Simple Summary
Prostate-specific membrane antigen positron emission tomography (PSMA PET) is a modern imaging modality used in the management of patients with prostate cancer with improved accuracy in detecting lymph nodes and distant disease spread. In this paper, we discuss how the increasing use of PSMA PET is changing clinical management in patients with prostate cancer, specifically those previously treated for localized disease and now presenting with recurrence or low-volume metastatic disease spread. We also discuss how PSMA PET is affecting clinical trial design and interpretation. More clinical trials are needed to investigate whether the use of PSMA PET translates into improved patient survival or quality of life.
Abstract
Prostate-specific membrane antigen (PSMA) positron emission tomography (PET) scans have higher sensitivity and specificity for detecting lymph nodes or metastatic disease relative to conventional imaging in prostate cancer staging. Since its FDA approval and incorporation into treatment guidelines, the use of PSMA PET has increased in patients undergoing initial staging, those with recurrence after initial definitive treatment, and patients with metastatic disease. Although the early detection of metastatic lesions is changing disease management, it is unclear whether this impact on management translates into clinical benefit. This review will summarize evidence pertaining to the change in patient management due to PSMA PET use and will discuss the implications of PSMA PET on treatment decisions in prostate cancer, particularly in the settings of biochemical recurrence and metachronous oligometastatic disease.
1. Introduction
Prostate cancer (PCa) is the most common cancer and the second leading cause of cancer-related deaths in males in the United States with 248,530 new cases and 34,130 deaths in 2021 [1]. Treatment decisions for patients with PCa are dependent on several factors, including disease stage, grade, risk grouping, patient characteristics (e.g., life expectancy, performance status, comorbidities, etc.), patient preferences, and available resources. Initial cancer staging, staging in the setting of recurrence, and monitoring of response to systemic therapy in patients with metastatic disease rely on information provided by imaging. Magnetic resonance imaging (MRI) and transrectal ultrasound (TRUS) are used for the detection of intra-prostatic disease, extra-capsular extension, and seminal vesicle involvement. Computed tomography (CT) and bone scans have been the main imaging modalities used for the detection of metastases. Positron emission tomography (PET) imaging tracers, such as 11C-Choline and 18F-Fluciclovine, outperform FDG PET in the detection of nodal and osseous disease [2]. More recently, however, prostate-specific membrane antigen (PSMA)-based tracers have been found to be more accurate for the detection of prostate cancer [3,4,5,6,7,8]. PSMA is a type II transmembrane glycoprotein that is overexpressed in prostate cancer. PSMA PET relies on small molecules (68Ga-PSMA-11 or 18F-DCFPyL) that bind with high affinity to the extracellular component of PSMA [9]. 68Ga-PSMA-11 PET obtained FDA approval both for the detection of suspected metastatic lesions at initial staging and in the setting of biochemical recurrence (BCR) following curative therapy in December 2020. Following FDA approval, PSMA PET use was endorsed by the National Comprehensive Cancer Network (NCCN) and the Society of Nuclear Medicine and Molecular Imaging (SNMMI) guidelines [10,11].
The influence of PSMA PET on the management of patients with PCa is undergoing extensive investigation. PSMA PET has higher sensitivity and specificity for detecting lymph node or metastatic disease and has a considerable impact on management decisions relative to conventional imaging [12]. Although PSMA PET is changing disease management, it is unclear whether this impact on management translates into clinical benefit; studies are ongoing to answer this question. In this review, we will summarize and discuss available evidence pertaining to the change in patient management due to PSMA PET use, specifically in patients previously treated for localized disease and now presenting with PSA recurrence, including those with metachronous oligometastasis (Figure 1). Given the differences in outcomes and possibly biology between patients with synchronous and metachronous low-volume metastatic disease [13], we will not discuss the use of PSMA PET in patients with newly diagnosed synchronous metastatic disease. We will also not discuss the impact of PSMA PET on newly diagnosed localized prostate cancer. Our goal was to write a narrative review of the literature, not a meta-analysis or a systematic review. We conducted our search on PubMed with the following strategy: prostatic OR “prostate cancer” AND “positron emission tomography’’ OR PET AND ‘‘prostate membrane specific antigen’’ OR PSMA AND management OR change OR impact. Abstracts were screened by the first co-authors and representative papers were included if relevant to the review’s subject. The search was limited to papers written in English and published between 2015–2022. Clinicaltrials.gov was used to select ongoing trials of PSMA PET. Tables were created to collect required information and to summarize the available evidence.
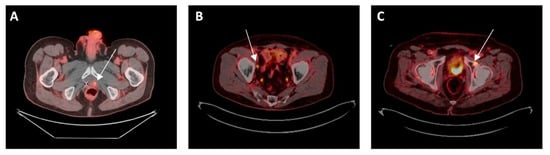
Figure 1.
(A) Local failure after prior radiotherapy: 72-year-old man with intermediate-risk prostate adenocarcinoma treated with radiation to the prostate with biochemical failure 5 years later. PSMA PET CT showed uptake in left prostate but no regional or distant disease. He received salvage brachytherapy. (B) Regional failure after prior prostatectomy: 75-year-old man with high-risk prostate cancer treated with radical prostatectomy, with post-op PSA of 0.17, then treated with pelvic and prostate bed radiation. He again had rising PSA and PSMA PET CT showed uptake in one right pelvic lymph node but no local or distant disease. He received stereotactic radiotherapy to the lymph node. (C) Metachronous oligometastasis: 72-year-old man with intermediate-risk prostate adenocarcinoma treated with prostatectomy with undetectable PSA post-op, which then increased a few years later and PSMA PET CT showed uptake in left acetabulum. He received SBRT to the bone lesion without hormone therapy.
2. PSMA PET in the Setting of Biochemical Recurrence (BCR) without Evidence of Distant Metastasis
While several factors may affect PSA levels [14], an increasing PSA after definitive management usually indicates recurrence. In patients treated with radical prostatectomy (RP), BCR is generally defined as a detectable PSA level ≥ 0.2 ng/mL with a second confirmatory level [15,16]. EAU guidelines state that a PSA level > 0.4 ng/mL and rising after RP is a better threshold for the prediction of further metastases [17]. Patients with BCR after RP without distant metastasis are often treated with salvage RT (SRT) with or without hormone therapy. In patients treated with radiotherapy (RT), BCR is defined as a rise of ≥2 ng/mL above nadir PSA [18]. The management of BCR after RT begins with confirming the absence of disseminated disease (i.e., the recurrence is confined to the pelvis), with those patients meeting this criterion receiving local salvage treatment (to the prostate or to the pelvic lymph nodes). Patients with distant recurrence (after initial RP or RT) are started on androgen deprivation therapy (ADT) with or without hormone intensification. Traditionally, disseminated disease is ruled out using bone scan or CT. PSMA PET has better detection rates than conventional imaging [19]. A recent systematic review and meta-analysis, including 1309 patients with BCR from 16 studies, showed that the detection rate of 68Ga-PSMA PET increased with PSA. For the PSA categories 0–0.2, 0.2–1, 1–2, and >2 ng/mL, 42%, 58%, 76%, and 95% of the scans, respectively, were positive [12].
