Multiparametric 18F-FDG PET/MRI-Based Radiomics for Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
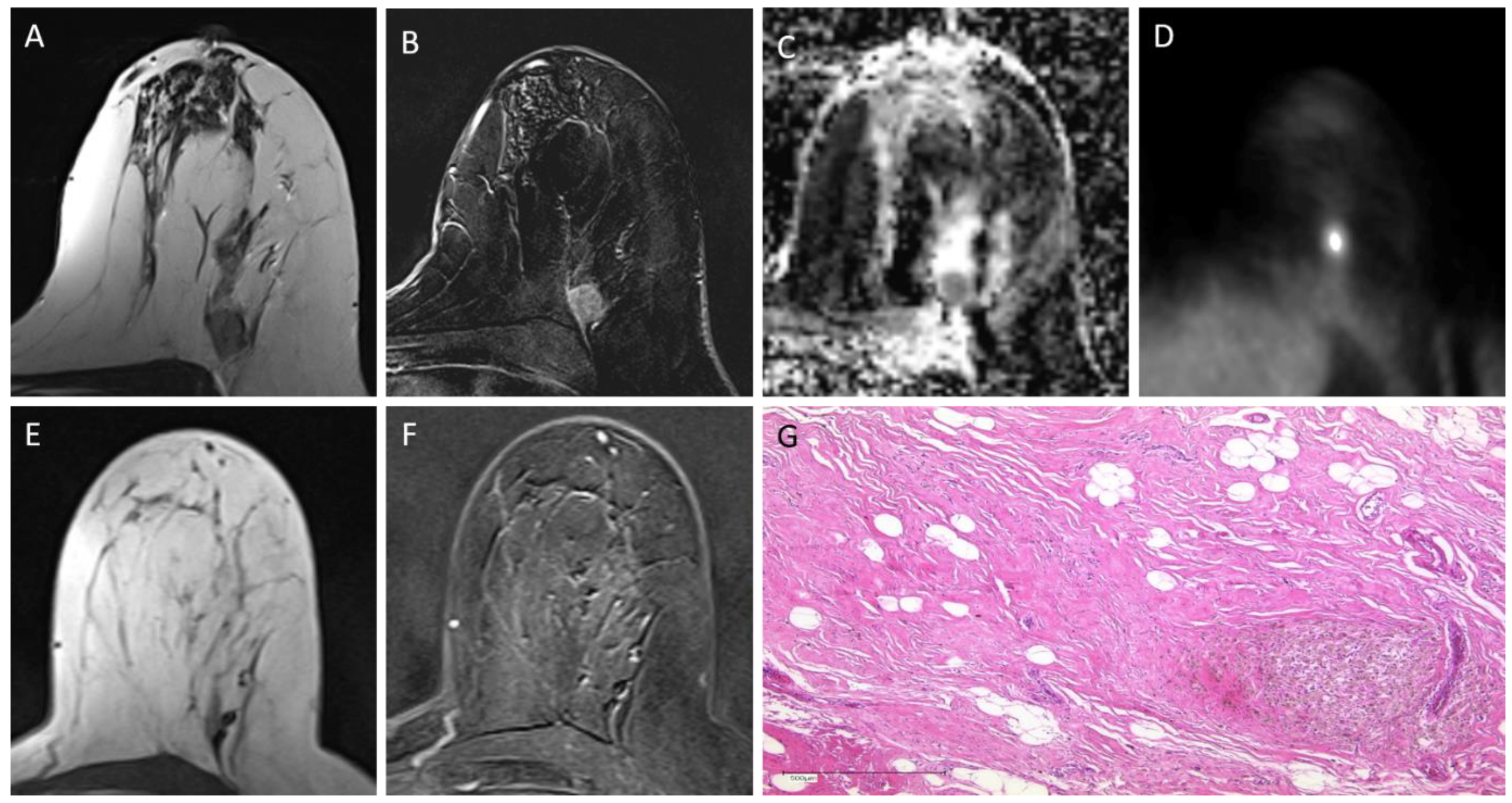
2.2. PET/MRI
2.3. Image Analysis
2.4. Radiomics Analysis
2.5. Reference Standard
2.6. Statistical Analysis and Predictive Model Building
3. Results
3.1. Patient Population and Breast Lesion Characteristics
3.2. Prediction of Pathological Response in Entire Cohort
3.3. Subgroup Analysis 1: Prediction of pCR in HR+/HER2−
3.4. Subgroup Analysis 2: Prediction of pCR in TN/HER2+
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, X.; Moran, M.S.; Zhang, N.; Haffty, B.; Yang, Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur. J. Cancer 2011, 47, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Luangdilok, S.; Samarnthai, N.; Korphaisarn, K. Association between Pathological Complete Response and Outcome Following Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer Patients. J. Breast Cancer 2014, 17, 376–385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Braman, N.M.; Etesami, M.; Prasanna, P.; Dubchuk, C.; Gilmore, H.; Tiwari, P.; Plecha, D.; Madabhushi, A. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res. 2017, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Valdora, F.; Houssami, N.; Rossi, F.; Calabrese, M.; Tagliafico, A.S. Rapid review: Radiomics and breast cancer. Breast Cancer Res. Treat. 2018, 169, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Burnside, E.S.; Huang, E.; Drukker, K.; Hoadley, K.A.; Fan, C.; Conzen, S.D.; Zuley, M.; Net, J.M.; et al. Quantitative MRI radiomics in the prediction of molecular classifications of breast cancer subtypes in the TCGA/TCIA data set. NPJ Breast Cancer 2016, 2, 16012. [Google Scholar] [CrossRef]
- Bitencourt, A.G.V.; Gibbs, P.; Rossi Saccarelli, C.; Daimiel, I.; Lo Gullo, R.; Fox, M.J.; Thakur, S.; Pinker, K.; Morris, E.A.; Morrow, M.; et al. MRI-based machine learning radiomics can predict HER2 expression level and pathologic response after neoadjuvant therapy in HER2 overexpressing breast cancer. eBioMedicine 2020, 61, 103042. [Google Scholar] [CrossRef]
- Leithner, D.; Bernard-Davila, B.; Martinez, D.F.; Horvat, J.V.; Jochelson, M.S.; Marino, M.A.; Avendano, D.; Ochoa-Albiztegui, R.E.; Sutton, E.J.; Morris, E.A.; et al. Radiomic Signatures Derived from Diffusion-Weighted Imaging for the Assessment of Breast Cancer Receptor Status and Molecular Subtypes. Mol. Imaging Biol. 2020, 22, 453–461. [Google Scholar] [CrossRef]
- Leithner, D.; Mayerhoefer, M.E.; Martinez, D.F.; Jochelson, M.S.; Morris, E.A.; Thakur, S.B.; Pinker, K. Non-Invasive Assessment of Breast Cancer Molecular Subtypes with Multiparametric Magnetic Resonance Imaging Radiomics. J. Clin. Med. 2020, 9, 1853. [Google Scholar] [CrossRef]
