Simple Summary
Lenvatinib is a systemic treatment for patients with advanced hepatocellular carcinoma (HCC). Stereotactic body radiation therapy (SBRT) is an advanced technique of hypofractionated external beam radiotherapy (EBRT) that can be applied in patients with HCC. The current study showed that the area under the concentration–time curve of lenvatinib concentration (AUClenvatinib) increased by 148.8% with radiotherapy (RT)2Gy×3f’x (EBRT for the whole liver), and 68.9% with RT9Gy×3f’× (SBRT targeting a 1.5 × 1.5 cm region in the center of the liver) in the sequential regimen compared to the concurrent regimen in rats. Additionally, the AUClenvatinib was decreased by 50% in the concurrent regimen of both RT techniques with lenvatinib compared to the control group. The biodistribution of lenvatinib in the organs at risk was markedly decreased in the concurrent regimens. The radiation–drug interactions were between lenvatinib and RT, and showed sequential preferably.
Abstract
Concurrent and sequential regimens involving radiotherapy (RT) and lenvatinib were designed with off-target or stereotactic body radiation therapy (SBRT) doses in a freely moving rat model to evaluate the effect of RT on the pharmacokinetics (PK) of lenvatinib. Liver RT concurrent with lenvatinib decreased the area under the concentration–time curve of lenvatinib concentration (AUClenvatinib) by 51.1% with three fractions of 2 Gy (RT2Gy×3f’x, p = 0.03), and 48.9% with RT9Gy×3f’x (p = 0.03). The AUClenvatinib increased by 148.8% (p = 0.008) with RT2Gy×3f’x, and 68.9% (p = 0.009) with RT9Gy×3f’x in the sequential regimen compared to the concurrent regimen. There were no differences in the AUClenvatinib between RT2Gy×3f’x and RT9Gy×3f’x in the concurrent or sequential regimen. Both the RT2Gy×3f’x and RT9Gy×3f’x concurrent regimens markedly decreased the biodistribution of lenvatinib in the heart, liver, lung, spleen, and kidneys, which ranged from 31% to 100% for RT2Gy×3f’x, and 11% to 100% for RT9Gy×3f’x, compared to the sham regimen. The PK and biodistribution of lenvatinib can be modulated by simultaneous off-target irradiation and SBRT doses. The timing of lenvatinib administration with respect to RT, impacted the PK and biodistribution of the drug. Additionally, off-target and SBRT doses had a similar ability to modulate the effect of systemic therapy.
1. Introduction
The incidence of hepatocellular carcinoma (HCC) and the associated mortality are increasing worldwide [1]. The oral multikinase inhibitor sorafenib (Nexavar, Bayer Pharma AG, Berlin, Germany) provides a clinically significant improvement in the overall survival of HCC patients [2,3]. Recently, the phase III REFLECT trial showed that lenvatinib (Eisai Inc., Woodcliff Lake, NJ, USA) was noninferior to sorafenib for the overall survival of patients with HCC [4]. Therefore, the National Comprehensive Cancer Network® Clinical Practice Guidelines in Oncology list both lenvatinib and sorafenib as first-line systemic treatments for HCC patients.
Stereotactic body radiation therapy (SBRT) is an advanced external beam radiotherapy (EBRT) technique, which can be considered as an alternative to ablation/embolization techniques, or used when these techniques fail or are contraindicated for HCC [5,6]. Recently, impressive benefits have been reported for radiotherapy (RT) combined with sorafenib in patients with unresectable HCC [7,8], but severe adverse effects have also been reported [9,10]. Moreover, interactions between RT and sorafenib, regardless of the target or off-target radiation dose, were confirmed by pharmacokinetics (PK) in a freely moving rat model [8].
Nuclear factor kappa B (NF-κB) modulates the expression of cytochrome P450 (CYP) 3A4 [11], increasing the expression of platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) [12]. NF-κB responds to environmental stress, including RT [13]. Additionally, the expression of CYP3A4 can be affected by RT [8]. Furthermore, lenvatinib targets: VEGFR1, 2, and 3; fibroblast growth factor receptor (FGFR) 1, 2, 3, and 4; PDGFR-alpha (α); the RET proto-oncogene; and c-kit [14,15], and is metabolized primarily in the liver, undergoing oxidative metabolism via CYP3A4 [16].
Taken together, these data suggest that there may be interactions between RT and lenvatinib. In the current study, the PK behavior of lenvatinib with different RT doses and schedules was evaluated. Furthermore, the biodistribution of lenvatinib with, and without, RT was evaluated to provide suggestions for clinical applications.
2. Materials and methods
2.1. Chemicals and Reagents
Lenvatinib and cyclosporin A were purchased from Sigma-Aldrich (St. Louis, MO, USA). As an internal standard, biochanin A was purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). Polyethylene glycol 400 (PEG 400) and heparin sodium were purchased from Sigma-Aldrich. Pentobarbital sodium was obtained from SCI Pharmtech (Taoyuan, Taiwan). The solvents and reagents for chromatography were purchased from J.T. Baker (Phillipsburg, NJ, USA) and Merck (Darmstadt, Germany). Standard solutions of lenvatinib and biochanin A in methanol were stored at −20 °C. Triply deionized water from Millipore (Bedford, MA, USA) was used for all preparations.
2.2. High-Performance Liquid Chromatography-Ultraviolet (HPLC-UV)
The HPLC system consisted of a chromatographic pump (LC−20AT), an online injector (SIL−20C) equipped with a 10 μL sample loop to inject the sample, and an ultraviolet detector (SPD-M20A). Lenvatinib and samples were separated on an Agilent ZORBAX SB-phenyl column (Agilent Technologies, Santa Clara, CA, USA, 150 × 4.6 mm i.d., particle size = 5 μm). The mobile phase for the lenvatinib group consisted of water and acetonitrile (30:70, v:v) at a flow rate of 1 mL/min. A wavelength of 251 nm was set for the optimal photodiode-array detection of lenvatinib. Lenvatinib had a retention time of 6.5 min, with good separation and no endogenous interference in the rat plasma samples, and the procedure exhibited good selectivity (Figure 1).