Three main studies have established the benefit of PSMA PET over conventional imaging in the recurrent setting. OSPREY is a phase 2/3 trial, which enrolled two cohorts of patients: cohort A included patients with high-risk prostate cancer, and cohort B included patients with metastatic or recurrent disease on conventional imaging. 18F-DCFPyL PET showed improvements in the specificity and positive predictive value when compared with conventional imaging [5]. CONDOR is a phase 3 study of patients with a median PSA level of 0.8 ng/mL after RP or RT and negative conventional imaging. 18F-DCFPyL PET improved disease localization in this cohort of patients [4]. Fendler et al. also prospectively assessed the accuracy of 68Ga-PSMA PET in patients with BCR after RT and/or RP and established high positive predictive value, detection rate, and inter-reader agreement for the localization of recurrent prostate cancer with PSMA PET [20]. It is, therefore, clear that PSMA PET has the potential of finding metastases or disease extensions that are not detected using conventional imaging. Table 1 summarizes a selected list of studies, which investigated the effect of PSMA PET on management decisions in patients with recurrent prostate cancer initially treated with RT or RP [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].

Table 1.
Selected studies on the impact of PSMA PET on treatment planning in patients with recurrence after definitive therapy.
2.1. Biochemical Recurrence after Radical Prostatectomy
In patients with BCR after RP, detecting the presence and site of recurrence is crucial for RT planning. Intuitively, the presence of distant disease obviates the need for pelvic or prostate bed RT, and the presence of local failure or lymph nodes has implications for radiation field design, RT dose, and hormone therapy use and duration. Since most SRT is delivered at relatively low PSA levels (<1 ng/mL), conventional imaging is unlikely to add any valuable information for treatment planning, and thus most patients would receive SRT. The ability of PSMA PET to detect disease at low PSA levels prior to SRT offers a unique opportunity for personalized therapy. This has been shown in several studies. In their retrospective analysis, Bottke et al. evaluated the impact of PSMA PET on SRT planning in 76 patients with BCR after RP [21]. PSMA PET led to a change in therapeutic target volumes in 28% of patients. Similarly, Farolfi et al. looked at 119 patients with BCR following RP and found that RT planning was modified in 30% of patients [44]. These two studies and several others had a median PSA in the 0.2–0.3 ng/mL range, highlighting the fact that PSMA PET leads to significant change in post-RP radiotherapy management even at low PSA levels. This is extremely important given that SRT yields better oncologic outcomes when delivered at lower PSA levels (ideally <0.2–0.5 ng/mL) [49]. In summary, PSMA PET influences SRT planning anywhere between 28–76% of patients with biochemically recurrent prostate cancer following RP. Notably, the change in management is not only limited to SRT planning volumes and dose levels in the prostate bed or pelvis but also includes adding, stopping, and/or completely switching between treatment modalities. Patients who were previously thought to be eligible to receive SRT, for example, may be found to harbor distant metastasis and no longer receive local SRT. Such changes allow some patients to avoid unnecessary treatments while helping others receive more tailored therapy.
2.2. Biochemical Recurrence after Radiation Therapy
Compared to surgery, fewer studies have investigated the effect of PSMA PET on the management of patients treated initially with RT as the primary definitive treatment and presenting with BCR. In a prospective study, Liu et al. investigated the influence of PSMA PET compared to conventional imaging in managing patients with radio-recurrent prostate cancer. 18F-DCFPyL PET identified extra-prostatic disease in twice as many men and detected a site of recurrence in 87% of men, compared with 67% with conventional imaging. Furthermore, 18F-DCFPyL PET identified potentially actionable disease (prostate-only recurrence or oligometastatic disease) in 75% of men and changed the proposed management in 43% of men [32]. Afaq et al. evaluated the change in management following PSMA PET in 68 patients treated with RP and 32 patients treated with RT and found that 50% of the RT group had a change in their intended management after PSMA PET [50]. Barbaud et al. also studied patients with BCR after prior RP or RT who underwent PSMA PET imaging following negative Choline-11 PET and found that PSMA PET led to a change in the management in 73.8% of patients, with nearly 50% receiving radiation field adjustments [33]. Interestingly, several studies found that patients primarily treated with RT have a significantly higher rate of PSMA PET positivity, compared to patients treated with RP [34,36,50]. This higher rate of disease localization in patients who meet Phoenix criteria suggests that there may be a role for PSMA PET-based interventions at earlier PSA (before the nadir + 2 threshold) in patients with radio-recurrence. Fendler et al. and Hope et al. also reported that PSMA PET prevented additional tests from being performed and reduced the number of patients with unknown disease location by 50% [36,37]. Several other studies, including small prospective trials, also show that PSMA PET influences treatment management in patients with BCR [37,38,39].
To summarize, PSMA PET influences management in patients with BCR following both RT and RP despite the heterogeneity and retrospective nature of the studies and the variation in the definition of “treatment change” among the different studies. In a meta-analysis of 15 studies, including 1163 patients in the recurrent and definitive settings, the pooled proportion of management changes was 54% with half of these changes entailing a new treatment modality [51]. Although it is unknown whether this translates to better oncologic outcomes or improved quality of life, PSMA PET provides useful information to physicians, which affects their decision-making process. It is important to mention the recently published EMPIRE-1 study in which 18F-Fluciclovine-PET-based post-prostatectomy RT planning significantly improved survival free from biochemical recurrence or persistence, compared to conventional imaging [52]. Despite the limitations of the study, the results give hope that PSMA PET will favorably impact treatment outcomes, given its superior sensitivity and specificity over 18F-Fluciclovine-PET. Randomized trials looking at outcomes in patients whose management is planned with PSMA PET vs. standard of care imaging are ongoing to determine whether these changes are beneficial or not, namely in terms of survival and quality of life.
