [CrossRef]
- Turiello, R.; Capone, M.; Morretta, E.; Monti, M.C.; Madonna, G.; Azzaro, R.; Del Gaudio, P.; Simeone, E.; Sorrentino, A.; Ascierto, P.A.; et al. Exosomal CD73 from serum of patients with melanoma suppresses lymphocyte functions and is associated with therapy resistance to anti-PD-1 agents. J. Immunother. Cancer 2022, 10, e004043. [Google Scholar] [CrossRef]
- Seifert, M.; Benmebarek, M.R.; Briukhovetska, D.; Markl, F.; Dorr, J.; Cadilha, B.L.; Jobst, J.; Stock, S.; Andreu-Sanz, D.; Lorenzini, T.; et al. Impact of the selective A2(A)R and A2(B)R dual antagonist AB928/etrumadenant on CAR T cell function. Br. J. Cancer 2022, 127, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Briceno, P.; Rivas-Yanez, E.; Rosemblatt, M.V.; Parra-Tello, B.; Farias, P.; Vargas, L.; Simon, V.; Cardenas, C.; Lladser, A.; Salazar-Onfray, F.; et al. CD73 Ectonucleotidase Restrains CD8+ T Cell Metabolic Fitness and Anti-tumoral Activity. Front. Cell Dev. Biol. 2021, 9, 638037. [Google Scholar] [CrossRef]
- McCaffery, I.; Laport, G.; Hotson, A.; Willingham, S.; Patnaik, A.; Beeram, M.; Miller, R. Biomarker and clinical activity of CPI-444, a novel small molecule inhibitor of A2A receptor (A2AR), in a Ph1b study in advanced cancers. Ann. Oncol. 2016, 27, vi124. [Google Scholar] [CrossRef]
- Harshman, L.C.; Chu, M.; George, S.; Hughes, B.G.M.; Carthon, B.C.; Fong, L.; Merchan, J.R.; Kwei, L.; Hotson, A.N.; Mobasher, M.; et al. Adenosine receptor blockade with ciforadenant +/- atezolizumab in advanced metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 129. [Google Scholar] [CrossRef]
- Fong, L.; Hotson, A.; Powderly, J.D.; Sznol, M.; Heist, R.S.; Choueiri, T.K.; George, S.; Hughes, B.G.M.; Hellmann, M.D.; Shepard, D.R.; et al. Adenosine 2A Receptor Blockade as an Immunotherapy for Treatment-Refractory Renal Cell Cancer. Cancer Discov. 2020, 10, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Willingham, S.B.; Ho, P.Y.; Hotson, A.; Hill, C.; Piccione, E.C.; Hsieh, J.; Liu, L.; Buggy, J.J.; McCaffery, I.; Miller, R.A. A2AR Antagonism with CPI-444 Induces Antitumor Responses and Augments Efficacy to Anti-PD-(L)1 and Anti-CTLA-4 in Preclinical Models. Cancer Immunol. Res. 2018, 6, 1136–1149. [Google Scholar] [CrossRef]
- Iannone, R.; Miele, L.; Maiolino, P.; Pinto, A.; Morello, S. Adenosine limits the therapeutic effectiveness of anti-CTLA4 mAb in a mouse melanoma model. Am. J. Cancer Res. 2014, 4, 172–181. [Google Scholar] [PubMed]
- Mittal, D.; Young, A.; Stannard, K.; Yong, M.; Teng, M.W.; Allard, B.; Stagg, J.; Smyth, M.J. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014, 74, 3652–3658. [Google Scholar] [CrossRef]
- Buisseret, L.; Rottey, S.; Bono, J.S.D.; Migeotte, A.; Delafontaine, B.; Manickavasagar, T.; Martinoli, C.; Wald, N.; Rossetti, M.; Gangolli, E.A.; et al. Phase 1 trial of the adenosine A2A receptor antagonist inupadenant (EOS-850): Update on tolerability, and antitumor activity potentially associated with the expression of the A2A receptor within the tumor. J. Clin. Oncol. 2021, 39, 2562. [Google Scholar] [CrossRef]
- Powderly, J.D.; Souza, P.L.d.; Gutierrez, R.; Horvath, L.; Seitz, L.; Ashok, D.; Park, A.; Walters, M.J.; Karakunnel, J.J.; Berry, W.; et al. AB928, a novel dual adenosine receptor antagonist, combined with chemotherapy or AB122 (anti-PD-1) in patients (pts) with advanced tumors: Preliminary results from ongoing phase I studies. J. Clin. Oncol. 2019, 37, 2604. [Google Scholar] [CrossRef]
- Murphy, K.M.; Nelson, C.A.; Sedy, J.R. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat. Rev. Immunol. 2006, 6, 671–681. [Google Scholar] [CrossRef]
