High-Dose-Rate Brachytherapy as an Organ-Sparing Treatment for Early Penile Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
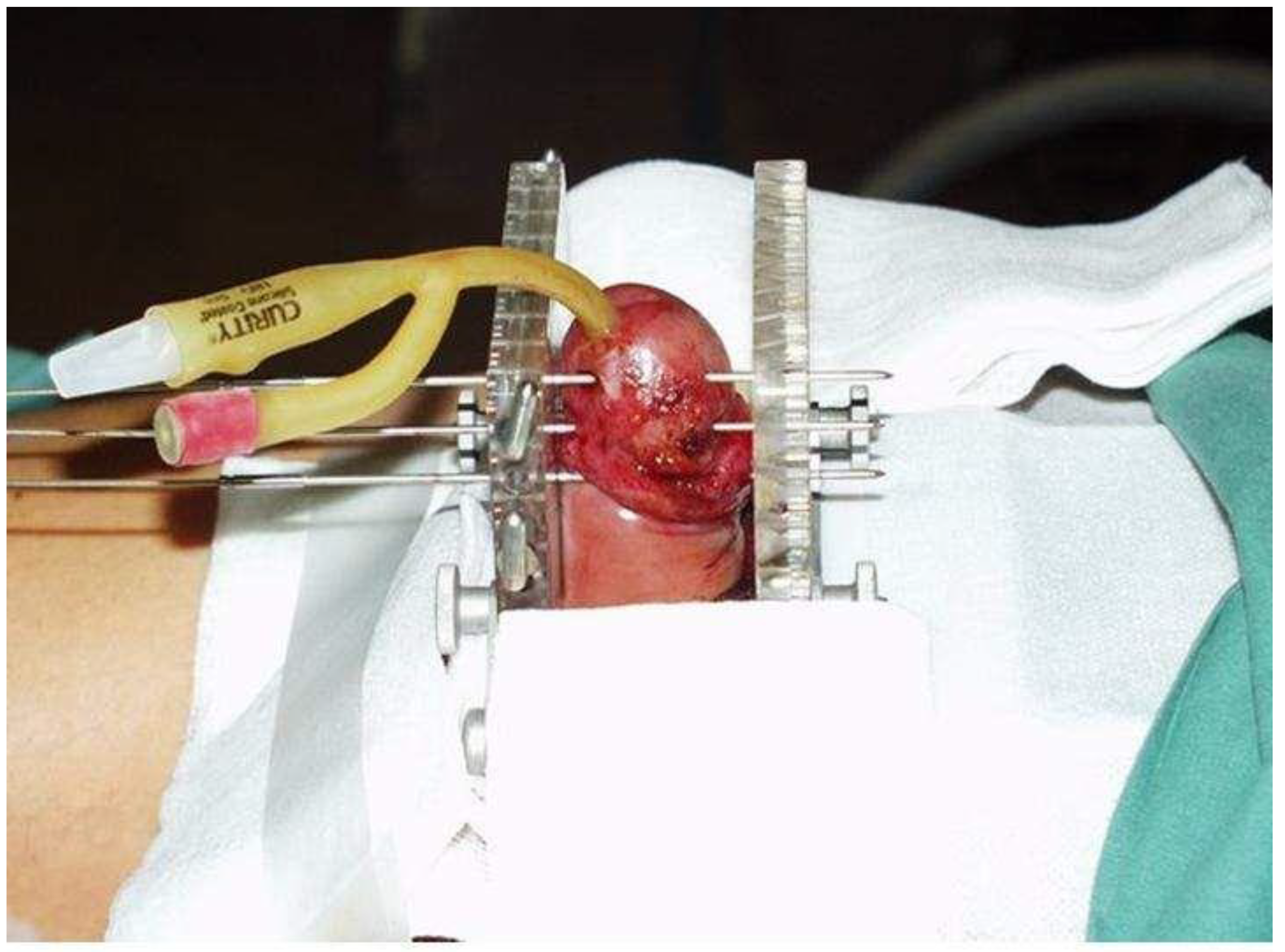
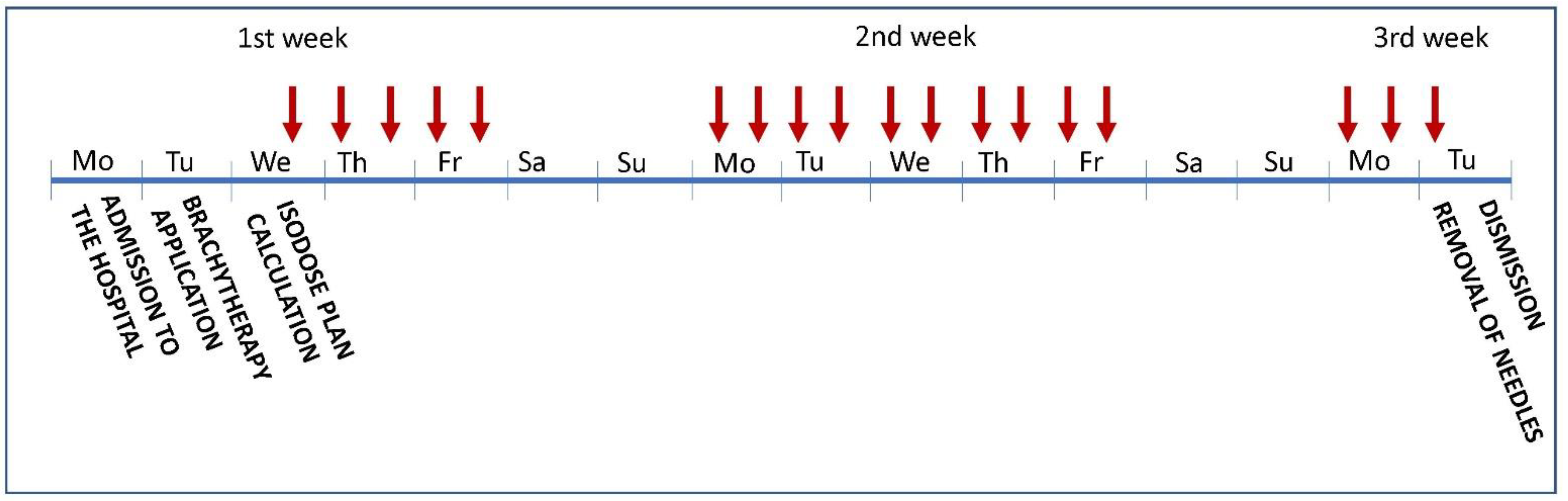
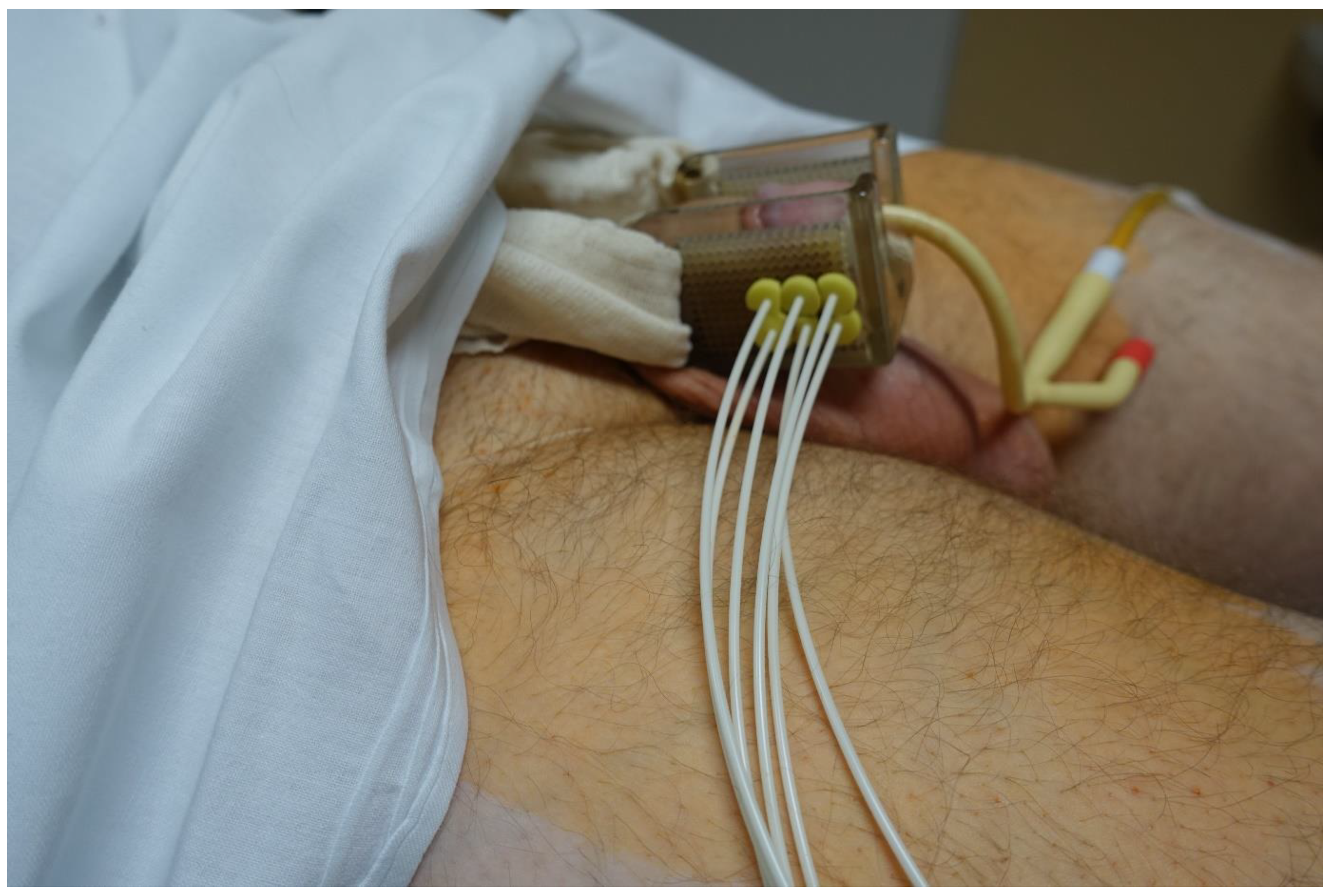
2.2. Brachytherapy
Brachytherapy Technique
2.3. Statistical Analysis
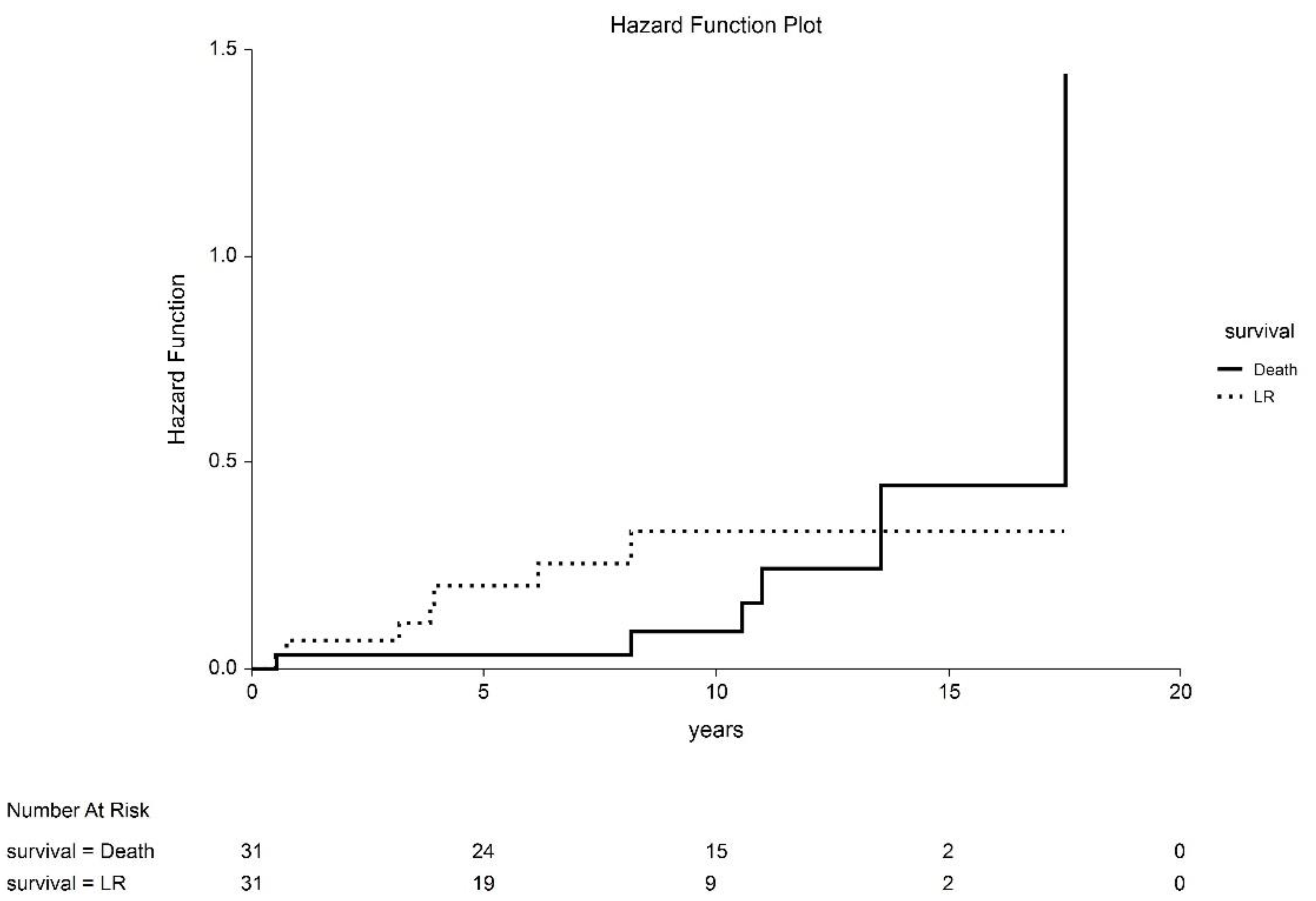
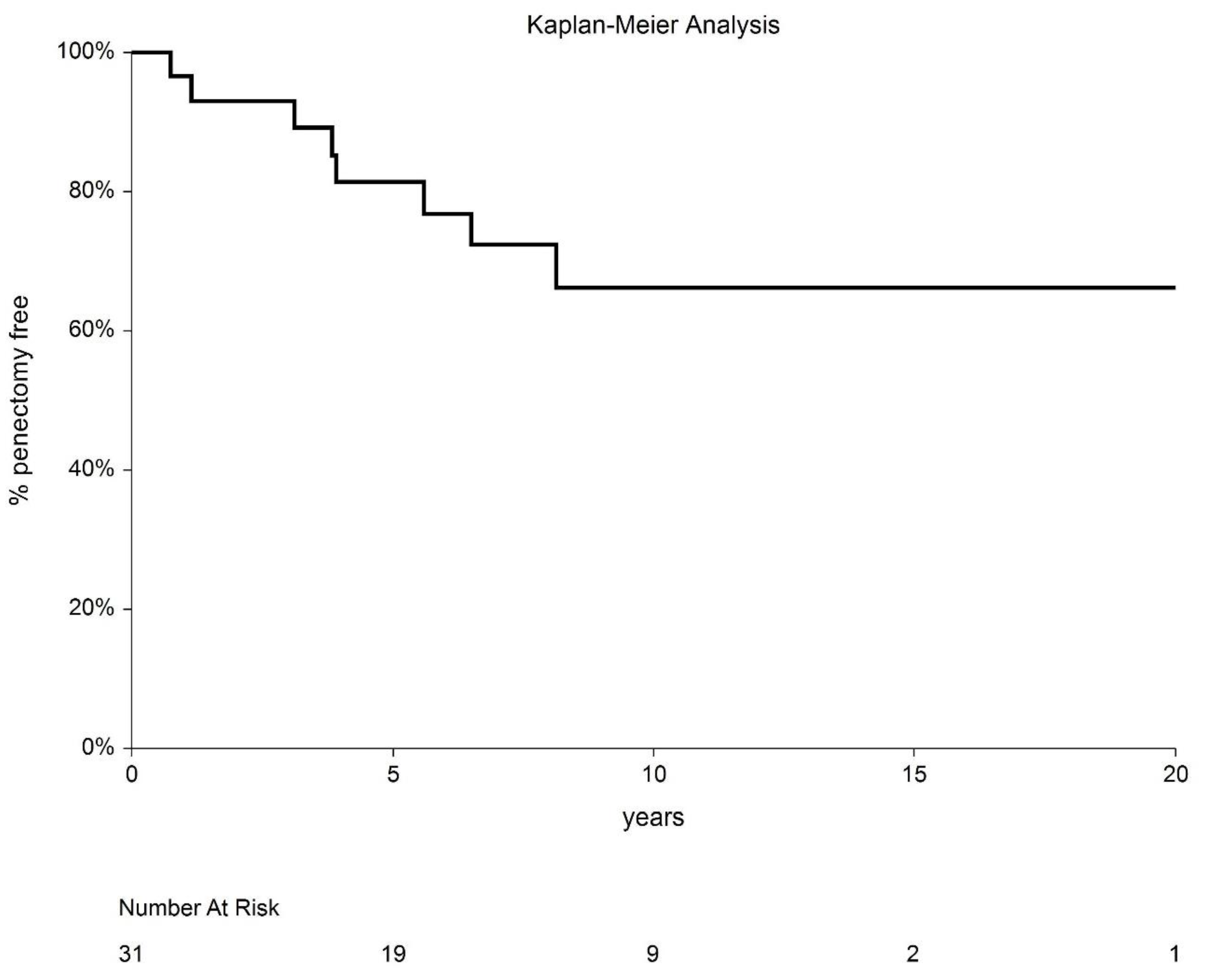
3. Results
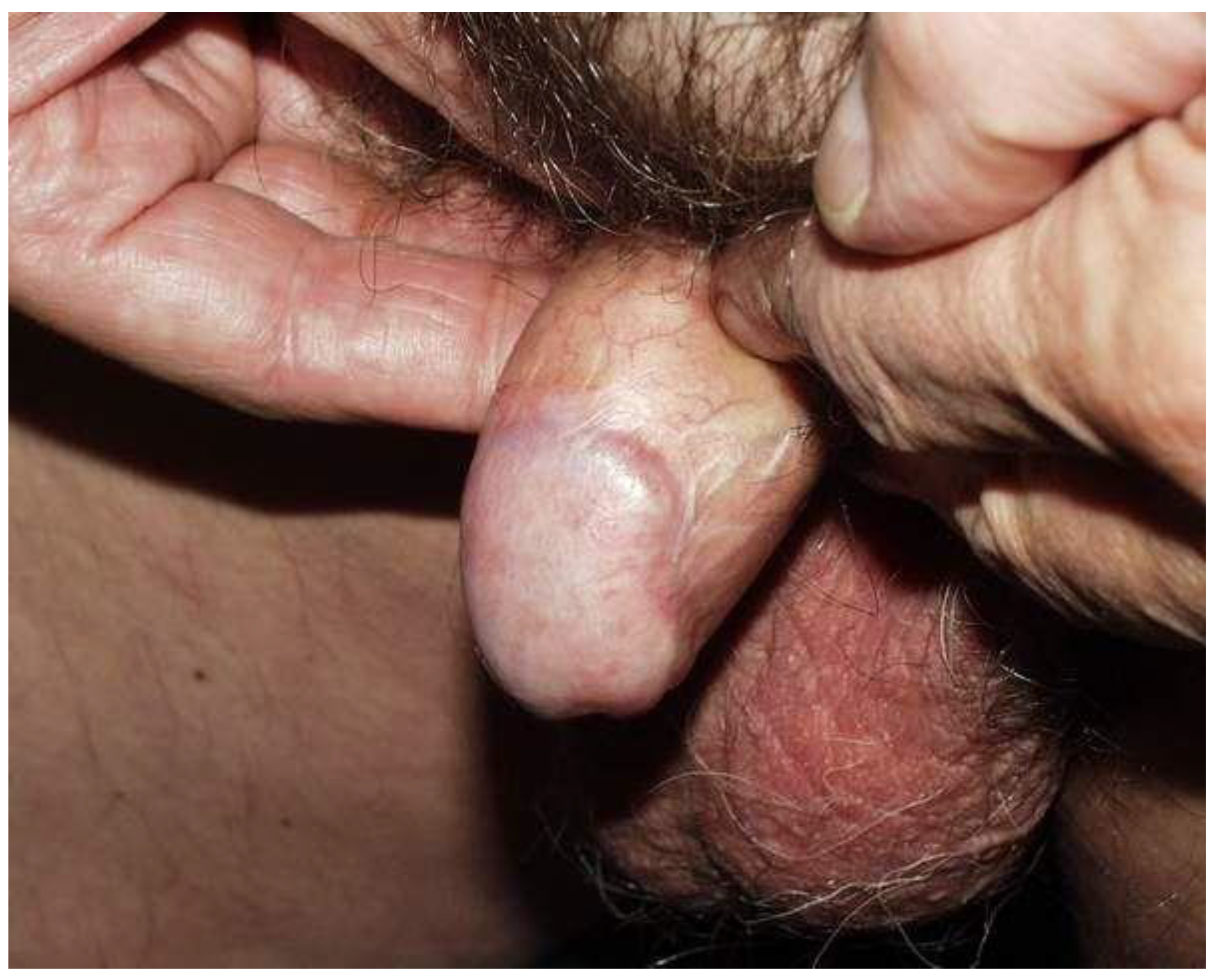
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crook, J.M.; Haie-Meder, C.; Demanes, D.J.; Mazeron, J.-J.; Martinez, A.A.; Rivard, M.J. American Brachytherapy Society–Groupe Européen de Curiethérapie–European Society of Therapeutic Radiation Oncology (ABS-GEC-ESTRO) Consensus Statement for Penile Brachytherapy. Brachytherapy 2013, 12, 191–198. [Google Scholar] [CrossRef]
- Fang, A.; Ferguson, J. Penile Sparing Techniques for Penile Cancer. Postgrad. Med. 2020, 132 (suppl. 4), 42–51. [Google Scholar] [CrossRef]
- Romero, F.R.; Richter Pereira dos Santos Romero, K.; de Mattos, M.A.E.; Camargo Garcia, C.R.; de Carvalho Fernandes, R.; Cardenuto Perez, M.D. Sexual Function after Partial Penectomy for Penile Cancer. Urology 2005, 66, 1292–1295. [Google Scholar] [CrossRef]