3. PSMA PET in the Setting of Metachronous Oligometastatic Disease
Oligometastatic prostate cancer (i.e., OMPC) includes patients who present with recurrence after initial definitive therapy with imaging showing limited metastatic involvement (i.e., metachronous), and patients presenting with de novo limited metastatic disease (i.e., synchronous). The distinction between synchronous and metachronous metastases is important as they may have different biologic and clinical characteristics. For the purpose of this paper, we will focus on metachronous oligometastatic disease. Whether OMPC is truly a distinct intermediate state between localized and widespread metastatic disease that is less aggressive and less likely to further metastasize is yet to be determined [53]. No consensus definition exists for OMPC. Traditionally, an accepted definition of OMPC is radiographic and is based on the number and location of metastatic lesions on conventional imaging [54]. The two most commonly referenced definitions of OMPC are derived from the “low volume” subgroup in CHAARTED [55], STAMPEDE [56], and GETUG-AFU 15 [57] or from the “non-high-risk” subgroup in LATITUDE [58]. However, the increasing use of PSMA PET and the increasing detection of small lesions that are otherwise not detected with conventional imaging are challenging these definitions. In addition to number and location, the genomic landscape of metastatic lesions also affect survival [59,60], and thus should also be considered in a more comprehensive definition for OMPC.
Two studies have defined the use of radiotherapy, specifically stereotactic body radiotherapy or SBRT, in patients with metachronous OMPC: STOMP and ORIOLE. The randomized phase II ORIOLE clinical trial compared outcomes of men with hormone-sensitive OMPC treated with metastasis-directed therapy (MDT) vs. observation [61]. MDT significantly decreased the progression of disease at 6 months relative to observation (19% vs. 61%, respectively). Although MDT was planned based on conventional imaging, a post hoc analysis revealed that patients who received consolidation to all detectable disease on PSMA PET had better progression-free survival (PFS) and distant metastasis-free survival than those who did not. These findings were validated in another prospective randomized phase II trial by Ost et al. (the STOMP trial), which looked at patients with recurrence after primary PCa treatment with three or fewer extracranial metastatic lesions on Choline PET (PSMA PET was not used). The median ADT-free survival was 21 months for the MDT group vs. 13 months for the surveillance group [62]. The benefit of MDT was maintained at longer follow-up with five-year data showing a 34% ADT-free survival for the MDT group vs. 8% for the surveillance group, as well as higher castrate-resistant prostate cancer (CRPC)-free survival (76% for MDT vs. 53% for surveillance). A combined analysis of STOMP and ORIOLE also showed that median PFS was prolonged with MDT, compared with observation [63].
Table 2 summarizes selected studies of PSMA PET staging and MDT in patients with OMPC [64,65,66,67,68,69,70,71,72]. The use of PSMA PET and MDT in the setting of OMPC has been investigated in a few prospective trials. In a single institution prospective study, Bowden et al. found that in a cohort of 176 patients, of whom 136 patients were initially staged with PSMA PET, median treatment escalation-free survival (TEFS) was 27.1 months. Patients staged with PSMA PET had a trend toward longer TEFS and lower treatment escalation rate at 2 years, although the difference was not statistically significant [64]. Similarly, Kneebone et al. found that biochemical disease-free survival was 11 months in patients with OMPC treated with MDT in 1–3 lesions detected on PSMA PET [65]. There were no local failures and toxicity was limited to grade 1 side effects. Several retrospective studies showcase the benefit of utilizing PSMA PET in patients with OMPC treated with MDT. Artigas et al. found an ADT-free survival of 74% at 2 years in 20 patients with hormone-sensitive OMPC. Biochemical response (defined as a PSA decrease by >50%) was seen in 70% of patients, and BCR-free survival was 53% at 2 years [66]. Guler et al. reported a PFS of 67% at 1 year for patients with castration-sensitive (CS) OMPC treated with MDT following PSMA PET. Notably, PFS was 0% for patients with castration-resistant (CR) OMPC, which they attributed to the more aggressive nature of CRPC [67]. Similar benefits were seen in other studies, including patients with castration resistance [68,69,70,71]. The case of castration resistance is particularly interesting given the upregulation of PSMA expression with the use of ADT, the possible association of PSMA expression with castration resistance, and the higher standardized uptake values (SUVmax) in patients with CRPC, compared to those with CSPC [73,74,75]. Finally, Deijen et al. retrospectively compared PSA response, PSA response duration, and ADT-free survival in patients with OMPC treated with PSMA PET- vs. Choline-11 PET-guided SBRT. PSMA PET led to improved ADT-free survival, as well as longer PSA response, compared to Choline-11 PET [72].

Table 2.
Selected studies investigating the use of metastasis-directed therapy in patients with oligometastatic prostate cancer staged with PSMA PET.
While the data are promising, these studies have several limitations. Namely, most of these studies are retrospective in nature, hence prone to a wide range of biases probably leading to an over-estimation of the effect size. Furthermore, patient and treatment characteristics are heterogeneous both among studies and within the same study. Caution is thus warranted in interpreting the results. Given all this information, PSMA PET potentially holds appreciable benefits in guiding MDT for patients with OMPC, and thus delaying the initiation of ADT or delaying the switching of systemic therapy at progression. Again, however, whether this has an impact on outcomes remains poorly understood and should be further investigated.
4. Discussion
Most of the current recommendations for the treatment of patients with prostate cancer are derived from older studies, before the widespread availability of PSMA PET. The increasing use of PSMA PET has caused a landscape shift and has led to new paradigms and disease presentations, which were not appreciated in the era of conventional imaging. Therefore, while findings are promising, it is still unclear how PSMA PET should change management and how to integrate these advances into modern clinical practice and future trial design. Interestingly, PSMA PET is having a significant impact on the interpretation of endpoints in clinical trials. For example, it is unclear how progression on PSMA PET should be exactly defined and whether it is clinically meaningful, especially when considering that PET-based metastatic progression happens before progression on conventional imaging. In patients enrolled on clinical trials, PSMA PET-based interventions may prolong conventional imaging-based metastasis endpoints and make interpretations of trial results difficult. Along the same lines, it is unclear whether progression on PSMA PET should lead to a change in therapy, such as adding or switching systemic therapies or adding MDT. Although a proposal for systemic therapy response–assessment criteria with PSMA PET has been published [76], more work needs to be done in this domain to connect the progression criteria with clinical meaningfulness.
