Cutaneous Adverse Reactions of Immunotherapy in Patients with Advanced Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Cutaneous irAEs in Melanoma Patients
3. Systemic Agents Used in the Treatment of Cutaneous Immune-Related Adverse Events
4. Cutaneous Immune-Related Adverse Events
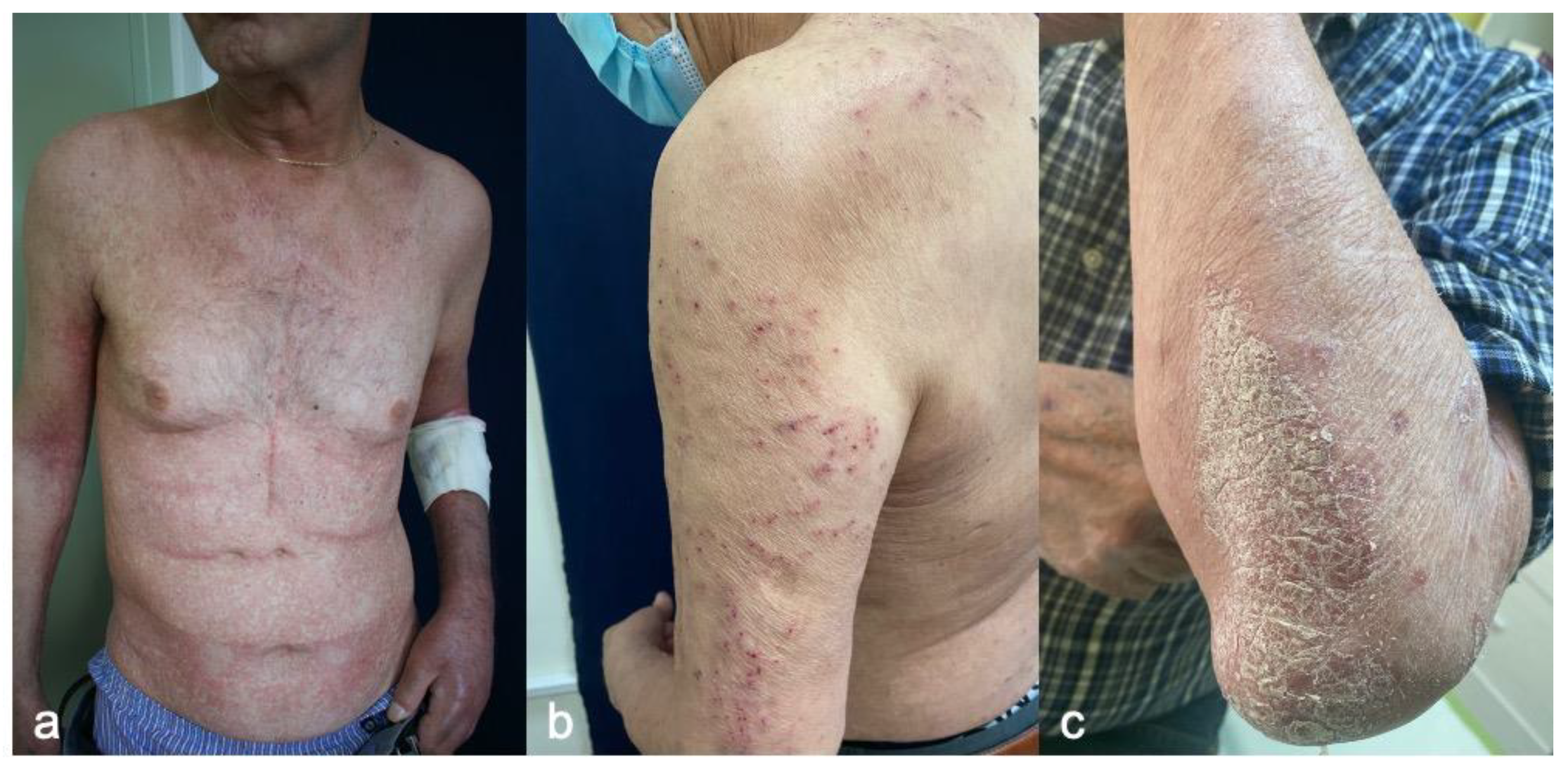
4.1. Morbilliform (Maculopapular) Rash
4.1.1. Incidence
4.1.2. Clinicopathological Characteristics
4.1.3. Management
4.2. Pruritus
4.2.1. Incidence
4.2.2. Clinicopathologic Characteristics
4.2.3. Treatment
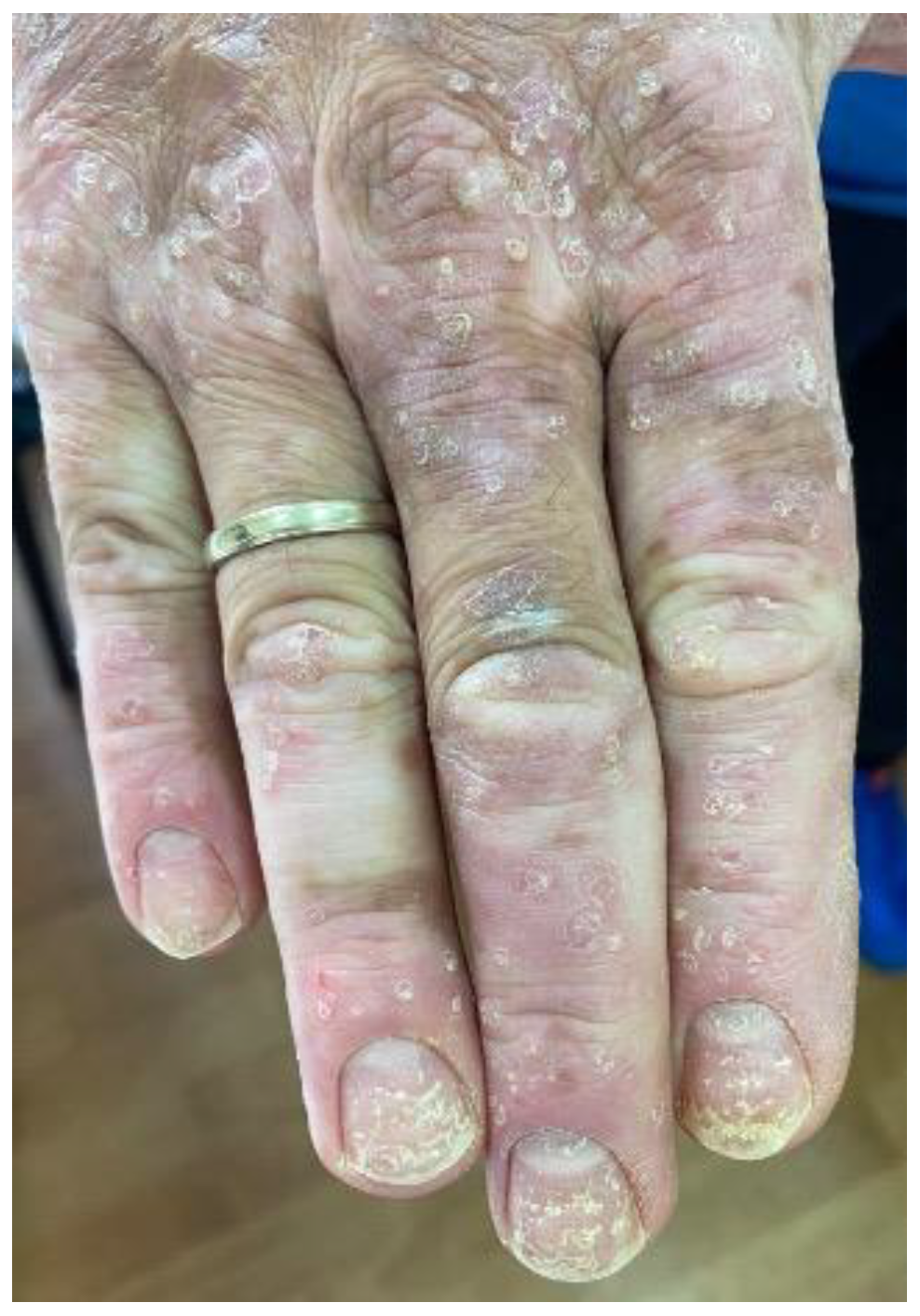
4.3. Psoriasiform Reactions
4.3.1. Incidence
4.3.2. Clinicopathologic Characteristics
4.3.3. Treatment
4.4. Lichen Planus-like Rash