- Demircioglu, A.; Grueneisen, J.; Ingenwerth, M.; Hoffmann, O.; Pinker-Domenig, K.; Morris, E.; Haubold, J.; Forsting, M.; Nensa, F.; Umutlu, L. A rapid volume of interest-based approach of radiomics analysis of breast MRI for tumor decoding and phenotyping of breast cancer. PLoS ONE 2020, 15, e0234871. [Google Scholar] [CrossRef]
- Pasha, N.; Turner, N.C. Understanding and overcoming tumor heterogeneity in metastatic breast cancer treatment. Nat. Cancer 2021, 2, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Mariani, O.; Meaudre, C.; Fuhrmann, L.; Xiao, H.; Naidoo, K.; Gillespie, A.; Roxanis, I.; Vincent-Salomon, A.; Haider, S.; et al. Assessment of the Molecular Heterogeneity of E-Cadherin Expression in Invasive Lobular Breast Cancer. Cancers 2022, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Umutlu, L.; Kirchner, J.; Bruckmann, N.M.; Morawitz, J.; Antoch, G.; Ingenwerth, M.; Bittner, A.K.; Hoffmann, O.; Haubold, J.; Grueneisen, J.; et al. Multiparametric Integrated 18F-FDG PET/MRI-Based Radiomics for Breast Cancer Phenotyping and Tumor Decoding. Cancers 2021, 13, 2928. [Google Scholar] [CrossRef] [PubMed]
- Romeo, V.; Clauser, P.; Rasul, S.; Kapetas, P.; Gibbs, P.; Baltzer, P.A.T.; Hacker, M.; Woitek, R.; Helbich, T.H.; Pinker, K. AI-enhanced simultaneous multiparametric 18F-FDG PET/MRI for accurate breast cancer diagnosis. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 596–608. [Google Scholar] [CrossRef]
- Kirchner, J.; Grueneisen, J.; Martin, O.; Oehmigen, M.; Quick, H.H.; Bittner, A.K.; Hoffmann, O.; Ingenwerth, M.; Catalano, O.A.; Heusch, P.; et al. Local and whole-body staging in patients with primary breast cancer: A comparison of one-step to two-step staging utilizing 18F-FDG-PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2328–2337. [Google Scholar] [CrossRef]
- Oehmigen, M.; Lindemann, M.E.; Lanz, T.; Kinner, S.; Quick, H.H. Integrated PET/MR breast cancer imaging: Attenuation correction and implementation of a 16-channel RF coil. Med. Phys. 2016, 43, 4808. [Google Scholar] [CrossRef]
- He, H.; Bai, Y.; Garcia, E.; Li, S. ADASYN: Adaptive Synthetic Sampling Approach for Imbalanced Learning. In Proceeding of the IEEE International Joint Conference on Neural Networks, Hong Kong, China, 1–8 June 2008; pp. 1322–1328. [Google Scholar] [CrossRef]