While not discussed in this review, PSMA PET imaging is indicated per the NCCN guidelines in the initial staging of patients with unfavorable intermediate- and high-risk disease. PSMA PET could be used for dose escalation to avid lesions, as per the FLAME study (which used MRI rather than PSMA PET) [77,78], given the high correlation between PET avidity and tumor aggressiveness on histopathology [79,80]. Although results from the PRIMARY clinical trial showed improved sensitivity and negative predictive value for the detection of clinically significant cancer with PSMA PET compared to MRI [81], it is unclear what added advantage exists for using PSMA PET images alone or in combination with MRI images over MRI alone for focal boosting or even for focal prostate treatments. More studies are needed in this setting. In the recurrent setting, some patients with BCR after RP will have a change in their treatment plan, including the addition of a new treatment modality or a complete change in modalities, based on PSMA PET findings. This is true even at low PSA values (0.2–0.5 ng/mL). Whether this change in treatment planning will improve outcomes remains to be proven. Nonetheless, PSMA PET opens the door for more personalized therapy, as opposed to a “one-size-fits-all” approach. In patients with BCR after RP and with a negative PSMA PET done at an appropriate PSA level, salvage RT should be offered without delay and patients should be counseled about the low detection rate at low PSA and the overall low sensitivity of PSMA PET imaging [7]. A negative PET should not be used to postpone salvage RT. Patients with BCR after prior definitive treatment with PSMA positive nodes in the pelvis may benefit from pelvic radiotherapy (plus boost to avid nodes) and long-term ADT (with or without intensified hormonal therapy). In patients treated with prior RP, the additional treatment of the prostatic fossa with radiation is also indicated in most cases. Patients with BCR after definitive treatment and PSMA-positive retroperitoneal nodes require ADT (with or without intensified hormonal therapy). Radiotherapy could be delivered with full pelvic and para-aortic fields or with SBRT to the involved nodes. PSMA PET studies on the patterns of failure after initial definitive treatment will continue to define consensus guidelines for pelvic radiotherapy volumes [82,83]. Clinicians evaluating PSMA PET images, however, should be aware of the potential pitfalls of PSMA PET imaging, including an 8% chance of false positive readings (e.g., within a previously irradiated prostate) and an 8% chance of false negative readings (e.g., urine activity or small size lesions) [84]. Interestingly, the biopsy of PSMA-positive lesions is rarely done in clinical practice, but clinicians should assess findings cautiously, especially when PSA or SUVmax are low, when there is only one lesion on the scan (e.g., solitary rib lesion), or when there is an absence of CT correlates. Such findings should raise suspicion for a false positive.
The increased sensitivity of PSMA PET leads to stage migration as patients previously thought to be at a low stage are upstaged based on new PSMA PET findings. This leads to improved survival in both stages: survival in the lower stage improves as the more advanced patients are upstaged and survival in the higher stage also increases when favorable patients enter this group. It is unclear how to exactly deal with stage migration when deciding on treatments, especially in patients considered for clinical trials. For example, how do we manage patients with BCR after prior RP and negative conventional imaging but with one small distant lesion on PSMA PET? Is biopsy always indicated in these situations? Should these patients receive salvage RT to the pelvis, or should they be treated with systemic therapy? If so, what is the most appropriate systemic therapy? Additionally, should they receive MDT? Finally, could such patients be enrolled on a trial of salvage radiotherapy that does not mandate molecular imaging? Currently, the clinical trial NRG GU-008, which randomizes patients with nodal involvement after prostatectomy to salvage radiation and long-term ADT with or without Apalutamide, excludes patients with M1 disease on molecular imaging. Similarly, it is unclear how to manage definitive patients with PSMA PET findings which are not corroborated by conventional imaging. Interestingly, the currently enrolling NRG GU-009 and NRG GU-010 allow patients who have bone metastases established only by Fluciclovine, Choline, or PSMA PET but are not definitive on bone scan or NaF PET to enroll. Finally, it is not clear how to specifically treat patients with metachronous OMPC with bone lesions per PSMA PET. Options include ADT alone, ADT with additional androgen receptor (AR)-targeted agents, MDT alone, MDT and ADT, or MDT and ADT plus additional AR-targeted agents. Currently, there is extreme heterogeneity in how these patients are being treated clinically, including patients progressing on clinical trials, which will make the future interpretation of data difficult. NRG GU-011 is trying to answer one part of this question.
This review has several limitations. First, we did not follow a systematic approach for the selection of papers as in a systematic review or meta-analysis. This means that there is the potential for selection bias. Second, we did not formally evaluate bias or heterogeneity. Third, the cohorts of patients included are not homogeneous. Although each section deals with a general presentation of disease (e.g., biochemical recurrence, OMPC, etc.), each study had its own inclusion and exclusion criteria, as well as its own definition of recurrence or OMPC. Thus, caution is warranted in drawing conclusions. Fourth, most of the included studies are retrospective in nature, and thus are inherently prone to certain biases, such as overestimation of effect size. Although a more systematic review should be conducted in the future, our work nonetheless provides readers with a summary (both quantitative and qualitative) of a large body of evidence relating to the effect of PSMA PET on management decisions. Finally, while we group all PSMA PET imaging modalities together, it is important to discuss the advantages and limitations offered by PSMA PET CT versus PSMA PET MRI. PSMA PET MRI combines the molecular data from PET with the superior anatomic details of the MRI. Although PSMA PET MRI shows good diagnostic performance, there is no clear data showing its superiority over PET CT [85]. PET CT scans are more widely available, less expensive, and the scans are faster and more comfortable for the patient but PET MRIs have better soft-tissue contrast.
5. Future Directions
As previously discussed, most of the available literature is retrospective in nature, severely limiting reliable conclusions. Several prospective trials are currently underway evaluating the role of PSMA PET imaging in the management of prostate cancer at initial staging, recurrence, and in (oligo)-metastatic disease. Importantly, many of these studies are comparing oncologic outcomes between standard of care vs. molecular imaging. A selected list of ongoing trials is presented in Table 3. NCT04794777 and NCT03582774 are randomized, open label, clinical trials that compare outcomes of patients presenting with BCR after RP, randomized to either standard SRT vs. individualized therapy based on PSMA PET. NCT04983095 is an open label, multi-center, randomized study that will look at patients with OMPC treated with SBRT and standard of care therapy vs. standard of care only. The PILLAR study (NCT03503344) is investigating the efficacy of apalutamide with or without MDT in treating participants with oligometastatic CRPC, with undetectable PSA at 6 months as the primary endpoint. Furthermore, other PSMA tracers are also being investigated, both in terms of metastasis detection rate and their effect on patient management. For example, NCT04978675, a prospective trial conducted at MD Anderson Cancer Center, is assessing the use of rh PSMA 7.3 PET/MRI in detecting recurrent prostate cancer, as well as its effect on salvage RT planning. Finally, as PSMA-targeted radioligand therapies (e.g., 177Lu-PSMA-617) show clinical benefit, the underlying resistance mechanisms and the role of PSMA PET imaging as a biomarker for patient selection will need to be further investigated in future studies [86,87,88,89].

Table 3.
Summary of relevant clinical trials using PSMA PET for patients with prostate cancer.