4.4.1. Incidence
4.4.2. Clinicopathologic Characteristics
4.4.3. Treatment
4.5. Eczematous Reactions
4.5.1. Incidence
4.5.2. Clinicopathologic Characteristics
4.5.3. Therapy
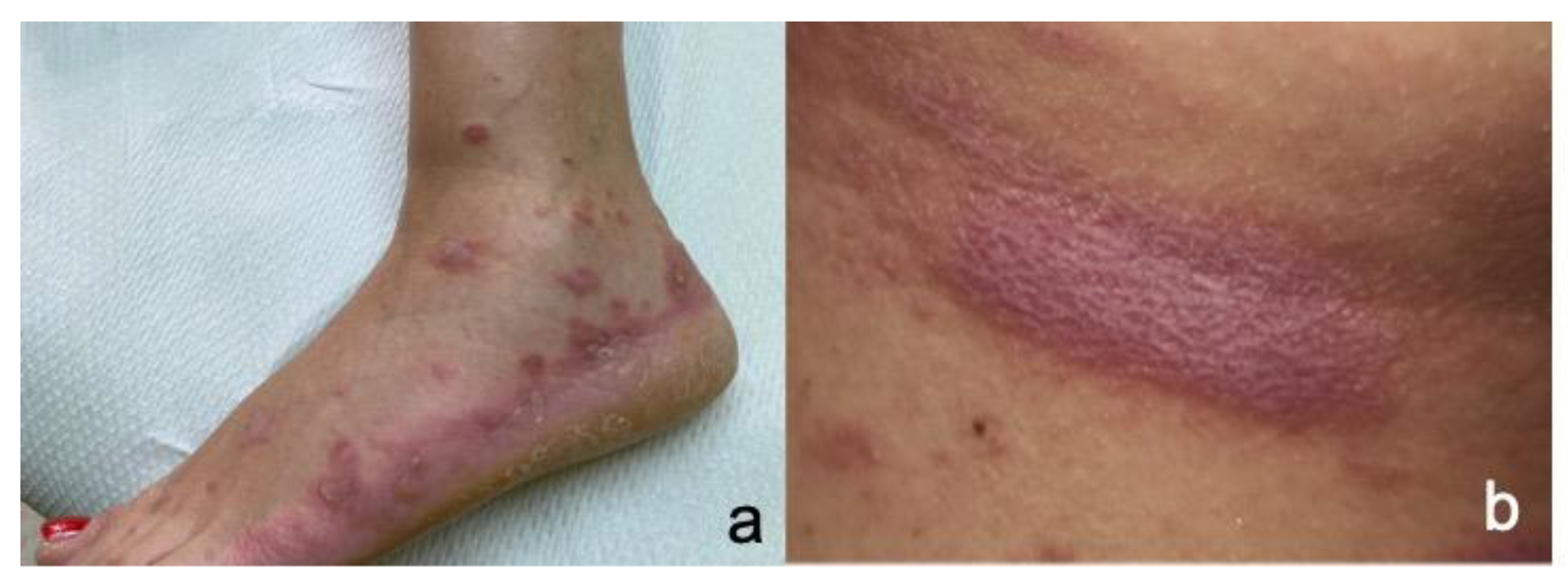
4.6. Bullous Pemphigoid (BP)
4.6.1. Incidence
4.6.2. Clinicopathologic Characteristics
4.6.3. Treatment
4.7. Vitiligo
4.7.1. Incidence
4.7.2. Clinicopathologic Characteristics
4.7.3. Treatment
4.8. Sarcoidosis
4.8.1. Incidence
4.8.2. Clinicopathologic Characteristics
4.8.3. Treatment
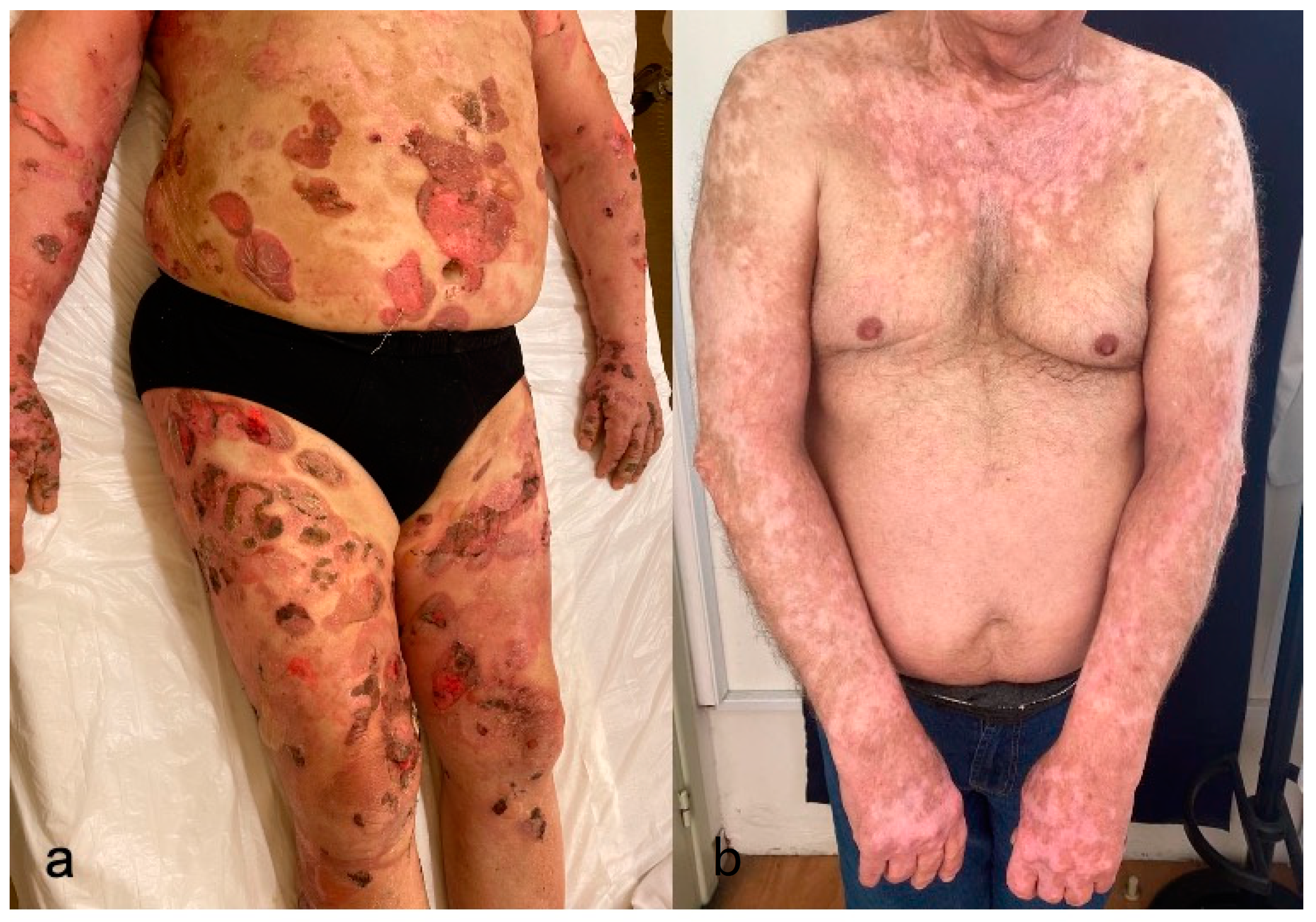
4.9. Severe Cutaneous Adverse Reactions
4.9.1. Incidence
4.9.2. Clinicopathologic Characteristics
4.9.3. Treatment
4.10. Other Reactions
5. Survival Related to Cutaneous Immune-Related Adverse Events
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujimura, T.; Muto, Y.; Asano, Y. Immunotherapy for Melanoma: The Significance of Immune Checkpoint Inhibitors for the Treatment of Advanced Melanoma. Int. J. Mol. Sci. 2022, 23, 15720. [Google Scholar] [CrossRef] [PubMed]
- Aroldi, F.; Middleton, M.R. Long-Term Outcomes of Immune Checkpoint Inhibition in Metastatic Melanoma. Am. J. Clin. Dermatol. 2022, 23, 331–338. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-SEER Research Data, 17 Registries (2000–2019) [Internet]. Division of Cancer Control and Population Sciences. 2022. Available online: https://seer.cancer.gov/statistics/ (accessed on 1 February 2023).
