1. Introduction
Brachytherapy (BT) is associated with a high conformity—a high dose to the tumor region and a steep dose drop-off, with consequential optimum protection of organs at risk (OAR) [
1,
2,
3]. BT for prostate cancer was evaluated in many studies, confirming an excellent local control and acceptable side effects [
4,
5,
6]. It can be applied as a permanent interstitial BT (LDR, low dose rate) and a temporary interstitial BT (HDR, high dose rate) and can also be applied alternatively in many centers [
7].
BT is a standard treatment method for prostate cancer in national and international guidelines [
1], with a considerably reduced integral dose in comparison to external beam radiotherapy (EBRT). Several studies compared the dosimetry between EBRT and BT [
8].
Our study included a large group of patients treated in clinical routine in the last years. A hydrogel spacer was only injected before HDR brachytherapy, as this treatment was usually applied as a boost to EBRT, thus resulting in a higher radiobiological dose and a larger risk for rectal toxicity.
The aim was to analyze and compare the dose distribution in (dose to the macroscopic tumor) and outside (dose to potential microscopic spread beyond the capsule) the prostate between LDR brachytherapy at different intervals (intraoperative, day 1, and day 30 dose distribution) and HDR brachytherapy. The impact of a hydrogel spacer and different prostate volumes was evaluated to specify the additional advantage in respect of the dose to the organs at risk (predominantly dose to the rectum) and the dose to the prostate (possibly improved coverage with a larger distance to an organ at risk). The dose distribution outside the prostate, comparison at different LDR brachytherapy planning and post-planning intervals, evaluation of the advantage of applying a hydrogel spacer, and consideration of different prostate volumes have not been considered in previously published literature and thus improve our knowledge in understanding differences between different brachytherapy techniques.
A literature review was performed to explain the clinical consequences that could result from the dosimetric findings in this study.
2. Materials and Methods
The intraoperative dose distribution of 102 LDR-BT (125-I sources with a source activity of 17.8 MBq [0.48 mCi]) patients was compared with the dose distribution of 105 HDR-BT (192-Ir source) patients (232 HDR-BT fractions). The injection of 10 mL hydrogel spacer (SpaceOAR
® System, Boston Scientific, Marlborough, MA, USA) was performed under transrectal ultrasound (TRUS) guidance after dissecting the space between the prostate and rectum with a saline/lidocaine solution under local anesthesia, as explained in detail in prior publications [
9]. A hydrogel spacer (10 mL) was only applied before HDR-BT, as HDR-BT was commonly applied as a boost to EBRT (resulting in a higher radiobiological dose), whereas LDR-BT was always performed as a monotherapy treatment in a single intervention.
The target volume for all patients was the prostate volume and the base of seminal vesicles without additional margins (CTV, clinical target volume = PTV, planning target volume). The extent of seminal vesicles included was based on individual patient factors, in particular the localization of the tumor in magnetic resonance imaging (MRI) and the localization of positive probes.
The prescription dose was 145 Gy for all LDR-BT patients, using 62 ± 10 (mean ± standard deviation) stranded sources and 21 ± 3 application needles. All patients have been treated with brachytherapy as monotherapy. The prescription dose was 9 Gy (151 fractions, treatment as a boost to EBRT, 1.8–2 Gy single fractions up to 50–50.4 Gy total dose) or 11.5 Gy (81 fractions, treatment as monotherapy in three fractions) for HDR-BT patients, using 14 ± 2 application needles. Treatment planning for LDR-BT was performed using VariSeed 9.0, and treatment planning for HDR-BT was performed using Vitesse 3.0 (Varian, Palo Alto, CA, USA).
