Intraarterial Therapies for the Management of Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
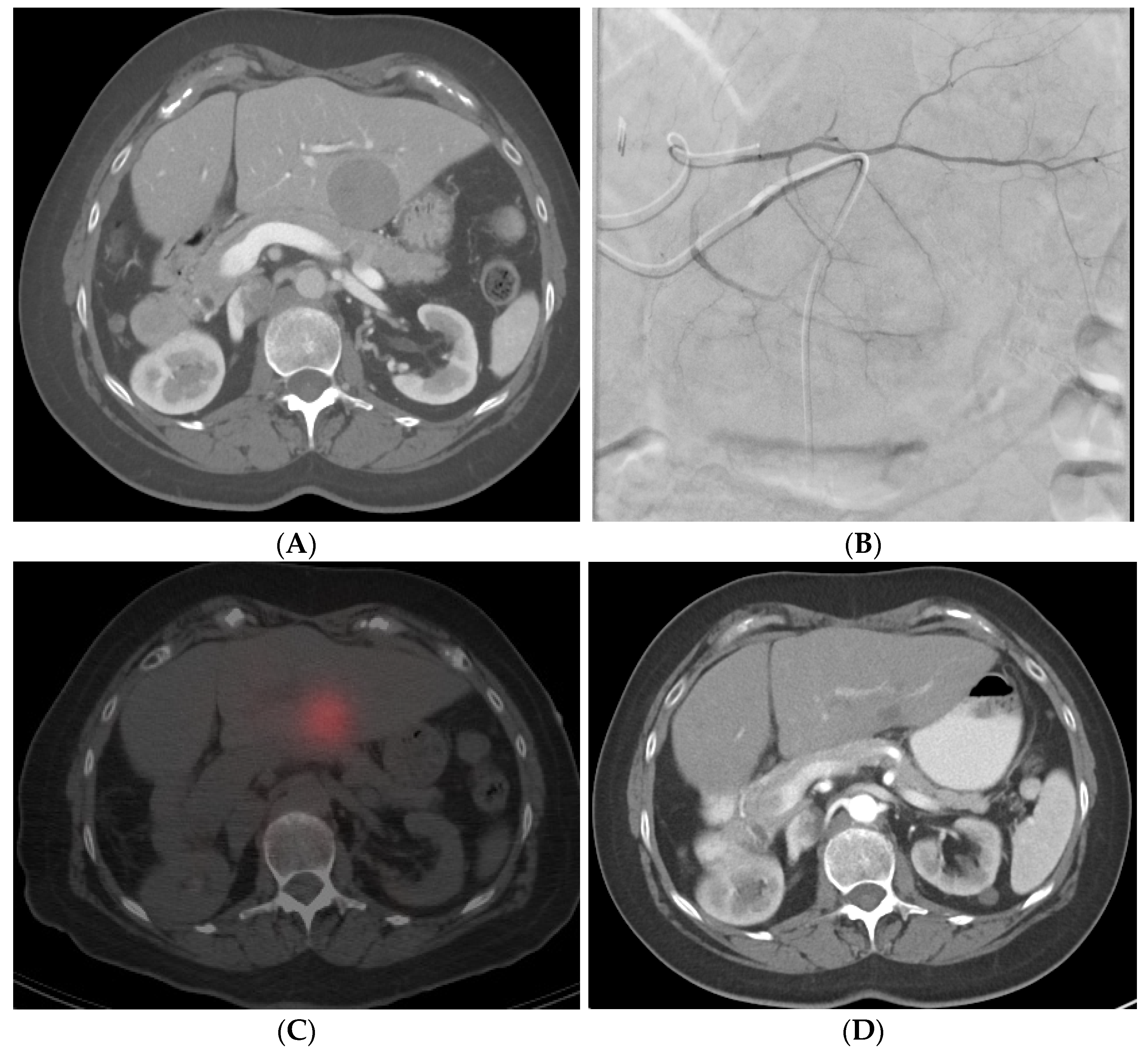
2. Methodology for Review
3. Treatment Options for Hepatocellular Carcinoma
3.1. Surgical and Systemic Therapies
3.2. Locoregional Therapies
4. Indications and Patient Selection for Treatment with Transarterial Therapies
5. BCLC Staging and Predictors of Response
5.1. Eastern Cooperative Oncology Group (ECOG)
5.2. Child–Pugh Classification
5.3. Albumin–Bilirubin (ALBI) Score
5.4. Platelet–ALBI (pALBI) Score
5.5. The Neutrophil-to-Lymphocyte Ratio (NLR)
5.6. MELD Score
5.7. Assessment for Retreatment with Transarterial Chemoembolization (ART) Score
5.8. ASARA Scoring System
5.9. Hepatoma Arterial-Embolization Prognostic (HAP) Score
5.10. Selection for Transarterial Chemoembolization Treatment (STATE) Score
5.11. Aspartate-Aminotransferase-to-Platelet Index (APRI) Score
5.12. Tumor Size, Tumor Number, Baseline AFP, Child–Pugh Class, and Objective Radiological Response (SNACOR) Score
5.13. Okuda Score
5.14. Cancer of the Liver Italian Program (CLIP) Score
6. Patient Preparation
6.1. Pretreatment Imaging
6.2. Pre-Procedural Labs and Tumor Markers
- Aminotransferase,
- Cholinesterase,
- Alkaline phosphatase,
- Gamma-glutamyl transferase,
- Bilirubin,
- Albumin,
- Prothrombin time,
- Creatinine,
- Electrolytes.
6.3. Pre-Procedural Preparation
- Biliary tree canulation/dilation/sphincterotomy;
- Presence of biliary prosthesis (e.g., plastic and metallic stents);
- Presence of a bilioenteric anastomosis;
- Presence of TIPS [87];
- Biliary or gallbladder stone.
7. Techniques: Angiography and Embolization Technique
7.1. Initial Angiography
7.2. Embolization Techniques
7.2.1. Bland Embolization (TAE)
7.2.2. Transarterial Chemoembolization (TACE)
7.2.3. Selective Internal Radiation Therapy (SIRT)
7.2.4. Hepatic Artery Infusion (HAI)
8. Mechanism of Action of Transarterial Therapies
8.1. Bland Embolization (TAE)
8.2. Transarterial Chemoembolization (TACE)
8.3. Drug-Eluting Beads–Transarterial Chemoembolization (DEB–TACE)
8.4. Selective Internal Radiation Therapy (SIRT)
8.5. Hepatic Artery Infusion (HAI)
9. Contraindications to Transarterial Embolization
9.1. Bland Embolization (TAE) and Transarterial Chemoembolization (TACE)
9.2. Selective Internal Radiation Therapy (SIRT)
10. Post-Procedural Follow-Up
11. Complications
11.1. Bland Embolization (TAE) and Transarterial Chemoembolization (TACE)
11.2. Selective Internal Radiation Therapy (SIRT)
12. Outcomes
12.1. Imaging Response Criteria
12.2. Overall Survival, Progression-Free Survival, and Hepatic Progression-Free Survival
13. New Developments and Future Directions
13.1. Newer Drug-Eluting Beads
13.2. Radiopaque Beads
13.3. Immunoembolization
13.4. Nanoparticles
13.5. Combination with Systemic Therapies
13.6. Combination with Percutaneous Therapies
13.7. Pre-Operative TACE
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gyves, J.W.; Ziessman, H.A.; Ensminger, W.D.; Thrall, J.H.; Niederhuber, J.E.; Keyes, J.W.; Walker, S. Definition of Hepatic Tumor Microcirculation by Single Photon Emission Computerized Tomography (SPECT). J. Nucl. Med. 1984, 25, 972–977. [Google Scholar] [PubMed]
- Bierman, H.R.; Byron, R.L.; Kelley, K.H.; Grady, A. Studies on the Blood Supply of Tumors in Man. III. Vascular Patterns of the Liver by Hepatic Arteriography in Vivo. J. Natl. Cancer Inst. 1951, 12, 107–131. [Google Scholar] [PubMed]
- Liapi, E.; Geschwind, J.-F.H. Intra-Arterial Therapies for Hepatocellular Carcinoma: Where Do We Stand? Ann. Surg. Oncol. 2010, 17, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef]
- OPTN/UNOS Policy Notice Modification to Hepatocellular Carcinoma (HCC) Extension Criteria. Available online: https://optn.transplant.hrsa.gov/media/2411/modification-to-hcc-auto-approval-criteria_policy-notice.pdf. (accessed on 28 April 2022).
