Sex Differences in the Effect of Vitamin D on Fatigue in Palliative Cancer Care—A Post Hoc Analysis of the Randomized, Controlled Trial ‘Palliative-D’
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of Fatigue
2.2. Ethical Considerations
2.3. Statistical Analysis
3. Results
3.1. Fatigue
3.2. Opioid-Induced Fatigue
3.3. QoL, Opioids, and Antibiotics
3.4. 25-Hydroxyvitamin D
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bower, J.E. Cancer-related fatigue-mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Ingham, G.; Urban, K.; Allingham, S.F.; Blanchard, M.; Marston, C.; Currow, D.C. The level of distress from fatigue reported in the final two months of life by a palliative care population; An Australian national prospective, consecutive case series. J. Pain Symptom Manag. 2020, 61, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Lundh Hagelin, C.; Wengström, Y.; Fürst, C.J. Patterns of fatigue related to advanced disease and radiotherapy in patients with cancer-a comparative cross-sectional study of fatigue intensity and characteristics. Support. Care Cancer 2009, 17, 519–526. [Google Scholar] [CrossRef]
- Al Maqbali, M.; Al Sinani, M.; Al Naamani, Z.; Al Badi, K.; Tanash, M.I. Prevalence of Fatigue in Patients with Cancer: A Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2021, 61, 167–189.e14. [Google Scholar] [CrossRef]
- Omdal, R. The biological basis of chronic fatigue: Neuroinflammation and innate immunity. Curr. Opin. Neurol. 2020, 33, 391–396. [Google Scholar] [CrossRef]
- Paulsen, Ø.; Laird, B.; Aass, N.; Lea, T.; Fayers, P.; Kaasa, S.; Klepstad, P. The relationship between pro-inflammatory cytokines and pain, appetite and fatigue in patients with advanced cancer. PLoS ONE 2017, 12, e0177620. [Google Scholar] [CrossRef]
- Xiao, C.; Eldridge, R.C.; Beitler, J.J.; Higgins, K.A.; Chico, C.E.; Felger, J.C.; Wommack, E.C.; Knobf, T.; Saba, N.F.; Shin, D.M.; et al. Association Among Glucocorticoid Receptor Sensitivity, Fatigue, and Inflammation in Patients with Head and Neck Cancer. Psychosom. Med. 2020, 82, 508–516. [Google Scholar] [CrossRef]