6. Conclusions
In conclusion, PSMA PET provides many answers and important information for clinicians when it comes to the management of patients with prostate cancer even at low PSA values where traditional imaging modalities are completely inadequate at detecting malignant lesions. However, PSMA also raises many questions, which remain unanswered. Larger randomized trials are needed to truly validate the effect of PSMA PET on patient outcomes in different disease settings.
Author Contributions
Conceptualization: O.M. and T.A.H.; writing—original draft preparation: O.M., K.E.L. and A.S.; writing—review and editing: O.M., A.S., K.E.L. and T.A.H.; supervision: O.M. and T.A.H.; project administration: O.M. and T.A.H.; funding acquisition: O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Niaz, M.J.; Sun, M.; Skafida, M.; Niaz, M.O.; Ivanidze, J.; Osborne, J.R.; O’Dwyer, E. Review of commonly used prostate specific PET tracers used in prostate cancer imaging in current clinical practice. Clin. Imaging 2021, 79, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Waller, J.; Flavell, R.; Heath, C.L. High Accuracy of PSMA PET in Initial Staging of High-Risk Prostate Cancer. Radiol. Imaging Cancer 2020, 2, e204025. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Rowe, S.P.; Gorin, M.A.; Saperstein, L.; Pouliot, F.; Josephson, D.; Wong, J.Y.C.; Pantel, A.R.; Cho, S.Y.; Gage, K.L.; et al. Diagnostic Performance of (18)F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin. Cancer Res. 2021, 27, 3674–3682. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with (18)F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Hope, T.A.; Eiber, M.; Armstrong, W.R.; Juarez, R.; Murthy, V.; Lawhn-Heath, C.; Behr, S.C.; Zhang, L.; Barbato, F.; Ceci, F.; et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021, 7, 1635–1642. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J.; Maurer, T.; Eiber, M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics 2018, 38, 200–217. [Google Scholar] [CrossRef]
- Jadvar, H.; Calais, J.; Fanti, S.; Feng, F.; Greene, K.L.; Gulley, J.L.; Hofman, M.; Koontz, B.F.; Lin, D.W.; Morris, M.J.; et al. Appropriate Use Criteria for Prostate-Specific Membrane Antigen PET Imaging. J. Nucl. Med. 2022, 63, 59–68. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Prostate Cancer (Version 1.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 16 September 2022).
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Francini, E.; Gray, K.P.; Xie, W.; Shaw, G.K.; Valenca, L.; Bernard, B.; Albiges, L.; Harshman, L.C.; Kantoff, P.W.; Taplin, M.E.; et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC). Prostate 2018, 78, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Crocetto, F.; Vito, C.D.; Martino, R.; Pandolfo, S.D.; Creta, M.; Aveta, A.; Buonerba, C.; Imbimbo, C. Clinical factors affecting prostate-specific antigen levels in prostate cancer patients undergoing radical prostatectomy: A retrospective study. Future Sci. OA 2021, 7, FSO643. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar] [CrossRef]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- EAU-EANM-ESTRO-ESUR-ISUP-SIOG. Guidelines on Prostate Cancer. 2022. Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 18 October 2022).
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Treglia, G.; Pereira Mestre, R.; Ferrari, M.; Bosetti, D.G.; Pascale, M.; Oikonomou, E.; De Dosso, S.; Jermini, F.; Prior, J.O.; Roggero, E.; et al. Radiolabelled choline versus PSMA PET/CT in prostate cancer restaging: A meta-analysis. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 127–139. [Google Scholar]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef]
- Bottke, D.; Miksch, J.; Thamm, R.; Krohn, T.; Bartkowiak, D.; Beer, M.; Bolenz, C.; Beer, A.J.; Prasad, V.; Wiegel, T. Changes of Radiation Treatment Concept Based on 68Ga-PSMA-11-PET/CT in Early PSA-Recurrences After Radical Prostatectomy. Front. Oncol. 2021, 11, 665304. [Google Scholar] [CrossRef]
- Calais, J.; Czernin, J.; Cao, M.; Kishan, A.U.; Hegde, J.V.; Shaverdian, N.; Sandler, K.; Chu, F.I.; King, C.R.; Steinberg, M.L.; et al. 68Ga-PSMA-11 PET/CT Mapping of Prostate Cancer Biochemical Recurrence After Radical Prostatectomy in 270 Patients with a PSA Level of Less Than 1.0 ng/mL: Impact on Salvage Radiotherapy Planning. J. Nucl. Med. 2018, 59, 230–237. [Google Scholar] [CrossRef]
- Bianchi, L.; Schiavina, R.; Borghesi, M.; Ceci, F.; Angiolini, A.; Chessa, F.; Droghetti, M.; Bertaccini, A.; Manferrari, F.; Marcelli, E.; et al. How does 68Ga-prostate-specific membrane antigen positron emission tomography/computed tomography impact the management of patients with prostate cancer recurrence after surgery? Int. J. Urol. 2019, 26, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Deandreis, D.; Guarneri, A.; Ceci, F.; Lillaz, B.; Bartoncini, S.; Oderda, M.; Nicolotti, D.G.; Pilati, E.; Passera, R.; Zitella, A.; et al. 68Ga-PSMA-11 PET/CT in recurrent hormone-sensitive prostate cancer (HSPC): A prospective single-centre study in patients eligible for salvage therapy. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Roberts, M.J.; Perera, M.; Williams, E.; Rhee, H.; Pryor, D.; Lehman, M.; Heathcote, P.; Wood, S.; Coucher, J.; et al. The clinical efficacy of PSMA PET/MRI in biochemically recurrent prostate cancer compared with standard of care imaging modalities and confirmatory histopathology: Results of a single-centre, prospective clinical trial. Clin. Exp. Metastasis 2020, 37, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Grubmuller, B.; Baltzer, P.; D’Andrea, D.; Korn, S.; Haug, A.R.; Hacker, M.; Grubmuller, K.H.; Goldner, G.M.; Wadsak, W.; Pfaff, S.; et al. 68Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy-diagnostic performance and impact on therapeutic decision-making. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Huits, T.H.; Luiting, H.B.; van der Poel, H.G.; Nandurkar, R.; Donswijk, M.; Schaake, E.; Vogel, W.; Roobol, M.J.; Wit, E.; Stricker, P.; et al. Distribution of prostate cancer recurrences on gallium-68 prostate-specific membrane antigen (68Ga-PSMA) positron-emission/computed tomography after radical prostatectomy with pathological node-positive extended lymph node dissection. Br. J. Urol. Int. 2020, 125, 876–883. [Google Scholar] [CrossRef]