- Opjordsmoen, S.; Waehre, H.; Aass, N.; Fossa, S.D. Sexuality in Patients Treated for Penile Cancer: Patients’ Experience and Doctors’ Judgement. Br. J. Urol. 1994, 73, 554–560. [Google Scholar] [CrossRef]
- Korzeniowski, M.A.; Crook, J.M. Contemporary Role of Radiotherapy in the Management of Penile Cancer. Transl. Androl. Urol. 2017, 6, 855–867. [Google Scholar] [CrossRef]
- Hakenberg, O.W.; Dräger, D.L.; Erbersdobler, A.; Naumann, C.M.; Jünemann, K.-P.; Protzel, C. The Diagnosis and Treatment of Penile Cancer. Dtsch. Arztebl. Int. 2018, 115, 646–652. [Google Scholar] [CrossRef]
- Azrif, M.; Logue, J.P.; Swindell, R.; Cowan, R.A.; Wylie, J.P.; Livsey, J.E. External-Beam Radiotherapy in T1-2 N0 Penile Carcinoma. Clin. Oncol. 2006, 18, 320–325. [Google Scholar] [CrossRef]
- Steel, G.G. The Dose-Rate Effect: Brachytherapy. In Basic Clinical Radiobiology; Steel, G.G., Ed.; Edward Arnold Publishers: London, UK, 1993. [Google Scholar]
- Martz, N.; Bodokh, Y.; Gautier, M.; Thamphya, B.; Schiappa, R.; Lam Cham Kee, D.; Chevallier, D.; Hannoun, A.; Chand, M.-E.; Hannoun-Levi, J.-M. High-Dose Rate Brachytherapy in Localized Penile Cancer: 5-Year Clinical Outcome Analysis. Clin. Transl. Radiat. Oncol. 2021, 27, 89–95. [Google Scholar] [CrossRef]