- Klasson, C.; Helde Frankling, M.; Lundh Hagelin, C.; Bjorkhem-Bergman, L. Fatigue in Cancer Patients in Palliative Care—A Review on Pharmacological Interventions. Cancers 2021, 13, 985. [Google Scholar] [CrossRef]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef]
- Mucke, M.; Mochamat Cuhls, H.; Peuckmann-Post, V.; Minton, O.; Stone, P.; Radbruch, L. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst. Rev. 2015, 5, CD006788. [Google Scholar]
- Hagelin, C.L.; Wengstrom, Y.; Ahsberg, E.; Furst, C.J. Fatigue dimensions in patients with advanced cancer in relation to time of survival and quality of life. Palliat. Med. 2009, 23, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.F.; Stewart, K.; Arseneault, R.; Moineddin, R.; Cellarius, V.; Librach, S.L.; Dudgeon, D. Women experience higher levels of fatigue than men at the end of life: A longitudinal home palliative care study. J. Pain Symptom Manag. 2007, 33, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Miaskowski, C. Gender differences in pain, fatigue, and depression in patients with cancer. JNCI Monogr. 2004, 2004, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helde Frankling, M.; Klasson, C.; Sandberg, C.; Nordström, M.; Warnqvist, A.; Bergqvist, J.; Bergman, P.; Björkhem-Bergman, L. ‘Palliative-D’—Vitamin D Supplementation to Palliative Cancer Patients: A Double Blind, Randomized Placebo-Controlled Multicenter Trial. Cancers 2021, 13, 3707. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Helde-Frankling, M.; Bergqvist, J.; Klasson, C.; Nordström, M.; Höijer, J.; Bergman, P.; Björkhem-Bergman, L. Vitamin D supplementation to palliative cancer patients: Protocol of a double-blind, randomised controlled trial ‘Palliative-D’. BMJ Support. Palliat. Care 2017, 7, 458–463. [Google Scholar] [CrossRef]
- Klasson, C.; Helde-Frankling, M.; Sandberg, C.; Nordström, M.; Lundh-Hagelin, C.; Björkhem-Bergman, L. Vitamin D and Fatigue in Palliative Cancer: A Cross-Sectional Study of Sex Difference in Baseline Data from the Palliative D Cohort. J. Palliat. Med. 2020, 24, 433–437. [Google Scholar] [CrossRef]