- Frampton, A.E.; Sivakumar, S. A New Combination Immunotherapy in Advanced Melanoma. N. Engl. J. Med. 2022, 386, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.E.; Park, J.J.W.; Wakade, D.; Chou, S.; Byth, K.; Fernandez-Penas, P. Cutaneous adverse events of anti-programmed death 1 antibodies combined with anti-cytotoxic T-lymphocyte-associated protein 4 therapy use in patients with metastatic melanoma. Melanoma Res. 2019, 29, 172–177. [Google Scholar] [CrossRef]
- Hassel, J.C.; Heinzerling, L.; Aberle, J.; Bahr, O.; Eigentler, T.K.; Grimm, M.O.; Grunwald, V.; Leipe, J.; Reinmuth, N.; Tietze, J.K.; et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat. Rev. 2017, 57, 36–49. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutierrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Wongvibulsin, S.; Pahalyants, V.; Kalinich, M.; Murphy, W.; Yu, K.H.; Wang, F.; Chen, S.T.; Reynolds, K.; Kwatra, S.G.; Semenov, Y.R. Epidemiology and risk factors for the development of cutaneous toxicities in patients treated with immune-checkpoint inhibitors: A United States population-level analysis. J. Am. Acad. Dermatol. 2022, 86, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Carlos, G.; Wakade, D.; Byth, K.; Kong, B.Y.; Chou, S.; Carlino, M.S.; Kefford, R.; Fernandez-Penas, P. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J. Am. Acad. Dermatol. 2016, 74, 455–461.e451. [Google Scholar] [CrossRef]
- Nikolaou, V.A.; Apalla, Z.; Carrera, C.; Fattore, D.; Sollena, P.; Riganti, J.; Segura, S.; Freites-Martinez, A.; Lallas, K.; Romano, M.C.; et al. Clinical associations and classification of immune checkpoint inhibitor-induced cutaneous toxicities: A multicentre study from the European Academy of Dermatology and Venereology Task Force of Dermatology for Cancer Patients. Br. J. Dermatol. 2022, 187, 962–969. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Shah, M.; Lopez-Olivo, M.A.; Suarez-Almazor, M.E. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann. Intern. Med. 2018, 168, 121–130. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Chen, X.; Li, L.; Song, W.; Li, W.; Zhao, Y.; Zhang, Y.; Han, F.; Lyu, Z.; et al. Analysis of characteristics and predictive factors of immune checkpoint inhibitor-related adverse events. Cancer Biol. Med. 2021, 18, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martinez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.C.; Pennell, N.A. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of Steroids use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 546. [Google Scholar] [CrossRef]
- Institute NC. Common Terminology Criteria for Adverse Events (CTCAE) 2021 [Updated 04/19/21]. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 1 February 2023).
- Amatore, F.; Villani, A.P.; Tauber, M.; Guillot, B.; Viguier, M.; Groupe de Recherche sur le Psoriasis de la Société Française de Dermatologie. French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults. Ann. Dermatol. Venereol. 2019, 146, 429–439. [Google Scholar] [CrossRef]
- Bertrand, F.; Montfort, A.; Marcheteau, E.; Imbert, C.; Gilhodes, J.; Filleron, T.; Rochaix, P.; Andrieu-Abadie, N.; Levade, T.; Meyer, N.; et al. TNFalpha blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat. Commun. 2017, 8, 2256. [Google Scholar] [CrossRef]
- Chen, A.Y.; Wolchok, J.D.; Bass, A.R. TNF in the era of immune checkpoint inhibitors: Friend or foe? Nat. Rev. Rheumatol. 2021, 17, 213–223. [Google Scholar] [CrossRef]
- Verheijden, R.J.; May, A.M.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.; van den Eertwegh, A.J.M.; de Groot, J.W.B.; Boers-Sonderen, M.J.; van der Hoeven, J.J.M.; Hospers, G.A.; et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin. Cancer Res. 2020, 26, 2268–2274. [Google Scholar] [CrossRef]
- Phillips, G.S.; Wu, J.; Hellmann, M.D.; Postow, M.A.; Rizvi, N.A.; Freites-Martinez, A.; Chan, D.; Dusza, S.; Motzer, R.J.; Rosenberg, J.E.; et al. Treatment Outcomes of Immune-Related Cutaneous Adverse Events. J. Clin. Oncol. 2019, 37, 2746–2758. [Google Scholar] [CrossRef]
- Blaise, M.; Cardot-Leccia, N.; Seitz-Polski, B.; Picard-Gauci, A.; Bertold, C.; Passeron, T.; Montaudie, H. Tocilizumab for Corticosteroid-Refractory Immune Checkpoint Inhibitor-Induced Generalized Morphea. JAMA Dermatol. 2023, 159, 112–114. [Google Scholar] [CrossRef]
- Klepper, E.M.; Robinson, H.N. Dupilumab for the treatment of nivolumab-induced bullous pemphigoid: A case report and review of the literature. Dermatol. Online J. 2021, 27, 6. [Google Scholar] [CrossRef]