- Fujiki, M.; Aucejo, F.; Kim, R. General Overview of Neo-Adjuvant Therapy for Hepatocellular Carcinoma before Liver Transplantation: Necessity or Option?: Neo-Adjuvant Therapy for Hepatocellular Carcinoma. Liver Int. 2011, 31, 1081–1089. [Google Scholar] [CrossRef]
- Pompili, M.; Mirante, V.G.; Rondinara, G.; Fassati, L.R.; Piscaglia, F.; Agnes, S.; Covino, M.; Ravaioli, M.; Fagiuoli, S.; Gasbarrini, G.; et al. Percutaneous Ablation Procedures in Cirrhotic Patients with Hepatocellular Carcinoma Submitted to Liver Transplantation: Assessment of Efficacy at Explant Analysis and of Safety for Tumor Recurrence. Liver Transplant. 2005, 11, 1117–1126. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Battiston, C.; Perrone, S.; Pulvirenti, A.; Regalia, E.; Romito, R.; Sarli, D.; Schiavo, M.; Garbagnati, F.; Marchianò, A.; et al. Radiofrequency Ablation of Small Hepatocellular Carcinoma in Cirrhotic Patients Awaiting Liver Transplantation: A Prospective Study. Ann. Surg. 2004, 240, 900–909. [Google Scholar] [CrossRef]
- Yao, F.Y.; Bass, N.M.; Nikolai, B.; Merriman, R.; Davern, T.J.; Kerlan, R.; Ascher, N.L.; Roberts, J.P. A Follow-up Analysis of the Pattern and Predictors of Dropout from the Waiting List for Liver Transplantation in Patients with Hepatocellular Carcinoma: Implications for the Current Organ Allocation Policy. Liver Transplant. 2003, 9, 684–692. [Google Scholar] [CrossRef]
- Tsochatzis, E.; Garcovich, M.; Marelli, L.; Papastergiou, V.; Fatourou, E.; Rodriguez-Peralvarez, M.L.; Germani, G.; Davies, N.; Yu, D.; Luong, T.V.; et al. Transarterial Embolization as Neo-Adjuvant Therapy Pretransplantation in Patients with Hepatocellular Carcinoma. Liver Int. 2013, 33, 944–949. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bruix, J. Systematic Review of Randomized Trials for Unresectable Hepatocellular Carcinoma: Chemoembolization Improves Survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Richard, H.M.; Silberzweig, J.E.; Mitty, H.A.; Lou, W.Y.; Ahn, J.; Cooper, J.M. Hepatic Arterial Complications in Liver Transplant Recipients Treated with Pretransplantation Chemoembolization for Hepatocellular Carcinoma. Radiology 2000, 214, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Graziadei, I.W.; Sandmueller, H.; Waldenberger, P.; Koenigsrainer, A.; Nachbaur, K.; Jaschke, W.; Margreiter, R.; Vogel, W. Chemoembolization Followed by Liver Transplantation for Hepatocellular Carcinoma Impedes Tumor Progression While on the Waiting List and Leads to Excellent Outcome. Liver Transplant. 2003, 9, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, P.H.; Ludkowski, M.; Forman, L.M.; Osgood, M.; Johnson, S.; Kugelmas, M.; Trotter, J.F.; Bak, T.; Wachs, M.; Kam, I.; et al. Hepatic Artery Chemoembolization for Hepatocellular Carcinoma in Patients Listed for Liver Transplantation. Am. J. Transplant. 2004, 4, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, D.; Svegliati-Baroni, G.; Candelari, R.; Mincarelli, C.; Mandolesi, A.; Bearzi, I.; Mocchegiani, F.; Vecchi, A.; Montalti, R.; Benedetti, A.; et al. Doxorubicin-Eluting Bead vs Conventional Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma before Liver Transplantation. World J. Gastroenterol 2013, 19, 5622–5632. [Google Scholar] [CrossRef]
- Kulik, L.M.; Atassi, B.; van Holsbeeck, L.; Souman, T.; Lewandowski, R.J.; Mulcahy, M.F.; Hunter, R.D.; Nemcek, A.A.; Abecassis, M.M.; Haines, K.G.; et al. Yttrium-90 Microspheres (TheraSphere) Treatment of Unresectable Hepatocellular Carcinoma: Downstaging to Resection, RFA and Bridge to Transplantation. J. Surg. Oncol. 2006, 94, 572–586. [Google Scholar] [CrossRef]
- Sandroussi, C.; Dawson, L.A.; Lee, M.; Guindi, M.; Fischer, S.; Ghanekar, A.; Cattral, M.S.; McGilvray, I.D.; Levy, G.A.; Renner, E.; et al. Radiotherapy as a Bridge to Liver Transplantation for Hepatocellular Carcinoma. Transplant. Int. 2010, 23, 299–306. [Google Scholar] [CrossRef]
- Lu, L.; Zeng, J.; Wen, Z.; Tang, C.; Xu, N. Transcatheter Arterial Chemoembolisation Followed by Three-Dimensional Conformal Radiotherapy versus Transcatheter Arterial Chemoembolisation Alone for Primary Hepatocellular Carcinoma in Adults. Cochrane Database Syst. Rev. 2019, 2, CD012244. [Google Scholar] [CrossRef]
- Toso, C.; Mentha, G.; Kneteman, N.M.; Majno, P. The Place of Downstaging for Hepatocellular Carcinoma. J. Hepatol 2010, 52, 930–936. [Google Scholar] [CrossRef]
- Yao, F.Y.; Fidelman, N. Reassessing the Boundaries of Liver Transplantation for Hepatocellular Carcinoma: Where Do We Stand with Tumor down-Staging? Hepatology 2016, 63, 1014–1025. [Google Scholar] [CrossRef]
- Parikh, N.D.; Waljee, A.K.; Singal, A.G. Downstaging Hepatocellular Carcinoma: A Systematic Review and Pooled Analysis. Liver Transplant. 2015, 21, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; Heimbach, J.K.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Wang, Z.; Murad, M.H.; Mohammed, K. Therapies for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation: A Systematic Review and Meta-Analysis. Hepatology 2018, 67, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after Sorafenib in Patients with Advanced Hepatocellular Carcinoma and Increased α-Fetoprotein Concentrations (REACH-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.-H.; Harding, J.J.; Merle, P.; et al. CheckMate 459: A Randomized, Multi-Center Phase III Study of Nivolumab (NIVO) vs Sorafenib (SOR) as First-Line (1L) Treatment in Patients (Pts) with Advanced Hepatocellular Carcinoma (AHCC). Ann. Oncol. 2019, 30, v874–v875. [Google Scholar] [CrossRef]
- Lencioni, R.A.; Allgaier, H.-P.; Cioni, D.; Olschewski, M.; Deibert, P.; Crocetti, L.; Frings, H.; Laubenberger, J.; Zuber, I.; Blum, H.E.; et al. Small Hepatocellular Carcinoma in Cirrhosis: Randomized Comparison of Radio-Frequency Thermal Ablation versus Percutaneous Ethanol Injection. Radiology 2003, 228, 235–240. [Google Scholar] [CrossRef]
- Huang, J.; Yan, L.; Cheng, Z.; Wu, H.; Du, L.; Wang, J.; Xu, Y.; Zeng, Y. A Randomized Trial Comparing Radiofrequency Ablation and Surgical Resection for HCC Conforming to the Milan Criteria. Ann. Surg. 2010, 252, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-T.; Lee, P.-H.; Tsang, Y.-M.; Lai, M.-Y.; Yang, P.-M.; Hu, R.-H.; Chen, P.-J.; Kao, J.-H.; Sheu, J.-C.; Lee, C.-Z.; et al. Percutaneous Ethanol Injection versus Surgical Resection for the Treatment of Small Hepatocellular Carcinoma: A Prospective Study. Ann. Surg. 2005, 242, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.K.C.; Chok, K.S.H.; Chan, A.C.Y.; Cheung, T.T.; Wong, T.C.L.; Fung, J.Y.Y.; Yuen, J.; Poon, R.T.P.; Fan, S.T.; Lo, C.M. Randomized Clinical Trial of Hepatic Resection versus Radiofrequency Ablation for Early-Stage Hepatocellular Carcinoma. Br. J. Surg. 2017, 104, 1775–1784. [Google Scholar] [CrossRef]
- Shibata, T.; Iimuro, Y.; Yamamoto, Y.; Maetani, Y.; Ametani, F.; Itoh, K.; Konishi, J. Small Hepatocellular Carcinoma: Comparison of Radio-Frequency Ablation and Percutaneous Microwave Coagulation Therapy. Radiology 2002, 223, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, N.; Shen, Q.; Cheng, W.; Qian, G.-J. Therapeutic Efficacy of Percutaneous Radiofrequency Ablation versus Microwave Ablation for Hepatocellular Carcinoma. PLoS ONE 2013, 8, e76119. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, D.E.; Kernagis, L.Y.; Soulen, M.C.; Geschwind, J.-F.H. Chemoembolization of Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2002, 13, S211–S221. [Google Scholar] [CrossRef]
- Ibrahim, S.-M.; Lewandowski, R.-J.; Sato, K.-T.; Gates, V.-L.; Kulik, L.; Mulcahy, M.-F.; Ryu, R.-K.; Omary, R.-A.; Salem, R. Radioembolization for the Treatment of Unresectable Hepatocellular Carcinoma: A Clinical Review. World J. Gastroenterol 2008, 14, 1664–1669. [Google Scholar] [CrossRef]
- Hawkins, M.A.; Dawson, L.A. Radiation Therapy for Hepatocellular Carcinoma: From Palliation to Cure. Cancer 2006, 106, 1653–1663. [Google Scholar] [CrossRef]
- Hoffe, S.E.; Finkelstein, S.E.; Russell, M.S.; Shridhar, R. Nonsurgical Options for Hepatocellular Carcinoma: Evolving Role of External Beam Radiotherapy. Cancer Control 2010, 17, 100–110. [Google Scholar] [CrossRef]
- Hasan, S.; Abel, S.; Verma, V.; Webster, P.; Arscott, W.T.; Wegner, R.E.; Kirichenko, A.; Simone II, C.B. Proton Beam Therapy versus Stereotactic Body Radiotherapy for Hepatocellular Carcinoma: Practice Patterns, Outcomes, and the Effect of Biologically Effective Dose Escalation. J. Gastrointest. Oncol. 2019, 10, 999–1009. [Google Scholar] [CrossRef]
- Andolino, D.L.; Johnson, C.S.; Maluccio, M.; Kwo, P.; Tector, A.J.; Zook, J.; Johnstone, P.A.S.; Cardenes, H.R. Stereotactic Body Radiotherapy for Primary Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e447–e453. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Jen, Y.-M.; Lee, M.-S.; Chang, L.-P.; Chen, C.-M.; Ko, K.-H.; Lin, K.-T.; Lin, J.-C.; Chao, H.-L.; Lin, C.-S.; et al. Stereotactic Body Radiation Therapy in Recurrent Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-K.; Kim, M.-S.; Cho, C.K.; Yang, K.M.; Yoo, H.J.; Kim, J.H.; Bae, S.H.; Jung, D.H.; Kim, K.B.; Lee, D.H.; et al. Stereotactic Body Radiation Therapy for Inoperable Hepatocellular Carcinoma as a Local Salvage Treatment after Incomplete Transarterial Chemoembolization. Cancer 2012, 118, 5424–5431. [Google Scholar] [CrossRef] [PubMed]