- Bruera, E.; Kuehn, N.; Miller, M.J.; Selmser, P.; Macmillan, K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J. Palliat. Care 1991, 7, 6–9. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present, and Future Developments. J. Pain Symptom Manag. 2017, 53, 630–643. [Google Scholar] [CrossRef] [Green Version]
- Bedard, G.; Zeng, L.; Zhang, L.; Lauzon, N.; Holden, L.; Tsao, M.; Danjoux, C.; Barnes, E.; Sahgal, A.; Poon, M.; et al. Minimal clinically important differences in the Edmonton symptom assessment system in patients with advanced cancer. J. Pain Symptom Manag. 2013, 46, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Shamieh, O.; Paiva, C.E.; Perez-Cruz, P.E.; Kwon, J.H.; Muckaden, M.A.; Park, M.; Yennu, S.; Kang, J.H.; Bruera, E. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: A prospective, multicenter study. Cancer 2015, 121, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Oldenmenger, W.H.; de Raaf, P.J.; de Klerk, C.; van der Rijt, C.C. Cut points on 0–10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: A systematic review. J. Pain Symptom Manag. 2013, 45, 1083–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selby, D.; Cascella, A.; Gardiner, K.; Do, R.; Moravan, V.; Myers, J.; Chow, E. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J. Pain Symptom Manag. 2010, 39, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Seow, H.; Sussman, J.; Martelli-Reid, L.; Pond, G.; Bainbridge, D. Do high symptom scores trigger clinical actions? An audit after implementing electronic symptom screening. J. Oncol. Pract. 2012, 8, e142–e148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundh Hagelin, C.; Klarare, A.; Fürst, C.J. The applicability of the translated Edmonton Symptom Assessment System: Revised [ESAS-r] in Swedish palliative care. Acta Oncol. 2018, 57, 560–562. [Google Scholar] [CrossRef]
- Groenvold, M.; Petersen, M.A.; Aaronson, N.K.; Arraras, J.I.; Blazeby, J.M.; Bottomley, A.; Fayers, P.M.; de Graeff, A.; Hammerlid, E.; Kaasa, S.; et al. The development of the EORTC QLQ-C15-PAL: A shortened questionnaire for cancer patients in palliative care. Eur. J. Cancer 2006, 42, 55–64. [Google Scholar] [CrossRef]
- Derogar, M.; van der Schaaf, M.; Lagergren, P. Reference values for the EORTC QLQ-C30 quality of life questionnaire in a random sample of the Swedish population. Acta Oncol. 2012, 51, 10–16. [Google Scholar] [CrossRef]