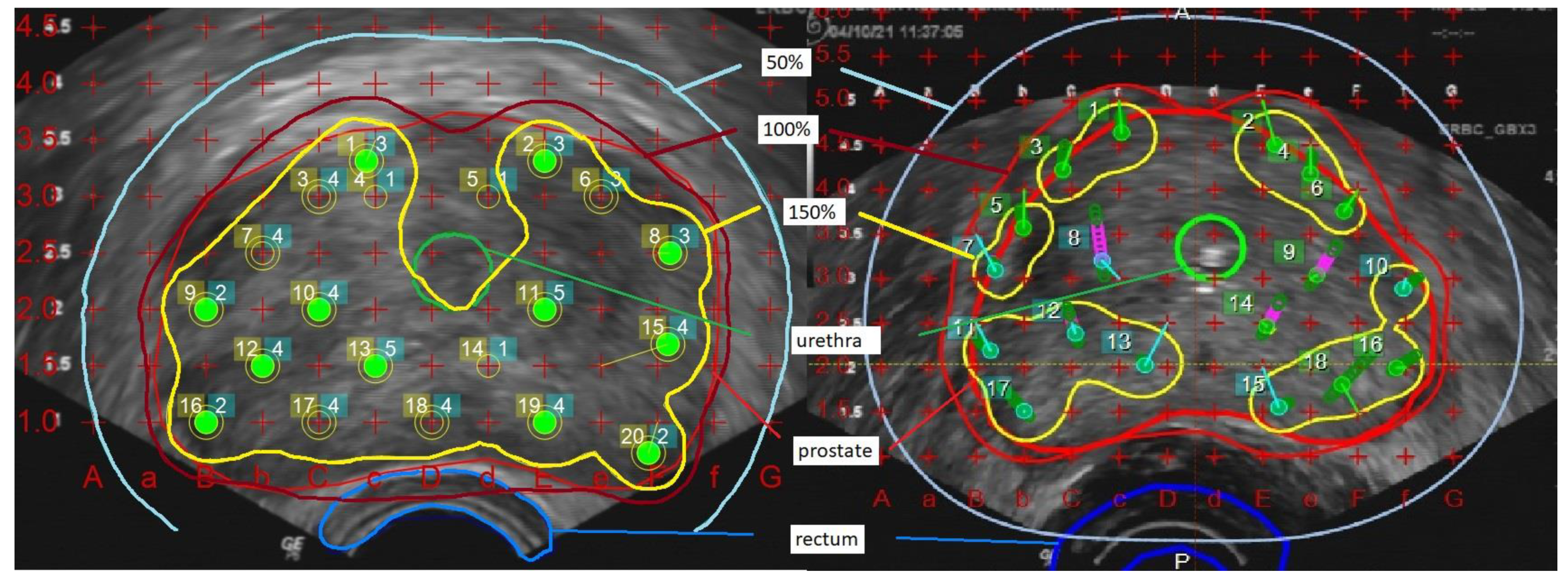
The comparison was based on dose levels relative to the respective prescription dose. For the analysis of the dose coverage outside the prostate, a 5 mm margin was added to prostate volume (PV+) as a surrogate of potential microscopic tumor extension and thus potential impact on tumor control. Prostate V100 and V150 denote the PV covered by 100% and 150% of the prescription dose, while prostate D90 and urethra D30 denote the maximum dose in 90% of the PV and 30% of the urethral volume and rectum D2 cm
3 and rectum D0.1 cm
3 the maximum dose to 2 cm
3 and 0.1 cm
3 of the rectal wall. Urethral volume corresponds to the volume of the transurethral catheter inside the prostate, and the rectum volume corresponds to the anterior rectal wall that could be defined in the transrectal ultrasound. For the comparison of different doses, all dose levels are reported as the percentage of the prescription dose, corresponding to the 100% dose level. Prostate D90 was required to receive at least 100% of the dose in treatment planning (example in
Figure 1).
Dose homogeneity indices were calculated to characterize and compare dose homogeneity: Dose Homogeneity Index DHI = (V100 − V150)/V100 (ratio of the target volume which receives a dose in the range of 1.0 to 1.5 times of the reference dose to the volume of the target that receives a dose greater than the reference dose); Overdose Volume Index ODI = V200/V100 (ratio of the target volume that receives a dose equal to or greater than 2.0 times of the reference dose to the volume of the target that receives a dose equal to or greater than the reference dose); Dose Nonuniformity Ratio DNR = V150/V100 (ratio of the target volume that receives a dose equal to or greater than 1.5 times of the reference dose to the volume of the target that receives a dose equal to or greater than the reference dose) [
10,
11].
Patients after LDR-BT received a post-planning CT on day 1 (with a urethral catheter) and day 30 after implantation. Urethral contour on day 30 was based on estimation.