- Bujold, A.; Massey, C.A.; Kim, J.J.; Brierley, J.; Cho, C.; Wong, R.K.S.; Dinniwell, R.E.; Kassam, Z.; Ringash, J.; Cummings, B.; et al. Sequential Phase I and II Trials of Stereotactic Body Radiotherapy for Locally Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2013, 31, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Lucatelli, P.; Burrel, M.; Guiu, B.; de Rubeis, G.; van Delden, O.; Helmberger, T. CIRSE Standards of Practice on Hepatic Transarterial Chemoembolisation. Cardiovasc. Interv. Radiol. 2021, 44, 1851–1867. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J. A Concise Review of Updated Guidelines Regarding the Management of Hepatocellular Carcinoma around the World: 2010–2016. Clin. Mol. Hepatol. 2016, 22, 7–17. [Google Scholar] [CrossRef]
- Torzilli, G.; Belghiti, J.; Kokudo, N.; Takayama, T.; Capussotti, L.; Nuzzo, G.; Vauthey, J.-N.; Choti, M.A.; De Santibanes, E.; Donadon, M.; et al. A Snapshot of the Effective Indications and Results of Surgery for Hepatocellular Carcinoma in Tertiary Referral Centers: Is It Adherent to the EASL/AASLD Recommendations?: An Observational Study of the HCC East-West Study Group. Ann. Surg. 2013, 257, 929–937. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and Response Criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Tonko, S.; Dufour, J.-F. Hepatology scores. Ther. Umsch. 2013, 70, 577–579. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of Liver Function in Patients with Hepatocellular Carcinoma: A New Evidence-Based Approach-the ALBI Grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Na, S.K.; Yim, S.Y.; Suh, S.J.; Jung, Y.K.; Kim, J.H.; Seo, Y.S.; Yim, H.J.; Yeon, J.E.; Byun, K.S.; Um, S.H. ALBI versus Child-Pugh Grading Systems for Liver Function in Patients with Hepatocellular Carcinoma. J. Surg. Oncol. 2018, 117, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.; Mouli, S.; Kulik, L.; Desai, K.; Thornburg, B.; Ganger, D.; Baker, T.; Abecassis, M.; Ralph Kallini, J.; Gabr, A.; et al. Independent Analysis of Albumin-Bilirubin Grade in a 765-Patient Cohort Treated with Transarterial Locoregional Therapy for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2016, 27, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Waked, I.; Berhane, S.; Toyoda, H.; Chan, S.L.; Stern, N.; Palmer, D.; Tada, T.; Yeo, W.; Mo, F.; Bettinger, D.; et al. Transarterial Chemo-Embolisation of Hepatocellular Carcinoma: Impact of Liver Function and Vascular Invasion. Br. J. Cancer 2017, 116, 448–454. [Google Scholar] [CrossRef]
- Huo, T.-I.; Liu, P.-H.; Hsu, C.-Y. ALBI Score as a Novel Tool in Staging and Treatment Planning for Hepatocellular Carcinoma: Is It Sufficient? Liver Cancer 2017, 6, 375–376. [Google Scholar] [CrossRef]
- Liu, P.-H.; Hsu, C.-Y.; Hsia, C.-Y.; Lee, Y.-H.; Chiou, Y.-Y.; Huang, Y.-H.; Lee, F.-Y.; Lin, H.-C.; Hou, M.-C.; Huo, T.-I. ALBI and PALBI Grade Predict Survival for HCC across Treatment Modalities and BCLC Stages in the MELD Era. J. Gastroenterol. Hepatol. 2017, 32, 879–886. [Google Scholar] [CrossRef]
- Petrie, H.T.; Klassen, L.W.; Kay, H.D. Inhibition of Human Cytotoxic T Lymphocyte Activity in Vitro by Autologous Peripheral Blood Granulocytes. J. Immunol. 1985, 134, 230–234. [Google Scholar]
- el-Hag, A.; Clark, R.A. Immunosuppression by Activated Human Neutrophils. Dependence on the Myeloperoxidase System. J. Immunol. 1987, 139, 2406–2413. [Google Scholar]
- Niemi, K.M.; Kero, M.; Kanerva, L.; Mattila, R. Epidermolysis Bullosa Simplex. A New Histologic Subgroup. Arch. Dermatol. 1983, 119, 138–141. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Zhang, X.; Zhao, S.; Hu, J.; Han, G.; Liu, L. The Neutrophil-to-Lymphocyte Ratio Is a Predictive Factor for the Survival of Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Ann. Transl. Med. 2020, 8, 541. [Google Scholar] [CrossRef]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; ter Borg, P.C. A Model to Predict Poor Survival in Patients Undergoing Transjugular Intrahepatic Portosystemic Shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, S.; Montano-Loza, A.J.; Salat, P.; McCarthy, M.; Kneteman, N.; Meza-Junco, J.; Owen, R. Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma: Predictors of Survival. Can. J. Gastroenterol. 2011, 25, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Sieghart, W.; Hucke, F.; Pinter, M.; Graziadei, I.; Vogel, W.; Müller, C.; Heinzl, H.; Trauner, M.; Peck-Radosavljevic, M. The ART of Decision Making: Retreatment with Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. Hepatology 2013, 57, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.H.; Abid, S.; Haq, T.U.; Awan, S. Role of Assessment for Retreatment with Transarterial Chemoembolization Score in Decision of Retreatment with Trans-Arterial Chemo-Embolization Sessions in Patients with Hepatocellular Carcinoma. J. Ayub Med. Coll. Abbottabad 2017, 29, 378–383. [Google Scholar] [PubMed]
- Jia, K.-F.; Wang, H.; Yu, C.-L.; Yin, W.-L.; Zhang, X.-D.; Wang, F.; Sun, C.; Shen, W. ASARA, a Prediction Model Based on Child-Pugh Class in Hepatocellular Carcinoma Patients Undergoing Transarterial Chemoembolization. Hepatobiliary Pancreat Dis. Int. 2022. [Google Scholar] [CrossRef]
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Beirne, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burroughs, A.K.; et al. A Simple Prognostic Scoring System for Patients Receiving Transarterial Embolisation for Hepatocellular Cancer. Ann. Oncol. 2013, 24, 2565–2570. [Google Scholar] [CrossRef]
- Georgiades, C.S.; Liapi, E.; Frangakis, C.; Park, J.; Kim, H.W.; Hong, K.; Geschwind, J.-F.H. Prognostic Accuracy of 12 Liver Staging Systems in Patients with Unresectable Hepatocellular Carcinoma Treated with Transarterial Chemoembolization. J. Vasc. Interv. Radiol. 2006, 17, 1619–1624. [Google Scholar] [CrossRef]
- Hucke, F.; Pinter, M.; Graziadei, I.; Bota, S.; Vogel, W.; Müller, C.; Heinzl, H.; Waneck, F.; Trauner, M.; Peck-Radosavljevic, M.; et al. How to STATE Suitability and START Transarterial Chemoembolization in Patients with Intermediate Stage Hepatocellular Carcinoma. J. Hepatol. 2014, 61, 1287–1296. [Google Scholar] [CrossRef]
- El Khaddari, S.; Gaudin, J.-L.; Abidi, H.; Picaud, G.; Rode, A.; Souquet, J.-C. Chemoembolization in hepatocellular carcinoma: Multivariate analysis of survival prog.gnostic factors after the first session. Gastroenterol. Clin. Biol. 2002, 26, 728–734. [Google Scholar]
- Wai, C.-T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.-F. A Simple Noninvasive Index Can Predict Both Significant Fibrosis and Cirrhosis in Patients with Chronic Hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Zhang, X.; Svn, Z.; Liv, M.; Liu, M.; Zhang, Y.; Sun, Q. Assessment of Prognostic Value of Aspartate Aminotransferase-to-Platelet Ratio Index in Patients With Hepatocellular Carcinoma: Meta-Analysis of 28 Cohort Studies. Front. Med. Lausanne 2021, 8, 756210. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.P.; Zhao, Q.Y.; Li, S.; Wang, H.; Wu, P.H. Non-invasive fibrosis indexes in predicting acute liver function deterioration after transcatheter arterial chemoembolization. Zhonghua Yi Xue Za Zhi 2016, 96, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Shim, J.H.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, K.M.; Lim, Y.-S.; Han, K.-H.; Lee, H.C. Risk Prediction for Patients with Hepatocellular Carcinoma Undergoing Chemoembolization: Development of a Prediction Model. Liver Int. 2016, 36, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Mähringer-Kunz, A.; Weinmann, A.; Schmidtmann, I.; Koch, S.; Schotten, S.; Pinto dos Santos, D.; Pitton, M.B.; Dueber, C.; Galle, P.R.; Kloeckner, R. Validation of the SNACOR Clinical Scoring System after Transarterial Chemoembolisation in Patients with Hepatocellular Carcinoma. BMC Cancer 2018, 18, 489. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Ohtsuki, T.; Obata, H.; Tomimatsu, M.; Okazaki, N.; Hasegawa, H.; Nakajima, Y.; Ohnishi, K. Natural History of Hepatocellular Carcinoma and Prognosis in Relation to Treatment. Study of 850 Patients. Cancer 1985, 56, 918–928. [Google Scholar] [CrossRef]
- Levy, I.; Sherman, M. Liver Cancer Study Group of the University of Toronto Staging of Hepatocellular Carcinoma: Assessment of the CLIP, Okuda, and Child-Pugh Staging Systems in a Cohort of 257 Patients in Toronto. Gut 2002, 50, 881–885. [Google Scholar] [CrossRef]
- A New Prognostic System for Hepatocellular Carcinoma: A Retrospective Study of 435 Patients: The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology 1998, 28, 751–755. [CrossRef]
- Li, L.; Gou, C.-Y.; Li, J.-Y.; Achakzai, R.; Li, X.-H. Cancer of the Liver Italian Program Score Helps Identify Potential Candidates for Transarterial Chemoembolization in Patients with Barcelona Clinic Liver Cancer Stage C. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 152–157. [Google Scholar] [CrossRef]
- Mitchell, D.G.; Bruix, J.; Sherman, M.; Sirlin, C.B. LI-RADS (Liver Imaging Reporting and Data System): Summary, Discussion, and Consensus of the LI-RADS Management Working Group and Future Directions. Hepatology 2015, 61, 1056–1065. [Google Scholar] [CrossRef]
- Lencioni, R.; Petruzzi, P.; Crocetti, L. Chemoembolization of Hepatocellular Carcinoma. Semin. Interv. Radiol. 2013, 30, 3–11. [Google Scholar] [CrossRef]