- Kellas-Ślęczka, S.; Białas, B.; Fijałkowski, M.; Wojcieszek, P.; Szlag, M.; Cholewka, A.; Wesołowski, M.; Ślęczka, M.; Krzysztofiak, T.; Larysz, D.; et al. Nineteen-Year Single-Center Experience in 76 Patients with Penile Cancer Treated with High-Dose-Rate Brachytherapy. Brachytherapy 2019, 18, 493–502. [Google Scholar] [CrossRef]
- Marbán, M.; Crook, J.; Keyes, M.; Dubash, R.; Batchelar, D. High-Dose-Rate Brachytherapy for Localized Penile Cancer: Evolution of a Technique. Brachytherapy 2020, 19, 201–209. [Google Scholar] [CrossRef]
- Sharma, D.N.; Joshi, N.P.; Gandhi, A.K.; Haresh, K.P.; Gupta, S.; Julka, P.K.; Rath, G.K. High-Dose-Rate Interstitial Brachytherapy for T1–T2-Stage Penile Carcinoma: Short-Term Results. Brachytherapy 2014, 13, 481–487. [Google Scholar] [CrossRef]
- Rouscoff, Y.; Falk, A.T.; Durand, M.; Gal, J.; Chand, M.-E.; Gautier, M.; Marsaud, A.; Chevallier, D.; Amiel, J.; Hannoun-Levi, J.-M. High-Dose Rate Brachytherapy in Localized Penile Cancer: Short-Term Clinical Outcome Analysis. Radiat. Oncol. 2014, 9, 142. [Google Scholar] [CrossRef]
- Petera, J.; Sirák, I.; Kašaová, L.; Mačingová, Z.; Paluska, P.; Zouhar, M.; Kutílek, P.; Brod’ák, M.; Vošmik, M. High–Dose Rate Brachytherapy in the Treatment of Penile Carcinoma—First Experience. Brachytherapy 2011, 10, 136–140. [Google Scholar] [CrossRef]