- Meijer, D.; van Leeuwen, P.J.; Oosterholt, P.M.J.; Bodar, Y.J.L.; van der Poel, H.G.; Hendrikse, N.H.; Donswijk, M.L.; Wondergem, M.; Vellekoop, A.E.; van Moorselaar, R.J.A.; et al. Management impact of (18)F-DCFPyL PET/CT in hormone-sensitive prostate cancer patients with biochemical recurrence after definitive treatment: A multicenter retrospective study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2960–2969. [Google Scholar] [CrossRef]
- Mena, E.; Lindenberg, M.L.; Shih, J.H.; Adler, S.; Harmon, S.; Bergvall, E.; Citrin, D.; Dahut, W.; Ton, A.T.; McKinney, Y.; et al. Clinical impact of PSMA-based (18)F-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 4–11. [Google Scholar] [CrossRef]
- Rousseau, C.; Le Thiec, M.; Ferrer, L.; Rusu, D.; Rauscher, A.; Maucherat, B.; Frindel, M.; Baumgartner, P.; Fleury, V.; Denis, A.; et al. Preliminary results of a 68Ga-PSMA PET/CT prospective study in prostate cancer patients with occult recurrence: Diagnostic performance and impact on therapeutic decision-making. Prostate 2019, 79, 1514–1522. [Google Scholar] [CrossRef]
- Tan, J.S.H.; Goh, C.X.Y.; Koh, Y.S.; Li, Y.; Tuan, J.K.L.; Chua, E.T.; Tan, T.W.K.; Wang, M.L.C.; Lee, L.S.; Tay, K.J.; et al. 68Gallium-labelled PSMA-PET/CT as a diagnostic and clinical decision-making tool in Asian prostate cancer patients following prostatectomy. Cancer Biol. Med. 2019, 16, 157–166. [Google Scholar] [CrossRef]
- Liu, W.; Zukotynski, K.; Emmett, L.; Chung, H.T.; Chung, P.; Wolfson, R.; Rachinsky, I.; Kapoor, A.; Metser, U.; Loblaw, A.; et al. A Prospective Study of 18F-DCFPyL PSMA PET/CT Restaging in Recurrent Prostate Cancer following Primary External Beam Radiotherapy or Brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 546–555. [Google Scholar] [CrossRef]
- Barbaud, M.; Frindel, M.; Ferrer, L.; Le Thiec, M.; Rusu, D.; Rauscher, A.; Maucherat, B.; Baumgartner, P.; Fleury, V.; Colombie, M.; et al. 68Ga-PSMA-11 PET-CT study in prostate cancer patients with biochemical recurrence and non-contributive 18F-Choline PET-CT: Impact on therapeutic decision-making and biomarker changes. Prostate 2019, 79, 454–461. [Google Scholar] [CrossRef]
- Cerci, J.J.; Fanti, S.; Lobato, E.E.; Kunikowska, J.; Alonso, O.; Medina, S.; Novruzov, F.; Lengana, T.; Granados, C.; Kumar, R.; et al. Diagnostic Performance and Clinical Impact of 68Ga-PSMA-11 PET/CT Imaging in Early Relapsed Prostate Cancer After Radical Therapy: A Prospective Multicenter Study (IAEA-PSMA Study). J. Nucl. Med. 2022, 63, 240–247. [Google Scholar] [CrossRef] [PubMed]
- De Bari, B.; Mazzola, R.; Aiello, D.; Aloi, D.; Gatta, R.; Corradini, S.; Salgarello, M.; Alongi, F. (68Ga)-PSMA-PET/CT for the detection of postoperative prostate cancer recurrence: Possible implications on treatment volumes for radiation therapy. Cancer Radiother. 2019, 23, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Ferdinandus, J.; Czernin, J.; Eiber, M.; Flavell, R.R.; Behr, S.C.; Wu, I.K.; Lawhn-Heath, C.; Pampaloni, M.H.; Reiter, R.E.; et al. Impact of 68Ga-PSMA-11 PET on the Management of Recurrent Prostate Cancer in a Prospective Single-Arm Clinical Trial. J. Nucl. Med. 2020, 61, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Aggarwal, R.; Chee, B.; Tao, D.; Greene, K.L.; Cooperberg, M.R.; Feng, F.; Chang, A.; Ryan, C.J.; Small, E.J.; et al. Impact of 68Ga-PSMA-11 PET on Management in Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2017, 58, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, E.; Wilson, D.; Lacroix-Poisson, F.; Krauze, A.; Chi, K.; Gleave, M.; McKenzie, M.; Tyldesley, S.; Goldenberg, S.L.; Benard, F. A Prospective Study on (18)F-DCFPyL PSMA PET/CT Imaging in Biochemical Recurrence of Prostate Cancer. J. Nucl. Med. 2019, 60, 1587–1593. [Google Scholar] [CrossRef]
- Afaq, A.; Payne, H.; Davda, R.; Hines, J.; Cook, G.J.R.; Meagher, M.; Priftakis, D.; Warbey, V.S.; Kelkar, A.; Orczyk, C.; et al. A Phase II, Open-label study to assess safety and management change using 68Ga-THP PSMA PET/CT in patients with high risk primary prostate cancer or biochemical recurrence after radical treatment: The PRONOUNCED study. J. Nucl. Med. 2021, 62, 1727–1734. [Google Scholar] [CrossRef]
- Albisinni, S.; Artigas, C.; Aoun, F.; Biaou, I.; Grosman, J.; Gil, T.; Hawaux, E.; Limani, K.; Otte, F.X.; Peltier, A.; et al. Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: Preliminary analysis of a multidisciplinary approach. BJU Int. 2017, 120, 197–203. [Google Scholar] [CrossRef]
- Bashir, U.; Tree, A.; Mayer, E.; Levine, D.; Parker, C.; Dearnaley, D.; Oyen, W.J.G. Impact of Ga-68-PSMA PET/CT on management in prostate cancer patients with very early biochemical recurrence after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 901–907. [Google Scholar] [CrossRef]
- Bluemel, C.; Linke, F.; Herrmann, K.; Simunovic, I.; Eiber, M.; Kestler, C.; Buck, A.K.; Schirbel, A.; Bley, T.A.; Wester, H.J.; et al. Impact of 68Ga-PSMA PET/CT on salvage radiotherapy planning in patients with prostate cancer and persisting PSA values or biochemical relapse after prostatectomy. EJNMMI Res. 2016, 6, 78. [Google Scholar] [CrossRef]
