Pulsed Microwave Liver Ablation: An Additional Tool to Treat Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
- −
- Patients not suitable for liver resection (due to performance status, portal hypertension, or the status of end-stage liver disease)
- −
- The absence of extrahepatic spread or vascular invasion
- −
- HCC < 70 mm
2.1. Pulsed Microwave Ablation
2.2. Radiological Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Population
3.2. Disease
3.3. Procedures
3.4. Complications According to Clavien–Dindo and Outcome
3.5. Follow-Up
4. Discussion
4.1. Safety and Efficacy
4.2. Survival
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lingiah, V.A.; Niazi, M.; Olivo, R.; Paterno, F.; Guarrera, J.V.; Pyrsopoulos, N.T. Liver Transplantation Beyond Milan Criteria. J. Clin. Transl. Hepatol. 2020, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Garuti, F.; Neri, A.; Avanzato, F.; Gramenzi, A.; Rampoldi, D.; Rucci, P.; Farinati, F.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.L.; et al. The changing scenario of hepatocellular carcinoma in Italy: An update. Liver Int. 2020, 41, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Cucchetti, A.; Erroi, V.; Garuti, F.; Odaldi, F.; Trevisani, F. Surveillance for early diagnosis of hepatocellular carcinoma: How best to do it? World J. Gastroenterol. 2013, 19, 8808–8821. [Google Scholar] [CrossRef]
- Finotti, M.; Vitale, A.; Volk, M.; Cillo, U. A 2020 update on liver transplant for hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 885–900. [Google Scholar] [CrossRef]
- Affonso, B.B.; Galastri, F.L.; da Motta Leal Filho, J.M.; Nasser, F.; Falsarella, P.M.; Cavalcante, R.N.; De Almeida, M.D.; Felga, G.E.G.; Valle, L.G.M.; Wolosker, N. Long-term outcomes of hepatocellular carcinoma that underwent chemoembolization for bridging or downstaging. World J. Gastroenterol. 2019, 25, 5687–5701. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Bin Riaz, I.; Naqvi, S.A.A.; Almquist, D.R.; Mina, S.; Almasri, J.; Shah, S.; Almader-Douglas, D.; Junior, P.L.S.U.; Mahipal, A.; et al. Systemic Therapy and Sequencing Options in Advanced Hepatocellular Carcinoma. JAMA Oncol. 2020, 6, e204930. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Ito, K.; Takemura, N.; Inagaki, F.; Mihara, F.; Kokudo, N. Difference in treatment algorithms for hepatocellular carcinoma between world’s principal guidelines. Glob. Health Med. 2020, 2, 282–291. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Burroughs, A.; Dufour, J.-F.; Galle, P.R.; Mazzaferro, V.M.; Piscaglia, F.; Raoul, J.L.; Sangro, B.; Bolondi, L. Heterogeneity of Patients with Intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a Subclassification to Facilitate Treatment Decisions. Semin. Liver Dis. 2012, 32, 348–359. [Google Scholar] [CrossRef]