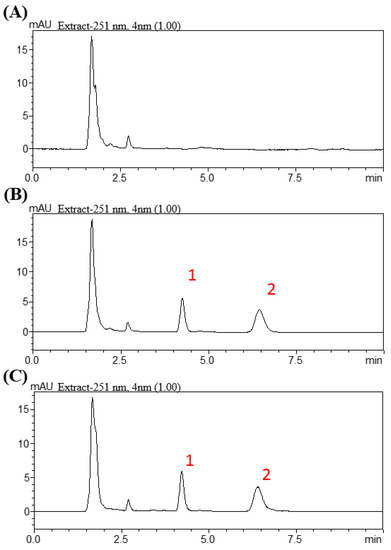
Figure 1.
Chromatograms resulting from high-performance liquid chromatography for lenvatinib. (A) Blank. (B) Blank spiked with lenvatinib (1 µg/mL) and biochanin A (0.75 µg/mL). (C) Plasma Scheme 60 min after lenvatinib administration (3 mg/kg, p.o.). Peak 1: biochanin A (0.75 µg/mL). Peak 2: lenvatinib (0.94 µg/mL).
2.3. Method Validation: Calibration Curve
The calibration curves covered a concentration range from 0.1 to 50 μg/mL. The linearity of the assay was determined using the coefficient of determination (r2) for the calibration curve, which should be greater than 0.995. The limit of detection (LOD) was defined as the concentration that generated a signal-to-noise ratio of 3, and the lower limit of quantification (LLOQ) was defined as the lowest concentration within the linear regression that yielded a signal-to-noise ratio of 10. A 0.01 mg/mL limit of quantification was identified as the lowest concentration on the calibration curve that could be measured routinely with acceptable bias and relative SD. Calibration standards for plasma samples were prepared by adding known amounts of lenvatinib (10 μL) into rat plasma blanks (40 μL each) to yield a range of 0.05–50 μg/mL. These mixtures were supplemented with 150 μL of internal standard solution (10 μg/mL).
2.4. Method Validation: Precision, Accuracy, and Recovery
The intra- and interassay variability for lenvatinib was determined by quantifying six replicates at concentrations of 0.05, 0.1, 0.5, 1, 5, and 10 μg/mL, using the above-described HPLC method on the same day, and on six consecutive days. The accuracy (% bias) was calculated from the nominal concentration (Cnom) and the mean value of observed concentrations (Cobs) as follows: accuracy (% bias) = ((Cnom − Cobs)/Cnom) × 100. The relative standard deviation (RSD) was calculated from the observed concentrations as follows: precision (% RSD) = (standard deviation (SD)/Cobs) × 100. The same data were used to determine both accuracy and precision. The relative error and coefficient of variation were maintained within ± 15%, except for the LLOQ, which was not permitted to exceed ± 20%. Recovery was assessed at three different concentrations (0.05, 0.5 and 10 μg/mL) by comparing the peak areas of the post-extraction spiked samples with those of the standard solution.
2.5. Experimental Animals and Drug Administration
2.5.1. Animals and Sample Preparation
The study protocol was reviewed and approved by the Institutional Animal Experimentation Committee of National Yang-Ming University, Taipei, Taiwan, and by the Institutional Animal Care and Use Committee (IACUC, approval number 1080314). Adult male Sprague-Dawley rats (300 ± 20 g body weight) were provided by the Laboratory Animal Center at National Yang-Ming University (Taipei, Taiwan). The animals had access to water and food ad libitum (laboratory rodent diet 5P14, PMI Feeds, Richmond, IN, USA) and lived in a pathogen-free environment, with a 12/12 h light–dark cycle. All animal experiments followed the guidelines and procedures for the care of laboratory animals at National Yang-Ming University.
2.5.2. Irradiation Technique
A freely moving rat model was designed for the current study [8]. The rats were anesthetized and immobilized on a board while undergoing computed tomography to localize the whole liver or a central area measuring 1.5 cm × 1.5 cm for the SBRT technique. For the whole liver field, the cranial margin was set 5 mm from the top of the diaphragm, and the caudal margin was set 5 mm lower than the liver margin. The whole liver was targeted for irradiation. The experimental animals were randomized into groups receiving three fractions of sham RT, RT2Gy, or RT9Gy, given concurrently or sequentially with lenvatinib. Data were collected from six rats per group.
2.5.3. Drug Delivery with Different Schedules and Doses of RT
A radiation dose of 2 Gy was considered the daily conventional EBRT dose or the off-target dose around the target that received an ablation RT dose. A dose of 9 Gy was used to simulate SBRT. The animals were divided into five groups, as follows: (A) sham group, lenvatinib (3 mg/kg) only for 3 days (d) with RT0Gy (lenvatinib×3d); (B) whole-liver (2 Gy, 3 fractions) RT2Gy×3f’x concurrent with 3 d of lenvatinib (3 mg/kg); (C) whole-liver RT2Gy×3f’x followed by 3 d of lenvatinib (3 mg/kg); (D) SBRT (9 Gy with 3 fractions) RT9Gy×3f’x concurrent with lenvatinib (3 mg/kg) for 3 d; and (E) RT9Gy×3f’x followed by 3 days of lenvatinib (3 mg/kg) for 3 d (Figure 2). Rats were initially anesthetized with pentobarbital (50 mg/kg, i.p.) and remained anesthetized throughout the experimental period. After surgery, the rats were placed in an experimental cage and allowed to recover for 1 day. Lenvatinib was dissolved in triply deionized water and administered [3 mg/kg, per os (p.o.)] to the rats (n = 6 per group).
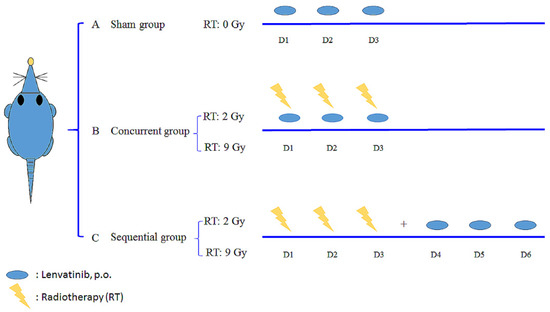
Figure 2.
Oral lenvatinib [3 mg/kg per os (p.o.), quaque die (q.d.)] with radiotherapy (RT) under different time schedules and RT doses. The rats were randomly divided into a sham group, lenvatinib (p.o., q.d. × 3 d) with RT0Gy (lenvatinib×3d); a concurrent group, lenvatinib×3d 1 h after RT2Gy in 3 fractions (RT2Gy×3f’x) and RT9Gy×3f’x; and a sequential group, lenvatinib×3d 24 h after RT2Gy×3f’x and RT9Gy×3f’x. Data are expressed as the mean ± standard error of the mean.