- Petera, J.; Odrážka, K.; Zouhar, M.; Bedrošová, J.; Doležel, M. High-Dose-Rate Interstitial Brachytherapy for the Treatment of Penile Carcinoma. Strahlenther. Onkol. 2004, 180, 123–125. [Google Scholar] [CrossRef]
- Chaudhary, A.J.; Ghosh, S.; Bhalavat, R.L.; Kulkarni, J.N.; Sequeira, B.V.E. Interstitial Brachytherapy in Carcinoma of the Penis. Strahlenther. Onkol. 1999, 175, 17–20. [Google Scholar] [CrossRef]
- de Crevoisier, R.; Slimane, K.; Sanfilippo, N.; Bossi, A.; Albano, M.; Dumas, I.; Wibault, P.; Fizazi, K.; Gerbaulet, A.; Haie-Meder, C. Long-Term Results of Brachytherapy for Carcinoma of the Penis Confined to the Glans (N- or NX). Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1150–1156. [Google Scholar] [CrossRef]
- Crook, J.; Ma, C.; Grimard, L. Radiation Therapy in the Management of the Primary Penile Tumor: An Update. World J. Urol. 2009, 27, 189–196. [Google Scholar] [CrossRef]
- Dalannes, M.; Malavaud, B.; Douchez, J.; Bonnet, J.; Daly, N.J. Iridium-192 Interstitial Therapy for Squamous Cell Carcinoma of the Penis. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 479–483. [Google Scholar] [CrossRef]
- Kiltie, A.E.; Elwell, C.; Close, H.J.; Ash, D.V. Iridium-192 Implantation for Node-Negative Carcinoma of the Penis: The Cookridge Hospital Experience. Clin. Oncol. 2000, 12, 25–31. [Google Scholar] [CrossRef]