- Finotti, M.; D’Amico, F.; Mulligan, D.; Testa, G. A review of the current and future role of robotic surgery in liver surgery and transplantation. Hepatobiliary Surg. Nutr. 2021. [Google Scholar] [CrossRef]
- Liang, P.; Wang, Y. Microwave Ablation of Hepatocellular Carcinoma. Oncology 2007, 72, 124–131. [Google Scholar] [CrossRef]
- Cillo, U.; Finotti, M.; Di Renzo, C.; Vitale, A.; Zanus, G.; Gringeri, E.; Bertacco, A.; Polacco, M.; D’Amico, F. Thoracoscopic Ablation of Critically Located Liver Tumors: A Safety and Efficacy Cohort Study. Front. Surg. 2021, 8, 49. [Google Scholar] [CrossRef]
- D’Amico, F.; Serafini, S.; Finotti, M.; Di Bello, M.; Di Renzo, C.; Cillo, U. One-lung ventilation to treat hepatic dome lesion—A further step towards minimally invasive surgery: A case report. J. Med Case Rep. 2019, 13, 83. [Google Scholar] [CrossRef]
- Bertacco, A.; D’Amico, F.; Romano, M.; Finotti, M.; Vitale, A.; Cillo, U. Liver radiofrequency ablation as emergency treatment for a ruptured hepatocellular carcinoma: A case report. J. Med Case Rep. 2017, 11, 54. [Google Scholar] [CrossRef][Green Version]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.-Y.; Lu, X.; Zhai, B. Laparoscopic Microwave Ablation of Hepatocellular Carcinoma at Liver Surface: Technique Effectiveness and Long-Term Outcomes. Technol. Cancer Res. Treat. 2019, 18, 1533033818824338. [Google Scholar] [CrossRef]
- Zanus, G.; Boetto, R.; Gringeri, E.; Vitale, A.; D’Amico, F.; Carraro, A.; Bassi, D.; Bonsignore, P.; Noaro, G.; Mescoli, C.; et al. Microwave Thermal Ablation for Hepatocarcinoma: Six Liver Transplantation Cases. Transplant. Proc. 2011, 43, 1091–1094. [Google Scholar] [CrossRef]
- Bedoya, M.; Del Rio, A.M.; Chiang, J.; Brace, C.L. Microwave ablation energy delivery: Influence of power pulsing on ablation results in anex vivoandin vivoliver model. Med Phys. 2014, 41, 123301. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.H.; Thompson, K.; McKillop, I.H.; Cochran, A.; Kirks, R.C.; Vrochides, D.; Martinie, J.B.; Swan, R.Z.; Iannitti, D.A. Operative microwave ablation for hepatocellular carcinoma: A single center retrospective review of 219 patients. J. Gastrointest. Oncol. 2017, 8, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.C.H.; Brace, C.L.; Hinshaw, J.L.; Quek, L.H.H.; Huang, I.K.H.; Kwan, J.; Lim, G.H.T.; Lee, F.T.; Pua, U. Microwave ablation of the liver in a live porcine model: The impact of power, time and total energy on ablation zone size and shape. Int. J. Hyperth. 2020, 37, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Prud’Homme, C.; Teriitehau, C.; Adam, J.; Tun, J.K.; Roux, C.; Hakime, A.; Delpla, A.; Deschamps, F.; de Baere, T.; Tselikas, L. Lung microwave ablation—An in vivo swine tumor model experiment to evaluate ablation zones. Int. J. Hyperth. 2020, 37, 879–886. [Google Scholar] [CrossRef]