2.5.4. Pretreatment with Ketoconazole for Drug Delivery with RT under Different Time Schedules and Doses
The effects of ketoconazole (KZT), an inhibitor of CYP3A [17] and P-gp [18], were investigated in rats treated with lenvatinib. The rats were pretreated with KZT (10 mg/kg, i.p.) 30 min before RT or lenvatinib. After 30 min, lenvatinib (3 mg/kg) was administered intragastrically. Blood samples were obtained according to a preset schedule.
2.5.5. Sample Preparation
Blood samples were collected in a heparin-rinsed vial via polyethylene tubing (PE-50) implanted into the jugular vein of each rat. Aliquots of 100–120 µL blood were collected at time intervals of 0, 5, 15, 30, 45, 60, 90, 120, 150, 180, 210, and 240 min following drug administration. At each time point, 200 μL of blood was drawn into heparin-rinsed Eppendorf tubes and then centrifuged at 13,000 rpm for 10 min at 4 °C to obtain plasma. Plasma was stored at −20 °C until analysis. Each collected blood sample was transferred to a heparinized microcentrifuge tube and centrifuged at 13,000 rpm for 10 min. The resulting plasma (50 μL) was then mixed with 150 μL of internal standard solution (10 μg/mL). The denatured protein precipitate was separated by vortexing for 20 s and centrifuged at 13,000 rpm for 10 min at 4 °C.
2.5.6. Organ Distribution
Six hours after lenvatinib administration×3d (3 mg/kg, p.o.), blood samples were collected as mentioned above. The brain, liver, heart, spleen, lung, and kidney were collected and weighed. These collected samples were stored at −20 °C until analysis.
2.5.7. Organ Samples
The thawed organ samples were homogenized in 50% aqueous acetonitrile (the ratio of sample weight and volume was 1:5), and the homogenate was centrifuged at 13,000× g for 10 min at 4 °C. The supernatant was collected, placed in brown Eppendorf tubes, and stored at −20 °C until analysis. Briefly, each organ sample (50 μL) was combined with 150 μL of IS solution (diethylstilbestrol) for protein precipitation. Finally, 20 μL of filtrate was subjected to HPLC analysis.
2.5.8. Hepatic and Renal Functions
Glutamic-pyruvic transaminase (GPT) and creatine were measured to examine the influence of different modalities on hepatic function and renal function by a standard colorimetric method using a Synchron L × 20 spectrophotometer (Beckman Coulter, Lakeview Pkwy S Dr, IN, USA) and manufacturer-supplied reagents.
2.6. Pharmacokinetics and Data Analysis
PK parameters, including the area under the concentration–time curve (AUC), clearance (CL), elimination half-life (t1/2), volume of distribution at steady state (Vss), and mean residence time (MRT), were calculated using the PK calculation software WinNonlin Standard Edition, Version 1.1 (Scientific Consulting, Apex, NC, USA) by a compartmental method.
2.7. Calculations and Data Analysis
All statistical calculations were performed with Statistical Product and Service Solutions (SPSS) for Windows, version 20.0 (SPSS, IBM, Armonk, NY, USA). All data are expressed as the mean ±SD. One-way analysis of variance (ANOVA) was used for comparisons between groups, and statistically significant differences were defined as * p < 0.05 or ** p < 0.01.
3. Results
3.1. Method of Validation for Linearity, Recovery, Precision, Accuracy, and Stability
In the current study, the LOD of lenvatinib in plasma was 0.5 μg/mL. The regression equation for lenvatinib in rat plasma was y = 1.6777 × −0.0751 (r2 = 1). The intraday accuracy (% bias) of lenvatinib ranged from 2.39 to 23.3%. The intraday precision (% RSD) ranged from 2.35 to 23.8%. The recovery rate of lenvatinib at 0.05 to 10 µg/mL ranged from 111.8% to 96.15%.
3.2. Both RT2Gy and RT9Gy Modulated the AUC of Lenvatinib in the Plasma of Freely Moving Rats
Compared to lenvatinib×3d, the AUClenvatinib and Cmax decreased by 51.1% (p = 0.03) and 58.3% (p = 0.03), respectively, with RT2Gy×3f’x in the concurrent regimen. However, the AUClenvatinib increased by 21.6% with RT2Gy×3f’x in the sequential regimen (p = 0.44). Additionally, compared to the concurrent regimen, the AUClenvatinib and Cmax increased by 148.8% (p = 0.008) and 154.5% (p = 0.008), respectively, in the sequential RT2Gy×3f’x regimen. Moreover, the AUClenvatinib did not differ between the concurrent RT2Gy×3f’x and RT9Gy×3f’x regimens (p = 0.87). (Figure 3A)
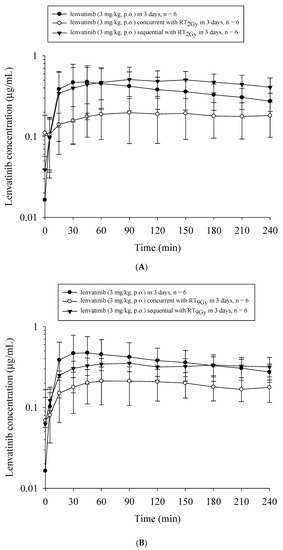
Figure 3.
The concentration–time curves of lenvatinib in the plasma of rats under different treatment schedules with or without radiotherapy (RT). The treated groups in (A) included the sham group, which received lenvatinib (p.o., q.d.×3d) with RT0 Gy (lenvatinib×3d); the concurrent group, which received lenvatinib×3d 1 h after 3 fractions of RT2Gy (RT2Gy×3f’x); and the sequential group, which received lenvatinib×3d 24 h after RT2Gy×3f’x. The treated groups in (B) included the sham group, which received lenvatinib×3d; the concurrent group, which received lenvatinib×3d 1 h after RT9 Gy in 3 fractions (RT9Gy×3f’x); and the sequential group, which received lenvatinib×3d 24 h after RT9Gy×3f’x. Data are expressed as the mean ± standard error of the mean (n = 6 per group).