- Boreta, L.; Gadzinski, A.J.; Wu, S.Y.; Xu, M.; Greene, K.; Quanstrom, K.; Nguyen, H.G.; Carroll, P.R.; Hope, T.A.; Feng, F.Y. Location of Recurrence by Gallium-68 PSMA-11 PET Scan in Prostate Cancer Patients Eligible for Salvage Radiotherapy. Urology 2019, 129, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Farolfi, A.; Ceci, F.; Castellucci, P.; Graziani, T.; Siepe, G.; Lambertini, A.; Schiavina, R.; Lodi, F.; Morganti, A.G.; Fanti, S. 68Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA <0.5 ng/mL. Efficacy and impact on treatment strategy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Habl, G.; Sauter, K.; Schiller, K.; Dewes, S.; Maurer, T.; Eiber, M.; Combs, S.E. 68Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: Individualized medicine or new standard in salvage treatment. Prostate 2017, 77, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Hegemann, N.S.; Eze, C.; Li, M.; Rogowski, P.; Schaefer, C.; Stief, C.; Buchner, A.; Zamboglou, C.; Fendler, W.P.; Ganswindt, U.; et al. Impact of 68Ga-PSMA PET/CT on the Radiotherapeutic Approach to Prostate Cancer in Comparison to CT: A Retrospective Analysis. J. Nucl. Med. 2019, 60, 963–970. [Google Scholar] [CrossRef]
- van Leeuwen, P.J.; Stricker, P.; Hruby, G.; Kneebone, A.; Ting, F.; Thompson, B.; Nguyen, Q.; Ho, B.; Emmett, L. 68Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016, 117, 732–739. [Google Scholar] [CrossRef]
- Zacho, H.D.; Nielsen, J.B.; Dettmann, K.; Haberkorn, U.; Langkilde, N.C.; Jensen, J.B.; Petersen, L.J. 68Ga-PSMA PET/CT in Patients With Biochemical Recurrence of Prostate Cancer: A Prospective, 2-Center Study. Clin. Nucl. Med. 2018, 43, 579–585. [Google Scholar] [CrossRef]
- Tendulkar, R.D.; Agrawal, S.; Gao, T.; Efstathiou, J.A.; Pisansky, T.M.; Michalski, J.M.; Koontz, B.F.; Hamstra, D.A.; Feng, F.Y.; Liauw, S.L.; et al. Contemporary Update of a Multi-Institutional Predictive Nomogram for Salvage Radiotherapy After Radical Prostatectomy. J. Clin. Oncol 2016, 34, 3648–3654. [Google Scholar] [CrossRef]
- Afaq, A.; Alahmed, S.; Chen, S.H.; Lengana, T.; Haroon, A.; Payne, H.; Ahmed, H.; Punwani, S.; Sathekge, M.; Bomanji, J. Impact of 68Ga-Prostate-Specific Membrane Antigen PET/CT on Prostate Cancer Management. J. Nucl. Med. 2018, 59, 89–92. [Google Scholar] [CrossRef]
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of 68Ga-PSMA PET on the Management of Patients with Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef]
- Jani, A.B.; Schreibmann, E.; Goyal, S.; Halkar, R.; Hershatter, B.; Rossi, P.J.; Shelton, J.W.; Patel, P.R.; Xu, K.M.; Goodman, M.; et al. (18)F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): A single centre, open-label, phase 2/3 randomised controlled trial. Lancet 2021, 397, 1895–1904. [Google Scholar] [CrossRef]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Vapiwala, N.; Schaeffer, E.M.; Ryan, C.J. Oligometastatic Prostate Cancer: A Shrinking Subset or an Opportunity for Cure? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. New Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Gravis, G.; Fizazi, K.; Joly, F.; Oudard, S.; Priou, F.; Esterni, B.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 149–158. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Ozguroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Gandaglia, G.; Karakiewicz, P.I.; Briganti, A.; Passoni, N.M.; Schiffmann, J.; Trudeau, V.; Graefen, M.; Montorsi, F.; Sun, M. Impact of the Site of Metastases on Survival in Patients with Metastatic Prostate Cancer. Eur. Urol. 2015, 68, 325–334. [Google Scholar] [CrossRef]
- Lussier, Y.A.; Xing, H.R.; Salama, J.K.; Khodarev, N.N.; Huang, Y.; Zhang, Q.; Khan, S.A.; Yang, X.; Hasselle, M.D.; Darga, T.E.; et al. MicroRNA expression characterizes oligometastasis(es). PLoS ONE 2011, 6, e28650. [Google Scholar] [CrossRef]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef]
- Deek, M.P.; Van der Eecken, K.; Sutera, P.; Deek, R.A.; Fonteyne, V.; Mendes, A.A.; Decaestecker, K.; Kiess, A.P.; Lumen, N.; Phillips, R.; et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J. Clin. Oncol. 2022, 40, 3377–3382. [Google Scholar] [CrossRef] [PubMed]
- Bowden, P.; See, A.W.; Frydenberg, M.; Haxhimolla, H.; Costello, A.J.; Moon, D.; Ruljancich, P.; Grummet, J.; Crosthwaite, A.; Pranavan, G.; et al. Fractionated stereotactic body radiotherapy for up to five prostate cancer oligometastases: Interim outcomes of a prospective clinical trial. Int. J. Cancer 2020, 146, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kneebone, A.; Hruby, G.; Ainsworth, H.; Byrne, K.; Brown, C.; Guo, L.; Guminski, A.; Eade, T. Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Detected via Prostate-specific Membrane Antigen Positron Emission Tomography. Eur. Urol. Oncol. 2018, 1, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Artigas, C.; Flamen, P.; Charlier, F.; Levillain, H.; Wimana, Z.; Diamand, R.; Albisinni, S.; Gil, T.; Velthoven, R.V.; Peltier, A.; et al. 68Ga-PSMA PET/CT-based metastasis-directed radiotherapy for oligometastatic prostate cancer recurrence after radical prostatectomy. World J. Urol. 2019, 37, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Guler, O.C.; Engels, B.; Onal, C.; Everaert, H.; Van den Begin, R.; Gevaert, T.; de Ridder, M. The feasibility of prostate-specific membrane antigen positron emission tomography(PSMA PET/CT)-guided radiotherapy in oligometastatic prostate cancer patients. Clin. Transl. Oncol. 2018, 20, 484–490. [Google Scholar] [CrossRef]
- Kalinauskaite, G.; Senger, C.; Kluge, A.; Furth, C.; Kufeld, M.; Tinhofer, I.; Budach, V.; Beck, M.; Hochreiter, A.; Grun, A.; et al. 68Ga-PSMA-PET/CT-based radiosurgery and stereotactic body radiotherapy for oligometastatic prostate cancer. PLoS ONE 2020, 15, e0240892. [Google Scholar] [CrossRef]