- Mazeron, J.J.; Langlois, D.; Lobo, P.A.; Huart, J.A.; Calitchi, E.; Lusinchi, A.; Raynal, M.; Le Bourgeois, J.P.; Abbou, C.C.; Pierquin, B. Interstitial Radiation Therapy for Carcinoma of the Penis Using Iridium 192 Wires: The Henri Mondor Experience (1970–1979). Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 1891–1895. [Google Scholar] [CrossRef]
- Rozan, R.; Albuisson, E.; Giraud, B.; Donnarieix, D.; Delannes, M.; Pigneux, J.; Hoffstetter, S.; Gerbaulet, A.; Chinet-Charrot, P.; Goupil, A.; et al. Interstitial Brachytherapy for Penile Carcinoma: A Multicentric Survey (259 Patients). Radiother. Oncol. 1995, 36, 83–93. [Google Scholar] [CrossRef]
- Soria, J.-C.; Fizazi, K.; Piron, D.; Kramar, A.; Gerbaulet, A.; Haie-Meder, C.; Perrin, J.-L.; Court, B.; Wibault, P.; Théodore, C. Squamous Cell Carcinoma of the Penis: Multivariate Analysis of Prognostic Factors and Natural History in a Monocentric Study with a Conservative Policy. Ann. Oncol. 1997, 8, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Cano, E.R.; Demanes, D.J.; Puthawala, A.A.; Vikram, B. The American Brachytherapy Society Recommendations for High-Dose-Rate Brachytherapy for Head-and-Neck Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Maddineni, S.B.; Lau, M.M.; Sangar, V.K. Identifying the needs of penile cancer sufferers: A systematic review of the quality of