Similarly, the AUClenvatinib and Cmax decreased by 48.9% (p = 0.03) and 56.1% (p = 0.04), respectively, with the concurrent RT9Gy×3f’x regimen. There was no statistically significant difference in the AUClenvatinib between the lenvatinib x 3d and sequential RT9Gy×3f’x groups. Intriguingly, the AUClenvatinib and Cmax increased by 68.9% (p = 0.009) and 68.7% (p = 0.02), respectively, with the sequential RT9Gy×3f’x group compared to the concurrent regimen. Furthermore, there were no statistically significant differences in the AUClenvatinib between the sequential RT2Gy×3f’x and RT9Gy×3f’x groups (p = 0.12). (Figure 3B)
Compared to RT concurrent or sequential with lenvatinib, the coadministration of ketoconazole with RT and lenvatinib mildly decreased the AUClenvatinib in all groups except for the concurrent RT9 Gy group, which showed a nonsignificant 28% increase (Figure 4 and Table 1).
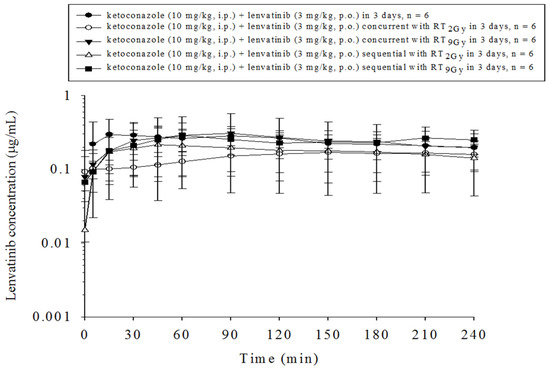
Figure 4.
The concentration–time curves of lenvatinib in the plasma of rats that received lenvatinib coadministered with ketoconazole with or without radiotherapy (RT) under different time courses. Data are expressed as the mean ± standard error of the mean (n = 6 per group).

Table 1.
Pharmacokinetic parameters of lenvatinib in rats after administration for 3 d (3 mg/kg, p.o.) with or without radiotherapy (RT, 2 Gy and 9 Gy) and ketoconazole (KTZ, 10 mg/kg, i.p., 30 min, before RT or lenvatinib).
3.3. Organ Distribution under Different RT and Lenvatinib Regimens
Both the RT2Gy×3f’x and RT9Gy×3f’x concurrent regimens markedly decreased the biodistribution of lenvatinib in the heart, liver, lung, spleen, and kidneys, which ranged from 31% to 100% for RT2Gy×3f’x, and 11% to 100% for RT9Gy×3f’x, compared to the sham regimen. In contrast, the RT2Gy×3f’x and RT9Gy×3f’x sequential regimens increased the biodistribution of lenvatinib in the heart, liver, spleen, and kidneys, which ranged from 31% to 143% for RT2Gy×3f’x and 27% to 100% for RT9Gy×3f’x, compared to the control regimen. However, the concentration of lenvatinib in the lung in the RT2Gy×3f’x and RT9Gy×3f’x sequential regimens was decreased by 15% and 31%, respectively, although the differences were not statistically significant. Intriguingly, lenvatinib was not detected in the brain. Additionally, the concentration of lenvatinib in the spleen increased more than 100% during the sequential regimen (Figure 5 and Table 2).
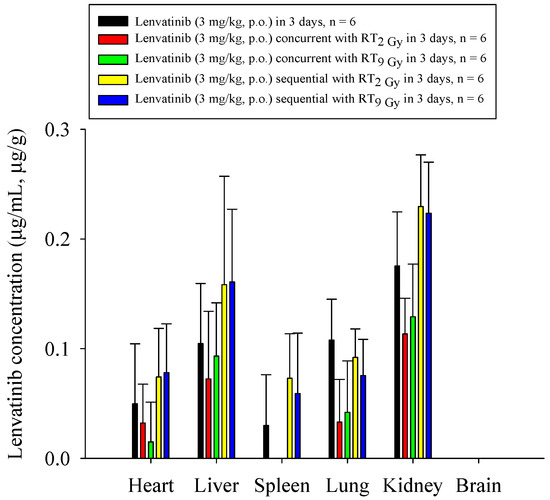
Figure 5.
The concentration of lenvatinib in the heart, liver, spleen, lung, kidney, and brain of rats after the administration of lenvatinib (3 mg/kg, p.o.) with or without radiotherapy. The lenvatinib concentrations in the organs are expressed in units of µg/mL or µg/g (n = 6 per group).

Table 2.
Concentrations of lenvatinib in the heart, liver, spleen, lung, kidney, and brain of rats after administration (3 mg/kg, p.o.) with or without radiotherapy.
4. Discussion
To our knowledge, our study is the first to show the interaction between RT and lenvatinib. The AUC of lenvatinib could be modulated by RT with off-target and SBRT doses. Additionally, RT concurrent or sequential with lenvatinib impacted the PK and biodistribution. Together, these data support the RT–PK phenomenon in our study.
Sorafenib is a multikinase inhibitor that targets the Raf/MAPK/ERK signaling pathway, interfering with the tyrosine kinases VEGFR-2/3, PDGFR-β, B-Raf, c-Raf, FGFR1, Flt3, c-KIT, RET, and p38 α, and induces tumor cell apoptosis in HCC [19,20]. Clinically, sorafenib provides overall survival benefits for patients with unresectable HCC, as was confirmed in the Sorafenib HCC Assessment Randomized Protocol (SHARP) [2] and Asia-Pacific [3] trials. Moreover, preclinical data from studies combining sorafenib and RT have suggested improved efficacy in HCC cell lines both in vitro and in vivo [21,22]. Additionally, the efficacy of RT with sorafenib in patients with unresectable HCC has been reported [7,8]. Moreover, the AUC of sorafenib can be modulated by RT [8].
Lenvatinib is an anticancer drug for the treatment of thyroid cancer [23], renal cancer [24], and HCC [4]. Lenvatinib concurrently targets: VEGFR1, 2, and 3; FGFR1, 2, 3 and 4; PDGFR-α; the RET proto-oncogene; and c-kit [14,15]. Due to these properties, lenvatinib is a candidate tumor inhibitor, acting in the same manner as sorafenib. Moreover, the REFLECT trial showed a noninferior median survival time for HCC patients treated with lenvatinib compared to sorafenib [4]. Therefore, the National Comprehensive Cancer Network Guidelines® list lenvatinib alongside sorafenib as a first-line treatment for patients with HCC.