- Schraml, C.; Kaufmann, S.; Rempp, H.; Syha, R.; Ketelsen, D.; Notohamiprodjo, M.; Nikolaou, K. Imaging of HCC-Current State of the Art. Diagnostics 2015, 5, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Tangkijvanich, P.; Anukulkarnkusol, N.; Suwangool, P.; Lertmaharit, S.; Hanvivatvong, O.; Kullavanijaya, P.; Poovorawan, Y. Clinical Characteristics and Prognosis of Hepatocellular Carcinoma: Analysis Based on Serum Alpha-Fetoprotein Levels. J. Clin. Gastroenterol. 2000, 31, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Kobayashi, A.; Ohya, A.; Takahashi, M.; Yokoyama, T.; Shimizu, A.; Motoyama, H.; Furusawa, N.; Notake, T.; Kitagawa, N.; et al. Assessment of Treatment Outcomes Based on Tumor Marker Trends in Patients with Recurrent Hepatocellular Carcinoma Undergoing Trans-Catheter Arterial Chemo-Embolization. Int. J. Clin. Oncol. 2014, 19, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Tan, Z.; Xiang, X.; Dan, Y.; Deng, G. Effectiveness of PIVKA-II in the Detection of Hepatocellular Carcinoma Based on Real-World Clinical Data. BMC Cancer 2017, 17, 608. [Google Scholar] [CrossRef] [PubMed]
- Chehab, M.A.; Thakor, A.S.; Tulin-Silver, S.; Connolly, B.L.; Cahill, A.M.; Ward, T.J.; Padia, S.A.; Kohi, M.P.; Midia, M.; Chaudry, G.; et al. Adult and Pediatric Antibiotic Prophylaxis during Vascular and IR Procedures: A Society of Interventional Radiology Practice Parameter Update Endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. J. Vasc. Interv. Radiol. 2018, 29, 1483–1501.e2. [Google Scholar] [CrossRef]
- Miura, J.T.; Rilling, W.S.; White, S.B.; Hieb, R.A.; Tutton, S.M.; Patel, P.J.; Gamblin, T.C.; Hohenwalter, E.J. Safety and Efficacy of Transarterial Chemoembolization in Patients with Transjugular Intrahepatic Portosystemic Shunts. HPB Oxf. 2015, 17, 707–712. [Google Scholar] [CrossRef][Green Version]
- Patel, I.J.; Rahim, S.; Davidson, J.C.; Hanks, S.E.; Tam, A.L.; Walker, T.G.; Wilkins, L.R.; Sarode, R.; Weinberg, I. Society of Interventional Radiology Consensus.s.s Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J. Vasc. Interv. Radiol. 2019, 30, 1168–1184.e1. [Google Scholar] [CrossRef]
- Kis, B.; Mills, M.; Hoffe, S.E. Hepatic Radioembolization from Transradial Access: Initial Experience and Comparison to Transfemoral Access. Diagn. Interv. Radiol. 2016, 22, 444–449. [Google Scholar] [CrossRef]
- Vuurmans, T.; Hilton, D. Brewing the Right Cocktail for Radial Intervention. Indian Heart J. 2010, 62, 221–225. [Google Scholar]
- Zimmermann, M.; Schulze-Hagen, M.; Pedersoli, F.; Isfort, P.; Heinzel, A.; Kuhl, C.; Bruners, P. Y90-Radioembolization via Variant Hepatic Arteries: Is There a Relevant Risk for Non-Target Embolization? WJR 2019, 11, 102–109. [Google Scholar] [CrossRef]
- Gaba, R.C. Chemoembolization Practice Patterns and Technical Methods Among Interventional Radiologists: Results of an Online Survey. Am. J. Roentgenol. 2012, 198, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Abdelmaksoud, M.H.K.; Louie, J.D.; Kothary, N.; Hwang, G.L.; Kuo, W.T.; Hofmann, L.V.; Hovsepian, D.M.; Sze, D.Y. Embolization of Parasitized Extrahepatic Arteries to Reestablish Intrahepatic Arterial Supply to Tumors before Yttrium-90 Radioembolization. J. Vasc. Interv. Radiol. 2011, 22, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Tacher, V.; Lin, M.; Duran, R.; Yarmohammadi, H.; Lee, H.; Chapiro, J.; Chao, M.; Wang, Z.; Frangakis, C.; Sohn, J.H.; et al. Comparison of Existing Response Criteria in Patients with Hepatocellular Carcinoma Treated with Transarterial Chemoembolization Using a 3D Quantitative Approach. Radiology 2016, 278, 275–284. [Google Scholar] [CrossRef]
- Noori, V.J.; Eldrup-Jørgensen, J. A Systematic Review of Vascular Closure Devices for Femoral Artery Puncture Sites. J. Vasc. Surg. 2018, 68, 887–899. [Google Scholar] [CrossRef]
- Rand, T.; Loewe, C.; Schoder, M.; Schmook, M.T.; Peck-Radosavljevic, M.; Kettenbach, J.; Wolf, F.; Schneider, B.; Lammer, J. Arterial Embolization of Unresectable Hepatocellular Carcinoma with Use of Microspheres, Lipiodol, and Cyanoacrylate. Cardiovasc. Interv. Radiol 2005, 28, 313–318. [Google Scholar] [CrossRef]
- Lencioni, R.; Crocetti, L. Local-Regional Treatment of Hepatocellular Carcinoma. Radiology 2012, 262, 43–58. [Google Scholar] [CrossRef]
- Datta, J.; Narayan, R.R.; Kemeny, N.E.; D’Angelica, M.I. Role of Hepatic Artery Infusion Chemotherapy in Treatment of Initially Unresectable Colorectal Liver Metastases: A Review. JAMA Surg. 2019, 154, 768. [Google Scholar] [CrossRef]
- Liu, Z.-C.; Lu, X.-F.; Yang, H.; Liu, H.-D.; Song, X.; Ning, S.-L.; Xu, Y.-F.; Chen, Y.-X. Clinical Outcomes of Patients with Severe Hepatic Hereditary Hemorrhagic Telangiectasia After Banding of the Hepatic Artery and Banding/Ligation of Branches of the Hepatic Artery. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-J.; He, M.-K.; Chen, H.-W.; Fang, W.-Q.; Zhou, Y.-M.; Xu, L.; Wei, W.; Zhang, Y.-J.; Guo, Y.; Guo, R.-P.; et al. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. JCO 2022, 40, 150–160. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, A.H.; Kim, K.A.; Won, J.Y.; Lee, D.Y.; Lee, K.-H. A Simplified Technique of Percutaneous Hepatic Artery Port-Catheter Insertion for the Treatment of Advanced Hepatocellular Carcinoma with Portal Vein Invasion. Korean J. Radiol. 2010, 11, 648. [Google Scholar] [CrossRef][Green Version]
- Seki, H.; Shiina, M. Placement of a Long Tapered Side-Hole Catheter in the Hepatic Artery: Technical Advantages, Catheter Stability, and Arterial Patency. Am. J. Roentgenol. 2006, 187, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, O.; Mvouama, S.; Pellegrinelli, J.; Guillen, K.; Manfredi, S.; Ghiringhelli, F.; Falvo, N.; Midulla, M.; Loffroy, R. Percutaneous Implantation of a Microcatheter-Port System for Hepatic Arterial Infusion Chemotherapy of Unresectable Liver Tumors: Technical Feasibility, Functionality, and Complications. Diagnostics 2021, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Fatourou, E.; O’Beirne, J.; Meyer, T.; Burroughs, A.K. Transarterial Chemoembolization and Bland Embolization for Hepatocellular Carcinoma. World J. Gastroenterol. 2014, 20, 3069–3077. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, J.-F.H.; Nezami, N. Combining Chemotherapy and Radiation Therapy for Liver Cancer: Is the Solution an Intraarterial Approach? Cardiovasc. Interv. Radiol. 2020, 43, 1538–1539. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL–EORTC Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W. The Current Practice of Transarterial Chemoembolization for the Treatment of Hepatocellular Carcinoma. Korean J. Radiol. 2009, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Peng, Z.; Zhang, Y.; Chen, M.; Li, J.; Guo, R.; Li, J.; Li, B.; Mei, J.; Feng, S.; et al. Lack of Response to Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma: Abandon or Repeat? Radiology 2021, 298, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, J.-H.; Ji, J.-S.; Zhong, B.-Y.; Zhou, G.-H.; Song, J.-J.; Hou, Z.-H.; Huang, P.; Zhang, S.; Li, Z.; et al. Imaging Changes and Clinical Complications After Drug-Eluting Bead Versus Conventional Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma: Multicenter Study. AJR Am. J. Roentgenol. 2021, 217, 933–943. [Google Scholar] [CrossRef]
- Saini, A.; Wallace, A.; Alzubaidi, S.; Knuttinen, M.G.; Naidu, S.; Sheth, R.; Albadawi, H.; Oklu, R. History and Evolution of Yttrium-90 Radioembolization for Hepatocellular Carcinoma. JCM 2019, 8, 55. [Google Scholar] [CrossRef]
- Vente, M.A.D.; Wondergem, M.; van der Tweel, I.; van den Bosch, M.A.A.J.; Zonnenberg, B.A.; Lam, M.G.E.H.; van Het Schip, A.D.; Nijsen, J.F.W. Yttrium-90 Microsphere Radioembolization for the Treatment of Liver Malignancies: A Structured Meta-Analysis. Eur. Radiol. 2009, 19, 951–959. [Google Scholar] [CrossRef]
- Gabr, A.; Riaz, A.; Johnson, G.E.; Kim, E.; Padia, S.; Lewandowski, R.J.; Salem, R. Correlation of Y90-Absorbed Radiation Dose to Pathological Necrosis in Hepatocellular Carcinoma: Confirmatory Multicenter Analysis in 45 Explants. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Sethi, I.; Villalobos, A.; Wagstaff, W.; Schuster, D.M.; Bercu, Z.; Brandon, D.; Kokabi, N. Determination of Tumour Dose Response Threshold and Implication on Survival in Patients with HCC Treated with Y90 Radiation Segmentectomy: A Simple Semi-Quantitative Analysis. Nucl. Med. Commun. 2021; Publish Ahead of Print. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, Q.; Zou, R.; Shen, J.; Fang, W.; Tan, G.; Zhou, Y.; Wu, X.; Xu, L.; Wei, W.; et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 953. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Yokosuka, O.; Ogasawara, S.; Obi, S.; Izumi, N.; Aikata, H.; Nagano, H.; Hatano, E.; Sasaki, Y.; et al. Sorafenib plus Low-Dose Cisplatin and Fluorouracil Hepatic Arterial Infusion Chemotherapy versus Sorafenib Alone in Patients with Advanced Hepatocellular Carcinoma (SILIUS): A Randomised, Open Label, Phase 3 Trial. Lancet Gastroenterol. Hepatol. 2018, 3, 424–432. [Google Scholar] [CrossRef]
- Zheng, K.; Zhu, X.; Fu, S.; Cao, G.; Li, W.-Q.; Xu, L.; Chen, H.; Wu, D.; Yang, R.; Wang, K.; et al. Sorafenib Plus Hepatic Arterial Infusion Chemotherapy versus Sorafenib for Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis: A Randomized Trial. Radiology 2022, 303, 455–464. [Google Scholar] [CrossRef]
- Raoul, J.-L.; Sangro, B.; Forner, A.; Mazzaferro, V.; Piscaglia, F.; Bolondi, L.; Lencioni, R. Evolving Strategies for the Management of Intermediate-Stage Hepatocellular Carcinoma: Available Evidence and Expert Opinion on the Use of Transarterial Chemoembolization. Cancer Treat. Rev. 2011, 37, 212–220. [Google Scholar] [CrossRef]