- Michelson, H.; Bolund, C.; Brandberg, Y. Multiple chronic health problems are negatively associated with health related quality of life (HRQoL) irrespective of age. Qual. Life Res. 2000, 9, 1093–1104. [Google Scholar] [CrossRef]
- Michelson, H.; Bolund, C.; Nilsson, B.; Brandberg, Y. Health-related quality of life measured by the EORTC QLQ-C30—Reference values from a large sample of Swedish population. Acta Oncol. 2000, 39, 477–484. [Google Scholar]
- Bergman, P.; Sperneder, S.; Höijer, J.; Bergqvist, J.; Björkhem-Bergman, L. Low vitamin D levels are associated with higher opioid dose in palliative cancer patients—Results from an observational study in Sweden. PLoS ONE 2015, 10, e0128223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björkhem-Bergman, L.; Bergman, P. Vitamin D and patients with palliative cancer. BMJ Support. Palliat. Care 2016, 6, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Helde-Frankling, M.; Björkhem-Bergman, L. Vitamin D in Pain Management. Int. J. Mol. Sci. 2017, 18, 2170. [Google Scholar] [CrossRef] [Green Version]
- Koole, J.L.; Bours, M.J.; van Roekel, E.H.; Breedveld-Peters, J.J.; van Duijnhoven, F.J.; van den Ouweland, J.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; Weijenberg, M.P. Higher serum vitamin D concentrations are longitudinally associated with better global quality of life and less fatigue in colorectal cancer survivors up to 2 years after treatment. Cancer Epidemiol. Prevent. Biomark. 2020, 29, 1135–1144. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Alonso, M.; Dusso, A.; Ariza, G.; Nabal, M. Vitamin D deficiency and its association with fatigue and quality of life in advanced cancer patients under palliative care: A cross-sectional study. Palliat. Med. 2016, 30, 89–96. [Google Scholar] [CrossRef]