Angiogenesis is initiated under inflammatory or hypoxic conditions [25]. VEGF is a cytokine that is associated with the formation of new blood vessels for tumors, increased tumor proliferation, and the growth of tumor cells [26]. Irradiation induces hypoxia and VEGF upregulation [27], as well as VEGFR2 receptors in the tumor endothelium [28], PDGF in endothelial cells [29], and PDGFR in fibroblasts [30]. Additionally, NF-κB responds to irradiation [31] and increases the expression of VEGF [12]. Agents that inhibit VEGF signaling can: “normalize” tumor blood vessels; create a radiosensitive microenvironment [27,32,33]; enhance radiation damage to endothelial cells by promoting vessel regression and tumor cell death; suppress waves of reoxygenation, and hence reduce radiotherapy resistance [34]; promote ceramide-mediated apoptosis, to enhance the effect of radiotherapy [35]; and reduce the acute mobilization of circulating endothelial cells and endothelial progenitor cells [27,32,33]. These data suggest that RT in combination with both VEGF and PDGF signaling inhibitors greatly enhances antiangiogenic and antitumor effects.
Moreover, RT induces inflammation and results in the recruitment of neutrophils, macrophages, plasma cells, and granulocytes to the target area [36,37,38]. Polymorphonuclear neutrophils (PMNs) promote the secretion of matrix metalloproteinase (MMP)−8, a member of the MMP family, when they are localized in inflammatory areas [39]. Collagenases (MMP−1, 8, and 13) are proteins associated with angiogenesis [40]. Additionally, MMP−8 secreted by irradiated nonparenchymal cells enhances the migration and invasion of HCC [41]. More importantly, MMP−8 has been demonstrated to play a major role in local RT-induced modulation of systemic PK of 5-fluorouracil (5-FU) [42]. It is reasonable to suspect that the inflammatory process induced by RT and the autocrine and paracrine functions of the VEGF and MMP families may jointly contribute to the RT-PK phenomenon.
Sorafenib [22] and regorafenib [43] selectively inhibit the radiation-induced activation of VEGFR and enhance the effectiveness of irradiation. Intriguingly, lenvatinib targets VEGFR and PDGFR, and significantly inhibits thyroid cancer growth when combined with RT [44]. Moreover, RT followed by sorafenib was associated with the greatest tumor growth delay, as reported by Plastaras et al. [45]. Additionally, the AUC of sorafenib can be upregulated by RT [8]. Interestingly, it has recently been reported that the AUC of regorafenib can be modulated by RT [46]. In other words, lenvatinib is a potential candidate for radiosensitization, and the PK of lenvatinib may be modulated by RT.
Both off-target and treatment doses have similar interactions with chemotherapy drugs, such as 5-FU [47,48] and cisplatin [49,50], and multikinase inhibitors, such as sorafenib [8]. Similarly, the current study also confirmed that off-target and SBRT doses were comparable in their ability to modulate the PK of lenvatinib. The AUClenvatinib decreased by approximately 50% with concurrent RT2Gy×3f’x and RT9Gy×3f’x. However, the AUClenvatinib increased by 149% with RT2Gy×3f’x, and by 69% with RT9Gy×3f’x in the sequential regimen compared to the concurrent regimen.
Similarly, the Cmax of lenvatinib decreased by 58% with RT2Gy×3f’x, and by 56% with RT9Gy×3f’x in the concurrent regimen. A decreased Cmax during the concurrent regimen indicates that both off-target and SBRT doses in local liver RT reduce the effect on the absorption of lenvatinib. In other words, the sequential regimen has a greater ability than the concurrent regimen to maintain the absorption of lenvatinib. The REFLECT trial noted improvements in progression-free survival, time to progression, and objective response with lenvatinib compared to sorafenib [4]. Accordingly, the concentration of lenvatinib in the liver was increased by 50% with the sequential RT regimen in the current study. RT was found to be at its most effective when administered during the “normalization window” of angiogenesis inhibitors [51], suggesting that the scheduling of the two modalities impacts the maximum clinical benefit. These results suggest that the combination of RT and lenvatinib affects the PK of lenvatinib in the plasma, and that sequential regimens may be more impactful than concurrent regimens.
Lenvatinib is a substrate for P-glycoprotein, and the oxidative metabolism of lenvatinib is mediated primarily by CYP3A4. However, recent results have shown no clinically important alterations in lenvatinib exposure following the coadministration of lenvatinib with rifampin [16] or ketoconazole [52]. Similarly, our study also noted minimal changes in systemic lenvatinib exposure following the coadministration of lenvatinib and RT with ketoconazole. In other words, oxidative metabolism does not appear to be a major pathway involved in the action of lenvatinib or its coadministration with RT. It is possible that multiple pathways are involved in lenvatinib metabolism, and the coadministration of lenvatinib with CYP3A4 inhibitors during RT is not likely to result in clinically important alterations in lenvatinib exposure.
Patients with HCC treated by SBRT have achieved encouraging outcomes [5,6]. SBRT delivers highly conformal dose distributions, allowing dose escalation, but this is at the expense of a larger volume around the target receiving low to moderate doses compared to three-dimensional RT [53]. Blettner et al. [54] reported a significant nonlinear dose–response relationship compatible with a cell-killing effect at high doses. Coppes et al. [55] reported that the out-of-field effect of radiation was similar to the in-field effect. Moreover, the AUCs of chemotherapy drugs and multikinase inhibitors can be modulated by low-dose RT in free-moving rat models [8,42,50]. Interestingly, there were no differences in the AUClenvatinib between the concurrent RT2Gy×3f’x and RT9Gy×3f’x groups. Similarly, there were no differences in the AUClenvatinib between the sequential RT2Gy×3f’x and RT9Gy×3f’x groups. These observations suggest the parallel impact of off-target and treatment irradiation doses, especially in the same regimen. For this reason, low-dose “bath” effects should be considered in advanced radiotherapy techniques concurrent or sequential with lenvatinib.