- Apte, A.P.; Iyer, A.; Crispin-Ortuzar, M.; Pandya, R.; van Dijk, L.V.; Spezi, E.; Thor, M.; Um, H.; Veeraraghavan, H.; Oh, J.H.; et al. Technical Note: Extension of CERR for computational radiomics: A comprehensive MATLAB platform for reproducible radiomics research. Med. Phys. 2018, 45, 3713–3720. [Google Scholar] [CrossRef]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Kaufmann, M.; Hortobagyi, G.N.; Goldhirsch, A.; Scholl, S.; Makris, A.; Valagussa, P.; Blohmer, J.-U.; Eiermann, W.; Jackesz, R.; Jonat, W.; et al. Recommendations From an International Expert Panel on the Use of Neoadjuvant (Primary) Systemic Treatment of Operable Breast Cancer: An Update. J. Clin. Oncol. 2006, 24, 1940–1949. [Google Scholar] [CrossRef]
- Sinn, H.P.; Schmid, H.; Junkermann, H.; Huober, J.; Leppien, G.; Kaufmann, M.; Bastert, G.; Otto, H.F. Histologic regression of breast cancer after primary (neoadjuvant) chemotherapy. Geburtshilfe Frauenheilkd 1994, 54, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Leithner, D.; Horvat, J.V.; Marino, M.A.; Bernard-Davila, B.; Jochelson, M.S.; Ochoa-Albiztegui, R.E.; Martinez, D.F.; Morris, E.A.; Thakur, S.; Pinker, K. Radiomic signatures with contrast-enhanced magnetic resonance imaging for the assessment of breast cancer receptor status and molecular subtypes: Initial results. Breast Cancer Res. 2019, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Lo Gullo, R.; Vincenti, K.; Rossi Saccarelli, C.; Gibbs, P.; Fox, M.J.; Daimiel, I.; Martinez, D.F.; Jochelson, M.S.; Morris, E.A.; Reiner, J.S.; et al. Diagnostic value of radiomics and machine learning with dynamic contrast-enhanced magnetic resonance imaging for patients with atypical ductal hyperplasia in predicting malignant upgrade. Breast Cancer Res. Treat. 2021, 187, 535–545. [Google Scholar] [CrossRef]
- Pinker, K.; Shitano, F.; Sala, E.; Do, R.K.; Young, R.J.; Wibmer, A.G.; Hricak, H.; Sutton, E.J.; Morris, E.A. Background, current role, and potential applications of radiogenomics. J. Magn. Reson. Imaging JMRI 2018, 47, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Asselain, B.; Barlow, W.; Bartlett, J.; Bergh, J.; Bergsten-Nordström, E.; Bliss, J.; Boccardo, F.; Boddington, C.; Bogaerts, J.; Bonadonna, G.; et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Newman, L.A.; Pernick, N.L.; Adsay, V.; Carolin, K.A.; Philip, P.A.; Sipierski, S.; Bouwman, D.L.; Kosir, M.A.; White, M.; Visscher, D.W. Histopathologic evidence of tumor regression in the axillary lymph nodes of patients treated with preoperative chemotherapy correlates with breast cancer outcome. Ann. Surg. Oncol. 2003, 10, 734–739. [Google Scholar] [CrossRef]
- McNeish, D.M. Using Lasso for Predictor Selection and to Assuage Overfitting: A Method Long Overlooked in Behavioral Sciences. Multivar. Behav. Res. 2015, 50, 471–484. [Google Scholar] [CrossRef]