- Barrios, D.M.; Phillips, G.S.; Geisler, A.N.; Trelles, S.R.; Markova, A.; Noor, S.J.; Quigley, E.A.; Haliasos, H.C.; Moy, A.P.; Schram, A.M.; et al. IgE blockade with omalizumab reduces pruritus related to immune checkpoint inhibitors and anti-HER2 therapies. Ann. Oncol. 2021, 32, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Abdat, R.; Waldman, R.A.; de Bedout, V.; Czernik, A.; McLeod, M.; King, B.; Gordon, S.; Ahmed, R.; Nichols, A.; Rothe, M.; et al. Dupilumab as a novel therapy for bullous pemphigoid: A multicenter case series. J. Am. Acad. Dermatol. 2020, 83, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Damsky, W.; Jilaveanu, L.; Turner, N.; Perry, C.; Zito, C.; Tomayko, M.; Leventhal, J.; Herold, K.; Meffre, E.; Bosenberg, M.; et al. B cell depletion or absence does not impede anti-tumor activity of PD-1 inhibitors. J. Immunother. Cancer 2019, 7, 153. [Google Scholar] [CrossRef]
- Sowerby, L.; Dewan, A.K.; Granter, S.; Gandhi, L.; LeBoeuf, N.R. Rituximab Treatment of Nivolumab-Induced Bullous Pemphigoid. JAMA Dermatol. 2017, 153, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Kosche, C.; Stout, M.; Sosman, J.; Lukas, R.V.; Choi, J.N. Dermatomyositis in a patient undergoing nivolumab therapy for metastatic melanoma: A case report and review of the literature. Melanoma Res. 2020, 30, 313–316. [Google Scholar] [CrossRef]
- Messer, A.; Drozd, B.; Glitza, I.C.; Lu, H.; Patel, A.B. Dermatomyositis associated with nivolumab therapy for melanoma: A case report and review of the literature. Dermatol. Online J. 2020, 26. [Google Scholar] [CrossRef]
- Apalla, Z.; Nikolaou, V.; Fattore, D.; Fabbrocini, G.; Freites-Martinez, A.; Sollena, P.; Lacouture, M.; Kraehenbuehl, L.; Stratigos, A.; Peris, K.; et al. European recommendations for management of immune checkpoint inhibitors-derived dermatologic adverse events. The EADV task force ‘Dermatology for cancer patients’ position statement. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 332–350. [Google Scholar] [CrossRef]
- Glinos, G.D.; Fisher, W.S.; Morr, C.S.; Seminario-Vidal, L. Nivolumab-induced psoriasis successfully treated with risankizumab-rzaa in a patient with stage III melanoma. JAAD Case Rep. 2021, 11, 74–77. [Google Scholar] [CrossRef]
- Takeda, K.; Yanagitani, N. Guselkumab for treating immune checkpoint inhibitor-induced psoriatic arthritis. Ann. Rheum. Dis. 2022, 81, 1479–1480. [Google Scholar] [CrossRef]
- Kang, J.H.; Bluestone, J.A.; Young, A. Predicting and Preventing Immune Checkpoint Inhibitor Toxicity: Targeting Cytokines. Trends Immunol. 2021, 42, 293–311. [Google Scholar] [CrossRef]
- Monsour, E.P.; Pothen, J.; Balaraman, R. A Novel Approach to the Treatment of Pembrolizumab-induced Psoriasis Exacerbation: A Case Report. Cureus 2019, 11, e5824. [Google Scholar] [CrossRef] [PubMed]
- Kost, Y.; Mattis, D.; Muskat, A.; Amin, B.; McLellan, B. Immune Checkpoint Inhibitor-Induced Psoriasiform, Spongiotic, and Lichenoid Dermatitis: A Novel Clinicopathological Pattern. Cureus 2022, 14, e28010. [Google Scholar] [CrossRef] [PubMed]
- Sanlorenzo, M.; Vujic, I.; Daud, A.; Algazi, A.; Gubens, M.; Luna, S.A.; Lin, K.; Quaglino, P.; Rappersberger, K.; Ortiz-Urda, S. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol. 2015, 151, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.L.; Tetzlaff, M.T.; Nagarajan, P.; Drucker, C.; Diab, A.; Hymes, S.R.; Duvic, M.; Hwu, W.J.; Wargo, J.A.; Torres-Cabala, C.A.; et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J. Cutan. Pathol. 2017, 44, 158–176. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef]
- Hwang, S.J.; Anforth, R.; Carlos, G.; Fernandez-Penas, P. Cutaneous Adverse Events of New Anti-melanoma Therapies: Classification and Management. Actas Dermosifiliogr. 2017, 108, 6–16. [Google Scholar] [CrossRef]
- Jaber, S.H.; Cowen, E.W.; Haworth, L.R.; Booher, S.L.; Berman, D.M.; Rosenberg, S.A.; Hwang, S.T. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch. Dermatol. 2006, 142, 166–172. [Google Scholar] [CrossRef]
- Malviya, N.; Tattersall, I.W.; Leventhal, J.; Alloo, A. Cutaneous immune-related adverse events to checkpoint inhibitors. Clin. Dermatol. 2020, 38, 660–678. [Google Scholar] [CrossRef]
- Mineiro Dos Santos Garrett, N.F.; Carvalho da Costa, A.C.; Barros Ferreira, E.; Damiani, G.; Diniz Dos Reis, P.E.; Inocencio Vasques, C. Prevalence of dermatological toxicities in patients with melanoma undergoing immunotherapy: Systematic review and meta-analysis. PLoS ONE 2021, 16, e0255716. [Google Scholar] [CrossRef]