Organ distributions decreased in the concurrent regimen and increased in the sequential regimen. In the sequential regimen, RT increased the concentration of lenvatinib by 50% in the heart and 30% in the kidneys. However, in the Phase III Study of (E7080) Lenvatinib in Differentiated Cancer of the Thyroid (SELECT), patients treated with lenvatinib experienced cardiac dysfunction (7%), nausea and vomiting (20–40%), stomatitis (20–30%), diarrhea (60%), fatigue (more than 50%), palmar-plantar erythrodysesthesia syndrome (70%), hemorrhagic events (35%), thrombocytopenia (8.8%), fistula formation (1.5%), reversible posterior leukoencephalopathy syndrome (0.4%), and thromboembolic events (5.4%), and one-third of patients experienced proteinuria of any grade [23,56]. Additionally, increased exposure to lenvatinib is correlated with an increased incidence and severity of treatment-emergent hypertension [57], which is a demonstrated class effect of agents targeting the VEGF/VEGFR signaling pathway [58]. Avoiding potential side effects through close follow-up is as important as pursuing better treatment effects with combined modalities. Intriguingly, lenvatinib was not detected in the brain. Lenvatinib is a substrate for P-glycoprotein [16], an efflux pump on the endothelial cells that comprise the blood–brain barrier (BBB), suggesting that lenvatinib may be unable to penetrate the BBB. In the development of new radiation-modulated strategies and the design of clinical trials, these unexpected biological enhancements of lenvatinib in the RT-PK phenomenon should be addressed cautiously, to avoid severe toxicity when RT and lenvatinib are used as synergistic tools in cancer treatment strategies.
There were some limitations to our study. First, the current study did not use orthotopic or heterotopic models, but used a freely moving Sprague-Dawley rat model to confirm the interaction between RT and lenvatinib. However, the differences in the PK profile of lenvatinib between healthy subjects and patients are small [59]. Therefore, it appears reasonable to use a nontumor model to evaluate the interaction between RT and lenvatinib.
Second, the current study did not include a disease model treated with lenvatinib and RT; therefore, we cannot report the treatment effects of the combination of RT and lenvatinib. However, the current analysis sheds light on the discrepancies of PK in the concurrent and sequential regimens of RT with lenvatinib, which may be useful for prospective clinical trial designs. Brade et al. [9] noted that concurrent SBRT and sorafenib may cause unpredictable toxicity. Our previous study also confirmed that the AUC of sorafenib was increased in concurrent regimens with SBRT and EBRT [8]. These data provide a preclinical proof of concept for the effects of RT impact multikinase inhibitor activity and the RT-PK phenomenon to support clinical practice, as well as the design and conduct of early-phase radiotherapy trials with targeted therapeutics.
Third, the possible mechanism was not examined in the current study, because the presence or absence of the RT-PK phenomenon in the context of lenvatinib plus RT could not be ensured before the study. However, we confirmed that the systemic PK of lenvatinib could be modulated by conventional EBRT and SBRT, as well as concurrent RT and sequential RT. The design of these combination regimens provides different scenarios in clinical practice. We realized that an in vitro study could not replicate or validate an in vivo study because the impacts of the local microenvironment and systemic modulators would be absent. Notably, the RT regimen affects P-gp activity, and the sequential RT regimen increases CYP3A4 activity to modulate the PK of sorafenib [8]. Therefore, there is a clear need for further in vitro studies to explore the mechanism of the RT-PK phenomenon of lenvatinib in the future; for example, by comparing various HCC tumor cell lines with nontumorigenic hepatic cells to assess cell viability, colony formation, cell cycle situations, DNA double-strand break, DNA repair, CYP 3A4 activity, and P-gp activity.
5. Conclusions
Taken together, these data support the concept of the interplay between RT and lenvatinib by suggesting that the systemic PK of lenvatinib can be modulated by irradiation. The timing of lenvatinib and RT doses affected the AUC and biodistribution of the drug during treatment. The AUClenvatinib was decreased by 50% in multiple fractions of RT concurrent with lenvatinib. Additionally, off-target and SBRT doses had a similar ability to modulate systemic therapy. Furthermore, CYP3A4-related oxidative metabolism does not appear to be a major pathway involved in the action of lenvatinib alone or coadministration with RT. There is a pressing need to incorporate our current understanding of the systemic effects of localized irradiation into future treatment strategies, and the current findings can serve as a starting point for the scientific community to explore the effects of combined lenvatinib and RT as synergistic tools in HCC treatment strategies.
Author Contributions
C.-H.H., Y.-J.C., T.-H.T. and L.-Y.W.: conceptualization, methodology. C.-H.H.: data curation, writing—original draft preparation. Y.-J.C., T.-H.T.: visualization, investigation. Y.-J.C., T.-H.T.: supervision. L.-Y.W.: writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Far Eastern Memorial Hospital (FEMH 107-2314-B-418-007; FEMH 108-2314-B-418-003-MY2), the Ministry of Science and Technology, Taiwan (MOST 107-2314-B-418-007; MOST 108-2314-B-418-003-MY2) and the NYMU-FEMH Joint Research Program (107DN20, 108DN31, 109D32).
Institutional Review Board Statement
All procedures were performed in studies approved by the Institutional Animal Experimentation Committee of National Yang Ming Chiao Tung University, Taipei, Taiwan, and by the Institutional Animal Care and Use Committee on 14 March 2019 (IACUC, approval number 1080314).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed are available from the corresponding author on reasonable request.