- Pasero, C.; Olive, D. Interfering with coinhibitory molecules: BTLA/HVEM as new targets to enhance anti-tumor immunity. Immunol. Lett. 2013, 151, 71–75. [Google Scholar] [CrossRef]
- Demerle, C.; Gorvel, L.; Olive, D. BTLA-HVEM Couple in Health and Diseases: Insights for Immunotherapy in Lung Cancer. Front. Oncol. 2021, 11, 682007. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.C.; Ware, C.F. The canonical and unconventional ligands of the herpesvirus entry mediator. Adv. Exp. Med. Biol. 2011, 691, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Freeman, G.J. The CD160, BTLA, LIGHT/HVEM pathway: A bidirectional switch regulating T-cell activation. Immunol. Rev. 2009, 229, 244–258. [Google Scholar] [CrossRef]
- Cai, G.; Anumanthan, A.; Brown, J.A.; Greenfield, E.A.; Zhu, B.; Freeman, G.J. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat. Immunol. 2008, 9, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Giustiniani, J.; Marie-Cardine, A.; Bensussan, A. A soluble form of the MHC class I-specific CD160 receptor is released from human activated NK lymphocytes and inhibits cell-mediated cytotoxicity. J. Immunol. 2007, 178, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Gauci, M.L.; Giustiniani, J.; Lepelletier, C.; Garbar, C.; Thonnart, N.; Dumaz, N.; Foussat, A.; Lebbe, C.; Bensussan, A.; Marie-Cardine, A. The soluble form of CD160 acts as a tumor mediator of immune escape in melanoma. Cancer Immunol. Immunother. 2022, 71, 2731–2742. [Google Scholar] [CrossRef]
- Wojciechowicz, K.; Spodzieja, M.; Lisowska, K.A.; Wardowska, A. The role of the BTLA-HVEM complex in the pathogenesis of autoimmune diseases. Cell. Immunol. 2022, 376, 104532. [Google Scholar] [CrossRef]
- Haymaker, C.L.; Wu, R.C.; Ritthipichai, K.; Bernatchez, C.; Forget, M.A.; Chen, J.Q.; Liu, H.; Wang, E.; Marincola, F.; Hwu, P.; et al. BTLA marks a less-differentiated tumor-infiltrating lymphocyte subset in melanoma with enhanced survival properties. Oncoimmunology 2015, 4, e1014246. [Google Scholar] [CrossRef] [PubMed]
- Malissen, N.; Macagno, N.; Granjeaud, S.; Granier, C.; Moutardier, V.; Gaudy-Marqueste, C.; Habel, N.; Mandavit, M.; Guillot, B.; Pasero, C.; et al. HVEM has a broader expression than PD-L1 and constitutes a negative prognostic marker and potential treatment target for melanoma. Oncoimmunology 2019, 8, e1665976. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, J.; Sun, Z.; Pagliano, O.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Olive, D.; Kuchroo, V.; Zarour, H.M. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012, 72, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Miselis, N.R.; Linn, D.; Restaino, C.; Baral, T.; Xia, J.; Ueda, R.; Sawant, A.; Baker, J.; Raghunathan, G.; Wang1, X.; et al. Abstract 577: Antagonism of the co-inhibitory receptor BTLA enhances efficacy of anti-PD-1 treatment in murine syngeneic tumor models. Cancer Res. 2017, 77, 577. [Google Scholar] [CrossRef]
- Schilder, R.J.; Powderly, J.D.; Park, H.; Bilen, M.A.; McKean, M.; May, R.; Feng, H.; Yao, S.; Keegan, P.; Naing, A. Phase Ia dose-escalation study of the anti-BTLA antibody icatolimab as a monotherapy in patients with advanced solid tumor. J. Clin. Oncol. 2022, 40, 2643. [Google Scholar] [CrossRef]