- Sieghart, W.; Hucke, F.; Peck-Radosavljevic, M. Transarterial Chemoembolization: Modalities, Indication, and Patient Selection. J. Hepatol 2015, 62, 1187–1195. [Google Scholar] [CrossRef]
- Kennedy, A.; Nag, S.; Salem, R.; Murthy, R.; McEwan, A.J.; Nutting, C.; Benson, A.; Espat, J.; Bilbao, J.I.; Sharma, R.A.; et al. Recommendations for Radioembolization of Hepatic Malignancies Using Yttrium-90 Microsphere Brachytherapy: A Consensus Panel Report from the Radioembolization Brachytherapy Oncology Consortium. Int. Radiat Oncol. Biol. Phys. 2007, 68, 13–23. [Google Scholar] [CrossRef]
- Maas, M.; Beets-Tan, R.; Gaubert, J.-Y.; Gomez Munoz, F.; Habert, P.; Klompenhouwer, L.G.; Vilares Morgado, P.; Schaefer, N.; Cornelis, F.H.; Solomon, S.B.; et al. Follow-up after Radiological Intervention in Oncology: ECIO-ESOI Evidence and Consensus-Based Recommendations for Clinical Practice. Insights Imaging 2020, 11, 83. [Google Scholar] [CrossRef]
- Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire A Comparison of Lipiodol Chemoembolization and Conservative Treatment for Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 1995, 332, 1256–1261. [CrossRef] [PubMed]
- Paye, F.; Farges, O.; Dahmane, M.; Vilgrain, V.; Flejou, J.F.; Belghiti, J. Cytolysis Following Chemoembolization for Hepatocellular Carcinoma. Br. J. Surg. 1999, 86, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, S.J.; Redhead, D.N.; Thomson, B.N.J.; Currie, E.J.; Parks, R.W.; Madhavan, K.K.; Garden, O.J. Postchemoembolisation Syndrome--Tumour Necrosis or Hepatocyte Injury? Br. J. Cancer 2003, 89, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Castells, A.; Bruix, J.; Ayuso, C.; Brú, C.; Montanyà, X.; Boix, L.; Rodès, J. Transarterial Embolization for Hepatocellular Carcinoma. Antibiotic Prophylaxis and Clinical Meaning of Postembolization Fever. J. Hepatol. 1995, 22, 410–415. [Google Scholar] [CrossRef]
- Leung, D.A.; Goin, J.E.; Sickles, C.; Raskay, B.J.; Soulen, M.C. Determinants of Postembolization Syndrome after Hepatic Chemoembolization. J. Vasc. Interv. Radiol. 2001, 12, 321–326. [Google Scholar] [CrossRef]
- Chan, A.O.; Yuen, M.-F.; Hui, C.-K.; Tso, W.-K.; Lai, C.-L. A Prospective Study Regarding the Complications of Transcatheter Intraarterial Lipiodol Chemoembolization in Patients with Hepatocellular Carcinoma. Cancer 2002, 94, 1747–1752. [Google Scholar] [CrossRef]
- Chung, J.W.; Park, J.H.; Han, J.K.; Choi, B.I.; Han, M.C.; Lee, H.S.; Kim, C.Y. Hepatic Tumors: Predisposing Factors for Complications of Transcatheter Oily Chemoembolization. Radiology 1996, 198, 33–40. [Google Scholar] [CrossRef]
- Garwood, E.R.; Fidelman, N.; Hoch, S.E.; Kerlan, R.K.; Yao, F.Y. Morbidity and Mortality Following Transarterial Liver Chemoembolization in Patients with Hepatocellular Carcinoma and Synthetic Hepatic Dysfunction. Liver Transplant. 2013, 19, 164–173. [Google Scholar] [CrossRef]
- Marelli, L.; Stigliano, R.; Triantos, C.; Senzolo, M.; Cholongitas, E.; Davies, N.; Tibballs, J.; Meyer, T.; Patch, D.W.; Burroughs, A.K. Transarterial Therapy for Hepatocellular Carcinoma: Which Technique Is More Effective? A Systematic Review of Cohort and Randomized Studies. Cardiovasc. Interv. Radiol. 2007, 30, 6–25. [Google Scholar] [CrossRef]
- Song, S.Y.; Chung, J.W.; Han, J.K.; Lim, H.G.; Koh, Y.H.; Park, J.H.; Lee, H.S.; Kim, C.Y. Liver Abscess after Transcatheter Oily Chemoembolization for Hepatic Tumors: Incidence, Predisposing Factors, and Clinical Outcome. Vasc. Interv. Radiol. 2001, 12, 313–320. [Google Scholar] [CrossRef]
- Kim, H.K.; Chung, Y.H.; Song, B.C.; Yang, S.H.; Yoon, H.K.; Yu, E.; Sung, K.B.; Lee, Y.S.; Lee, S.G.; Suh, D.J. Ischemic Bile Duct Injury as a Serious Complication after Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. J. Clin. Gastroenterol. 2001, 32, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-C.; Perng, W.-C.; Chen, C.-W.; Chian, C.-F.; Peng, C.-K.; Su, W.-L. Acute Respiratory Distress Syndrome after Transcatheter Arterial Chemoembolization of Hepatocellular Carcinomas. Am. J. Med. Sci. 2009, 338, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Kwok, P.C.H.; Lam, T.W.; Lam, C.L.; Lai, A.K.H.; Lo, H.Y.; Chan, S.C.H. Rare Pulmonary Complications after Transarterial Chemoembolisation for Hepatocellular Carcinoma: Two Case Reports. Hong Kong J. 2003, 9, 457–460. [Google Scholar]
- Kim, J.-T.; Heo, S.-H.; Choi, S.-M.; Lee, S.-H.; Park, M.-S.; Kim, B.-C.; Kim, Y.; Kim, M.-K.; Cho, K.-H. Cerebral Embolism of Iodized Oil (Lipiodol) after Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma. J. Neuroimaging 2009, 19, 394–397. [Google Scholar] [CrossRef]
- Jouneau, S.; Vauléon, E.; Caulet-Maugendre, S.; Polard, E.; Volatron, A.-C.; Meunier, C.; Tattevin, P.; Montani, D.; Garin, E.; Raoul, J.-L.; et al. 131I-Labeled Lipiodol-Induced Interstitial Pneumonia: A Series of 15 Cases. Chest 2011, 139, 1463–1469. [Google Scholar] [CrossRef]
- Brown, D.B.; Chapman, W.C.; Cook, R.D.; Kerr, J.R.; Gould, J.E.; Pilgram, T.K.; Darcy, M.D. Chemoembolization of Hepatocellular Carcinoma: Patient Status at Presentation and Outcome over 15 Years at a Single Center. AJR Am. J. Roentgenol 2008, 190, 608–615. [Google Scholar] [CrossRef]
- Toyoda, H.; Kumada, T.; Tada, T.; Sone, Y.; Fujimori, M. Transarterial Chemoembolization for Hepatitis B Virus-Associated Hepatocellular Carcinoma: Improved Survival after Concomitant Treatment with Nucleoside Analogues. J. Vasc. Interv. Radiol. 2012, 23, 317–322.e1. [Google Scholar] [CrossRef]
- Perrillo, R.P. Reactivated Hepatitis B Due to Medical Interventions: The Clinical Spectrum Expands. Antivir. Ther. 2011, 16, 947–949. [Google Scholar] [CrossRef]
- Atassi, B.; Bangash, A.K.; Lewandowski, R.J.; Ibrahim, S.; Kulik, L.; Mulcahy, M.F.; Murthy, R.; Ryu, R.K.; Sato, K.T.; Miller, F.H.; et al. Biliary Sequelae Following Radioembolization with Yttrium-90 Microspheres. J. Vasc. Interv. Radiol. 2008, 19, 691–697. [Google Scholar] [CrossRef]
- Riaz, A.; Lewandowski, R.J.; Kulik, L.M.; Mulcahy, M.F.; Sato, K.T.; Ryu, R.K.; Omary, R.A.; Salem, R. Complications Following Radioembolization with Yttrium-90 Microspheres: A Comprehensive Literature Review. J. Vasc. Interv. Radiol. 2009, 20, 1121–1130; quiz 1131. [Google Scholar] [CrossRef]
- Riaz, A.; Awais, R.; Salem, R. Side Effects of Yttrium-90 Radioembolization. Front. Oncol. 2014, 4, 198. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Lewandowski, R.J.; Atassi, B.; Gordon, S.C.; Gates, V.L.; Barakat, O.; Sergie, Z.; Wong, C.-Y.O.; Thurston, K.G. Treatment of Unresectable Hepatocellular Carcinoma with Use of 90Y Microspheres (TheraSphere): Safety, Tumor Response, and Survival. J. Vasc. Interv. Radiol. 2005, 16, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.G.E.H.; Abdelmaksoud, M.H.K.; Chang, D.T.; Eclov, N.C.; Chung, M.P.; Koong, A.C.; Louie, J.D.; Sze, D.Y. Safety of 90Y Radioembolization in Patients Who Have Undergone Previous External Beam Radiation Therapy. Int. J. Radiat Oncol. Biol. Phys. 2013, 87, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, T.F.; Saleem, S.; Atassi, B.; Reda, E.; Lewandowski, R.J.; Yaghmai, V.; Miller, F.; Ryu, R.K.; Ibrahim, S.; Sato, K.T.; et al. Fibrosis, Portal Hypertension, and Hepatic Volume Changes Induced by Intra-Arterial Radiotherapy with 90yttrium Microspheres. Dig. Dis. Sci. 2008, 53, 2556–2563. [Google Scholar] [CrossRef]
- Ayav, A.; Habib, N.; Jiao, L.R. Portal Hypertension Secondary to 90Yttrium Microspheres: An Unknown Complication. J. Clin. Oncol. 2005, 23, 8275–8276. [Google Scholar] [CrossRef]
- Carr, B.I.; Metes, D.M. Peripheral Blood Lymphocyte Depletion after Hepatic Arterial 90Yttrium Microsphere Therapy for Hepatocellular Carcinoma. Int. J. Radiat Oncol. Biol. Phys. 2012, 82, 1179–1184. [Google Scholar] [CrossRef]
- Currie, B.M.; Hoteit, M.A.; Ben-Josef, E.; Nadolski, G.J.; Soulen, M.C. Radioembolization-Induced Chronic Hepatotoxicity: A Single-Center Cohort Analysis. J. Vasc. Interv. Radiol. 2019, 30, 1915–1923. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J. Modified RECIST (MRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 052–060. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M.; Llovet, J.M.; Beaugrand, M.; Lencioni, R.; Burroughs, A.K.; Christensen, E.; Pagliaro, L.; Colombo, M.; Rodés, J. Clinical Management of Hepatocellular Carcinoma. Conclusions of the Barcelona-2000 EASL Conference. J. Hepatol. 2001, 35, 421–430. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Tirkes, T.; Hollar, M.A.; Tann, M.; Kohli, M.D.; Akisik, F.; Sandrasegaran, K. Response Criteria in Oncologic Imaging: Review of Traditional and New Criteria. RadioGraphics 2013, 33, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- James, K.; Eisenhauer, E.; Christian, M.; Terenziani, M.; Vena, D.; Muldal, A.; Therasse, P. Measuring Response in Solid Tumors: Unidimensional Versus Bidimensional Measurement. JNCI J. Natl. Cancer Inst. 1999, 91, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M. Management of Hepatocellular Carcinoma. Hepatology 2005, 42, 1208–1236. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Hoogstraten, B.; Staquet, M.; Winkler, A. Reporting Results of Cancer Treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef]
- Lim, H.K.; Han, J.K. Hepatocellular Carcinoma: Evaluation of Therapeutic Response to Interventional Procedures. Abdom. Imaging 2002, 27, 168–179. [Google Scholar] [CrossRef]