Statistical analysis was performed using IBM SPSS Statistics 28.0 software (IBM, Armonk, NY, USA). An unpaired t-test was applied to compare volumes and dose levels of different techniques, and a paired t-test to compare volumes and dose levels in LDR-BT patients at different intervals. A chi-square test was applied to compare categorical variables. All p-values reported are two-sided; p < 0.05 is considered significant.
3. Results
As demonstrated in
Table 1, patients treated with LDR-BT as a monotherapy are low-risk or favorable intermediate-risk patients who tended to be younger than patients treated with HDR-BT. Many HDR-BT patients are patients with considerable risk factors and more aggressive tumors.
The comparison of volume and dose values is shown in
Table 2. HDR-BT patients had a slightly larger PV but considerably larger PV+. The only not significantly different value comparing both methods was prostate V100, whereas prostate D90 was slightly higher in LDR-BT (mean difference of 4%). LDR-BT was characterized by a considerably more inhomogeneous dose distribution, characterized by a prostate V150 twice as high compared to HDR-BT. Accordingly, the Dose Homogeneity Index is less than half in LDR-BT compared to HDR-BT. The Overdose Volume Index and Dose Nonuniformity Ratio are about twice as high in LDR-BT compared to HDR-BT. As a consequence of higher doses within the prostate, considerably higher doses to the urethra resulted (mean difference of 32%).
The injection of a hydrogel spacer between the prostate and anterior rectal wall before HDR-BT resulted in considerably lower doses to the rectal wall. A mean rectum 2 cm3 reduction of about 30% and a mean reduction of the maximum dose (rectum 0.1 cm3) of about 70% could be achieved in comparison to LDR-BT patients.
Comparing day 1 and day 30 post-planning CT, a significantly lower prostate V150 (55 ± 9% on day 1 and 61 ± 10% on day 30) and prostate D90 (98 ± 8% on day 1 and 102 ± 10% on day 30) resulted on day 1, respectively (p < 0.01 for all comparisons). Urethra D30 was also significantly lower on day 1 (114 ± 18% on day 1 and 125 ± 21% on day 30; p < 0.01). However, the dose to the rectum increased considerably (rectum 2 cm3 of 77 ± 18% on day 1 and 88 ± 21% on day 30; p < 0.01). Thus, though differences between LDR-BT and HDR-BT were still highly significant, differences respecting dose inhomogeneity and dose to the urethra decreased in post-planning CT evaluations. However, differences respecting the dose to the rectal wall increased.
In
Table 3, the impact of prostate volume on dose distribution is demonstrated (LDR-BT: 52 patients < 41 cm
3; 50 patients ≥ 41 cm
3; HDR-BT: 115 fractions < 41 cm
3; 117 fractions ≥ 41 cm
3). The dose to the prostate was found to be higher in smaller prostates for LDR-BT but not for HDR-BT. PV + 5 mm D90 was identical for smaller prostates comparing LDR-BT vs. HDR-BT. A difference resulted only in larger prostate volumes, with a considerably higher PV + 5 mm D90 in HDR-BT. PV + 5 mm D90 in HDR-BT was higher for larger prostates in comparison to smaller prostates. However, dose inhomogeneity and dose to the urethra were similar in smaller and larger prostates for both BT techniques; rectal doses tended to be higher in larger prostates (differences between LDR-BT and HDR-BT dose distribution independent of prostate volume). Dose indices were independent of the prostate volume.
4. Discussion
4.1. Dose Distribution
An adequate radiotherapy dose is needed to achieve adequate local tumor control for prostate cancer [
12,
13]. Brachytherapy, either as monotherapy or a boost to external beam radiotherapy, is a method of dose escalation for prostate cancer. Several studies, including randomized controlled trials, reported superior local tumor control and biochemical control compared to EBRT alone [
6,
14,
15,
16,
17]. Higher rectal toxicity rates in combined modality treatments are the rationale for using a hydrogel spacer in this patient group.
In a systemic review including 14 clinical trials with 3534 patients who have been treated with HDR-BT as monotherapy, Viani et al. reported excellent results and effectiveness not only for local control after 5 years but also comparable toxicity rates to EBRT. The mean 5-year biochemical recurrence-free survival (bRFS) for low, intermediate, and high-risk patients were 98%, 94%, and 91%, respectively [
18].