- Donaldson, M.; Owen, J.L.; Chae, Y.K.; Choi, J.N. Management of Persistent Pruritus and Lichenoid Reaction Secondary to Nivolumab with Narrowband Ultraviolet B Phototherapy. Front. Oncol. 2018, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Fujimoto, D.; Nakamura, A.; Nagano, T.; Uehara, K.; Imai, Y.; Tomii, K. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer 2017, 109, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Vincenzi, B.; Guida, F.M.; Imperatori, M.; Schiavon, G.; Venditti, O.; Frezza, A.M.; Berti, P.; Tonini, G. Aprepitant for management of severe pruritus related to biological cancer treatments: A pilot study. Lancet Oncol. 2012, 13, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Coleman, E.; Ko, C.; Dai, F.; Tomayko, M.M.; Kluger, H.; Leventhal, J.S. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J. Am. Acad. Dermatol. 2019, 80, 990–997. [Google Scholar] [CrossRef]
- Nikolaou, V.; Sibaud, V.; Fattore, D.; Sollena, P.; Ortiz-Brugues, A.; Giacchero, D.; Romano, M.C.; Riganti, J.; Lallas, K.; Peris, K.; et al. Immune checkpoint-mediated psoriasis: A multicenter European study of 115 patients from the European Network for Cutaneous Adverse Event to Oncologic Drugs (ENCADO) group. J. Am. Acad. Dermatol. 2021, 84, 1310–1320. [Google Scholar] [CrossRef]
- Ellis, S.R.; Vierra, A.T.; Millsop, J.W.; Lacouture, M.E.; Kiuru, M. Dermatologic toxicities to immune checkpoint inhibitor therapy: A review of histopathologic features. J. Am. Acad. Dermatol. 2020, 83, 1130–1143. [Google Scholar] [CrossRef]
- Dulos, J.; Carven, G.J.; van Boxtel, S.J.; Evers, S.; Driessen-Engels, L.J.; Hobo, W.; Gorecka, M.A.; de Haan, A.F.; Mulders, P.; Punt, C.J.; et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J. Immunother. 2012, 35, 169–178. [Google Scholar] [CrossRef]
- Perez-Ruiz, E.; Minute, L.; Otano, I.; Alvarez, M.; Ochoa, M.C.; Belsue, V.; de Andrea, C.; Rodriguez-Ruiz, M.E.; Perez-Gracia, J.L.; Marquez-Rodas, I.; et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 2019, 569, 428–432. [Google Scholar] [CrossRef]
- Ameri, A.H.; Foreman, R.K.; Vedak, P.; Chen, S.; Miller, D.M.; Demehri, S. Hypertrophic Lichen Planus with Histological Features of Squamous Cell Carcinoma Associated with Immune Checkpoint Blockade Therapy. Oncologist 2020, 25, 366–368. [Google Scholar] [CrossRef]
- Masterson, W.M.; Brown, A.M.; Al Ameri, M.A.; Patel, A.B. A retrospective chart review of management strategies for lichenoid eruptions associated with immune-checkpoint inhibitor therapy from a single institution. Cancer Treat. Res. Commun. 2022, 30, 100506. [Google Scholar] [CrossRef]
- Quach, H.T.; Johnson, D.B.; LeBoeuf, N.R.; Zwerner, J.P.; Dewan, A.K. Cutaneous adverse events caused by immune checkpoint inhibitors. J. Am. Acad. Dermatol. 2021, 85, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Fattore, D.; Panariello, L.; Annunziata, M.C.; Fabbrocini, G. Prurigo nodularis and pembrolizumab: A therapeutic challenge. Eur. J. Cancer 2019, 110, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Vivar, K.L.; Deschaine, M.; Messina, J.; Divine, J.M.; Rabionet, A.; Patel, N.; Harrington, M.A.; Seminario-Vidal, L. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J. Cutan. Pathol. 2017, 44, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.T.; Khanna, T.; Antonov, N.; Audrey-Bayan, C.; Geskin, L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int. J. Dermatol. 2018, 57, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Schindler, K.; Querfeld, C.; Busam, K.; Cunningham, J.; Page, D.B.; Postow, M.A.; Weinstein, A.; Lucas, A.S.; Ciccolini, K.T.; et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol. Res. 2016, 4, 383–389. [Google Scholar] [CrossRef]
- Molina, G.E.; Reynolds, K.L.; Chen, S.T. Diagnostic and therapeutic differences between immune checkpoint inhibitor-induced and idiopathic bullous pemphigoid: A cross-sectional study. Br. J. Dermatol. 2020, 183, 1126–1128. [Google Scholar] [CrossRef]
- Siegel, J.; Totonchy, M.; Damsky, W.; Berk-Krauss, J.; Castiglione, F., Jr.; Sznol, M.; Petrylak, D.P.; Fischbach, N.; Goldberg, S.B.; Decker, R.H.; et al. Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: A retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J. Am. Acad. Dermatol. 2018, 79, 1081–1088. [Google Scholar] [CrossRef]
- Yun, S.J.; Oh, I.J.; Park, C.K.; Kim, Y.C.; Kim, H.B.; Kim, H.K.; Hong, A.R.; Kim, I.Y.; Ahn, S.J.; Na, K.J.; et al. Vitiligo-like depigmentation after pembrolizumab treatment in patients with non-small cell lung cancer: A case report. Transl. Lung Cancer Res. 2020, 9, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Billon, E.; Walz, J.; Brunelle, S.; Thomassin, J.; Salem, N.; Guerin, M.; Vicier, C.; Dermeche, S.; Albiges, L.; Tantot, F.; et al. Vitiligo Adverse Event Observed in a Patient With Durable Complete Response After Nivolumab for Metastatic Renal Cell Carcinoma. Front. Oncol. 2019, 9, 1033. [Google Scholar] [CrossRef]