- Nowak, A.; Boesch, L.; Andres, E.; Battegay, E.; Hornemann, T.; Schmid, C.; Bischoff-Ferrari, H.A.; Suter, P.M.; Krayenbuehl, P.-A. Effect of vitamin D3 on self-perceived fatigue: A double-blind randomized placebo-controlled trial. Medicine 2016, 95, e5353. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Sherman, A.; Monari-Sparks, M.J.; Schweiker, O.; Hunter, K. Correction of Low Vitamin D Improves Fatigue: Effect of Correction of Low Vitamin D in Fatigue Study (EViDiF Study). N. Am. J. Med. Sci. 2014, 6, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.; Gonzalez, A.; Yue, J.; Wu, X.; Qiu, M.; Gui, L.; Zhu, S.; Huang, L. Efficacy and Safety of Vitamin D Supplementation in Patients with Systemic Lupus Erythematosus: A Meta-analysis of Randomized Controlled Trials. Am. J. Med. Sci. 2019, 358, 104–114. [Google Scholar] [CrossRef]
- Jagannath, V.A.; Filippini, G.; Di Pietrantonj, C.; Asokan, G.V.; Robak, E.W.; Whamond, L.; Robinson, S.A. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst. Rev. 2018, 9, Cd008422. [Google Scholar] [CrossRef]
- Martinez-Alonso, M.; Dusso, A.; Ariza, G.; Nabal, M. The effect on quality of life of vitamin D administration for advanced cancer treatment (VIDAFACT study): Protocol of a randomised controlled trial. BMJ Open 2014, 4, e006128. [Google Scholar] [CrossRef] [Green Version]
- Schöttker, B.; Kuznia, S.; Laetsch, D.C.; Czock, D.; Kopp-Schneider, A.; Caspari, R.; Brenner, H. Protocol of the VICTORIA study: Personalized vitamin D supplementation for reducing or preventing fatigue and enhancing quality of life of patients with colorectal tumor-randomized intervention trial. BMC Cancer 2020, 20, 739. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and immune function: An overview. Proc. Nutr. Soc. 2012, 71, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Smith, C.; Jensen, M.; Holick, M.F.; Sauter, E.R. Vitamin D favorably alters the cancer promoting prostaglandin cascade. Anticancer Res. 2013, 33, 3861–3866. [Google Scholar] [PubMed]
- Hock, A.D. Review: Vitamin D3 deficiency results in dysfunctions of immunity with severe fatigue and depression in a variety of diseases. In Vivo 2014, 28, 133–145. [Google Scholar] [PubMed]
- Lerchbaum, E.; Pilz, S.; Trummer, C.; Rabe, T.; Schenk, M.; Heijboer, A.C.; Obermayer-Pietsch, B. Serum vitamin D levels and hypogonadism in men. Andrology 2014, 2, 748–754. [Google Scholar] [CrossRef]