life, psychosexual and psychosocial literature in penile cancer. BMC Urol. 2009, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Francis, A.; Hagenauer, A.; Hirsh, A.; Kaminsky, D.; Traughber, B.; Abouassaly, R.; Ellis, R. The Role of Brachytherapy in Organ Preservation for Penile Cancer: A Meta-Analysis and Review of the Literature. Brachytherapy 2015, 14, 517–524. [Google Scholar] [CrossRef]


| Characteristic | n |
|---|---|
| Patients | 31 |
| Median age, years (range) | 58.5 (33–72) |
| Tumor localization Glans Corpus | 1 30 |
| TNM TisN0M0 (recurrence after surgery) T1 N0M0 T1N1M0 | 6 24 1 |
| Largest dimension, mm (range) | 10.5 (5–30) |
| Median depth of invasion below the basement membrane at T1, mm (range) | 2.0 (0.2–6) |
| Histology, squamous cell carcinoma | 31 |
| Tumor grade G1 G2 G3 | 22 8 1 |
| Combined treatment Adjuvant irradiation of inguinal nodes Inguinal lymphadenectomy at the time of biopsy Inguinal lymphadenectomy after brachytherapy | 1 1 1 |
| Median follow-up, months (range) | 117 (5–210) |
| Characteristic | n |
|---|---|
| Number of catheters, median (range) | 5 (2–12) |
| Number of planes, median (range) | 2 (1–3) |
| Mean V100, cm3 (range) | 6.56 (2.12–11) |
| Mean V150, cm3 (range) | 2.26 (0.27–5) |
| Mean V200, cm3 (range) | 1.14 (0–3) |
| Mean DHI (range) | 0.64 (0.46–0.89) |