- Chapiro, J.; Lin, M.; Duran, R.; Schernthaner, R.E.; Geschwind, J.-F. Assessing Tumor Response after Loco-Regional Liver Cancer Therapies: The Role of 3D MRI. Expert Rev. Anticancer Ther. 2015, 15, 199–205. [Google Scholar] [CrossRef]
- Zhao, Y.; Duran, R.; Bai, W.; Sahu, S.; Wang, W.; Kabus, S.; Lin, M.; Han, G.; Geschwind, J.-F. Which Criteria Applied in Multi-Phasic CT Can Predict Early Tumor Response in Patients with Hepatocellular Carcinoma Treated Using Conventional TACE: RECIST, MRECIST, EASL or QEASL? Cardiovasc. Interv. Radiol. 2018, 41, 433–442. [Google Scholar] [CrossRef]
- Chapiro, J.; Duran, R.; Lin, M.; Schernthaner, R.E.; Wang, Z.; Gorodetski, B.; Geschwind, J.-F. Identifying Staging Markers for Hepatocellular Carcinoma before Transarterial Chemoembolization: Comparison of Three-Dimensional Quantitative versus Non-Three-Dimensional Imaging Markers. Radiology 2015, 275, 438–447. [Google Scholar] [CrossRef]
- Chapiro, J.; Wood, L.D.; Lin, M.; Duran, R.; Cornish, T.; Lesage, D.; Charu, V.; Schernthaner, R.; Wang, Z.; Tacher, V.; et al. Radiologic-Pathologic Analysis of Contrast-Enhanced and Diffusion-Weighted MR Imaging in Patients with HCC after TACE: Diagnostic Accuracy of 3D Quantitative Image Analysis. Radiology 2014, 273, 746–758. [Google Scholar] [CrossRef]
- O, J.H.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, J.; Nezami, N.; Laage-Gaupp, F.; Chapiro, J.; De Lin, M.; Duncan, J. Liver Tissue Classification Using an Auto-Context-Based Deep Neural Network with a Multi-Phase Training Framework. Patch Based Tech. Med. Imaging 2018, 11075, 59–66. [Google Scholar] [CrossRef]
- Abajian, A.; Murali, N.; Savic, L.J.; Laage-Gaupp, F.M.; Nezami, N.; Duncan, J.S.; Schlachter, T.; Lin, M.; Geschwind, J.-F.; Chapiro, J. Predicting Treatment Response to Intra-Arterial Therapies for Hepatocellular Carcinoma with the Use of Supervised Machine Learning—An Artificial Intelligence Concept. J. Vasc. Interv. Radiol. 2018, 29, 850–857.e1. [Google Scholar] [CrossRef]
- Miszczuk, M.A.; Chapiro, J.; Geschwind, J.-F.H.; Thakur, V.; Nezami, N.; Laage-Gaupp, F.; Kulon, M.; van Breugel, J.M.M.; Fereydooni, A.; Lin, M.; et al. Lipiodol as an Imaging Biomarker of Tumor Response After Conventional Transarterial Chemoembolization: Prospective Clinical Validation in Patients with Primary and Secondary Liver Cancer. Transl. Oncol. 2020, 13, 100742. [Google Scholar] [CrossRef] [PubMed]
- Nezami, N.; VAN Breugel, J.M.M.; Konstantinidis, M.; Chapiro, J.; Savic, L.J.; Miszczuk, M.A.; Rexha, I.; Lin, M.; Hong, K.; Georgiades, C. Lipiodol Deposition and Washout in Primary and Metastatic Liver Tumors After Chemoembolization. In Vivo 2021, 35, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Borde, T.; Nezami, N.; Gaupp, F.L.; Savic, L.J.; Taddei, T.; Jaffe, A.; Strazzabosco, M.; Lin, M.; Duran, R.; Georgiades, C.; et al. Optimization of the BCLC Staging System for Locoregional Therapy for Hepatocellular Carcinoma by Using Quantitative Tumor Burden Imaging Biomarkers at MRI. Radiology 2022, 304, 228–237. [Google Scholar] [CrossRef]
- Letzen, B.S.; Malpani, R.; Miszczuk, M.; de Ruiter, Q.M.B.; Petty, C.W.; Rexha, I.; Nezami, N.; Laage-Gaupp, F.; Lin, M.; Schlachter, T.R.; et al. Lipiodol as an Intra-Procedural Imaging Biomarker for Liver Tumor Response to Transarterial Chemoembolization: Post-Hoc Analysis of a Prospective Clinical Trial. Clin. Imaging 2021, 78, 194–200. [Google Scholar] [CrossRef]
- Weiss, C.R.; Nezami, N. One Step Closer to Precision Medicine for Transarterial Therapy of HCC. Radiology 2020, 297, 235–236. [Google Scholar] [CrossRef]
- Martin, S.P.; Fako, V.; Dang, H.; Dominguez, D.A.; Khatib, S.; Ma, L.; Wang, H.; Zheng, W.; Wang, X.W. PKM2 Inhibition May Reverse Therapeutic Resistance to Transarterial Chemoembolization in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 99. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Villanueva, A. Randomized Trials and Endpoints in Advanced HCC: Role of PFS as a Surrogate of Survival. J. Hepatol. 2019, 70, 1262–1277. [Google Scholar] [CrossRef]
- Beaver, J.A.; Howie, L.J.; Pelosof, L.; Kim, T.; Liu, J.; Goldberg, K.B.; Sridhara, R.; Blumenthal, G.M.; Farrell, A.T.; Keegan, P.; et al. A 25-Year Experience of US Food and Drug Administration Accelerated Approval of Malignant Hematology and Oncology Drugs and Biologics: A Review. JAMA Oncol. 2018, 4, 849. [Google Scholar] [CrossRef]
- Kemp, R.; Prasad, V. Surrogate Endpoints in Oncology: When Are They Acceptable for Regulatory and Clinical Decisions, and Are They Currently Overused? BMC Med. 2017, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.M.; Doepker, M.P.; Kim, Y.; Perez, M.C.; Gandle, C.; Thomas, K.L.; Choi, J.; Shridhar, R.; Zager, J.S. Hepatic Progression-Free and Overall Survival After Regional Therapy to the Liver for Metastatic Melanoma. Am. J. Clin. Oncol. 2018, 41, 747–753. [Google Scholar] [CrossRef]
- Lin, D.Y.; Liaw, Y.F.; Lee, T.Y.; Lai, C.M. Hepatic Arterial Embolization in Patients with Unresectable Hepatocellular Carcinoma--a Randomized Controlled Trial. Gastroenterology 1988, 94, 453–456. [Google Scholar] [CrossRef]
- Meyer, T.; Kirkwood, A.; Roughton, M.; Beare, S.; Tsochatzis, E.; Yu, D.; Davies, N.; Williams, E.; Pereira, S.P.; Hochhauser, D.; et al. A Randomised Phase II/III Trial of 3-Weekly Cisplatin-Based Sequential Transarterial Chemoembolisation vs Embolisation Alone for Hepatocellular Carcinoma. Br. J. Cancer 2013, 108, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Llovet, J.M.; Castells, A.; Montañá, X.; Brú, C.; Ayuso, M.C.; Vilana, R.; Rodés, J. Transarterial Embolization versus Symptomatic Treatment in Patients with Advanced Hepatocellular Carcinoma: Results of a Randomized, Controlled Trial in a Single Institution. Hepatology 1998, 27, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Tzeng, W.S.; Pan, H.B.; Yang, C.F.; Lai, K.H. Transcatheter Arterial Embolization with or without Cisplatin Treatment of Hepatocellular Carcinoma. A Randomized Controlled Study. Cancer 1994, 74, 2449–2453. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial Embolisation or Chemoembolisation versus Symptomatic Treatment in Patients with Unresectable Hepatocellular Carcinoma: A Randomised Controlled Trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Malagari, K.; Pomoni, M.; Kelekis, A.; Pomoni, A.; Dourakis, S.; Spyridopoulos, T.; Moschouris, H.; Emmanouil, E.; Rizos, S.; Kelekis, D. Prospective Randomized Comparison of Chemoembolization with Doxorubicin-Eluting Beads and Bland Embolization with BeadBlock for Hepatocellular Carcinoma. Cardiovasc. Intervent. Radiol. 2010, 33, 541–551. [Google Scholar] [CrossRef]
- Prajapati, H.J.; Xing, M.; Spivey, J.R.; Hanish, S.I.; El-Rayes, B.F.; Kauh, J.S.; Chen, Z.; Kim, H.S. Survival, Efficacy, and Safety of Small versus Large Doxorubicin Drug-Eluting Beads TACE Chemoembolization in Patients with Unresectable HCC. AJR Am. J. Roentgenol. 2014, 203, W706–W714. [Google Scholar] [CrossRef]
- Covey, A.M.; Maluccio, M.A.; Schubert, J.; BenPorat, L.; Brody, L.A.; Sofocleous, C.T.; Getrajdman, G.I.; Fong, Y.; Brown, K.T. Particle Embolization of Recurrent Hepatocellular Carcinoma after Hepatectomy. Cancer 2006, 106, 2181–2189. [Google Scholar] [CrossRef]
- Lo, C.-M.; Ngan, H.; Tso, W.-K.; Liu, C.-L.; Lam, C.-M.; Poon, R.T.-P.; Fan, S.-T.; Wong, J. Randomized Controlled Trial of Transarterial Lipiodol Chemoembolization for Unresectable Hepatocellular Carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, K.; Arii, S.; Ikai, I.; Omata, M.; Okita, K.; Ichida, T.; Matsuyama, Y.; Nakanuma, Y.; Kojiro, M.; Makuuchi, M.; et al. Prospective Cohort Study of Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma in 8510 Patients. Gastroenterology 2006, 131, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective Randomized Study of Doxorubicin-Eluting-Bead Embolization in the Treatment of Hepatocellular Carcinoma: Results of the PRECISION V Study. Cardiovasc. Interv. Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef]
- Facciorusso, A.; Di Maso, M.; Muscatiello, N. Drug-Eluting Beads versus Conventional Chemoembolization for the Treatment of Unresectable Hepatocellular Carcinoma: A Meta-Analysis. Dig. Liver Dis. 2016, 48, 571–577. [Google Scholar] [CrossRef]
- On Behalf of the PRECISION ITALIA STUDY GROUP; Golfieri, R.; Giampalma, E.; Renzulli, M.; Cioni, R.; Bargellini, I.; Bartolozzi, C.; Breatta, A.D.; Gandini, G.; Nani, R.; et al. Randomised Controlled Trial of Doxorubicin-Eluting Beads vs Conventional Chemoembolisation for Hepatocellular Carcinoma. Br. J. Cancer 2014, 111, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, A.; Kolligs, F.; Dollinger, M.; Schott, E.; Wege, H.; Bitzer, M.; Gog, C.; Lammert, F.; Schuchmann, M.; Walter, C.; et al. TACE plus Sorafenib for the Treatment of Hepatocellular Carcinoma: Results of the Multicenter, Phase II SOCRATES Trial. Cancer Chemother. Pharmacol. 2014, 74, 947–954. [Google Scholar] [CrossRef]
- Jin, B.; Wang, D.; Lewandowski, R.J.; Riaz, A.; Ryu, R.K.; Sato, K.T.; Larson, A.C.; Salem, R.; Omary, R.A. Chemoembolization Endpoints: Effect on Survival Among Patients With Hepatocellular Carcinoma. Am. J. Roentgenol. 2011, 196, 919–928. [Google Scholar] [CrossRef]