In the present study, treatment planning was based on the same average relative prostate V100 (92%). The crucial difference between the techniques was a considerably higher prostate V150 in LDR-BT in comparison to HDR-BT, consequently a lower dose homogeneity and a higher overdose volume, also leading to a considerably higher dose to the urethra. The dose to the rectal wall can be substantially reduced after a hydrogel spacer injection.
Technical or anatomical differences might result in dosimetric differences. Though different mean needle numbers and activities have been used in prior comparisons of HDR-BT vs. LDR-BT dose distributions [
19,
20], the results of our study are well supported. A former study compared the dose distribution in ultrasound-based planning for prostate cancer brachytherapy as monotherapy with single fraction HDR-BT (19 Gy prescription doses, 192-Ir, and a mean needle number of 18—in contrast to 14 needles in this study) in comparison to LDR-BT (145 Gy, I-125, a median seed activity of 0.56 mCi, and a mean source number of 47—in contrast to 0.48 mCi and 62 in this study). As in our study, relative prostate V150 in LDR-BT was about twice as high as prostate V150 in HDR-BT and urethra D30 was found >10% higher [
19]. Mean prostate V150 in our LDR-BT patients corresponded to the objective in a recently published consensus statement (≤65%) [
2]. In the publication by Solanki et al., the mean prostate V150 was found to be only about 50% higher in LDR-BT (mean 40%) in comparison to HDR-BT (mean 27%). However, the mean prostate V100 in LDR-BT was only 82% vs. 96% in HDR-BT, thus considerably lower in comparison to our study [
20].
Anatomical differences respecting prostate volume have been analyzed in our study in a large patient number. Studies comparing the dose to potential microscopic tumor spread and including postimplant dosimetry have not been published before. A hydrogel spacer has not been used in prior comparisons, and increasing the difference in the dose to the rectum between LDR-BT and HDR-BT has been found to be higher in LDR-BT even without a spacer [
19,
20].
Nevertheless, a hydrogel spacer (HDR-BT) did not result in an improved PTV coverage that could be assumed with a larger distance between the prostate and anterior rectal wall, as reported in a recently published study using stereotactic body radiotherapy [
21]. A similar prostate coverage can be explained by the planning process within our center that is focusing on an adequate, but not increased, minimum dose to the prostate. However, a higher dose resulted in larger prostates with a margin of 5 mm, which is comparable to the PTV definition in EBRT that includes the prostate with a margin. In contrast to EBRT [
22,
23], potential mitigation of prostate or seminal vesicle motion during treatment in patients with a spacer is less relevant in brachytherapy, as needles and sources are implanted during continuous prostate visualization in TRUS.
In spite of the dose distribution differences reported in our study, additional technical and radiobiological differences need to be considered. In contrast to HDR-BT, the dose in LDR-BT is delivered over a period of many weeks. With increasing and decreasing prostate edema, sources are slightly displaced and dose analyses on day 1 and day 30 differ from intraoperative dose planning [
24,
25,
26].
HDR-BT dose is delivered in a short period and is thus more consistent. However, fractionation concepts often differ between centers. A higher prostate V150 in LDR-BT might be associated with improved tumor control or increased urinary toxicity. Though the dose distribution is important to guide our treatments, the most important results for our clinical practice and guidelines are long-term clinical results.
4.2. Association of Dosimetric Results with Clinical Results
Several clinical studies analyzing differences between LDR-BT vs. HDR-BT have been published. Burchardt et al. evaluated differences in a PSA bounce between LDR-BT and HDR-BT and found a similar rate (20–30%) but significantly earlier and shorter PSA bounces after HDR-BT in comparison to LDR-BT [
27].
Grills et al. evaluated the potential for differing toxicities between LDR-BT and HDR-BT (38 Gy delivered in four fractions) after a median follow up of 35 months [
28]. HDR-BT was associated with decreased rates of acute urinary frequency, urgency, and dysuria compared with LDR-BT. Chronic urinary frequency, urgency, and Grade 2 rectal toxicities were also decreased with HDR. The treatment methods maintained the same biochemical control. A phase 2 randomized pilot study compared PSA outcomes, quality of life, and late toxicity between LDR-BT and HDR-BT (single 19 Gy fraction). Quality of life, including IPSS (International Prostate Symptom Score) and EPIC 26 (Expanded Prostate Cancer Index Composite) urinary irritative scores were significantly more favorable for patients treated with HDR-BT over the first 36 months [
3,
29]. This can well be the consequence of a lower relative dose to the urethra, as reported in this study. However, a significantly larger proportion of patients after LDR-BT reached PSA < 0.04 ng/mL. This can indicate a larger radio-biologically effective dose, possibly also a result of a considerably larger prostate V150.