- Teulings, H.E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J. Clin. Oncol. 2015, 33, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Wolner, Z.J.; Marghoob, A.A.; Pulitzer, M.P.; Postow, M.A.; Marchetti, M.A. A case report of disappearing pigmented skin lesions associated with pembrolizumab treatment for metastatic melanoma. Br. J. Dermatol. 2018, 178, 265–269. [Google Scholar] [CrossRef]
- Larsabal, M.; Marti, A.; Jacquemin, C.; Rambert, J.; Thiolat, D.; Dousset, L.; Taieb, A.; Dutriaux, C.; Prey, S.; Boniface, K.; et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J. Am. Acad. Dermatol. 2017, 76, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Babai, S.; Voisin, A.L.; Bertin, C.; Gouverneur, A.; Le-Louet, H. Occurrences and Outcomes of Immune Checkpoint Inhibitors-Induced Vitiligo in Cancer Patients: A Retrospective Cohort Study. Drug Saf. 2020, 43, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Reule, R.B.; North, J.P. Cutaneous and pulmonary sarcoidosis-like reaction associated with ipilimumab. J. Am. Acad. Dermatol. 2013, 69, e272–e273. [Google Scholar] [CrossRef] [PubMed]
- Suozzi, K.C.; Stahl, M.; Ko, C.J.; Chiang, A.; Gettinger, S.N.; Siegel, M.D.; Bunick, C.G. Immune-related sarcoidosis observed in combination ipilimumab and nivolumab therapy. JAAD Case Rep. 2016, 2, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, C.M.; Haun, P.; English, J., 3rd; Rosenbach, M. Immune checkpoint inhibitors and the development of granulomatous reactions. J. Am. Acad. Dermatol. 2019, 81, 1165–1175. [Google Scholar] [CrossRef]
- Tetzlaff, M.T.; Nelson, K.C.; Diab, A.; Staerkel, G.A.; Nagarajan, P.; Torres-Cabala, C.A.; Chasen, B.A.; Wargo, J.A.; Prieto, V.G.; Amaria, R.N.; et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: A marker of therapy response in a subset of melanoma patients. J. Immunother. Cancer 2018, 6, 14. [Google Scholar] [CrossRef]
- Maloney, N.J.; Ravi, V.; Cheng, K.; Bach, D.Q.; Worswick, S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: A systematic review. Int. J. Dermatol. 2020, 59, e183–e188. [Google Scholar] [CrossRef]
- Raschi, E.; Antonazzo, I.C.; La Placa, M.; Ardizzoni, A.; Poluzzi, E.; De Ponti, F. Serious Cutaneous Toxicities with Immune Checkpoint Inhibitors in the U.S. Food and Drug Administration Adverse Event Reporting System. Oncologist 2019, 24, e1228–e1231. [Google Scholar] [CrossRef]
- Page, B.; Borradori, L.; Beltraminelli, H.; Yawalkar, N.; Hunger, R.E. Acute generalized exanthematous pustulosis associated with ipilimumab and nivolumab. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e256–e257. [Google Scholar] [CrossRef]
- Tang, K.; Seo, J.; Tiu, B.C.; Le, T.K.; Pahalyants, V.; Raval, N.S.; Ugwu-Dike, P.O.; Zubiri, L.; Naranbhai, V.; Carrington, M.; et al. Association of Cutaneous Immune-Related Adverse Events With Increased Survival in Patients Treated With Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Therapy. JAMA Dermatol. 2022, 158, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cuevas, S.; Lopez-Chavira, A.; Zepeda del Rio, G.; Cuadra-Garcia, I.; Fernandez-Diez, J. Prognostic significance of cutaneous depigmentation in Mexican patients with malignant melanoma. Arch. Med. Res. 1998, 29, 155–158. [Google Scholar] [PubMed]
- Arpaia, N.; Cassano, N.; Vena, G.A. Regressing cutaneous malignant melanoma and vitiligo-like depigmentation. Int. J. Dermatol. 2006, 45, 952–956. [Google Scholar] [CrossRef]
- Francisco, G.; Rao, B.K.; Victor, F.C. Two reports of malignant melanoma arising within a new vitiligo-like depigmented patch. JAAD Case Rep. 2018, 4, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Lommerts, J.E.; Bekkenk, M.W.; Luiten, R.M. Vitiligo induced by immune checkpoint inhibitors in melanoma patients: An expert opinion. Expert Opin. Drug Saf. 2021, 20, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Teramoto, Y.; Asami, Y.; Matsuya, T.; Adachi, J.I.; Nishikawa, R.; Yamamoto, A. Nivolumab Therapy for Treatment-Related Vitiligo in a Patient With Relapsed Metastatic Melanoma. JAMA Dermatol. 2017, 153, 942–944. [Google Scholar] [CrossRef]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef]
- Thompson, L.L.; Chang, M.S.; Polyakov, N.J.; Blum, A.E.; Josephs, N.; Krasnow, N.A.; Yoon, J.; Li, E.B.; Molina, G.E.; Said, J.T.; et al. Prognostic significance of cutaneous immune-related adverse events in patients with melanoma and other cancers on immune checkpoint inhibitors. J. Am. Acad. Dermatol. 2022, 86, 886–889. [Google Scholar] [CrossRef]
- Rofe, O.; Bar-Sela, G.; Keidar, Z.; Sezin, T.; Sadik, C.D.; Bergman, R. Severe bullous pemphigoid associated with pembrolizumab therapy for metastatic melanoma with complete regression. Clin. Exp. Dermatol. 2017, 42, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Kittai, A.S.; Oldham, H.; Cetnar, J.; Taylor, M. Immune Checkpoint Inhibitors in Organ Transplant Patients. J. Immunother. 2017, 40, 277–281. [Google Scholar] [CrossRef]