- Radosevic, A.; Prieto, D.; Burdío, F.; Berjano, E.; Prakash, P.; Trujillo, M. Short pulsed microwave ablation: Computer modeling and ex vivo experiments. Int. J. Hyperth. 2021, 38, 409–420. [Google Scholar] [CrossRef]
- Filippiadis, D. Continuous versus pulsed microwave ablation in the liver: Any difference in intraoperative pain scores? Ann. Gastroenterol. 2020, 34, 80–84. [Google Scholar] [CrossRef]
- Harari, C.M.; Magagna, M.; Bedoya, M.; Lee, F.T.; Lubner, M.G.; Hinshaw, J.L.; Ziemlewicz, T.; Brace, C.L. Microwave Ablation: Comparison of Simultaneous and Sequential Activation of Multiple Antennas in Liver Model Systems. Radiology 2016, 278, 95–103. [Google Scholar] [CrossRef]
- Forner, A.; Gilabert, M.; Bruix, J.; Raoul, J.-L. Treatment of intermediate-stage hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2014, 11, 525–535. [Google Scholar] [CrossRef]
- Reig, M.; Darnell, A.; Forner, A.; Rimola, J.; Ayuso, C.; Bruix, J. Systemic Therapy for Hepatocellular Carcinoma: The Issue of Treatment Stage Migration and Registration of Progression Using the BCLC-Refined RECIST. Semin. Liver Dis. 2014, 34, 444–455. [Google Scholar] [CrossRef]
- Bruix, J.; Reig, M.; Rimola, J.; Forner, A.; Burrel, M.; Vilana, R.; Ayuso, C. Clinical decision making and research in hepatocellular carcinoma: Pivotal role of imaging techniques. Hepatology 2011, 54, 2238–2244. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022. [Google Scholar] [CrossRef]
- Cillo, U.; Bertacco, A.; Fasolo, E.; Carandina, R.; Vitale, A.; Zanus, G.; Gringeri, E.; D’Amico, F.; Bassi, D.; Neri, D.; et al. Videolaparoscopic microwave ablation in patients with HCC at a European high-volume center: Results of 815 procedures. J. Surg. Oncol. 2019, 120, 956–965. [Google Scholar] [CrossRef]
- Imamura, H.; Takami, Y.; Ryu, T.; Wada, Y.; Sasaki, S.; Ureshino, H.; Saitsu, H. Feasibility and safety of surgical microwave ablation for hepatocellular carcinoma in elderly patients: A single center analysis in Japan. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Swan, R.Z.; Sindram, D.; Martinie, J.B.; Iannitti, D.A. Operative Microwave Ablation for Hepatocellular Carcinoma: Complications, Recurrence, and Long-Term Outcomes. J. Gastrointest. Surg. 2013, 17, 719–729. [Google Scholar] [CrossRef]
- Chong, C.C.; Lee, K.F.; Cheung, S.Y.; Chu, C.C.; Fong, A.K.; Wong, J.; Hui, J.W.; Fung, A.K.; Lok, H.T.; Lo, E.Y.; et al. Prospective double-blinded randomized controlled trial of Microwave versus RadioFrequency Ablation for hepatocellular carcinoma (McRFA trial). HPB 2020, 22, 1121–1127. [Google Scholar] [CrossRef]
- Livraghi, T.; Meloni, F.; Solbiati, L.; Zanus, G.; Collaborative Italian Group Using AMICA system. Complications of Microwave Ablation for Liver Tumors: Results of a Multicenter Study. Cardiovasc. Interv. Radiol. 2011, 35, 868–874. [Google Scholar] [CrossRef]