- Spira, D.; Buchmann, N.; Konig, M.; Rosada, A.; Steinhagen-Thiessen, E.; Demuth, I.; Norman, K. Sex-specific differences in the association of vitamin D with low lean mass and frailty: Results from the Berlin Aging Study II. Nutrition 2019, 62, 1–6. [Google Scholar] [CrossRef]
- Anic, G.M.; Albanes, D.; Rohrmann, S.; Kanarek, N.; Nelson, W.G.; Bradwin, G.; Rifai, N.; McGlynn, K.A.; Platz, E.A.; Mondul, A.M. Association between serum 25-hydroxyvitamin D and serum sex steroid hormones among men in NHANES. Clin. Endocrinol. 2016, 85, 258–266. [Google Scholar] [CrossRef]
- De Angelis, C.; Galdiero, M.; Pivonello, C.; Garifalos, F.; Menafra, D.; Cariati, F.; Salzano, C.; Galdiero, G.; Piscopo, M.; Vece, A.; et al. The role of vitamin D in male fertility: A focus on the testis. Rev. Endocr. Metab. Disord. 2017, 18, 285–305. [Google Scholar] [CrossRef]
- Aversa, A.; Morgentaler, A. The practical management of testosterone deficiency in men. Nat. Rev. Urol. 2015, 12, 641–650. [Google Scholar] [CrossRef]
- Helde Frankling, M.; Klasson, C.; Björkhem-Bergman, L. Successful Strategies and Areas of Improvement-Lessons Learned from Design and Conduction of a Randomized Placebo-Controlled Trial in Palliative Care, ‘Palliative-D’. Life 2021, 11, 1233. [Google Scholar] [CrossRef] [PubMed]
| Variable | Vitamin D | Placebo | |||
|---|---|---|---|---|---|
| Men (n = 34) | Women (n = 33) | Men (n = 40) | Women (n = 43) | ||
| Age, years | 68 (60–75) | 69 (59–75) | 70 (64–73) | 68 (59–73) | |
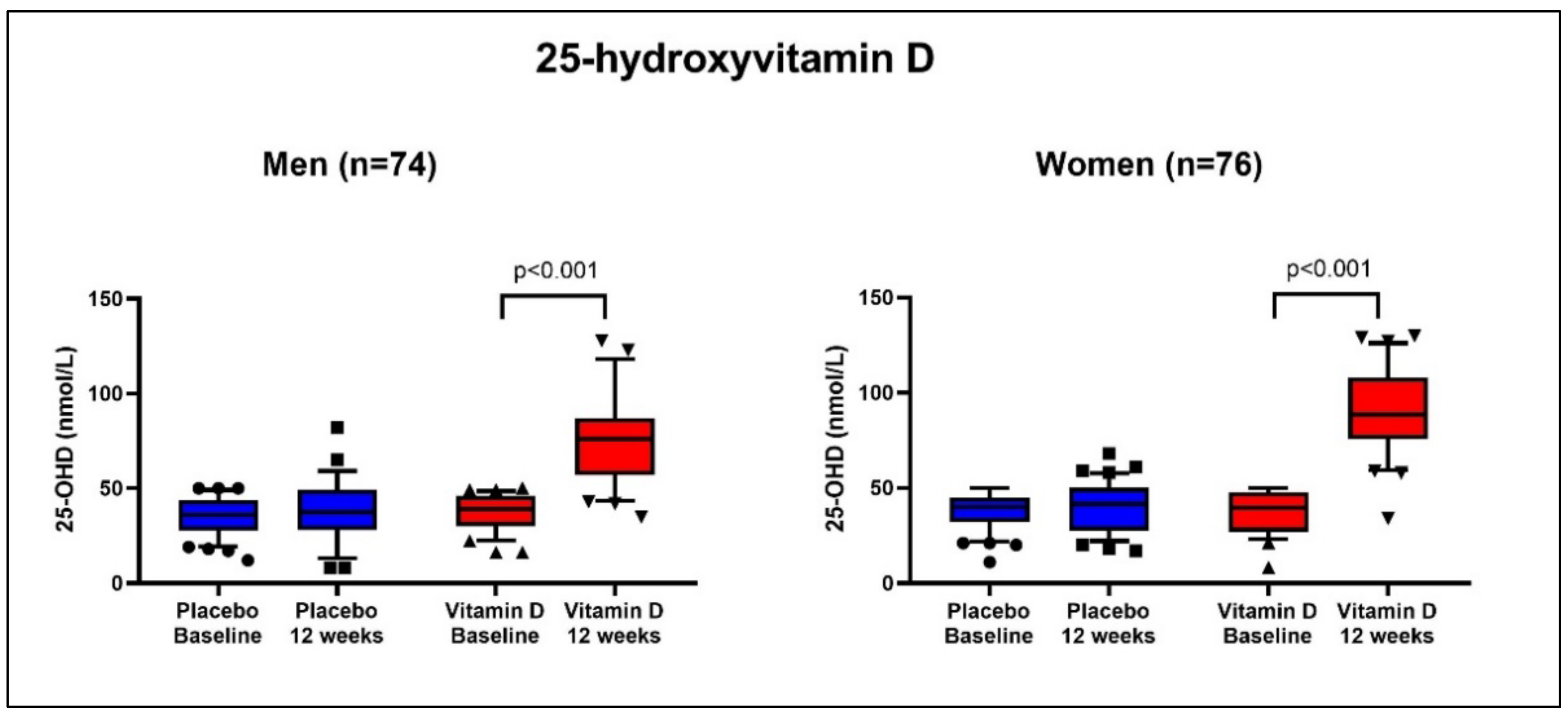
| 25-OHD, nmol/L | 39 (30–46) | 40 (27–48) | 36 (28–44) | 40 (32–45) | |
| Fentanyl dose, µg/h | 0 (0–25) | 0 (0–37) | 0 (0–22) | 0 (0–12) | |
| No. days on antibiotics/month | 0 (0–0) | 0 (0–3) | 0 (0–4) | 0 (0–0) | |
| Albumin, g/L | 32 (27–36) | 32 (29–36) | 31 (28–36) | 32 (28–34) | |
| Calcium, mmol/L | 2.31 (2.21–2.38) | 2.31 (2.23–2.42) | 2.30 (2.22–2.36) | 2.33 (2.26–2.41) | |
| Creatinine, µmol/L | 80 (68–97) | 61 (55–77) | 77 (67–96) | 64 (55–77) | |
| CRP, mg/L | 4 (1–31) | 7 (2–23) | 8 (3–29) | 5 (2–14) | |
| Type of cancer, No. patients | … … | … … | |||
| Brain | 0 | 1 | 1 | 0 | |
| Breast | 0 | 4 | 0 | 10 | |
| Upper gastrointestinal | 10 | 6 | 13 | 6 | |
| Lower gastrointestinal | 12 | 9 | 10 | 10 | |