- Kontos, F.; Michelakos, T.; Kurokawa, T.; Sadagopan, A.; Schwab, J.H.; Ferrone, C.R.; Ferrone, S. B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clin. Cancer Res. 2021, 27, 1227–1235. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, C.; Liu, Z.; Yang, M.; Tang, X.; Wang, Y.; Zheng, M.; Huang, J.; Zhong, K.; Zhao, S.; et al. B7-H3-Targeted CAR-T Cells Exhibit Potent Antitumor Effects on Hematologic and Solid Tumors. Mol. Ther. Oncolytics 2020, 17, 180–189. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.; Xia, Y.; Wang, Y.; Wang, Y.; Shi, Y.; Xing, H.; Qu, T.; Wang, Y.; Ma, W. Immune checkpoint of B7-H3 in cancer: From immunology to clinical immunotherapy. J. Hematol. Oncol. 2022, 15, 153. [Google Scholar] [CrossRef]
- Hashiguchi, M.; Kobori, H.; Ritprajak, P.; Kamimura, Y.; Kozono, H.; Azuma, M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc. Natl. Acad. Sci. USA 2008, 105, 10495–10500. [Google Scholar] [CrossRef]
- Kobori, H.; Hashiguchi, M.; Piao, J.; Kato, M.; Ritprajak, P.; Azuma, M. Enhancement of effector CD8+ T-cell function by tumour-associated B7-H3 and modulation of its counter-receptor triggering receptor expressed on myeloid cell-like transcript 2 at tumour sites. Immunology 2010, 130, 363–373. [Google Scholar] [CrossRef] [PubMed]
- King, R.G.; Herrin, B.R.; Justement, L.B. Trem-like transcript 2 is expressed on cells of the myeloid/granuloid and B lymphoid lineage and is up-regulated in response to inflammation. J. Immunol. 2006, 176, 6012–6021. [Google Scholar] [CrossRef] [PubMed]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Leitner, J.; Klauser, C.; Pickl, W.F.; Stockl, J.; Majdic, O.; Bardet, A.F.; Kreil, D.P.; Dong, C.; Yamazaki, T.; Zlabinger, G.; et al. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur. J. Immunol. 2009, 39, 1754–1764. [Google Scholar] [CrossRef]
- Yan, R.; Yang, S.; Gu, A.; Zhan, F.; He, C.; Qin, C.; Zhang, X.; Feng, P. Murine b7-h3 is a co-stimulatory molecule for T cell activation. Monoclon. Antib. Immunodiagn. Immunother. 2013, 32, 395–398. [Google Scholar] [CrossRef]
- Vigdorovich, V.; Ramagopal, U.A.; Lazar-Molnar, E.; Sylvestre, E.; Lee, J.S.; Hofmeyer, K.A.; Zang, X.; Nathenson, S.G.; Almo, S.C. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure 2013, 21, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.V.; Nguyen, T.; Li, Z.; Yang, Y.; Duong, J.; Wang, Y.; Dong, C. Murine B7-H3 is a negative regulator of T cells. J. Immunol. 2004, 173, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.K.; Gajewska, B.U.; Okada, H.; Gronski, M.A.; Bertram, E.M.; Dawicki, W.; Duncan, G.S.; Bukczynski, J.; Plyte, S.; Elia, A.; et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 2003, 4, 899–906. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Jia, L.; Kim, J.K.; Li, J.; Deng, P.; Zhang, W.; Krebsbach, P.H.; Wang, C.Y. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell 2021, 28, 1597–1613.e7. [Google Scholar] [CrossRef]
- Hagelstein, I.; Engel, M.; Hinterleitner, C.; Manz, T.; Marklin, M.; Jung, G.; Salih, H.R.; Zekri, L. B7-H3-targeting Fc-optimized antibody for induction of NK cell reactivity against sarcoma. Front. Immunol. 2022, 13, 1002898. [Google Scholar] [CrossRef]
- Gao, W.; Wen, H.; Liang, L.; Dong, X.; Du, R.; Zhou, W.; Zhang, X.; Zhang, C.; Xiang, R.; Li, N. IL20RA signaling enhances stemness and promotes the formation of an immunosuppressive microenvironment in breast cancer. Theranostics 2021, 11, 2564–2580. [Google Scholar] [CrossRef]
- Cao, S.; Peterson, S.M.; Muller, S.; Reichelt, M.; McRoberts Amador, C.; Martinez-Martin, N. A membrane protein display platform for receptor interactome discovery. Proc. Natl. Acad. Sci. USA 2021, 118, e2025451118. [Google Scholar] [CrossRef]
- Feng, R.; Chen, Y.; Liu, Y.; Zhou, Q.; Zhang, W. The role of B7-H3 in tumors and its potential in clinical application. Int. Immunopharmacol. 2021, 101, 108153. [Google Scholar] [CrossRef]