- Lu, M.-D.; Xu, H.-X.; Xie, X.-Y.; Yin, X.-Y.; Chen, J.-W.; Kuang, M.; Xu, Z.-F.; Liu, G.-J.; Zheng, Y.-L. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: A retrospective comparative study. J. Gastroenterol. 2005, 40, 1054–1060. [Google Scholar] [CrossRef]
- Ohmoto, K.; Yoshioka, N.; Tomiyama, Y.; Shibata, N.; Kawase, T.; Yoshida, K.; Kuboki, M.; Yamamoto, S. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J. Gastroenterol. Hepatol. 2009, 24, 223–227. [Google Scholar] [CrossRef]
- Ding, J.; Jing, X.; Liu, J.; Wang, Y.; Wang, F.; Wang, Y.; Du, Z. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur. J. Radiol. 2013, 82, 1379–1384. [Google Scholar] [CrossRef]
- Vogl, T.J.; Farshid, P.; Naguib, N.N.N.; Zangos, S.; Bodelle, B.; Paul, J.; Mbalisike, E.C.; Beeres, M.; Nour-Eldin, N.-E.A. Ablation therapy of hepatocellular carcinoma: A comparative study between radiofrequency and microwave ablation. Gastrointest. Radiol. 2015, 40, 1829–1837. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, Y.; He, W.; Zou, R.; Qiu, J.; Shen, J.; Yang, Z.; Zhang, Y.; Wang, C.; Wang, Y.; et al. Microwave vs radiofrequency ablation for hepatocellular carcinoma within the Milan criteria: A propensity score analysis. Aliment. Pharmacol. Ther. 2018, 48, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shen, Q.; Liu, P.; Wu, P.; Lu, Z.; Huang, B.; Qian, G.; Chen, Y. Microwave ablation for the treatment of hepatocellular carcinoma that met up-to-seven criteria: Feasibility, local efficacy and long-term outcomes. Eur. Radiol. 2017, 27, 3877–3887. [Google Scholar] [CrossRef] [PubMed]
- Santambrogio, R.; Chiang, J.; Barabino, M.; Meloni, F.M.; Bertolini, E.; Melchiorre, F.; Opocher, E. Comparison of Laparoscopic Microwave to Radiofrequency Ablation of Small Hepatocellular Carcinoma (≤3 cm). Ann. Surg. Oncol. 2016, 24, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Morales, R.R.; Zanus, G.; Farinati, F.; Burra, P.; Angeli, P.; Frigo, A.C.; Del Poggio, P.; Rapaccini, G.; Di Nolfo, M.A.; et al. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: A multicentre, cohort study. Lancet Oncol. 2011, 12, 654–662. [Google Scholar] [CrossRef]
- Vitale, A.; Finotti, M.; Trevisani, F.; Farinati, F.; Giannini, E.G. Treatment allocation in patients with hepatocellular carcinoma: Need for a paradigm shift? Liver Cancer Int. 2021. [Google Scholar] [CrossRef]
- Vitale, A.; Trevisani, F.; Farinati, F.; Cillo, U. Treatment of Hepatocellular Carcinoma in the Precision Medicine Era: From Treatment Stage Migration to Therapeutic Hierarchy. Hepatology 2020, 72, 2206–2218. [Google Scholar] [CrossRef]