- Pal, M.; Mather, P.M. Support vector machines for classification in remote sensing. Int. J. Remore Sens. 2005, 26, 1007–1011. [Google Scholar] [CrossRef]
- Cain, E.H.; Saha, A.; Harowicz, M.R.; Marks, J.R.; Marcom, P.K.; Mazurowski, M.A. Multivariate machine learning models for prediction of pathologic response to neoadjuvant therapy in breast cancer using MRI features: A study using an independent validation set. Breast Cancer Res. Treat. 2019, 173, 455–463. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, J.; Wang, Y.; Xu, X.; Zhang, Y.; Zhang, Y.; Liu, G.; Cheng, J. Textural features of 18F-FDG PET after two cycles of neoadjuvant chemotherapy can predict pCR in patients with locally advanced breast cancer. Ann. Nucl. Med. 2017, 31, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, X.; Xu, C.; Liu, C.; Zheng, C.; Fulham, M.J.; Feng, D.; Wang, L.; Song, S.; Huang, G. 18F-FDG PET/CT radiomic predictors of pathologic complete response (pCR) to neoadjuvant chemotherapy in breast cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, H.-A.; Kim, W.; Lim, I.; Lee, I.; Byun, B.H.; Noh, W.C.; Seong, M.-K.; Lee, S.-S.; Kim, B.I.; et al. Early prediction of neoadjuvant chemotherapy response for advanced breast cancer using PET/MRI image deep learning. Sci. Rep. 2020, 10, 21149. [Google Scholar] [CrossRef] [PubMed]

| Total Patients | Number of Patients | |
|---|---|---|
| Menopause status | ||
| Pre | 38 | |
| Peri | 4 | |
| Post | 31 | |
| Ki67 | ||
| Negative < 15% | 4 | |
| Positive > 15% | 69 | |
| PR status | ||
| Positive | 48 | |
| Negative | 25 | |
| ER status | ||
| Positive | 47 | |
| Negative | 26 | |
| HER2neu expression | ||
| 0 | 29 | |
| 1+ | 22 | |
| 2+ | 6 | |
| 3+ | 16 | |
| Tumor grade | ||
| G1 | 1 | |
| G2 | 37 | |
| G3 | 35 | |
| Subtype | ||
| Basal-like/ triple-negative | 19 | |
| Luminal A | 10 | |
| Luminal B | 42 | |
| Her2-enriched | 2 | |
| Histology | ||
| NST | 69 | |
| Lobular invasive | 3 | |
| Other | 1 | |
| Response (Regression criteria of Sinn) | ||
| 0 | 4 | |
| 1 | 31 | |
| 2 | 4 | |
| 3 | 3 | |
| 4 | 31 | |
| h | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) |
|---|---|---|---|---|---|---|
| ADC | 0.72 (0.61–0.83) | 71.4 (55.4–84.3) | 69.0 (52.9–82.4) | 69.8 (58.6–79.0) | 70.7 (59.0–80.3) | 70.2 (59.3–79.7) |
| T2 | 0.70 (0.58–0.82) | 76.2 (60.6–88.0) | 69.0 (52.9–82.4) | 71.1 (60.3–80.0) | 74.4 (61.9–83.8) | 72.6 (61.8–81.8) |
| Dynamic 1 | 0.69 (0.58–0.81) | 66.7 (50.5–80.4) | 69.0 (52.9–82.4) | 68.3 (56.6–78.0) | 67.4 (56.3–76.9) | 67.9 (56.8–77.6) |
| Dynamic 2 | 0.66 (0.54–0.78) | 71.4 (55.4–84.3) | 59.5 (43.3–74.4) | 63.8 (53.9–72.7) | 67.6 (54.9–78.1) | 65.5 (54.3–75.5) |