- Wang, J.; Chong, K.K.; Nakamura, Y.; Nguyen, L.; Huang, S.K.; Kuo, C.; Zhang, W.; Yu, H.; Morton, D.L.; Hoon, D.S. B7-H3 associated with tumor progression and epigenetic regulatory activity in cutaneous melanoma. J. Investig. Dermatol. 2013, 133, 2050–2058. [Google Scholar] [CrossRef]
- Lee, Y.H.; Martin-Orozco, N.; Zheng, P.; Li, J.; Zhang, P.; Tan, H.; Park, H.J.; Jeong, M.; Chang, S.H.; Kim, B.S.; et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017, 27, 1034–1045. [Google Scholar] [CrossRef]
- Flem-Karlsen, K.; Tekle, C.; Andersson, Y.; Flatmark, K.; Fodstad, O.; Nunes-Xavier, C.E. Immunoregulatory protein B7-H3 promotes growth and decreases sensitivity to therapy in metastatic melanoma cells. Pigment Cell Melanoma Res. 2017, 30, 467–476. [Google Scholar] [CrossRef]
- Flem-Karlsen, K.; Tekle, C.; Oyjord, T.; Florenes, V.A.; Maelandsmo, G.M.; Fodstad, O.; Nunes-Xavier, C.E. p38 MAPK activation through B7-H3-mediated DUSP10 repression promotes chemoresistance. Sci. Rep. 2019, 9, 5839. [Google Scholar] [CrossRef] [PubMed]
- Tekle, C.; Nygren, M.K.; Chen, Y.W.; Dybsjord, I.; Nesland, J.M.; Maelandsmo, G.M.; Fodstad, O. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int. J. Cancer 2012, 130, 2282–2290. [Google Scholar] [CrossRef]
- Ma, J.; Shang, T.; Ma, P.; Sun, X.; Zhao, J.; Sun, X.; Zhang, M. Bispecific anti-CD3 x anti-B7-H3 antibody mediates T cell cytotoxic ability to human melanoma in vitro and in vivo. Investig. New Drugs 2019, 37, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tang, X.; Zhang, Z.; Gu, L.; Wei, H.; Zhao, S.; Zhong, K.; Mu, M.; Huang, C.; Jiang, C.; et al. Tandem CAR-T cells targeting CD70 and B7-H3 exhibit potent preclinical activity against multiple solid tumors. Theranostics 2020, 10, 7622–7634. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Prawira, A.; Antonia, S.; Rahma, O.; Tolcher, A.; Cohen, R.B.; Lou, Y.; Hauke, R.; Vogelzang, N.; Zandberg, D.P.; et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: Interim results from a multicenter phase I/II trial. J. Immunother. Cancer 2022, 10, e004424. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.R.; Albert, C.M.; Taylor, M.; Wilson, A.; Rawlings-Rhea, S.; Huang, W.; Seidel, K.; Narayanaswany, P.; Wu, V.; Brown, C.; et al. STRIVE-02: A first-in-human phase 1 trial of systemic B7H3 CAR T cells for children and young adults with relapsed/refractory solid tumors. J. Clin. Oncol. 2022, 40, 10011. [Google Scholar] [CrossRef]
- Scribner, J.A.; Brown, J.G.; Son, T.; Chiechi, M.; Li, P.; Sharma, S.; Li, H.; De Costa, A.; Li, Y.; Chen, Y.; et al. Preclinical Development of MGC018, a Duocarmycin-based Antibody-drug Conjugate Targeting B7-H3 for Solid Cancer. Mol. Cancer Ther. 2020, 19, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Powderly, J.D.; Spira, A.I.; Bakkacha, O.; Loo, D.; Bohac, G.C.; Sharma, M. Phase 1 dose escalation study of MGC018, an anti-B7-H3 antibody-drug conjugate (ADC), in patients with advanced solid tumors. J. Clin. Oncol. 2021, 39, 2631. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.X.; Chen, Y.X.; Wu, H.X.; Yin, L.; Zhao, Q.; Luo, H.Y.; Zeng, Z.L.; Qiu, M.Z.; Xu, R.H. Integrated analysis of single-cell and bulk RNA sequencing data reveals a pan-cancer stemness signature predicting immunotherapy response. Genome Med. 2022, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, A.; Vollmer, S.; Tietze, J.; Galinski, A.; Heppt, M.V.; Burdek, M.; Berking, C.; Prinz, J.C. Clonality of CD4(+) Blood T Cells Predicts Longer Survival With CTLA4 or PD-1 Checkpoint Inhibition in Advanced Melanoma. Front. Immunol. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Fairfax, B.P.; Taylor, C.A.; Watson, R.A.; Nassiri, I.; Danielli, S.; Fang, H.; Mahe, E.A.; Cooper, R.; Woodcock, V.; Traill, Z.; et al. Peripheral CD8(+) T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat. Med. 2020, 26, 193–199. [Google Scholar] [CrossRef]