- Vitale, A.; Farinati, F.; Finotti, M.; Di Renzo, C.; Brancaccio, G.; Piscaglia, F.; Cabibbo, G.; Caturelli, E.; Missale, G.; Marra, F.; et al. Overview of Prognostic Systems for Hepatocellular Carcinoma and ITA.LI.CA External Validation of MESH and CNLC Classifications. Cancers 2021, 13, 1673. [Google Scholar] [CrossRef]
| Variables | Results |
|---|---|
| Age, mean ± SD (range); median | 68.2 ± 9.2 (36–88); 69 |
| Sex (M/F), n (%) | 88 (77.9)/25 (22.1) |
| Etiology, n (%) | |
| Alcohol-related | 22/113 (19.5) |
| Alcohol-related and metabolic | 5/113 (4.4) |
| Viral | 68/113 (60.2) |
| HCV | 43/113 (38.1) |
| HCV + HIV | 2/113 (1.8) |
| HCV + ETOH | 2/113 (1.8) |
| HBV | 17/113 (15.0) |
| HBV + ETOH | 4/113 (3.5) |
| Metabolic | 11/113 (9.7) |
| Other | 7/113 (6.2) |
| BCLC, n (%) | |
| 0 | 11 (10) |
| A | 59 (52) |
| B | 33 (29) |
| C | 6 (5) |
| D | 2 (2) |
| NR | 2 (2) |
| MELD, Mean ± SD (Range) | Results |
|---|---|
| Considering procedures | 9.4 ± 3.1 (6–21) |
| Considering patients (MELD score at the first procedure) | 9.4 ± 3.2 (6–21) |
| Child–Pugh, n (%) | |
| A5 | 52 (46.0) |
| A6 | 40 (35.4) |
| B7 | 13 (11.5) |
| B8 | 2 (1.8) |
| B9 | 1 (0.9) |
| C10 | 2 (1.8) |
| C11 | 1 (0.9) |
| *NR = not reported/missing data | 2 (1.8) |
| Portal thrombosis, n (%) | 10 (8.1) |
| CEA (ng/mL), mean ± SD (range); median | 3.4 ± 4.9 (0.5–49.4); 2.3 |
| CA 19.9 (U/mL), mean ± SD (range); median | 28.7 ± 37.3 (0.6–296); 18 |
| AFP (ng/mL), mean ± SD (range) | 51.0 ± 138.1 (1–851) |
| Ascites, n (%) | 33 (26.4) |
| Portal hypertension, n (%) | 83 (67.5) |
| *NR = not reported/missing data |
| Lesions’ size (mm), mean ± SD (range); median | 17.3 ± 11.7 (7–70); 13 |
| Clusters of diameters, n (%) | |
| <3 cm | 253/286 (88.5) |
| 3–5 cm | 29/286 (10.1) |
| >5 cm | 4/286 (1.4) |
| Site of lesions, n (%) | |
| Posterior segments (s1, s6, s7, s8) | 132 (47.2) |
| Anterior segments and left lobe (s2, s3, s4a, s4b, s5) | 154 (53.8) |
| Multinodular disease, n (%) | 90 (78.9) |
| Number of lesions in patients, mean ± SD (range); median | 3.5 ± 2.2 (1–10); 3 |
| Previous surgical treatments (n°), mean ± SD (range); median | 1.9 ± 2.2 (0–11); 3 |
| Previous loco-regional treatments (n°), mean ± SD (range) | |
| PEI | 0.1 ± 0.7 (0–6) |
| RFA/MWA | 0.5 ± 0.8 (0–4) |
| TACE | 0.7 ± 0.9 (0–4) |
| Previous resection (n°), mean ± SD (range); median | 0.2 ± 0.5 (0–2) |
| Surgical approach, n (%) | |
| VLS | 105 (83.3) |
| Laparotomic | 14 (11.1) |
| Percutaneous | 7 (5.6) |
| Time of ablation (min), mean ± SD (range); median | 8.0 ± 7.0 (1–40); 6.0 |
| Probe power (W), mean ± SD (range); median | 81.2 ± 19.7 (40–120); 75 |
| Associated resection, n (%) | 24 (18.6) |
| Major | 1 (0.8) |
| Minor | 23 (18.0) |
| Hospital stay (days), mean ± SD (range); median | |
| All | 4.96 (1–26); 4 |
| VLS (105) | 4.84 (1–26); 4 |
| Open (14) | 8.15 (4–20); 7 |
| Percutaneous (7) | 1.0 (1); 1 |