- Habbel, V.S.A.; Zeile, M.; Stavrou, G.A.; Wacker, F.; Brüning, R.; Oldhafer, K.-J.; Rodt, T. Correlation between SACE (Subjective Angiographic Chemoembolization Endpoint) Score and Tumor Response and Its Impact on Survival after DEB-TACE in Patients with Hepatocellular Carcinoma. Abdom. Radiol. 2019, 44, 3463–3479. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, Multicentre Prospective Trial of Transarterial Chemoembolisation (TACE) plus Sorafenib as Compared with TACE Alone in Patients with Hepatocellular Carcinoma: TACTICS Trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef]
- Georgiades, C.S.; Hong, K.; D’Angelo, M.; Geschwind, J.-F.H. Safety and Efficacy of Transarterial Chemoembolization in Patients with Unresectable Hepatocellular Carcinoma and Portal Vein Thrombosis. J. Vasc. Interv. Radiol. 2005, 16, 1653–1659. [Google Scholar] [CrossRef]
- Ha, B.Y.; Ahmed, A.; Sze, D.Y.; Razavi, M.K.; Simpson, N.; Keeffe, E.B.; Nguyen, M.H. Long-Term Survival of Patients with Unresectable Hepatocellular Carcinoma Treated with Transcatheter Arterial Chemoinfusion. Aliment. Pharmacol. Ther. 2007, 26, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Prapong, W.; Sze, D.Y.; So, S.K.; Razavi, M.K. Treatment of Hepatocellular Carcinoma with Sub-Selective Transcatheter Arterial Oily Chemoinfusion. Tech. Vasc. Interv. Radiol. 2002, 5, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, H.-K.; Kim, S.Y.; Kim, K.M.; Ko, G.-Y.; Gwon, D.I.; Sung, K.-B. Transcatheter Arterial Chemoembolization vs. Chemoinfusion for Unresectable Hepatocellular Carcinoma in Patients with Major Portal Vein Thrombosis. Aliment. Pharmacol. Ther. 2009, 29, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Hilgard, P.; Hamami, M.; Fouly, A.E.; Scherag, A.; Müller, S.; Ertle, J.; Heusner, T.; Cicinnati, V.R.; Paul, A.; Bockisch, A.; et al. Radioembolization with Yttrium-90 Glass Microspheres in Hepatocellular Carcinoma: European Experience on Safety and Long-Term Survival. Hepatology 2010, 52, 1741–1749. [Google Scholar] [CrossRef]
- Sangro, B.; Carpanese, L.; Cianni, R.; Golfieri, R.; Gasparini, D.; Ezziddin, S.; Paprottka, P.M.; Fiore, F.; Van Buskirk, M.; Bilbao, J.I.; et al. Survival after Yttrium-90 Resin Microsphere Radioembolization of Hepatocellular Carcinoma across Barcelona Clinic Liver Cancer Stages: A European Evaluation. Hepatology 2011, 54, 868–878. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, B.J.; Kim, Y.H.; Han, K.-H.; Cho, S.B.; Cho, K.R.; Uhm, S.-H.; Choe, J.-G.; Choi, J.Y.; Chun, H.J.; et al. Radioembolization With Yttrium-90 Resin Microspheres in Hepatocellular Carcinoma: A Multicenter Prospective Study. Am. J. Clin. Oncol. 2015, 38, 495–501. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.J.; Mulcahy, M.F.; Riaz, A.; Ryu, R.K.; Ibrahim, S.; Atassi, B.; Baker, T.; Gates, V.; Miller, F.H.; et al. Radioembolization for Hepatocellular Carcinoma Using Yttrium-90 Microspheres: A Comprehensive Report of Long-Term Outcomes. Gastroenterology 2010, 138, 52–64. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.J.; Kulik, L.; Wang, E.; Riaz, A.; Ryu, R.K.; Sato, K.T.; Gupta, R.; Nikolaidis, P.; Miller, F.H.; et al. Radioembolization Results in Longer Time-to-Progression and Reduced Toxicity Compared with Chemoembolization in Patients with Hepatocellular Carcinoma. Gastroenterology 2011, 140, 497–507.e2. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Bhoori, S.; Romito, R.; Chiesa, C.; Morosi, C.; Maccauro, M.; Marchianò, A.; Bongini, M.; Lanocita, R.; et al. Yttrium-90 Radioembolization for Intermediate-Advanced Hepatocellular Carcinoma: A Phase 2 Study. Hepatology 2013, 57, 1826–1837. [Google Scholar] [CrossRef]
- Garin, E.; Lenoir, L.; Edeline, J.; Laffont, S.; Mesbah, H.; Porée, P.; Sulpice, L.; Boudjema, K.; Mesbah, M.; Guillygomarc’h, A.; et al. Boosted Selective Internal Radiation Therapy with 90Y-Loaded Glass Microspheres (B-SIRT) for Hepatocellular Carcinoma Patients: A New Personalized Promising Concept. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1057–1068. [Google Scholar] [CrossRef]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.-P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and Safety of Selective Internal Radiotherapy with Yttrium-90 Resin Microspheres Compared with Sorafenib in Locally Advanced and Inoperable Hepatocellular Carcinoma (SARAH): An Open-Label Randomised Controlled Phase 3 Trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Gabr, A.; Abouchaleh, N.; Ali, R.; Al Asadi, A.; Mora, R.A.; Kulik, L.; Ganger, D.; Desai, K.; Thornburg, B.; et al. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology 2018, 287, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Padia, S.A.; Geisinger, M.A.; Newman, J.S.; Pierce, G.; Obuchowski, N.A.; Sands, M.J. Effectiveness of Coil Embolization in Angiographically Detectable versus Non-Detectable Sources of Upper Gastrointestinal Hemorrhage. J. Vasc. Interv. Radiol. 2009, 20, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Biederman, D.M.; Titano, J.J.; Korff, R.A.; Fischman, A.M.; Patel, R.S.; Nowakowski, F.S.; Lookstein, R.A.; Kim, E. Radiation Segmentectomy versus Selective Chemoembolization in the Treatment of Early-Stage Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2018, 29, 30–37.e2. [Google Scholar] [CrossRef]
- Mehta, N.; Frenette, C.; Tabrizian, P.; Hoteit, M.; Guy, J.; Parikh, N.; Ghaziani, T.T.; Dhanasekaran, R.; Dodge, J.L.; Natarajan, B.; et al. Downstaging Outcomes for Hepatocellular Carcinoma: Results From the Multicenter Evaluation of Reduction in Tumor Size before Liver Transplantation (MERITS-LT) Consortium. Gastroenterology 2021, 161, 1502–1512. [Google Scholar] [CrossRef]
- Katsanos, K.; Kitrou, P.; Spiliopoulos, S.; Maroulis, I.; Petsas, T.; Karnabatidis, D. Comparative Effectiveness of Different Transarterial Embolization Therapies Alone or in Combination with Local Ablative or Adjuvant Systemic Treatments for Unresectable Hepatocellular Carcinoma: A Network Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2017, 12, e0184597. [Google Scholar] [CrossRef]
- Lewis, S.R.; Pritchard, M.W.; Evans, D.J.; Butler, A.R.; Alderson, P.; Smith, A.F.; Roberts, I. Colloids versus Crystalloids for Fluid Resuscitation in Critically Ill People. Cochrane Database Syst. Rev. 2018, 8, 1465–1858. [Google Scholar] [CrossRef]
- Lewis, A.L.; Taylor, R.R.; Hall, B.; Gonzalez, M.V.; Willis, S.L.; Stratford, P.W. Pharmacokinetic and Safety Study of Doxorubicin-Eluting Beads in a Porcine Model of Hepatic Arterial Embolization. J. Vasc. Interv. Radiol. 2006, 17, 1335–1343. [Google Scholar] [CrossRef]
- Tang, Y.; Taylor, R.R.; Gonzalez, M.V.; Lewis, A.L.; Stratford, P.W. Evaluation of Irinotecan Drug-Eluting Beads: A New Drug–Device Combination Product for the Chemoembolization of Hepatic Metastases. J. Control. Release 2006, 116, e55–e56. [Google Scholar] [CrossRef]
- Ashrafi, K.; Tang, Y.; Britton, H.; Domenge, O.; Blino, D.; Bushby, A.J.; Shuturminska, K.; den Hartog, M.; Radaelli, A.; Negussie, A.H.; et al. Characterization of a Novel Intrinsically Radiopaque Drug-Eluting Bead for Image-Guided Therapy: DC Bead LUMITM. J. Control. Release 2017, 250, 36–47. [Google Scholar] [CrossRef]
- Sharma, K.V.; Dreher, M.R.; Tang, Y.; Pritchard, W.; Chiesa, O.A.; Karanian, J.; Peregoy, J.; Orandi, B.; Woods, D.; Donahue, D.; et al. Development of “Imageable” Beads for Transcatheter Embolotherapy. J. Vasc. Interv. Radiol. 2010, 21, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, A.; Thanoo, B.C.; Rathinam, K.; Mohanty, M. Preparation and Evaluation of Radiopaque Hydrogel Microspheres Based on PHEMA/Iothalamic Acid and PHEMA/Iopanoic Acid as Particulate Emboli. J. Biomed. Mater. Res. 1990, 24, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Thanoo, B.C.; Jayakrishnan, A. Radiopaque Hydrogel Microspheres. J. Microencapsul. 1989, 6, 233–244. [Google Scholar] [CrossRef]
- Horák, D.; Metalová, M.; Svec, F.; Drobník, J.; Kálal, J.; Borovicka, M.; Adamyan, A.A.; Voronkova, O.S.; Gumargalieva, K.Z. Hydrogels in Endovascular Embolization. III. Radiopaque Spherical Particles, Their Preparation and Properties. Biomaterials 1987, 8, 142–145. [Google Scholar] [CrossRef]
- Pesapane, F.; Nezami, N.; Patella, F.; Geschwind, J.F. New Concepts in Embolotherapy of HCC. Med. Oncol. 2017, 34, 58. [Google Scholar] [CrossRef] [PubMed]
- Duran, R.; Sharma, K.; Dreher, M.R.; Ashrafi, K.; Mirpour, S.; Lin, M.; Schernthaner, R.E.; Schlachter, T.R.; Tacher, V.; Lewis, A.L.; et al. A Novel Inherently Radiopaque Bead for Transarterial Embolization to Treat Liver Cancer—A Pre-Clinical Study. Theranostics 2016, 6, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.; Langman, M.; Werner-Zwanziger, U.; Abraham, R.J.; Boyd, D. Mixture Designs to Assess Composition–Structure–Property Relationships in SiO2–CaO–ZnO–La2O3–TiO2–MgO–SrO–Na2O Glasses: Potential Materials for Embolization. J. Biomater. Appl. 2013, 28, 416–433. [Google Scholar] [CrossRef]
- Yamamoto, A.; Chervoneva, I.; Sullivan, K.L.; Eschelman, D.J.; Gonsalves, C.F.; Mastrangelo, M.J.; Berd, D.; Shields, J.A.; Shields, C.L.; Terai, M.; et al. High-Dose Immunoembolization: Survival Benefit in Patients with Hepatic Metastases from Uveal Melanoma. Radiology 2009, 252, 290–298. [Google Scholar] [CrossRef]
- Berg, M.; Wingender, G.; Djandji, D.; Hegenbarth, S.; Momburg, F.; Hämmerling, G.; Limmer, A.; Knolle, P. Cross-Presentation of Antigens from Apoptotic Tumor Cells by Liver Sinusoidal Endothelial Cells Leads to Tumor-Specific CD8+ T Cell Tolerance. Eur. J. Immunol. 2006, 36, 2960–2970. [Google Scholar] [CrossRef]