- Sacco, A.; Forgione, L.; Carotenuto, M.; Luca, A.; Ascierto, P.A.; Botti, G.; Normanno, N. Circulating Tumor DNA Testing Opens New Perspectives in Melanoma Management. Cancers 2020, 12, 2914. [Google Scholar] [CrossRef] [PubMed]
- Forschner, A.; Battke, F.; Hadaschik, D.; Schulze, M.; Weissgraeber, S.; Han, C.T.; Kopp, M.; Frick, M.; Klumpp, B.; Tietze, N.; et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma—Results of a prospective biomarker study. J. Immunother. Cancer 2019, 7, 180. [Google Scholar] [CrossRef]
| Drug | Format | ClinicalTrials.gov Identifier | Phase | Indication | Intervention | Target |
|---|---|---|---|---|---|---|
| Relatlimab (BMS-986016) | Fully human IgG4κ monoclonal antibody | NCT05704933 | I | Neoadjuvant therapy for surgically resectable melanoma brain metastases | Relatlimab + Nivolumab vs. Nivolumab + Ipilimumab | Anti-LAG-3 + Anti- PD-1 vs. Anti-PD-1 + Anti-CTLA-4 |
| NCT05629546 | I | Advanced or metastatic melanoma, after progression on ICI therapy | Memory-like natural killer cells (autologous or allogeneic) with Relatlimab + Nivolumab | ML NKs with Anti-LAG-3 + Anti- PD-1 | ||
| NCT04935229 | I | Metastatic uveal melanoma in the liver | Pressure-enabled hepatic artery infusion of SD-101, alone or in combination with Nivolumab, Nivolumab + Ipilimumab, or Relatlimab + Nivolumab | PEDD/HAI TLR9-agonist +/− Anti-PD-1, Anti-PD-1 + Anti-CTLA-4 or Anti-LAG-3 + Anti-PD-1 | ||
| NCT01968109 | I/IIa | Advanced solid tumors, including melanoma that is immunotherapy-naïve and immunotherapy-experienced | Single-agent Relatlimab or Relatlimab + Nivolumab | Anti-LAG-3 or Anti-LAG-3 + Anti-PD-1 | ||
| NCT03978611 | I/IIa | Unresectable or metastatic melanoma, after progression on anti-PD-1 ICI therapy | Relatlimab + Ipilimumab | Anti-LAG-3 + Anti-CTLA-4 | ||
| NCT02465060 | II | Solid tumors, including melanoma, after progression ≥ one line of standard treatment or for which no agreed-upon treatment approach exists, including melanoma | Subprotocol Z1M (LAG-3 expression ≥ 1%); Relatlimab + Nivolumab | Anti-LAG-3 + Anti-PD-1 | ||
| NCT05704647 | II | Active melanoma brain metastases | Relatlimab + Nivolumab | Anti-LAG-3 + Anti- PD-1 | ||
| NCT05428007 | II | Unresectable clinical-stage-III–IV melanoma | Sarilumab, Ipilimumab and Relatlimab + Nivolumab | Anti-IL-6R, Anti-CTLA-4 and Anti-LAG-3 + Anti- PD-1 | ||
| NCT05418972 | II | Neoadjuvant +/− adjuvant therapy for high risk, clinical-stage-II cutaneous melanoma | pre-surgery +/− post-surgery Relatlimab + Nivolumab | Anti-LAG-3 + Anti- PD-1 | ||
| NCT03743766 | II | Metastatic melanoma naïve to prior immunotherapy in the metastatic setting | Nivolumab, Relatlimab, or Nivolumab + Relatlimab followed by Nivolumab + Relatlimab | Anti-PD-1, Anti-LAG-3 or Anti-LAG-3 + Anti-PD-1 | ||
| NCT04552223 | II | Metastatic uveal melanoma | Relatlimab + Nivolumab | Anti-LAG-3 + Anti- PD-1 | ||
| NCT05077280 | II | Metastatic uveal melanoma | Concurrent stereotactic radiotherapy with Relatlimab + Nivolumab | SBRT with Anti-LAG-3 + Anti-PD-1 | ||
| NCT03470922 | II/III | Previously untreated metastatic or unresectable melanoma | Relatlimab + Nivolumab vs. Nivolumab | Anti-LAG-3 + Anti- PD-1 vs. Anti-PD-1 | ||