| Hospital stay for patients scored Clavien–Dindo 1, 2, and 3 | |
| n = 41 (32.5%) | 5.09 (1–26); 4 |
| 12-Month LTP | n = 286 |
| 0 | 182 (90.1%) |
| 1 | 20 (9.9%) |
| 12-month LTP according to nodule size (mm) | n = 286—p = 0.0956 |
| <30 (n = 253) | 15 (8.4%) |
| 30–50 (n = 29) | 4 (21.1%) |
| >50 (n = 4) | 1 (25%) |
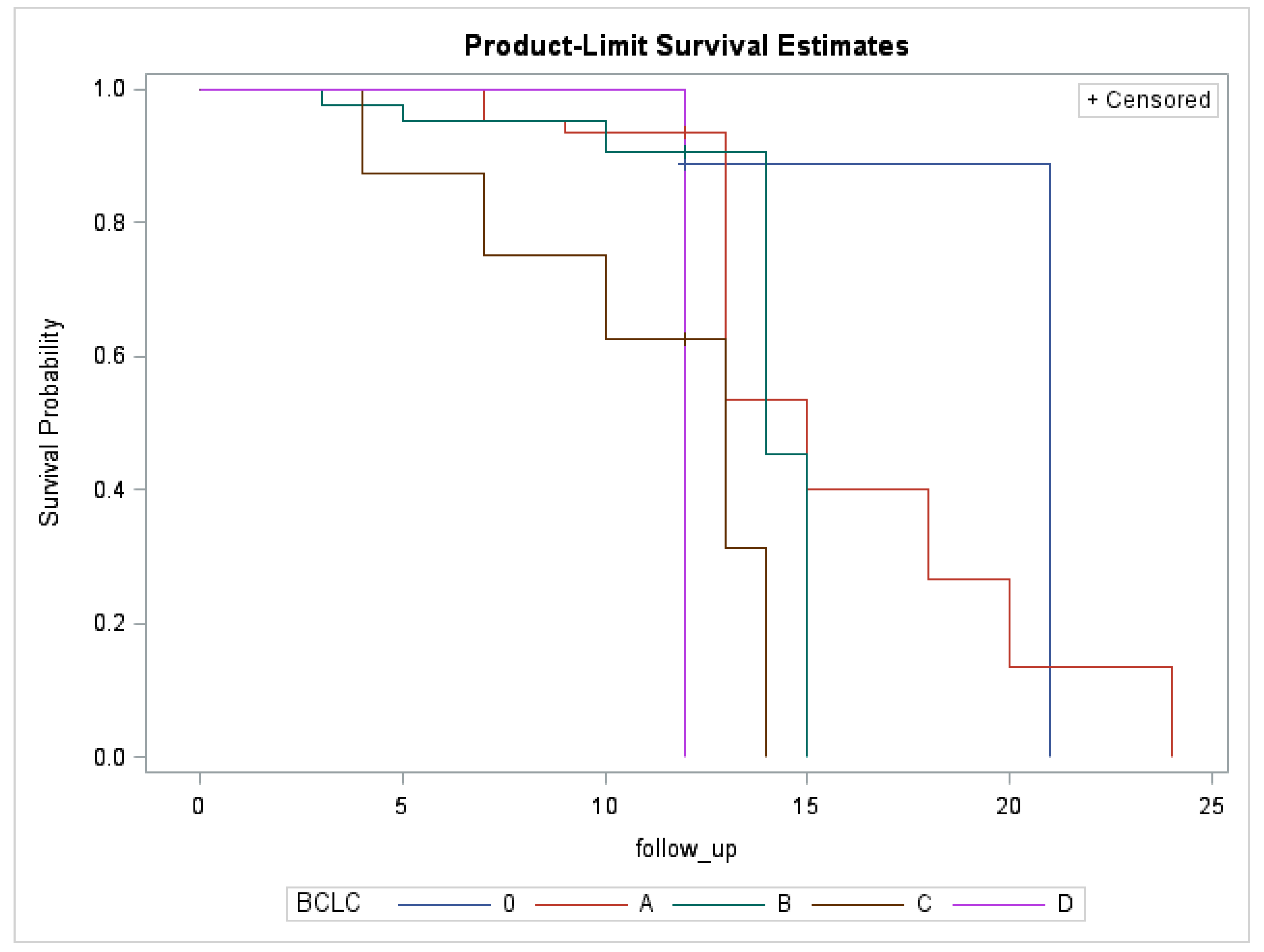
| 12-month LTP according to the BCLC stage | n = 286—p = 0.0111 |
| 0 (n = 13) | 1 (9.1%) |
| A (n = 113) | 14 (16.9%) |
| B (n = 137) | 4 (3.9%) |
| C (n = 15) | 0 (0.0%) |
| D (n = 8) | 1 (50.0%) |
| 12-month IHR | n = 126 |
| 0 | 70 (72.2%) |
| 1 | 27 (27.8%) |
| 12-month IHR according to nodule size (mm) | n = 126—p = 0.7636 |
| <30 (n = 103) | 22 (27.8%) |
| 30–50 (n = 21) | 4 (25.0%) |
| >50 (n = 2) | 1 (50.0%) |
| Author, Year | Procedures (n°) | Diameter (cm) | Follow-Up (months) | LTP (%) |
|---|---|---|---|---|
| Lu MD et al., 2005 [37] | 49 | ≤3 cm 3–5 cm | 25.1 | 6.8% 30.0% |
| Ohmoto K et al., 2009 [38] | 49 | ≤2 cm | 12 | 13% |
| Ding J et al., 2013 [39] | 113 | ≤3 cm 3–5 cm | 18.3 | 7.3% 21.2% |
| Vogl TJ et al., 2015 [40] | 28 | ≤5 cm | 12 | 11.1% |
| Li W et al., 2017 [41] | 60 | ≤3 cm | 12 | 14.9% |
| Xu Y et al., 2017 [42] | 294 | ≤3 cm 3–5 cm 5–6 cm | 12 | 10.6% 16.9% 28.6% |
| Baker EH et al., 2017 [22] | 219 | 1–6 cm (median 3.2 cm) | 9.9 | 8.5% |
| Santambrogio et al., 2017 [43] | 60 | ≤3 cm | 26.9 | 8.3% |
| Liu W et al., 2018 [41] | 126 | ≤3 cm | 36.8 | 11.7% |
| Cillo U et al., 2019 [32] | 815 | ≤5 cm | 6 | 23.1% |
| Our experience—2020 | 126 | All <3 cm 3–5 cm >5 cm | 12 | 9.9% 8.4% 21.1% 25% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanus, G.; Tagliente, G.; Rossi, S.; Bonis, A.; Zambon, M.; Scopelliti, M.; Brizzolari, M.; Grossi, U.; Romano, M.; Finotti, M. Pulsed Microwave Liver Ablation: An Additional Tool to Treat Hepatocellular Carcinoma. Cancers 2022, 14, 748. https://doi.org/10.3390/cancers14030748
Zanus G, Tagliente G, Rossi S, Bonis A, Zambon M, Scopelliti M, Brizzolari M, Grossi U, Romano M, Finotti M. Pulsed Microwave Liver Ablation: An Additional Tool to Treat Hepatocellular Carcinoma. Cancers. 2022; 14(3):748. https://doi.org/10.3390/cancers14030748
Chicago/Turabian StyleZanus, Giacomo, Giovanni Tagliente, Serena Rossi, Alessandro Bonis, Mattia Zambon, Michele Scopelliti, Marco Brizzolari, Ugo Grossi, Maurizio Romano, and Michele Finotti. 2022. "Pulsed Microwave Liver Ablation: An Additional Tool to Treat Hepatocellular Carcinoma" Cancers 14, no. 3: 748. https://doi.org/10.3390/cancers14030748
APA StyleZanus, G., Tagliente, G., Rossi, S., Bonis, A., Zambon, M., Scopelliti, M., Brizzolari, M., Grossi, U., Romano, M., & Finotti, M. (2022). Pulsed Microwave Liver Ablation: An Additional Tool to Treat Hepatocellular Carcinoma. Cancers, 14(3), 748. https://doi.org/10.3390/cancers14030748