| Study | Patients, n | Dose, Gy | Mean FU, Months (Range) | 5y-LRFS, % | 5-y CSS, % | Complication Rates, Necrosis/Stenosis, % | % Penile Preservation/Years |
|---|---|---|---|---|---|---|---|
| LDR-BT | |||||||
| Chaudhary et al. [16] | 23 | 50 | 21 (4–117) | 70 | n.s. | 0/9 | 70/8 |
| de Crevoisier et al. [17] | 144 | 65 | 68 (6–348) | 80 | 92 | 26/ 29 | 72/10 |
| Crook et al. [18] | 67 | 60 | 48 (6–194) | 87 | 83.6 | 12/ 9 | 88/5 |
| Delannes et al. [19] | 51 | 50-65 | 65 (12–144) | 86 | 85 | 23/45 | 75/5 |
| Kiltie et al. [20] | 31 | 63.5 | 61.5 | 81 | 85.4 | 8/44 | 75/5 |
| Mazeron et al. [21] | 50 | 60–70 | 36–96+ | 78 | 6/19 | 74/5 | |
| Rozan et al. [22] | 184 | 63 | 139 | 86 | 88 | 21/45 | 78/10 |
| Soria et al. [23] | 102 | 61–70 | 111 | 77 | 72 | n.s. | 72/6 |
| HDR-BT | |||||||
| Martz et al. [9] | 29 | 35-39/9/F/5d BID | 72.4 | 86 | 82 | 10.3/7 | 79.3/5 |
| Kellas-Slezcka et al. [10] | 76 | 42.8/48.2 3-3.5/F BID | 76 (7–204) | 65.6 | 85 | 2.6/1.3 | 66.9/10 |
| Marbán et al. [11] | 7 | 38.4/12 F—53.04/17F BID | 90 | 86 | 86 | 43/43 | 86/7.5 |
| Sharma et al. [12] | 14 | 42-51/14-17 F BID | 22 | 86 | n.s. | 0/0 | 93/3 |
| Rouscoff et al. [13] | 12 | 36/9 F/5d— 39/9 F/5d BID | 27 (5.1–83) | 83 | 100 | 9/9 | 92/5 |