- Oehus, A.K.; Kroeze, S.G.C.; Schmidt-Hegemann, N.S.; Vogel, M.M.E.; Kirste, S.; Becker, J.; Burger, I.A.; Derlin, T.; Bartenstein, P.; Eiber, M.; et al. Efficacy of PSMA ligand PET-based radiotherapy for recurrent prostate cancer after radical prostatectomy and salvage radiotherapy. BMC Cancer 2020, 20, 362. [Google Scholar] [CrossRef]
- Onal, C.; Ozyigit, G.; Oymak, E.; Guler, O.C.; Tilki, B.; Hurmuz, P.; Akyol, F. Stereotactic radiotherapy to oligoprogressive lesions detected with 68Ga-PSMA-PET/CT in castration-resistant prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3683–3692. [Google Scholar] [CrossRef]
- Hurmuz, P.; Onal, C.; Ozyigit, G.; Igdem, S.; Atalar, B.; Sayan, H.; Akgun, Z.; Kurt, M.; Ozkok, H.B.; Selek, U.; et al. Treatment outcomes of metastasis-directed treatment using 68Ga-PSMA-PET/CT for oligometastatic or oligorecurrent prostate cancer: Turkish Society for Radiation Oncology group study (TROD 09-002). Strahlenther. Onkol. 2020, 196, 1034–1043. [Google Scholar] [CrossRef]
- Deijen, C.L.; Vrijenhoek, G.L.; Schaake, E.E.; Vogel, W.V.; Moonen, L.M.F.; Pos, F.J.; van der Poel, H.G.; Borst, G.R. PSMA-11-PET/CT versus choline-PET/CT to guide stereotactic ablative radiotherapy for androgen deprivation therapy deferral in patients with oligometastatic prostate cancer. Clin. Transl. Radiat. Oncol. 2021, 30, 1–6. [Google Scholar] [CrossRef]
- Wright, G.L., Jr.; Grob, B.M.; Haley, C.; Grossman, K.; Newhall, K.; Petrylak, D.; Troyer, J.; Konchuba, A.; Schellhammer, P.F.; Moriarty, R. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 1996, 48, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Truillet, C.; Ehman, E.C.; Afshar-Oromieh, A.; Aggarwal, R.; Ryan, C.J.; Carroll, P.R.; Small, E.J.; Evans, M.J. 68Ga-PSMA-11 PET Imaging of Response to Androgen Receptor Inhibition: First Human Experience. J. Nucl. Med. 2017, 58, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Meller, B.; Bremmer, F.; Sahlmann, C.O.; Hijazi, S.; Bouter, C.; Trojan, L.; Meller, J.; Thelen, P. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Fanti, S.; Hadaschik, B.; Herrmann, K. Proposal for Systemic-Therapy Response-Assessment Criteria at the Time of PSMA PET/CT Imaging: The PSMA PET Progression Criteria. J. Nucl. Med. 2020, 61, 678–682. [Google Scholar] [CrossRef]
- Kerkmeijer, L.G.W.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; van der Voort van Zijp, J.; van Vulpen, M.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 787–796. [Google Scholar] [CrossRef]
- Zamboglou, C.; Spohn, S.K.B.; Ruf, J.; Benndorf, M.; Gainey, M.; Kamps, M.; Jilg, C.; Gratzke, C.; Adebahr, S.; Schmidtmayer-Zamboglou, B.; et al. PSMA-PET- and MRI-Based Focal Dose Escalated Radiation Therapy of Primary Prostate Cancer: Planned Safety Analysis of a Nonrandomized 2-Armed Phase 2 Trial (ARO2020-01). Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 1025–1035. [Google Scholar] [CrossRef]
- Zamboglou, C.; Drendel, V.; Jilg, C.A.; Rischke, H.C.; Beck, T.I.; Schultze-Seemann, W.; Krauss, T.; Mix, M.; Schiller, F.; Wetterauer, U.; et al. Comparison of 68Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics 2017, 7, 228–237. [Google Scholar] [CrossRef]
- Zamboglou, C.; Schiller, F.; Fechter, T.; Wieser, G.; Jilg, C.A.; Chirindel, A.; Salman, N.; Drendel, V.; Werner, M.; Mix, M.; et al. 68Ga-HBED-CC-PSMA PET/CT Versus Histopathology in Primary Localized Prostate Cancer: A Voxel-Wise Comparison. Theranostics 2016, 6, 1619–1628. [Google Scholar] [CrossRef]
- Emmett, L.; Buteau, J.; Papa, N.; Moon, D.; Thompson, J.; Roberts, M.J.; Rasiah, K.; Pattison, D.A.; Yaxley, J.; Thomas, P.; et al. The Additive Diagnostic Value of Prostate-specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study. Eur. Urol. 2021, 80, 682–689. [Google Scholar] [CrossRef]
- Schiller, K.; Sauter, K.; Dewes, S.; Eiber, M.; Maurer, T.; Gschwend, J.; Combs, S.E.; Habl, G. Patterns of failure after radical prostatectomy in prostate cancer-implications for radiation therapy planning after Ga-68-PSMA-PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1656–1662. [Google Scholar] [CrossRef]
- Hall, W.A.; Paulson, E.; Davis, B.J.; Spratt, D.E.; Morgan, T.M.; Dearnaley, D.; Tree, A.C.; Efstathiou, J.A.; Harisinghani, M.; Jani, A.B.; et al. NRG Oncology Updated International Consensus Atlas on Pelvic Lymph Node Volumes for Intact and Postoperative Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 174–185. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Simko, J.P.; Kurhanewicz, J.; Santos, R.D.; Feng, F.Y.; Reiter, R.E.; Rettig, M.B.; Nickols, N.G.; et al. False positive PSMA PET for tumor remnants in the irradiated prostate and other interpretation pitfalls in a prospective multi-center trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, C.; Fernandez-Pascual, E.; Arcaniolo, D.; Emberton, M.; Sanchez-Salas, R.; Artigas Guix, C.; Bianco, F.; Cathcart, P.; Murphy, D.G.; Counago, F.; et al. The Role of Prostate-specific Membrane Antigen Positron Emission Tomography/Magnetic Resonance Imaging in Primary and Recurrent Prostate Cancer: A Systematic Review of the Literature. Eur. Urol. Focus 2022, 8, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Violet, J.; Sandhu, S.; Hicks, R.J.; Ravi Kumar, A.S.; Iravani, A.; Kong, G.; Akhurst, T.; Thang, S.P.; Murphy, D.G.; et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2322–2327. [Google Scholar] [CrossRef]
- Loizzo, D.; Pandolfo, S.D.; Rogers, D.; Cerrato, C.; di Meo, N.A.; Autorino, R.; Mirone, V.; Ferro, M.; Porta, C.; Stella, A.; et al. Novel Insights into Autophagy and Prostate Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 3826. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).