In a study comparing LDR-BT vs. HDR-BT boost for external beam radiotherapy, acute urinary toxicity (patient-reported using IPSS and EPIC scores, but also physician-assessed Grade 2 or greater genitourinary toxicity) has been found to be significantly lower (again resulting from a lower dose to the urethra) after HDR-BT boost, though the effect size diminished over time [
30].
We have found a significantly higher dose around the prostate (PV + 5 mm D90) in larger prostates—a dose that might cover microscopic tumors around the prostate. In a publication by Le et al. [
31], the biochemical control 6 years after HDR-BT was found to be significantly higher for patients with prostate volumes ≥ 60 cm
3 in comparison to volumes < 60 cm
3 (91% vs. 78%;
p < 0.01), though dose prescription to the prostate was found to be comparable. The findings in our analysis could explain these clinical results.
Experience with the hydrogel spacer to reduce the dose to the rectal wall and thus reduce toxicity has also increased considerably in recent years, in particular for EBRT. A systemic review showed not only a significant reduction in the dose to the rectal wall—as demonstrated for our patients in this study—but also reduced the late gastrointestinal and genitourinary toxicity rates [
32]. Long-term studies showed significantly improved bowel quality of life for patients treated with a hydrogel spacer [
33]. Studies evaluating the advantage of a hydrogel spacer for prostate cancer BT in combination with EBRT or BT as monotherapy have also been published recently [
34,
35,
36,
37,
38]. A spacer can be advised especially for patients with a higher risk of rectal toxicity [
39,
40].
5. Conclusions
In comparison to HDR-BT, LDR-BT was characterized by a more inhomogeneous dose inside the prostate, a higher dose to the urethra, and a lower dose around the prostate in larger prostate volumes. The dose to the rectal wall could be considerably reduced by applying a hydrogel spacer. However, the prostate volume dose coverage, as determined by prostate V100 and prostate D90, has not been improved with a larger distance between the prostate and anterior rectal wall.
These results explain the clinical differences that have been found in other studies: comparable tumor control (with comparable prostate V100 and dose drop-off around the prostate), higher acute urinary toxicity rates (dose to the urethra), and lower PSA nadir (larger V150 volume) in LDR-BT; higher tumor control in larger prostates after HDR-BT (higher dose around the prostate); and lower rectal toxicity resulting from the application of a hydrogel spacer (considerably lower rectal dose).
Author Contributions
Conceptualization, H.H. (Hathal Haddad) and M.P.; methodology, H.H. (Hathal Haddad) and M.P.; validation, H.H. (Hathal Haddad) and M.P.; formal analysis, H.H. (Hathal Haddad) and M.P.; investigation, H.H. (Hathal Haddad), H.H. (Horst Hermani), H.H. (Herbert Hanitzsch), A.H. and M.P.; resources, M.P.; data curation, H.H. (Hathal Haddad); writing—original draft preparation, H.H. (Hathal Haddad) and M.P.; writing—review and editing, H.H. (Horst Hermani), H.H. (Herbert Hanitzsch), A.H. and M.P.; resources, M.P.; supervision, M.P.; project administration, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study due to local law (retrospective anonymous plan evaluation).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study for the treatment.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available, as patient consent has not been given for the publication of individual details.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Henry, A.; Pieters, B.R.; Andre Siebert, F.; Hoskin, P. GEC-ESTRO ACROP prostate brachytherapy guidelines. Radiother. Oncol. 2022, 167, 244–251. [Google Scholar] [CrossRef]