| NCT05625399 | III | Previously untreated metastatic or unresectable melanoma | Relatlimab + Nivolumab | Anti-LAG-3 + Anti- PD-1 | ||
| NCT05002569 | III | Adjuvant therapy for completely resected clinical-stage-III–IV melanoma | Relatlimab + Nivolumab vs. Nivolumab | Anti-LAG-3 + Anti- PD-1 vs. Anti-PD-1 | ||
| Fianlimab (REGN3767) | Fully human IgG4κ monoclonal antibody | NCT03005782 | I | Advanced malignancies, including melanoma | Fianlimab +/− Cemiplimab | Anti-LAG-3 +/− Anti-PD-1 |
| NCT05352672 | III | Previously untreated unresectable locally advanced or metastatic melanoma | Fianlimab + Cemiplimab vs. Pembrolizumab or Cemiplimab | Anti-LAG-3 + Anti-PD-1 vs. Anti-PD-L1 or Anti-PD-1 | ||
| NCT05608291 | III | Adjuvant therapy for completely resected high-risk melanoma | Fianlimab + Cemiplimab vs. Pembrolizumab | Anti-LAG-3 + Anti-PD-1 vs. Anti-PD-L1 | ||
| LBL-007 | Fully human IgG4κ monoclonal antibody | NCT04640545 | I | Unresectable or metastatic melanoma | LBL-007 + Toripalimab or LBL-007 + Toripalimab and Axitinib Tablets | Anti-LAG-3 + Anti-PD-1 or Anti-LAG-3 + Anti-PD-1 + TKI |
| INCAGN02385 | Humanized Fc-engineered IgG1κ monoclonal antibody | NCT04370704 | I/II | Advanced melanoma | INCAGN02385 + INCAGN02390 or INCAGN02385 + INCAGN02390 + INCMGA00012 | Anti-LAG-3 + Anti-TIM-3 or Anti-LAG-3 + Anti-TIM-3 + Anti-PD-1 |
| RO7247669 (RG-6139) | Fc-inert IgG1-based bispecific antibody | NCT04140500 | I | Advanced and/or metastatic solid tumors, including melanoma | single-agent RO7247669 | Anti-(PD-1 × LAG-3) |
| NCT05116202 | Ib/II | Immunotherapy-naïve, stage-III/IV melanoma | Nivolumab + Ipilimumab or RO7247669 or Atezolizumab + Tiragolumab or RO7247669 + Tiragolumab | Anti-PD-1 + Anti -CTLA-4 or Anti-(PD-1 × LAG-3) or Anti-PD-L1 + Anti-TIGIT or Anti-(PD-1 × LAG-3) + Anti-TIGIT | ||
| NCT05419388 | II | Previously untreated unresectable or metastatic melanoma | Single-agent RO7247669 | Anti-(PD-1 × LAG-3) | ||
| XmAb®22841 (Bavunalimab) | Fc-engineered half-IgG1κ/scFv bispecific antibody | NCT03849469 | I | Advanced solid tumors including melanoma | XmAb22841 +/− Pembrolizumab | Anti-(CTLA-4 × LAG-3) +/− Anti-PD-L1 |
| NCT05695898 | Ib/II | Metastatic melanoma refractory to prior ICI therapy with and without CNS disease | XmAb22841 + XmAb23104 | Anti-(CTLA-4 × LAG-3) + Anti-(PD-1 × ICOS) | ||
| EMB-02 | FIT-Ig® bispecific antibody | NCT04618393 | I/II | Advanced solid tumors, including locally advanced/metastatic melanoma with >1 prior therapy (PD-1/L1 +/− CTLA-4 ICI) | Single-agent EMB-02 | Anti-(PD-1 × LAG-3) |
| Drug | Format | ClinicalTrials.gov Identifier | Phase | Indication | Intervention | Target |
|---|---|---|---|---|---|---|
| Tiragolumab (RG6058, MTIG7192A) | Fully human IgG1 monoclonal anitbody | NCT05483400 | II | Anti-PD-1-resistant melanoma | Tiragolumab + Atezolizumab | Anti-TIGIT + anti-PD-1 |
| NCT05116202 | II | Immunotherapy-naïve stage-III/IV melanoma | Tiragolumab + Atezolizumab or Tiragolumab + RO7247669 | Anti-TIGIT + anti-PD-1 or anti-TIGIT + anti-PD1-LAG3 | ||
| NCT05060003 | II | Circulating tumor DNA+ stage-II melanoma | Tiragolumab + Atezolizumab | Anti-TIGIT + anti-PD-1 | ||
| NCT03554083 | II | High-risk stage-III melanoma | Tiragolumab + Atezolizumab | Anti-TIGIT + anti-PD-1 | ||
| Domvanalimab (AB154) | Humanized IgG1 monoclonal antibody | NCT05130177 | II | Anti-PD-1 relapsed/refractory melanoma | Domvanalimab + Zimberelimab | Anti-TIGIT + anti-PD-1 |