| Dynamic 3 | 0.71 (0.60–0.82) | 47.6 (32.0–64.6) | 78.6 (63.2–89.7) | 69.0 (53.5–81.1) | 60.0 (51.9–67.6) | 63.1 (51.9–73.4) |
| Dynamic 4 | 0.71 (0.60–0.82) | 38.1 (23.6–54.4) | 92.9 (80.5–98.5) | 84.2 (62.7–94.4) | 60.0 (53.8–65.9) | 65.5 (54.3–75.5) |
| Dynamic 5 | 0.72 (0.61–0.84) | 69.0 (52.9–82.4) | 71.4 55.4–84.3) | 70.7 (59.0–80.3) | 69.8 (58.6–79.0) | 70.2 (59.3–79.7) |
| All Dynamics | 0.69 (0.57–0.81) | 66.7 (50.5–80.4) | 76.2 (60.6–88.0) | 73.7 (61.0–83.4) | 69.6 (59.1–78.4) | 71.4 (60.5–80.8) |
| All MR | 0.76 (0.66–0.86) | 71.4 (55.4–84.3) | 69.0 (52.9–82.4) | 69.8 (58.6–79.0) | 70.7 (59.0–80.3) | 70.2 (59.3–79.7) |
| PET | 0.77 (0.66–0.87) | 81.0 (65.9–91.4) | 71.4 (55.4–84.3) | 73.9 (63.2–82.4) | 78.9 (66.1–87.8) | 76.2 (65.7–84.8) |
| All MR and PET | 0.80 (0.70–0.89) | 81.0 (65.9–91.4) | 73.8 (58.0–86.1) | 75.6 (64.6–84.0) | 79.5 (66.9–88.1) | 77.4 (67.0–85.8) |
| Images | AUC | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|
| ADC | 0.64 (0.48–0.79) | 70.4 (49.8–86.3) | 63.0 (42.4–80.6) | 65.5 (52.3–76.7) | 68.0 (52.6–80.3) | 66.7 (52.5–78.9) |
| T2 | 0.85 (0.74–0.95) | 66.7 (46.0–83.5) | 92.6 (75.7–99.1) | 90.0 (69.8–97.2) | 73.5 (61.7–82.7) | 79.6 (66.5–89.4) |
| Dynamic 1 | 0.79 (0.67–0.91) | 70.4 (49.8–86.3) | 74.1 (53.7–88.9) | 73.1 (57.8–84.3) | 71.4 (57.3–82.3) | 72.2 (58.4–83.5) |
| Dynamic 2 | 0.66 (0.51–0.81) | 59.3 (38.8–77.6) | 70.4 (49.8–86.3) | 66.7 (50.8–79.5) | 63.3 (50.8–74.3) | 64.8 (50.6–77.3) |
| Dynamic 3 | 0.69 (0.54–0.84) | 85.2 (66.3–95.8) | 59.3 (38.8–77.6) | 67.6 (56.4–77.2) | 80.0 (60.6–91.2) | 72.2 (58.4–83.5) |
| Dynamic 4 | 0.72 (0.58–0.86) | 66.7 (46.0–83.5) | 77.8 (57.7–91.4) | 75.0 (58.5–86.5) | 70.0 (56.9–80.5) | 72.2 (58.4–83.5) |
| Dynamic 5 | 0.65 (0.50–0.81) | 81.5 (61.9–93.7) | 63.0 (42.4–80.6) | 68.8 (56.6–78.8) | 77.3 (59.4–88.8) | 72.2 (58.4–83.5) |
| All Dynamics | 0.89 (0.80–0.98) | 92.6 (75.7–99.1) | 74.1 (53.7–88.9) | 78.1 (65.2–87.2) | 90.9 (72.1–97.5) | 83.3 (70.7–92.1) |
| All MR | 0.89 (0.79–0.98) | 81.5 (61.9–93.7) | 85.2 (66.3–95.8) | 84.6 (68.6–93.3) | 82.1 (67.3–91.2) | 83.3 (70.7–92.1) |
| PET | 0.90 (0.81–0.98) | 85.2 (66.3–95.8) | 85.2 (66.3–95.8) | 85.2 (69.7–93.5) | 85.2 (69.7–93.5) | 85.2 (72.9–93.4) |
| All MR and PET | 0.94 (0.88–1.00) | 85.2 (66.3–95.8) | 85.2 (66.3–95.8) | 85.2 (69.7–93.5) | 85.2 (69.7–93.5) | 85.2 (72.9–93.4) |
| Images | AUC | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|
| ADC | 0.64 (0.43–0.85) | 82.4 (56.6–96.2) | 60.0 (32.3–83.7) | 70.0 (54.7–81.8) | 75.0 (49.8–90.1) | 71.9 (53.5–86.3) |
| T2 | 0.75 (0.57–0.93) | 76.5 (50.1–93.2) | 66.7 (38.4–88.2) | 72.2 (54.8–84.8) | 71.4 (49.7–86.4) | 71.9 (53.3–86.3) |