| Gynecological | 0 | 7 | 0 | 8 | |
| Hematological | 1 | 1 | 1 | 0 | |
| Lung | 4 | 5 | 6 | 6 | |
| Prostate | 7 | 0 | 7 | 0 | |
| Sarcoma | 0 | 0 | 0 | 3 | |
| Urinary tract | 0 | 0 | 3 | 0 | |
| ESAS fatigue | 4 (1–5) | 3 (1–6) | 3 (1–5) | 3 (2–5) | |
| ESAS QoL | 3 (1–5) | 4 (2–6) | 4 (2–5) | 4 (2–5) | |
| EORTC QLQ-C15-PAL, Q11 | 2 (2–3) | 2 (2–3) | 3 (2–3) | 2 (2–3) | |
| EORTC QLQ-C15-PAL, Q15 | 4 (3–6) | 5 (4–5) | 4 (3–5) | 4 (3–5) | |
| Men n = 74 | ||
|---|---|---|
| Placebo n = 40, Vitamin D n = 34 | Unadjusted Model | Adjusted Model |
| Variable | β (95% CI) p-Value | β (95% CI) p-Value |
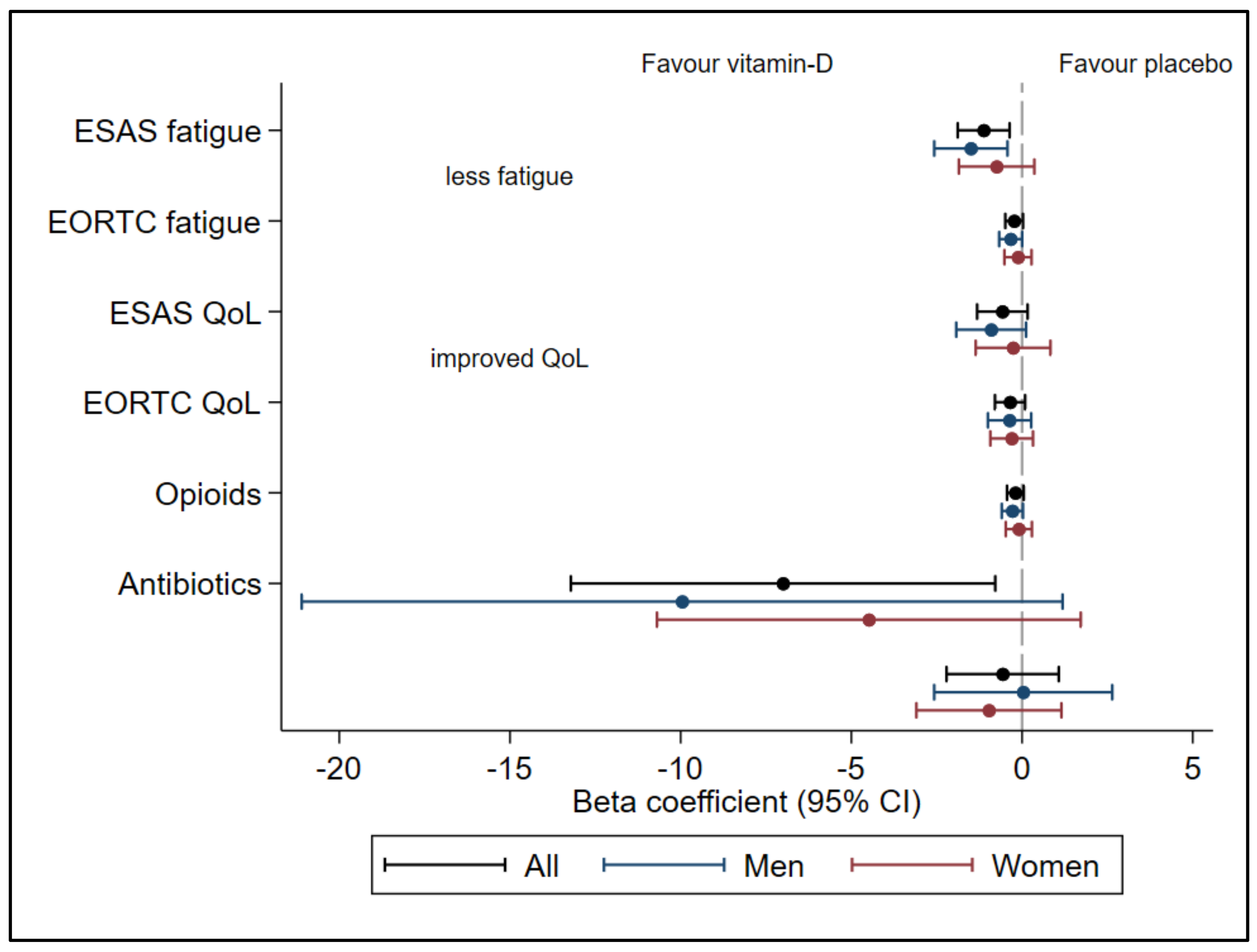
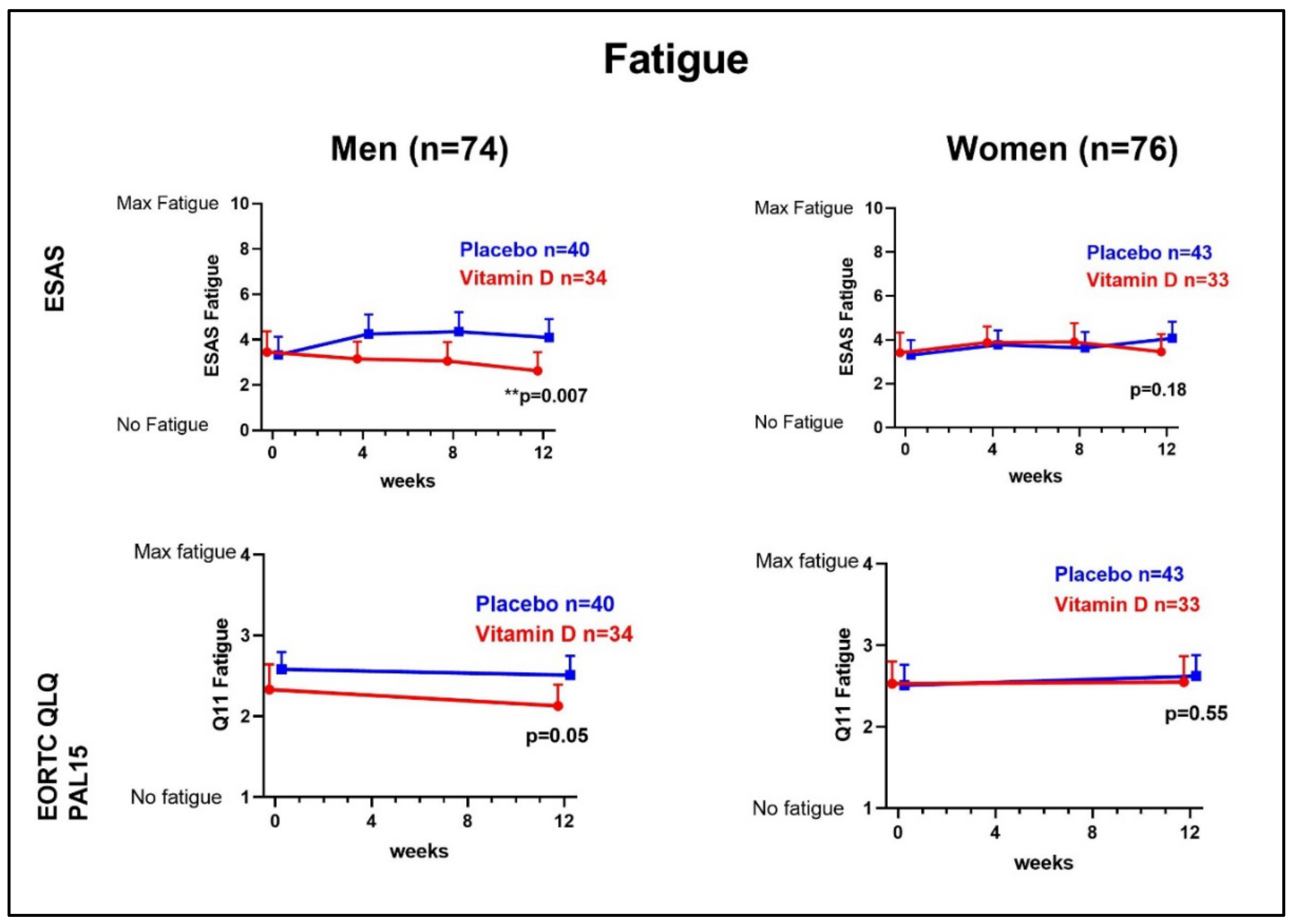
| Fatigue ESAS (0–10) | −1.50 (−2.57 to −0.43) | −1.47 (−2.59 to −0.35) |
| ** 0.007 | * 0.01 | |
| Fatigue EORTC QLQ-C15-PAL Q11 (1–4) | −0.33 (−0.67 to 0.03) | −0.38 (−0.74 to 0.03) |
| 0.05 | * 0.04 | |
| Fatigue EORTC QLQ-C15-PAL Q11 + Q7 (1–4) | −0.28 (−0.59 to 0.03) | −0.33 (−0.65 to −0.01) |
| 0.07 | * 0.046 | |
| QoL ESAS (0–10) | −0.90 (−1.93 to 0.12) | −1.03 (−2.11 to 0.06) |
| 0.08 | 0.06 | |
| QoL EORTC QLQ-C15-PAL Q15 (1–7) | 0.37 (−0.27 to 1.00) 0.25 | 0.41 (−0.26 to 1.08) 0.23 |
| Opioid doses (µg fentanyl/h) | −9.96 (−21.10 to 1.19) 0.08 | −5.67 (−17.34 to 5.99) 0.34 |
| Antibiotics (days/month) | 0.04 (−2.57 to 2.64) 0.98 | 0.99 (−1.68 to 3.67) 0.46 |
| Women n = 76 | ||
| Placebo n = 43 Vitamin D n = 33 | Unadjusted Model | Adjusted Model |
| Fatigue ESAS (0–10) | −0.75 (−1.85 to 0.36) 0.18 | −0.71 (−1.87 to 0.45) 0.23 |
| Fatigue EORTC QLQ-C15-PAL Q11 (1–4) | −0.12 (−0.52 to 0.28) 0.55 | −0.15 (−0.56 to 0.27) 0.49 |
| Fatigue EORTC QLQ-C15-PAL Q11 + Q7 (1–4) | −0.09 (−0.48 to 0.29) 0.62 | −0.11 (−0.51 to 0.29) 0.57 |
| QoL SAS (0–10) | −0.26 (−1.36 to 0.83) 0.63 | −0.33 (−1.49 to 0.83) 0.57 |
| QoL EORTC QLQ-C15-PAL Q15 (1–7) | 0.30 (−0.32 to 0.93) 0.34 | 0.34 (−0.31 to 1.00) 0.30 |
| Opioid doses (µg fentanyl/h) | −4.49 (−10.69 to 1.72) 0.15 | −3.39 (−9.70 to 2.93) 0.29 |
| Antibiotics (days/month) | −0.97 (−3.10 to 1.15) 0.37 | −0.97 (3.17 to 1.23) 0.38 |
| Men n = 74 | ||
|---|---|---|
| Placebo n = 40, Vitamin D n = 34 | Unadjusted Model | Opioid-Adjusted Model |