- King, M.T.; Keyes, M.; Frank, S.J.; Crook, J.M.; Butler, W.M.; Rossi, P.J.; Cox, B.W.; Showalter, T.N.; Mourtada, F.; Potters, L.; et al. Low dose rate brachytherapy for primary treatment of localized prostate cancer: A systemic review and executive summary of an evidence-based consensus statement. Brachytherapy 2021, 20, 1114–1129. [Google Scholar] [CrossRef]
- Hathout, L.; Mahmoud, O.; Wang, Y.; Vergalasova, I.; Barkati, M.; Despres, P.; Martin, A.G.; Foster, W.; Lacroix, F.; Delouya, G.; et al. A Phase 2 Randomized Pilot Study Comparing High-Dose-Rate Brachytherapy and Low-Dose-Rate Brachytherapy as Monotherapy in Localized Prostate Cancer. Adv. Radiat. Oncol. 2019, 4, 631–640. [Google Scholar] [CrossRef]
- Giberti, C.; Chiono, L.; Gallo, F.; Schenone, M.; Gastaldi, E. Radical retropubic prostatectomy versus brachytherapy for low-risk prostatic cancer: A prospective study. World J. Urol. 2009, 27, 607–612. [Google Scholar] [CrossRef]
- Grimm, P.D.; Blasko, J.C.; Sylvester, J.E.; Meier, R.M.; Cavanagh, W. 10-year biochemical (prostate-specific antigen) control of prostate cancer with (125) I brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 31–40. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Rojas, A.M.; Ostler, P.J.; Bryant, L.; Lowe, G.J. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: Mature 12-year results. Radiother. Oncol. 2021, 154, 214–219. [Google Scholar] [CrossRef]
- Skowronek, J. Low-dose-rate or high-dose-rate brachytherapy in treatment of prostate cancer-between options. J. Contemp. Brachytherapy 2013, 5, 33–41. [Google Scholar] [CrossRef]
- Major, T.; Frohlich, G.; Agoston, P.; Polgar, C.; Takacsi-Nagy, Z. The value of brachytherapy in the age of advanced external beam radiotherapy: A review of the literature in terms of dosimetry. Strahlenther. Onkol. 2022, 198, 93–109. [Google Scholar] [CrossRef]
- Hatiboglu, G.; Pinkawa, M.; Vallee, J.P.; Hadaschik, B.; Hohenfellner, M. Application technique: Placement of a prostate-rectum spacer in men undergoing prostate radiation therapy. BJU Int. 2012, 110, E647–E652. [Google Scholar] [CrossRef]
- Anbumani, S.; Nambiraj, N.A.; Dayalan, S.; Ganesh, K.; Anchineyan, P.; Bilimagga, R.S. A brachytherapy plan evaluation tool for interstitial applications. Adv. Bioinform. 2014, 2014, 376207. [Google Scholar] [CrossRef]
- Saw, C.B.; Suntharalingam, N.; Wu, A. Concept of dose nonuniformity in interstitial brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 519–527. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Fractionation and protraction for radiotherapy of prostate carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 1095–1101. [Google Scholar] [CrossRef]
- Fowler, J.; Chappell, R.; Ritter, M. Is alpha/beta for prostate tumours really low? Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 1021–1031. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Rojas, A.M.; Bownes, P.J.; Lowe, G.J.; Ostler, P.J.; Bryant, L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother. Oncol. 2012, 103, 217–222. [Google Scholar] [CrossRef]
- Grimm, P.; Billiet, I.; Bostwick, D.; Dicker, A.P.; Frank, S.; Immerzeel, J.; Keyes, M.; Kupelian, P.; Lee, W.R.; Machtens, S.; et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012, 109 (Suppl. S1), 22–29. [Google Scholar] [CrossRef]
- Goy, B.W.; Burchette, R. Ten-year treatment complication outcomes of radical prostatectomy vs external beam radiation vs brachytherapy for 1503 patients with intermediate risk prostate cancer. Brachytherapy 2021, 20, 1083–1089. [Google Scholar] [CrossRef]