- Weiskirch, L.M.; Bar-Dagan, Y.; Mokyr, M.B. Transforming Growth Factor-Beta-Mediated down-Regulation of Antitumor Cytotoxicity of Spleen Cells from MOPC-315 Tumor-Bearing Mice Engaged in Tumor Eradication Following Low-Dose Melphalan Therapy. Cancer Immunol. Immunother. 1994, 38, 215–224. [Google Scholar] [CrossRef]
- Kambayashi, T.; Alexander, H.R.; Fong, M.; Strassmann, G. Potential Involvement of IL-10 in Suppressing Tumor-Associated Macrophages. Colon-26-Derived Prostaglandin E2 Inhibits TNF-Alpha Release via a Mechanism Involving IL-10. J. Immunol. 1995, 154, 3383–3390. [Google Scholar] [PubMed]
- Bot, F.J.; van Eijk, L.; Schipper, P.; Löwenberg, B. Human Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Stimulates Immature Marrow Precursors but No CFU-GM, CFU-G, or CFU-M. Exp. Hematol. 1989, 17, 292–295. [Google Scholar] [PubMed]
- Albert, M.L.; Sauter, B.; Bhardwaj, N. Dendritic Cells Acquire Antigen from Apoptotic Cells and Induce Class I-Restricted CTLs. Nature 1998, 392, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.G.; McCaughan, G.W.; Bertolino, P. Intrahepatic Immunity: A Tale of Two Sites? Trends Immunol. 2005, 26, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-Y.; Jiang, G.; Yang, C.-S.; Tang, J.-Q.; Wei, Z.-P.; Liu, Y.-Q. Application of Nanotechnology in the Diagnosis and Therapy of Hepatocellular Carcinoma. PRA 2016, 11, 322–331. [Google Scholar] [CrossRef] [PubMed]
- European Society of Radiology (ESR) Medical Imaging in Personalised Medicine: A White Paper of the Research Committee of the European Society of Radiology (ESR). Insights Imaging 2015, 6, 141–155. [CrossRef] [PubMed]
- Degrauwe, N.; Hocquelet, A.; Digklia, A.; Schaefer, N.; Denys, A.; Duran, R. Theranostics in Interventional Oncology: Versatile Carriers for Diagnosis and Targeted Image-Guided Minimally Invasive Procedures. Front. Pharmacol. 2019, 10, 450. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Sechi, M.; Sanna, V.; Pala, N. Targeted Therapy Using Nanotechnology: Focus on Cancer. IJN 2014, 467. [Google Scholar] [CrossRef]
- Mohapatra, A.; Uthaman, S.; Park, I.-K. External and Internal Stimuli-Responsive Metallic Nanotherapeutics for Enhanced Anticancer Therapy. Front. Mol. Biosci. 2021, 7, 597634. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Geschwind, J.-F.; Liapi, E.; Salem, R. Transcatheter Intraarterial Therapies: Rationale and Overview. Radiology 2011, 259, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Yi, M.; Li, N.; Wu, K.; Wu, K. Advances of Targeted Therapy for Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 719896. [Google Scholar] [CrossRef]
- Ding, X.; Sun, W.; Li, W.; Shen, Y.; Guo, X.; Teng, Y.; Liu, X.; Zheng, L.; Li, W.; Chen, J. Transarterial Chemoembolization plus Lenvatinib versus Transarterial Chemoembolization plus Sorafenib as First-Line Treatment for Hepatocellular Carcinoma with Portal Vein Tumor Thrombus: A Prospective Randomized Study. Cancer 2021, 127, 3782–3793. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, J.; Li, H.-Y.; Wang, Z.-H.; Wu, J. Immunotherapy for Advanced Hepatocellular Carcinoma, Where Are We? Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2020, 1874, 188441. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 Expression in the Tumor Microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef]
- Niu, M.; Yi, M.; Li, N.; Luo, S.; Wu, K. Predictive Biomarkers of Anti-PD-1/PD-L1 Therapy in NSCLC. Exp. Hematol. Oncol. 2021, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Bucalau, A.-M.; Tancredi, I.; Verset, G. In the Era of Systemic Therapy for Hepatocellular Carcinoma Is Transarterial Chemoembolization Still a Card to Play? Cancers 2021, 13, 5129. [Google Scholar] [CrossRef]
- Fong, Z.V.; Palazzo, F.; Needleman, L.; Brown, D.B.; Eschelman, D.J.; Chojnacki, K.A.; Yeo, C.J.; Rosato, E.L. Combined Hepatic Arterial Embolization and Hepatic Ablation for Unresectable Colorectal Metastases to the Liver. Am. Surg. 2012, 78, 1243–1248. [Google Scholar] [CrossRef]
- Dawoud, M.A.; Mohamed, R.E.; El Waraki, M.S.; Gabr, A.M. Single-Session Combined Radiofrequency Ablation and Transarterial Chemoembolization in the Treatment of Hepatocellular Carcinoma. Egypt J. Radiol. Nucl. Med. 2017, 48, 935–946. [Google Scholar] [CrossRef]
- Yamashita, Y.-I.; Takeishi, K.; Tsuijita, E.; Yoshiya, S.; Morita, K.; Kayashima, H.; Iguchi, T.; Taketomi, A.; Shirabe, K.; Maehara, Y. Beneficial Effects of Preoperative Lipiodolization for Resectable Large Hepatocellular Carcinoma (≥5 Cm in Diameter). J. Surg. Oncol. 2012, 106, 498–503. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.-D.; Lu, L.; Wu, H.; Yu, J.-J.; Zhang, W.-G.; Pawlik, T.M.; Zhang, Y.-M.; Zhou, Y.-H.; Gu, W.-M.; et al. Preoperative Transcatheter Arterial Chemoembolization for Surgical Resection of Huge Hepatocellular Carcinoma (≥ 10 Cm): A Multicenter Propensity Matching Analysis. Hepatol. Int. 2019, 13, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Hwang, S.; Lee, Y.-J.; Kim, K.-H.; Ko, G.-Y.; Gwon, D.I.; Ahn, C.-S.; Moon, D.-B.; Ha, T.-Y.; Song, G.-W.; et al. Prognostic Effect of Preoperative Sequential Transcatheter Arterial Chemoembolization and Portal Vein Embolization for Right Hepatectomy in Patients with Solitary Hepatocellular Carcinoma. Korean J. Hepatobiliary Pancreat. Surg. 2015, 19, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-P.; Lai, E.C.H.; Li, A.-J.; Fu, S.-Y.; Zhou, J.-P.; Pan, Z.-Y.; Lau, W.Y.; Wu, M.-C. A Prospective, Randomized, Controlled Trial of Preoperative Transarterial Chemoembolization for Resectable Large Hepatocellular Carcinoma. Ann. Surg. 2009, 249, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Hasegawa, H.; Kinoshita, H.; Furukawa, M.; Imaoka, S.; Takasaki, K.; Kakumoto, Y.; Saitsu, H.; Yamada, R.; Oosaki, Y.; et al. A Prospective Randomized Trial of the Preventive Effect of Pre-Operative Transcatheter Arterial Embolization against Recurrence of Hepatocellular Carcinoma. Jpn J. Cancer Res. 1996, 87, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-F.; Guo, R.-P.; Zou, R.-H.; Shen, J.-X.; Wei, W.; Li, S.-H.; OuYang, H.-Y.; Zhu, H.-B.; Xu, L.; Lao, X.-M.; et al. Efficacy and Safety of Preoperative Chemoembolization for Resectable Hepatocellular Carcinoma with Portal Vein Invasion: A Prospective Comparative Study. Eur. Radiol. 2016, 26, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
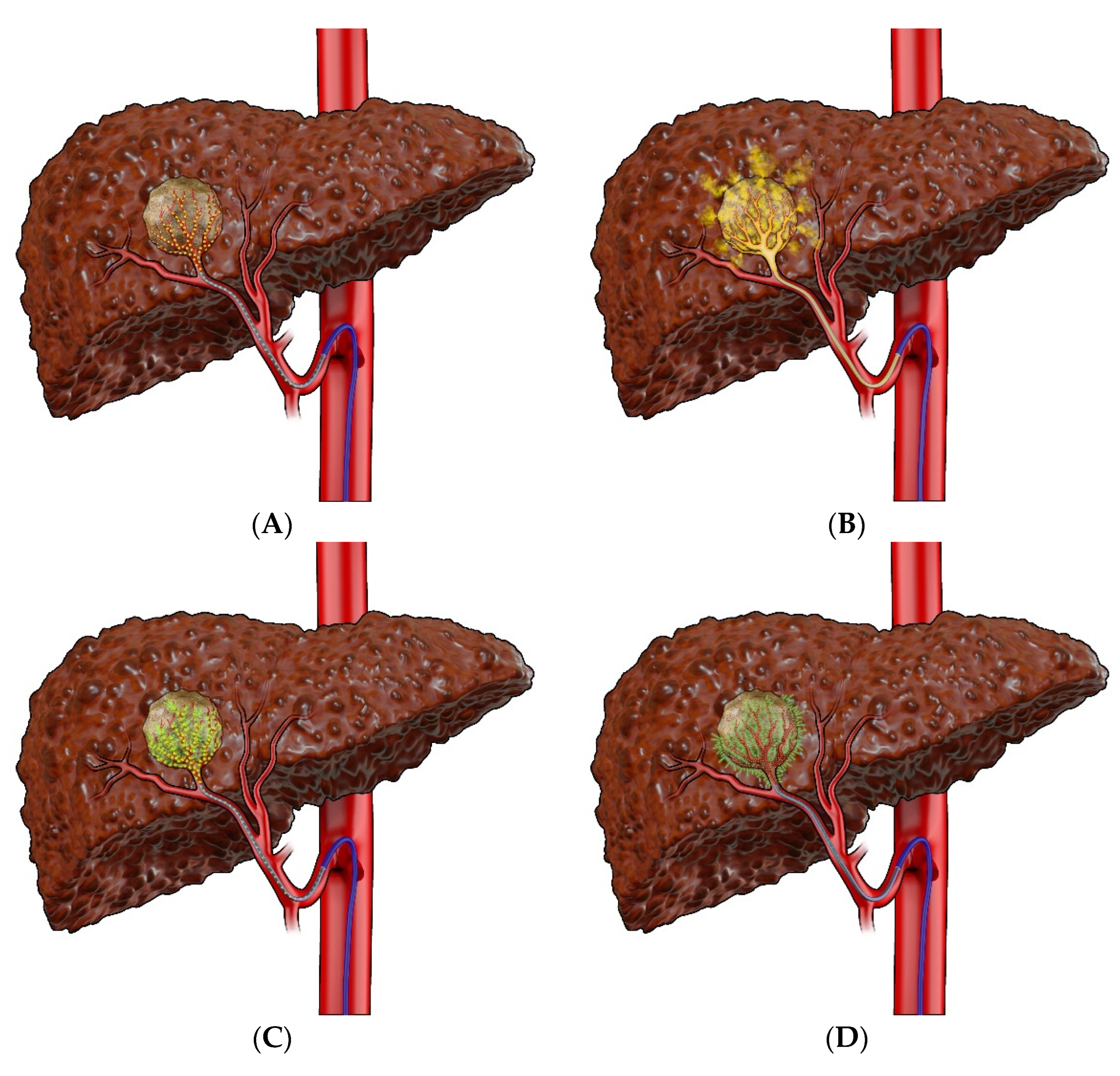
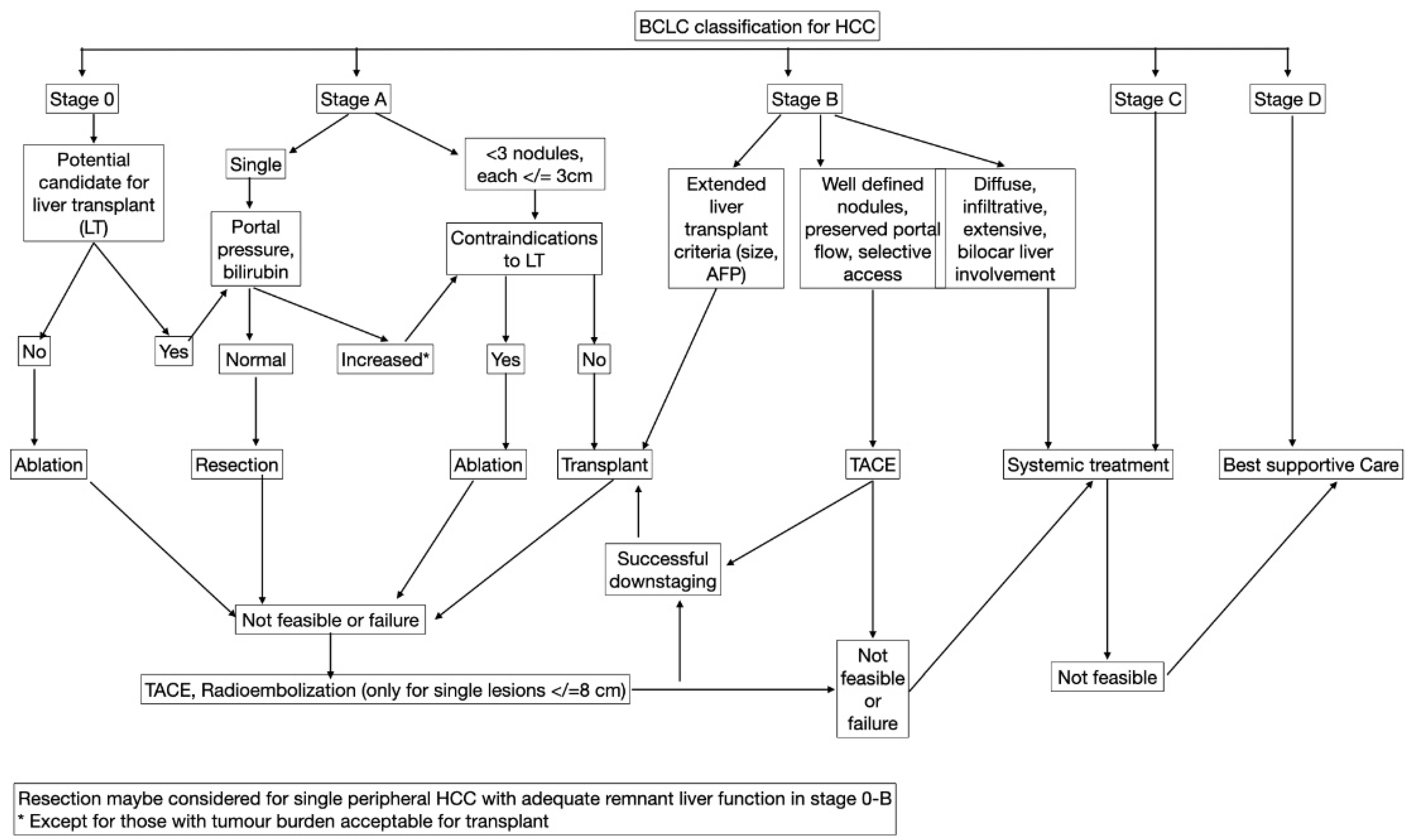

| Stages | Characteristics |
|---|---|
| Very early stage (0) | Single lesion ≤2 cm Preserved liver function Performance status: 0 (fully active) |
| Early stage (A) | Single lesion or ≤3 nodules each ≤3 cm in size Preserved liver function Performance status: 0 |
| Intermediate stage (B) | Multinodular Preserved liver function Performance status: 0 |
| Advanced stage (C) | Portal invasion and/or extrahepatic spread Preserved liver function Performance status: 1 (cannot do heavy physical work) or 2 (up and about more than half the day, can look after self but cannot work) |
| Terminal stage (D) | Any tumor burden End-stage liver function Performance status: 3 (in bed or a chair for more than half the day and need help to look after self) or 4 (in bed or chair all the time needing complete care) |
| Grade | ECOG Performance Status |
|---|---|
| 0 | Fully active |
| 1 | Cannot do heavy physical work |
| 2 | Up and about more than half the day, can look after self but cannot work |
| 3 | In bed or a chair for more than half the day and need help to look after self |
| 4 | In bed or chair all the time needing complete care |
| 5 | Dead |
| 6 | Fully active |
| Parameter | Points Assigned | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Ascites | Absent | Slight | Moderate |
| Serum bilirubin | <2 mg dL−1 (<34.2 micromol L−1) | 2 to 3 mg dL−1 (34.2 to 51.3 micromol L−1) | >3 mg dL−1 (>51.3 micromol L−1) |
| Serum albumin | >3.5 mg dL−1 (35 g L−1) | 2.8 to 3.5 g dL−1 (28 to 35 g L−1) | <2.8 g dL−1 (<28 g L−1) |
| Prothrombin time or INR | <4 or <1.7 | 4 to 6 or 1.7 to 2.3 | >6 or >2.3 |
| Encephalopathy | None | Grade 1 to 2 | Grade 3 to 4 |
| ALBI Grade | Score |
|---|---|
| Grade 1 | ≤2.6 |
| Grade 2 | >2.6 to 1.39 |
| Grade 3 | >1.39 |
| pALBI Grade | Score |
|---|---|
| Grade 1 | ≤−2.53 |
| Grade 2 | >−2.53 and ≤−2.09 |
| Grade 3 | >−2.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, T.; Shrigiriwar, A.; Habibollahi, P.; Cristescu, M.; Liddell, R.P.; Chapiro, J.; Inglis, P.; Camacho, J.C.; Nezami, N. Intraarterial Therapies for the Management of Hepatocellular Carcinoma. Cancers 2022, 14, 3351. https://doi.org/10.3390/cancers14143351
Garg T, Shrigiriwar A, Habibollahi P, Cristescu M, Liddell RP, Chapiro J, Inglis P, Camacho JC, Nezami N. Intraarterial Therapies for the Management of Hepatocellular Carcinoma. Cancers. 2022; 14(14):3351. https://doi.org/10.3390/cancers14143351
Chicago/Turabian StyleGarg, Tushar, Apurva Shrigiriwar, Peiman Habibollahi, Mircea Cristescu, Robert P. Liddell, Julius Chapiro, Peter Inglis, Juan C. Camacho, and Nariman Nezami. 2022. "Intraarterial Therapies for the Management of Hepatocellular Carcinoma" Cancers 14, no. 14: 3351. https://doi.org/10.3390/cancers14143351
APA StyleGarg, T., Shrigiriwar, A., Habibollahi, P., Cristescu, M., Liddell, R. P., Chapiro, J., Inglis, P., Camacho, J. C., & Nezami, N. (2022). Intraarterial Therapies for the Management of Hepatocellular Carcinoma. Cancers, 14(14), 3351. https://doi.org/10.3390/cancers14143351