| Variable | β (95% CI) p-Value | β (95% CI) p-Value |
| Fatigue ESAS (0–10) | −1.50 (−2.57 to −0.43) | −1.42 (−2.51 to −0.34) |
| ** 0.007 | * 0.01 | |
| Fatigue EORTC QLQ-C15-PAL Q11 (1–4) | −0.33 (−0.67 to 0.03) 0.05 | −0.30 (−0.64 to 0.03) 0.08 |
| Fatigue EORTC QLQ-C15-PAL Q11 + Q7 (1–4) | −0.28 (−0.59 to 0.03) 0.07 | −0.26 (−0.57 to 0.05) 0.10 |
| Women n = 76 | ||
| Placebo n = 43 Vitamin D n = 33 | Unadjusted Model | Opioid-Adjusted Model |
| Fatigue ESAS (0–10) | −0.75 (−1.85 to 0.36) 0.18 | −0.75 (−1.86 to 0.36) 0.18 |
| Fatigue EORTC QLQ-C15-PAL Q11 (1–4) | −0.12 (−0.52 to 0.28) 0.55 | −0.12 (−0.52 to 0.28) 0.56 |
| Fatigue EORTC QLQ-C15-PALQ11 + Q7 (1–4) | −0.09 (−0.48 to 0.29) 0.62 | −0.09 (−0.48 to 0.29) 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klasson, C.; Helde Frankling, M.; Warnqvist, A.; Sandberg, C.; Nordström, M.; Lundh-Hagelin, C.; Björkhem-Bergman, L. Sex Differences in the Effect of Vitamin D on Fatigue in Palliative Cancer Care—A Post Hoc Analysis of the Randomized, Controlled Trial ‘Palliative-D’. Cancers 2022, 14, 746. https://doi.org/10.3390/cancers14030746
Klasson C, Helde Frankling M, Warnqvist A, Sandberg C, Nordström M, Lundh-Hagelin C, Björkhem-Bergman L. Sex Differences in the Effect of Vitamin D on Fatigue in Palliative Cancer Care—A Post Hoc Analysis of the Randomized, Controlled Trial ‘Palliative-D’. Cancers. 2022; 14(3):746. https://doi.org/10.3390/cancers14030746
Chicago/Turabian StyleKlasson, Caritha, Maria Helde Frankling, Anna Warnqvist, Carina Sandberg, Marie Nordström, Carina Lundh-Hagelin, and Linda Björkhem-Bergman. 2022. "Sex Differences in the Effect of Vitamin D on Fatigue in Palliative Cancer Care—A Post Hoc Analysis of the Randomized, Controlled Trial ‘Palliative-D’" Cancers 14, no. 3: 746. https://doi.org/10.3390/cancers14030746
APA StyleKlasson, C., Helde Frankling, M., Warnqvist, A., Sandberg, C., Nordström, M., Lundh-Hagelin, C., & Björkhem-Bergman, L. (2022). Sex Differences in the Effect of Vitamin D on Fatigue in Palliative Cancer Care—A Post Hoc Analysis of the Randomized, Controlled Trial ‘Palliative-D’. Cancers, 14(3), 746. https://doi.org/10.3390/cancers14030746