- Chan, L.; Hwang, S.J.E.; Byth, K.; Kyaw, M.; Carlino, M.S.; Chou, S.; Fernandez-Penas, P. Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J. Am. Acad. Dermatol. 2020, 82, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.L.; Comito, F.; Agraso Busto, S.; Harland, C.; Turajlic, S.; Larkin, J.; Heelan, K.; Fearfield, L. Cutaneous toxicities in patients with melanoma receiving checkpoint inhibitor therapy: A retrospective review. The experience of a single large specialist institution. Clin. Exp. Dermatol. 2021, 46, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Hassel, J.C.; Kripp, M.; Al-Batran, S.; Hofheinz, R.D. Treatment of epidermal growth factor receptor antagonist-induced skin rash: Results of a survey among German oncologists. Onkologie 2010, 33, 94–98. [Google Scholar] [CrossRef] [PubMed]
| Systemic Treatment | Indications | Dose | Comments |
|---|---|---|---|
| Glucocorticosteroids [11,12,13,16] |
| Grade 2: prednisone (or equivalent) 0.5–1 mg/kg tapered over at least 4 weeks Grade 3: (prednisone 1 to 2 mg/kg/d or methylprednisolone 1 to 2 mg/kg/d). |
|
| Methotrexate [17] |
| 10–25 mg oral or subcutaneous once weekly |
|
| Acitretin |
| 25–30 mg oral daily |
|
| Anti-TNF [18,19,20] |
| Infliximab 5 mg/kg infusion Adalimumab |
|
| Anti-IL6 [22] |
| Tocilizumab 162 mg subcutaneous injection every 2 weeks |
|
| Dupilumab [21,23,24] |
| 600 mg loading dose and 300 mg subcutaneous injection administered every other week thereafter |
|
| Omalizumab [25] |
| 300-mg monthly injections |
|
| Rituximab [26,27] |
| 375 mg/m2 once weekly for 4 weeks |
|
| IVIG [28,29] |
| BP: IVIG 1–2 g/kg every 4 weeks (along with steroids) Dermatomyositis: 0.4 mg/kg daily for 5 days monthly SJS/TEN: 1–1.5 g/kg single infusion |
|
| Apremilast [21,30] |
| 30 mg twice daily |
|
| Anti-IL23 [30] |
| Guselkumab: 100 mg subcutaneous injection at weeks 0 and 4 and then every 8 weeks. Risankizumab: 150 mg subcutaneous injection at weeks 0 and 4 and then every 12 weeks Tildrakizumab: 100 mg subcutaneous injection at weeks 0, 4 and every 12 weeks thereafter. |
|
| Anti-IL12/23 [33] |
| Ustekinumab: 45 mg subcutaneous injection at weeks 0 and 4 and then every 12 weeks |
|
| Anti-IL17 [34,35] |
| Ixekizumab: 160 mg by subcutaneous at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then every 4 weeks. Secukinumab: 300 mg subcutaneous injection at weeks 0, 1, 2, 3 and 4 and then monthly. Brodalumab: 210 mg subcutaneous injection at weeks 0, 1, and 2 and then every 2 weeks. Bimekizumab: 320 mg subcutaneous injection at weeks 0, 4, 8, 12, 16 and every 8 weeks thereafter. |
|
| Cutaneous Toxicity | Clinical Presentation | Treatment |
|---|---|---|
| Alopecia areata | Non-scarring round patches of alopecia Alopecia totalis: Entire scalp and eyebrow involvement Alopecia universalis: Loss of total body hair. |
|
| Scleroderma | Skin tightening with thickening. Digital swelling. Xerosis. Periungual erythema. Fatigue. Muscle atrophy/weakness. Raynaud phenomenon |
|
| Leukocytoclastic vasculitis | Palpable purpura on the extremities |
|
| Acne-like lesions | Follicular papules and pustules located on the efface and trunk Rosacea like reactions |
|
| Neutrophilic Dermatoses Sweet’s syndromePyoderma gagrenosum | Violaceous, edematous tender papules and plaques Head and neck, extremities Fever, malaise, arthralgia Pustules that progress to ulcers with violaceous and undermined borders, often painful May occur at sites of trauma |
|
| SICA syndrome | Dry mouth, dry eyes |
|
| Grover Disease (Transient acantholytic dermatosis) | Pruritic erythematous papules, keratotic papules or papilovesicular eruption distributed on the central chest and back |
|
| Ichthyosis | Symmetric fish-like scaling, platelike brow scales on the limps, rough, dry skin on the trunk. Skin folds are spared |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaou, V.; Tsimpidakis, A.; Stratigos, A. Cutaneous Adverse Reactions of Immunotherapy in Patients with Advanced Melanoma. Cancers 2023, 15, 2084. https://doi.org/10.3390/cancers15072084
Nikolaou V, Tsimpidakis A, Stratigos A. Cutaneous Adverse Reactions of Immunotherapy in Patients with Advanced Melanoma. Cancers. 2023; 15(7):2084. https://doi.org/10.3390/cancers15072084
Chicago/Turabian StyleNikolaou, Vasiliki, Antonis Tsimpidakis, and Alexander Stratigos. 2023. "Cutaneous Adverse Reactions of Immunotherapy in Patients with Advanced Melanoma" Cancers 15, no. 7: 2084. https://doi.org/10.3390/cancers15072084
APA StyleNikolaou, V., Tsimpidakis, A., & Stratigos, A. (2023). Cutaneous Adverse Reactions of Immunotherapy in Patients with Advanced Melanoma. Cancers, 15(7), 2084. https://doi.org/10.3390/cancers15072084