| Dynamic 1 | 0.75 (0.57–0.93 | 82.4 (56.6–96.2) | 46.7 (21.3–73.4) | 63.6 (50.9–74.7) | 70.0 (42.2–88.2) | 65.6 (46.8–81.4) |
| Dynamic 2 | 0.74 (0.55–0.93) | 82.4 (56.6–96.2) | 73.3 (44.9–92.2) | 77.8 (59.5–89.3) | 78.6 (55.7–91.5) | 78.1 (60.0–90.7) |
| Dynamic 3 | 0.82 (0.65–0.98) | 88.2 (63.6–98.5) | 80.0 (51.9–95.7) | 83.3 (64.2–93.3) | 85.7 (61.4–95.8) | 84.4 (67.2–94.7) |
| Dynamic 4 | 0.83 (0.68–0.98) | 82.4 (56.6–92.4) | 73.3 (44.9–92.2) | 77.8 (59.5–89.3) | 78.6 (55.7–91.5) | 78.1 (60.0–90.7) |
| Dynamic 5 | 0.59 (0.38–0.80) | 64.7 (33.3–85.8) | 66.7 (38.4–88.2) | 68.8 (49.8–83.0) | 62.5 (44.4–77.7) | 65.6 (46.8–81.4) |
| All Dynamics | 0.92 (0.82–1.00) | 88.2 (63.6–98.5) | 86.7 (59.5–98.3) | 88.2 (67.1–96.5) | 86.7 (63.5–96.0) | 87.5 (71.0–96.5) |
| All MR | 0.92 (0.82–1.00) | 88.2 (63.6–98.5) | 86.7 (59.5–98.3) | 88.2 (67.1–96.5) | 86.7 (63.5–96.0) | 87.5 (71.0–96.5) |
| PET | 0.67 (0.48–0.86) | 70.6 (44.0–89.7) | 60.0 (32.3–83.7) | 66.7 (50.0–80.0) | 64.3 (43.6–80.7) | 65.6 (46.8–81.4) |
| All MR and PET | 0.92 (0.82–1.00) | 88.2 (63.6–98.5) | 86.7 (59.5–98.3) | 88.2 (67.1–96.5) | 86.7 (63.5–96.0) | 87.5 (71.0–96.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umutlu, L.; Kirchner, J.; Bruckmann, N.-M.; Morawitz, J.; Antoch, G.; Ting, S.; Bittner, A.-K.; Hoffmann, O.; Häberle, L.; Ruckhäberle, E.; et al. Multiparametric 18F-FDG PET/MRI-Based Radiomics for Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancers 2022, 14, 1727. https://doi.org/10.3390/cancers14071727
Umutlu L, Kirchner J, Bruckmann N-M, Morawitz J, Antoch G, Ting S, Bittner A-K, Hoffmann O, Häberle L, Ruckhäberle E, et al. Multiparametric 18F-FDG PET/MRI-Based Radiomics for Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancers. 2022; 14(7):1727. https://doi.org/10.3390/cancers14071727
Chicago/Turabian StyleUmutlu, Lale, Julian Kirchner, Nils-Martin Bruckmann, Janna Morawitz, Gerald Antoch, Saskia Ting, Ann-Kathrin Bittner, Oliver Hoffmann, Lena Häberle, Eugen Ruckhäberle, and et al. 2022. "Multiparametric 18F-FDG PET/MRI-Based Radiomics for Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer" Cancers 14, no. 7: 1727. https://doi.org/10.3390/cancers14071727
APA StyleUmutlu, L., Kirchner, J., Bruckmann, N.-M., Morawitz, J., Antoch, G., Ting, S., Bittner, A.-K., Hoffmann, O., Häberle, L., Ruckhäberle, E., Catalano, O. A., Chodyla, M., Grueneisen, J., Quick, H. H., Fendler, W. P., Rischpler, C., Herrmann, K., Gibbs, P., & Pinker, K. (2022). Multiparametric 18F-FDG PET/MRI-Based Radiomics for Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancers, 14(7), 1727. https://doi.org/10.3390/cancers14071727