| Vibostolimab (MK-7684) | Humanized IgG1 monoclonal antibody | NCT04303169 | I/II | Pre-surgery stage-III melanoma | Vibostolimab + Pembrolizumab | Anti-TIGIT + anti-PD-1 |
| NCT04305054 | I/II | Advanced melanoma | Vibostolimab + Pembrolizumab or Vibostolimab + Pembrolizumab+ Favezelimab | Anti-TIGIT + anti-PD-1 or anti-TIGIT + anti-PD-1 + anti-LAG3 | ||
| NCT04305041 | I/II | Anti-PD-1 refractory melanoma | Vibostolimab + Pembrolizumab+ Quavonlimab | Anti-TIGIT + anti-PD-1 + anti-CTLA-4 | ||
| NCT05665595 | III | High-risk stage-II–IV melanoma | Vibostolimab + Pembrolizumab vs. Pembrolizumab | Anti-TIGIT + anti-PD-1 | ||
| EOS-448 | Fully human IgG1 monoclonal antibody | NCT05060432 | I/II | Advanced solid tumors | EOS-448 + Pembrolizumab or EOS-448 + Inupadenant or EOS-448 + Dostarlimab or EOS-448 + Dostarlimab+ Inupadenant | Anti-TIGIT + anti-PD-1 or anti-TIGIT+ anti-A2AR or anti-TIGIT + anti-PD-1 or anti-TIGIT + anti-PD-1 + anti-A2AR |
| Drug | Format | ClinicalTrials.gov | Phase | Indication | Intervention | Target |
|---|---|---|---|---|---|---|
| Cobolimab (TSR-022) | Humanized IgG4 monoclonal antibody | NCT02817633 | I | Advanced solid tumors including melanoma | Cobolimab or Cobolimab + nivolumab or cobilimab + TSR-042 or cobolimab + TSR-042 and TSR-033 or cobolimab + TSR-042 and docetaxel or Cobolimab + TSR-042, pemetrexed and cisplatin or Cobolimab + TSR-042, pemetrexed and carboplatin | Anti-TIM-3 or anti-TIM-3 + anti-PD-1 or anti-TIM-3 + anti-PD-1 and anti-LAG-3 or anti-TIM-3 + anti-PD-1 and chemotherapy |
| NCT04139902 | II | Pre-surgery regionally advanced or oligometastatic melanoma | Cobolimab + Dostarlimab vs. Dostarlimab | Anti-TIM-3 + anti-PD-1 vs. anti-PD-1 | ||
| Sabatolimab (MBG453) | Humanized anti-TIM-3 IgG4 | NCT02608268 | I/II | Advanced solid tumors including melanoma | Sabatolimab + PDR001 or Sabatolimab | Anti-TIM-3 + anti-PD-1 or anti-TIM-3 |
| INCAGN02390 | Fully human Fc-engineered IgG1κ monoclonal antibody | NCT04370704 | I | Advanced solid tumors including melanoma | INCAGN02390 + INCAGN02385 or INCAGN02390 + INCAGN02385 + INCMGA00012 | Anti-TIM-3 + anti-LAG-3 or anti-TIM-3 + anti-LAG-3 + anti-PD-1 |
| RO7121661 | CrossMabVH-VL bispecific antibody | NCT03708328 | I | Advanced solid tumors including melanoma | RO7121661 | Anti-PD-1/TIM-3 |
| TQB2618 | N/A | NCT05451407 | Ib | Advanced melanoma | TQB2618 + Terprizumab | Anti-TIM-3 + anti-PD-1 |
| Target | Drug | Format | ClinicalTrials.gov Identifier | Phase | Indications | Intervention | Target |
|---|---|---|---|---|---|---|---|
| VISTA | KVA12123 | Fully human IgG1 monoclonal antibody | NCT05708950 | I/II | Advanced solid tumors | KVA12123 or KVA12123 + pembrolizuman | Anti-VISTA or anti-VISTA + anti-PD-1 |
| W0180 | Humanized IgG1 monoclonal antibdoy | NCT04564417 | I | Locally advanced or metastatic solid tumors | W0180 or W0180 + pembrolizumab | Anti-VISTA or anti-VISTA + anti-PD-1 | |
| CI-8993 | Fully human IgG2 monoclonal antibody | NCT04475523 | I | Locally advanced or metastatic solid tumors | CI-8993 | Anti-VISTA | |
| HMBD-002 | Humanized IgG4 | NCT05082610 | I | Advanced solid tumors | HMBD-002 or HMBD-002 + pembrolizumab | Anti-VISTA or anti-VISTA + anti-PD-1 | |
| IDO | IO102-IO103 | IDO/PD-L1 dual peptide vaccine | NCT05280314 | II | Pre-surgery and post-surgery stage IIIB, IIIC, or IIID melanoma or oligometastatic IV melanoma | IO102-IO103 + pembrolizumab | IDO/PD-L1 vaccine + anti-PD-1 |
| NCT05155254 | III | Previously untreated unresectable or metastatic melanoma | IO102-IO103 + pembrolizumab or pembrolizumab | IDO/PD-L1 vaccine + anti-PD-1 or anti-PD-1 | |||