- Haddad, H.; Hermani, H.; Bischoff, P.; Hanitzsch, H.; Heidrich, A.; Schaefer, A.; Kovacs, A.; Pinkawa, M. Permanent interstitial brachytherapy for prostate cancer implementing neoadjuvant prostatic artery embolization. Brachytherapy 2022, 21, 308–316. [Google Scholar] [CrossRef]
- Viani, G.A.; Arruda, C.V.; Assis Pellizzon, A.C.; De Fendi, L.I. HDR brachytherapy as monotherapy for prostate cancer: A systematic review with meta-analysis. Brachytherapy 2021, 20, 307–314. [Google Scholar] [CrossRef]
- Major, T.; Polgar, C.; Jorgo, K.; Stelczer, G.; Agoston, P. Dosimetric comparison between treatment plans of patients treated with low-dose-rate vs. high-dose-rate interstitial prostate brachytherapy as monotherapy: Initial findings of a randomized clinical trial. Brachytherapy 2017, 16, 608–615. [Google Scholar] [CrossRef]
- Solanki, A.A.; Mysz, M.L.; Patel, R.; Surucu, M.; Kang, H.; Plypoo, A.; Bajaj, A.; Korpics, M.; Martin, B.; Hentz, C.; et al. Transitioning From a Low-Dose-Rate to a High-Dose-Rate Prostate Brachytherapy Program: Comparing Initial Dosimetry and Improving Workflow Efficiency Through Targeted Interventions. Adv. Radiat. Oncol. 2019, 4, 103–111. [Google Scholar] [CrossRef]
- Alongi, F.; Rigo, M.; Figlia, V.; Cuccia, F.; Giaj-Levra, N.; Nicosia, L.; Ricchetti, F.; Vitale, C.; Sicignano, G.; De Simone, A.; et al. Rectal spacer hydrogel in 1.5T MR-guided and daily adapted SBRT for prostate cancer: Dosimetric analysis and preliminary patient-reported outcomes. Br. J. Radiol. 2021, 94, 20200848. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, R.; Sicignano, G.; Cuccia, F.; Vitale, C.; Rigo, M.; Giaj-Levra, N.; Nicosia, L.; Figlia, V.; Ricchetti, F.; Attina, G.; et al. Impact of hydrogel peri-rectal spacer insertion on seminal vesicles intrafraction motion during 1.5 T-MRI-guided adaptive stereotactic body radiotherapy for localized prostate cancer. Br. J. Radiol. 2021, 94, 20210521. [Google Scholar] [CrossRef] [PubMed]
- Pinkawa, M.; Piroth, M.D.; Holy, R.; Escobar-Corral, N.; Caffaro, M.; Djukic, V.; Klotz, J.; Eble, M.J. Spacer stability and prostate position variability during radiotherapy for prostate cancer applying a hydrogel to protect the rectal wall. Radiother. Oncol. 2013, 106, 220–224. [Google Scholar] [CrossRef]
- Pinkawa, M.; Asadpour, B.; Gagel, B.; Piroth, M.D.; Borchers, H.; Jakse, G.; Eble, M.J. Evaluation of source displacement and dose-volume changes after permanent prostate brachytherapy with stranded seeds. Radiother. Oncol. 2007, 84, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Pinkawa, M.; Asadpour, B.; Piroth, M.D.; Gagel, B.; Klotz, J.; Fischedick, K.; Borchers, H.; Jakse, G.; Eble, M.J. Rectal dosimetry following prostate brachytherapy with stranded seeds--comparison of transrectal ultrasound intra-operative planning (day 0) and computed tomography-postplanning (day 1 vs. day 30) with special focus on sources placed close to the rectal wall. Radiother. Oncol. 2009, 91, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Pinkawa, M.; Gagel, B.; Asadpour, B.; Piroth, M.D.; Klotz, J.; Borchers, H.; Jakse, G.; Eble, M.J. Seed displacements after permanent brachytherapy for prostate cancer in dependence on the prostate level. Strahlenther. Onkol. 2008, 184, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, W.; Skowronek, J. Time to PSA rise differentiates the PSA bounce after HDR and LDR brachytherapy of prostate cancer. J. Contemp. Brachytherapy 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Grills, I.S.; Martinez, A.A.; Hollander, M.; Huang, R.; Goldman, K.; Chen, P.Y.; Gustafson, G.S. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J. Urol. 2004, 171, 1098–1104. [Google Scholar] [CrossRef]