Acknowledgments
We thank Ya-Yu Yang for performing the laboratory work.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Munoz-Schuffenegger, P.; Barry, A.; Atenafu, E.G.; Kim, J.; Brierley, J.; Ringash, J.; Brade, A.; Dinniwell, R.; Wong, R.K.S.; Cho, C.; et al. Stereotactic body radiation therapy for hepatocellular carcinoma with Macrovascular invasion. Radiother. Oncol. 2020, 156, 120–126. [Google Scholar] [CrossRef]
- Wahl, D.R.; Stenmark, M.H.; Tao, Y.; Pollom, E.L.; Caoili, E.M.; Lawrence, T.S.; Schipper, M.J.; Feng, M. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J. Clin. Oncol. 2016, 34, 452–459. [Google Scholar] [CrossRef]
- Chen, S.W.; Lin, L.C.; Kuo, Y.C.; Liang, J.A.; Kuo, C.C.; Chiou, J.F. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1041–1047. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Chen, Y.J.; Tsai, T.H.; Wang, L.Y.; Tai, H.C.; Huang, H.L.; Huang, Y.C. Robust combination of liver stereotactic body radiotherapy modulates pharmacokinetics of sorafenib toward preferable parameters. Sci. Rep. 2020, 10, 9575. [Google Scholar] [CrossRef]
- Brade, A.M.; Ng, S.; Brierley, J.; Kim, J.; Dinniwell, R.; Ringash, J.; Wong, R.R.; Cho, C.; Knox, J.; Dawson, L.A. Phase 1 Trial of Sorafenib and Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 580–587. [Google Scholar] [CrossRef]
- Goody, R.B.; Brade, A.M.; Wang, L.; Craig, T.; Brierley, J.; Dinniwell, R.; Wong, R.K.S.; Cho, C.; Kim, J.; Kassam, Z.; et al. Phase I trial of radiation therapy and sorafenib in unresectable liver metastases. Radiother. Oncol. 2017, 123, 234–239. [Google Scholar] [CrossRef]
- Kuo, M.T.; Liu, Z.; Wei, Y.; Lin-Lee, Y.C.; Tatebe, S.; Mills, G.B.; Unate, H. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene 2002, 21, 1945–1954. [Google Scholar] [CrossRef]
- Eyries, M.; Collins, T.; Khachigian, L.M. Modulation of growth factor gene expression in vascular cells by oxidative stress. Endothel. J. Endothel. Cell Res. 2004, 11, 133–139. [Google Scholar] [CrossRef]
- Sukhatme, V.P.; Cao, X.M.; Chang, L.C.; Tsai-Morris, C.H.; Stamenkovich, D.; Ferreira, P.C.; Cohen, D.R.; Edwards, S.A.; Shows, T.B.; Curran, T.; et al. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell 1988, 53, 37–43. [Google Scholar] [CrossRef]
- Matsui, J.; Funahashi, Y.; Uenaka, T.; Watanabe, T.; Tsuruoka, A.; Asada, M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008, 14, 5459–5465. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Matsui, J.; Matsushima, T.; Obaishi, H.; Miyazaki, K.; Nakamura, K.; Tohyama, O.; Semba, T.; Yamaguchi, A.; Hoshi, S.S.; et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc. Cell 2014, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, R.C.; Aluri, J.; Fan, J.; Martinez, G.; Thompson, G.A.; Ren, M. Effect of rifampicin on the pharmacokinetics of lenvatinib in healthy adults. Clin. Investig. 2014, 34, 651–659. [Google Scholar] [CrossRef][Green Version]
- Achira, M.; Suzuki, H.; Ito, K.; Sugiyama, Y. Comparative studies to determine the selective inhibitors for P-glycoprotein and cytochrome P4503A4. AAPS PharmSci 1999, 1, E18. [Google Scholar] [CrossRef][Green Version]
- Thummel, K.E.; Wilkinson, G.R. In vitro and in vivo drug interactions involving human CYP3A. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 389–430. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Carter, C.; Lynch, M.; Lowinger, T.; Dumas, J.; Smith, R.A.; Schwartz, B.; Simantov, R.; Kelley, S. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 835–844. [Google Scholar] [CrossRef]
- Wild, A.T.; Gandhi, N.; Chettiar, S.T.; Aziz, K.; Gajula, R.P.; Williams, R.D.; Kumar, R.; Taparra, K.; Zeng, J.; Cades, J.A.; et al. Concurrent versus sequential sorafenib therapy in combination with radiation for hepatocellular carcinoma. PLoS ONE 2013, 8, e65726. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gu, K.; Yu, Z.; Yuan, D.; He, M.; Ma, N.; Lai, S.; Zhao, J.; Ren, Z.; Zhang, X.; et al. Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo. Cancer Lett 2013, 329, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Glen, H.; Michaelson, M.D.; Molina, A.; Eisen, T.; Jassem, J.; Zolnierek, J.; Maroto, J.P.; Mellado, B.; et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015, 16, 1473–1482. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G. Matrix metalloproteinases in angiogenesis: A moving target for therapeutic intervention. J. Clin. Investig. 1999, 103, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Dvorak, H.F.; Mukhopadhyay, D. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) peceptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J. Biol. Chem. 2001, 276, 26969–26979. [Google Scholar] [CrossRef]
- Wachsberger, P.; Burd, R.; Dicker, A.P. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: Exploring mechanisms of interaction. Clin. Cancer Res. 2003, 9, 1957–1971. [Google Scholar]
- Abdollahi, A.; Lipson, K.E.; Han, X.; Krempien, R.; Trinh, T.; Weber, K.J.; Hahnfeldt, P.; Hlatky, L.; Debus, J.; Howlett, A.R.; et al. SU5416 and SU6668 attenuate the angiogenic effects of radiation-induced tumor cell growth factor production and amplify the direct anti-endothelial action of radiation in vitro. Cancer Res. 2003, 63, 3755–3763. [Google Scholar]
- Timke, C.; Zieher, H.; Roth, A.; Hauser, K.; Lipson, K.E.; Weber, K.J.; Debus, J.; Abdollahi, A.; Huber, P.E. Combination of vascular endothelial growth factor receptor/platelet-derived growth factor receptor inhibition markedly improves radiation tumor therapy. Clin. Cancer Res. 2008, 14, 2210–2219. [Google Scholar] [CrossRef]
- Li, M.; Ping, G.; Plathow, C.; Trinh, T.; Lipson, K.E.; Hauser, K.; Krempien, R.; Debus, J.; Abdollahi, A.; Huber, P.E. Small molecule receptor tyrosine kinase inhibitor of platelet-derived growth factor signaling (SU9518) modifies radiation response in fibroblasts and endothelial cells. BMC Cancer 2006, 6, 79. [Google Scholar] [CrossRef]
- Li, N.; Karin, M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc. Natl. Acad. Sci. USA 1998, 95, 13012–13017. [Google Scholar] [CrossRef]
- Jain, R.K.; Duda, D.G.; Clark, J.W.; Loeffler, J.S. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat. Clin. Pract. Oncol. 2006, 3, 24–40. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.S. Radiation combined with antiangiogenic and antivascular agents. Semin. Radiat. Oncol. 2006, 16, 45–50. [Google Scholar] [CrossRef]