| CD27 | Varlimumab (CDX-1127) | Fully human IgG1 monoclonal antibody | NCT03617328 | I/II | Stage-II–IV melanoma | Varlimumab + 6MHP + Montanide ISA-51 + polyCLC or 6MHP + Montanide ISA-51 + polyCLC | Anti-CD27 + multipeptide vaccine + local adjuvant + local adjuvant or multipeptide vaccine + local adjuvant + local |
| CD70 | Anti-hCD70 CAR T-cells | Anti-hCD70 CAR T-cells | NCT02830724 | I/II | Unresectable solid tumors | Anti-hCD70 CAR + cyclophosphamide + flutarabine + aldesleukin | Anti-hCD70 CAR + alkylating agent + antimetabolite + recombinant IL-2 |
| Adenosine A2A receptor | Inupadenant | A2A receptor antagonist | NCT05117177 | I | Advanced solid tumors | Inupadenant | A2Ar antagonist |
| NCT05060432 | I/II | Advanced solid tumors | Inupadenant + EOS-448 or Inupadenant + dostarlimab or Inupadenant + dostarlimab + EOS-448 | A2Ar antagonist + anti-TIGIT or A2Ar antagonist + anti-PD-1 or A2Ar antagonist + anti-TIGIT + anti-PD-1 | |||
| BTLA | Icatolimab (JS004, TAB004) | Humanized IgG4 monoclonal antibody | NCT04773951 | I | Advanced solid tumors | Icatolimab | Anti-BTLA |
| NCT04137900 | I | Advanced solid tumors | Icatolimab + toripalimab or icatolimab | Anti-BTLA + anti-PD-1 or anti-BTLA |
| Drug | Format | ClinicalTrials.gov Identifier | Phase | Indication | Intervention | Target |
|---|---|---|---|---|---|---|
| Vobramitamab duocarmazine (MGC018) | Humanized IgG1 Anti-B7-H3 antibody-drug conjugate | NCT01391143 | I | Advanced solid tumors, including melanoma | Enoblituzumab | B7-H3 ADCC |
| NCT03729596 | I/II | Advanced solid tumors, including melanoma | Vobramitamab duocarmazine or Vobramitamab duocarmazine + retifanlimab | Anti-B7-H3 ADC or Anti-B7-H3 ADC + Anti-PD-1 | ||
| TAA06 | B7-H3-Targeted CAR-T Cells | NCT05190185 | I | Advanced solid tumors, including melanoma | TAA06 injection | B7-H3-Targeted CAR-T Cells |
| 4-1BBζ B7H3-EGFRt-DHFR | B7-H3-Targeted CAR-T Cells | NCT04483778 | I | Solid tumors, including melanoma, in children and young adults | 4-1BBζ B7H3-EGFRt-DHFR or 4-1BBζ B7H3-EGFRt-DHFR + 4-1BBζ CD19-Her2tG | B7-H3-Targeted CAR-T Cells |
| B7-H3-CAR T cells | B7-H3-Targeted CAR-T Cells | NCT04897321 | I | Recurrent/refractory B7-H3 positive solid tumors, including melanoma, after lymphodepleting chemotherapy | B7-H3-CAR T cells + Fludarabine + Cyclophosphamide + MESNA | B7-H3-Targeted CAR-T Cells + chemotherapy + chemotherapy protective agent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziogas, D.C.; Theocharopoulos, C.; Lialios, P.-P.; Foteinou, D.; Koumprentziotis, I.-A.; Xynos, G.; Gogas, H. Beyond CTLA-4 and PD-1 Inhibition: Novel Immune Checkpoint Molecules for Melanoma Treatment. Cancers 2023, 15, 2718. https://doi.org/10.3390/cancers15102718
Ziogas DC, Theocharopoulos C, Lialios P-P, Foteinou D, Koumprentziotis I-A, Xynos G, Gogas H. Beyond CTLA-4 and PD-1 Inhibition: Novel Immune Checkpoint Molecules for Melanoma Treatment. Cancers. 2023; 15(10):2718. https://doi.org/10.3390/cancers15102718
Chicago/Turabian StyleZiogas, Dimitrios C., Charalampos Theocharopoulos, Panagiotis-Petros Lialios, Dimitra Foteinou, Ioannis-Alexios Koumprentziotis, Georgios Xynos, and Helen Gogas. 2023. "Beyond CTLA-4 and PD-1 Inhibition: Novel Immune Checkpoint Molecules for Melanoma Treatment" Cancers 15, no. 10: 2718. https://doi.org/10.3390/cancers15102718
APA StyleZiogas, D. C., Theocharopoulos, C., Lialios, P.-P., Foteinou, D., Koumprentziotis, I.-A., Xynos, G., & Gogas, H. (2023). Beyond CTLA-4 and PD-1 Inhibition: Novel Immune Checkpoint Molecules for Melanoma Treatment. Cancers, 15(10), 2718. https://doi.org/10.3390/cancers15102718