| Petera et al. [14] | 10 | 54/18 F/9d BID | 20 (3.4–90.6) | No recurrence during FU | 100% during FU | 0/0 | 100% during FU |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohanková, D.; Sirák, I.; Vošmik, M.; Kašaová, L.; Grepl, J.; Paluska, P.; Holub, L.; Špaček, J.; Hodek, M.; Kopeček, M.; et al. High-Dose-Rate Brachytherapy as an Organ-Sparing Treatment for Early Penile Cancer. Cancers 2022, 14, 6248. https://doi.org/10.3390/cancers14246248
Pohanková D, Sirák I, Vošmik M, Kašaová L, Grepl J, Paluska P, Holub L, Špaček J, Hodek M, Kopeček M, et al. High-Dose-Rate Brachytherapy as an Organ-Sparing Treatment for Early Penile Cancer. Cancers. 2022; 14(24):6248. https://doi.org/10.3390/cancers14246248
Chicago/Turabian StylePohanková, Denisa, Igor Sirák, Milan Vošmik, Linda Kašaová, Jakub Grepl, Petr Paluska, Lukáš Holub, Jiří Špaček, Miroslav Hodek, Martin Kopeček, and et al. 2022. "High-Dose-Rate Brachytherapy as an Organ-Sparing Treatment for Early Penile Cancer" Cancers 14, no. 24: 6248. https://doi.org/10.3390/cancers14246248
APA StylePohanková, D., Sirák, I., Vošmik, M., Kašaová, L., Grepl, J., Paluska, P., Holub, L., Špaček, J., Hodek, M., Kopeček, M., & Petera, J. (2022). High-Dose-Rate Brachytherapy as an Organ-Sparing Treatment for Early Penile Cancer. Cancers, 14(24), 6248. https://doi.org/10.3390/cancers14246248