- Harada, H.; Inoue, M.; Itasaka, S.; Hirota, K.; Morinibu, A.; Shinomiya, K.; Zeng, L.; Ou, G.; Zhu, Y.; Yoshimura, M.; et al. Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate towards tumour blood vessels. Nat. Commun. 2012, 3, 783. [Google Scholar] [CrossRef]
- Kolesnick, R.; Fuks, Z. Radiation and ceramide-induced apoptosis. Oncogene 2003, 22, 5897–5906. [Google Scholar] [CrossRef]
- Garnett, C.T.; Palena, C.; Chakraborty, M.; Tsang, K.Y.; Schlom, J.; Hodge, J.W. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004, 64, 7985–7994. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, P.; Libert, C. Matrix metalloproteinase-8: Cleavage can be decisive. Cytokine Growth Factor Rev. 2006, 17, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Dejonckheere, E.; Van Lint, P.; Demeestere, D.; Van Wonterghem, E.; Vanlaere, I.; Puimege, L.; Van Hauwermeiren, F.; De Rycke, R.; Mc Guire, C.; et al. Matrix metalloprotease 8-dependent extracellular matrix cleavage at the blood-CSF barrier contributes to lethality during systemic inflammatory diseases. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 9805–9816. [Google Scholar] [CrossRef]
- Weiss, S.J.; Peppin, G.; Ortiz, X.; Ragsdale, C.; Test, S.T. Oxidative autoactivation of latent collagenase by human neutrophils. Science 1985, 227, 747–749. [Google Scholar] [CrossRef]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yin, Y.; Wang, X.; Wu, Z.; Liu, Y.; Zhang, F.; Lin, J.; Huang, Z.; Zhou, L. Sublethal irradiation promotes the metastatic potential of hepatocellular carcinoma cells. Cancer Sci. 2021, 112, 265–274. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Liu, C.Y.; Hsieh, Y.J.; Tai, H.C.; Wang, L.Y.; Tsai, T.H.; Chen, Y.J. Matrix metalloproteinase-8 mediates the unfavorable systemic impact of local irradiation on pharmacokinetics of anti-cancer drug 5-Fluorouracil. PLoS ONE 2011, 6, e21000. [Google Scholar] [CrossRef][Green Version]
- Mehta, M.; Griffith, J.; Panneerselvam, J.; Babu, A.; Mani, J.; Herman, T.; Ramesh, R.; Munshi, A. Regorafenib sensitizes human breast cancer cells to radiation by inhibiting multiple kinases and inducing DNA damage. Int. J. Radiat. Biol. 2020, 1–12. [Google Scholar] [CrossRef]
- Suzuki, K.; Iwai, H.; Utsunomiya, K.; Kono, Y.; Kobayashi, Y.; Van Bui, D.; Sawada, S.; Yun, Y.; Mitani, A.; Kondo, N.; et al. Combination therapy with lenvatinib and radiation significantly inhibits thyroid cancer growth by uptake of tyrosine kinase inhibitor. Exp. Cell Res. 2021, 398, 112390. [Google Scholar] [CrossRef]
- Plastaras, J.P.; Kim, S.H.; Liu, Y.Y.; Dicker, D.T.; Dorsey, J.F.; McDonough, J.; Cerniglia, G.; Rajendran, R.R.; Gupta, A.; Rustgi, A.K.; et al. Cell cycle dependent and schedule-dependent antitumor effects of sorafenib combined with radiation. Cancer Res. 2007, 67, 9443–9454. [Google Scholar] [CrossRef]
- Tsai, T.H.; Chen, Y.J.; Wang, L.Y.; Hsieh, C.H. Effect of Synchronous Versus Sequential Regimens on the Pharmacokinetics and Biodistribution of Regorafenib with Irradiation. Pharmaceutics 2021, 13, 386. [Google Scholar] [CrossRef]
- Liu, J.H.; Tsai, T.H.; Chen, Y.J.; Wang, L.Y.; Liu, H.Y.; Hsieh, C.H. Local Irradiation Modulates the Pharmacokinetics of Metabolites in 5-Fluorouracil-Radiotherapy-Pharmacokinetics Phenomenon. Front. Pharmacol. 2020, 11, 141. [Google Scholar] [CrossRef]
- Chen, Y.J.; Tsai, T.H.; Wang, L.Y.; Hsieh, C.H. Local Radiotherapy Affects Drug Pharmacokinetics-Exploration of a Neglected but Significant Uncertainty of Cancer Therapy. Technol. Cancer Res. Treat. 2017, 16, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Hou, M.L.; Chiang, M.H.; Tai, H.C.; Tien, H.J.; Wang, L.Y.; Tsai, T.H.; Chen, Y.J. Head and neck irradiation modulates pharmacokinetics of 5-fluorouracil and cisplatin. J. Transl. Med. 2013, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Chen, Y.J.; Hou, M.L.; Wang, L.Y.; Tai, H.C.; Hsieh, C.H. Pelvic irradiation modulates the pharmacokinetics of cisplatin in the plasma and lymphatic system. Am. J. Transl. Res. 2015, 7, 375–384. [Google Scholar]
- Zhang, X.; Li, Y.; Huang, Q.; Wang, H.; Yan, B.; Dewhirst, M.W.; Li, C.Y. Increased resistance of tumor cells to hyperthermia mediated by integrin-linked kinase. Clin. Cancer Res. 2003, 9, 1155–1160. [Google Scholar]
- Shumaker, R.; Aluri, J.; Fan, J.; Martinez, G.; Thompson, G.A.; Ren, M. Effects of Ketoconazole on the Pharmacokinetics of Lenvatinib (E7080) in Healthy Participants. Clin. Pharmacol. Drug Dev. 2015, 4, 155–160. [Google Scholar] [CrossRef]
- Purdy, J.A. Dose to normal tissues outside the radiation therapy patient’s treated volume: A review of different radiation therapy techniques. Health Phys. 2008, 95, 666–676. [Google Scholar] [CrossRef]
- Blettner, M.; Boice, J.D., Jr. Radiation dose and leukaemia risk: General relative risk techniques for dose-response models in a matched case-control study. Stat. Med. 1991, 10, 1511–1526. [Google Scholar] [CrossRef] [PubMed]
- Coppes, R.P.; Muijs, C.T.; Faber, H.; Gross, S.; Schippers, J.M.; Brandenburg, S.; Langendijk, J.A.; van Luijk, P. Volume-dependent expression of in-field and out-of-field effects in the proton-irradiated rat lung. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Takahashi, S. Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. Semin. Oncol. 2019, 46, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Tahara, M.; Robinson, B.; Francis, S.; Brose, M.S.; Habra, M.A.; Newbold, K.; Kiyota, N.; Dutcus, C.E.; Mathias, E.; et al. Treatment-emergent hypertension and efficacy in the phase 3 Study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer 2018, 124, 2365–2372. [Google Scholar] [CrossRef]
- Launay-Vacher, V.; Deray, G. Hypertension and proteinuria: A class-effect of antiangiogenic therapies. Anticancer Drugs 2009, 20, 81–82. [Google Scholar] [CrossRef]
- Gupta, A.; Jarzab, B.; Capdevila, J.; Shumaker, R.; Hussein, Z. Population pharmacokinetic analysis of lenvatinib in healthy subjects and patients with cancer. Br. J. Clin. Pharmacol. 2016, 81, 1124–1133. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).