- Reynaud, T.; Hathout, L.; Carignan, D.; Barkati, M.; Martin, A.G.; Foster, W.; Lacroix, F.; Delouya, G.; Taussky, D.; Morton, G.; et al. PSA outcomes and late toxicity of single-fraction HDR brachytherapy and LDR brachytherapy as monotherapy in localized prostate cancer: A phase 2 randomized pilot study. Brachytherapy 2021, 20, 1090–1098. [Google Scholar] [CrossRef]
- Dhere, V.R.; Fischer-Valuck, B.W.; Goyal, S.; Liu, Y.; Morgan, T.M.; Ghavidel, E.; Moghanaki, D.M.; Hershatter, B.W.; Patel, P.R.; Jani, A.B.; et al. Patient-reported outcomes after Low-dose-rate versus High-dose-rate brachytherapy boost in combination with external beam radiation for intermediate and high risk prostate cancer. Brachytherapy 2021, 20, 1130–1138. [Google Scholar] [CrossRef]
- Le, H.; Rojas, A.; Alonzi, R.; Hughes, R.; Ostler, P.; Lowe, G.; Bryant, L.; Hoskin, P. The influence of prostate volume on outcome after high-dose-rate brachytherapy alone for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 270–274. [Google Scholar] [CrossRef]
- Armstrong, N.; Bahl, A.; Pinkawa, M.; Ryder, S.; Ahmadu, C.; Ross, J.; Bhattacharyya, S.; Woodward, E.; Battaglia, S.; Binns, J.; et al. SpaceOAR Hydrogel Spacer for Reducing Radiation Toxicity During Radiotherapy for Prostate Cancer. A Systematic Review. Urology 2021, 156, e74–e85. [Google Scholar] [CrossRef] [PubMed]
- Pinkawa, M.; Berneking, V.; Schlenter, M.; Krenkel, B.; Eble, M.J. Quality of Life After Radiation Therapy for Prostate Cancer With a Hydrogel Spacer: 5-Year Results. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.; Bolton, D.; Lim Joon, D.; Chan, Y.; Lawrentschuk, N.; Ho, H.; Spencer, S.; Wasiak, J.; Guerrieri, M.; Ow, D.; et al. High dose rate brachytherapy boost for prostate cancer: Biochemical control and the impact of transurethral resection of the prostate and hydrogel spacer insertion on toxicity outcomes. J. Med. Imaging Radiat. Oncol. 2019, 63, 415–421. [Google Scholar] [CrossRef]
- Cousins, M.M.; Heckman, P.; Short, E.; Narayana, V.; Bryant, A.K.; Evans, C.; Hixson, G.; Hurley, P.; McLaughlin, P.W. Rectal sparing in prostate radiotherapy with combination-brachytherapy and hydrogel spacer. Brachytherapy 2022, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Teyateeti, A.; Grossman, C.; Kollmeier, M.A.; Fiasconaro, M.; Hopkins, M.; McBride, S.; Gorovets, D.; Shasha, D.; Cohen, G.; Zhang, Z.; et al. Influence of hydrogel spacer placement with prostate brachytherapy on rectal and urinary toxicity. BJU Int. 2022, 129, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.J.; Marcus, D.M.; Adrian Hall, W.; Agarwal, M. Hydrogel spacers and prostate brachytherapy. Brachytherapy 2022, 21, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Loon, W.; Tacey, M.; Bolton, D.; Tan, A.; Chan, Y.; Cham, C.W.; Ho, H.; Guerrieri, M.; Foroudi, F.; et al. Impact of hydrogel and hyaluronic acid rectal spacer on rectal dosimetry and toxicity in low-dose-rate prostate brachytherapy: A multi-institutional analysis of patients' outcomes. J. Contemp. Brachytherapy 2021, 13, 605–614. [Google Scholar] [CrossRef]
- Tohidinezhad, F.; Willems, Y.; Berbee, M.; Limbergen, E.V.; Verhaegen, F.; Dekker, A.; Traverso, A. Prediction models for brachytherapy-induced rectal toxicity in patients with locally advanced pelvic cancers: A systematic review. J. Contemp. Brachytherapy 2022, 14, 411–422. [Google Scholar] [CrossRef]
- Vanneste, B.G.; Hoffmann, A.L.; Van Lin, E.N.; Van De Voorde, L.; Pinkawa, M.; Lambin, P. Who will benefit most from hydrogel rectum spacer implantation in prostate cancer radiotherapy? A model-based approach for patient selection. Radiother. Oncol. 2016, 121, 118–123. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).