The Biological Roles and Molecular Mechanisms of Long Non-Coding RNA MEG3 in the Hallmarks of Cancer
Abstract
Simple Summary
Abstract
1. Introduction
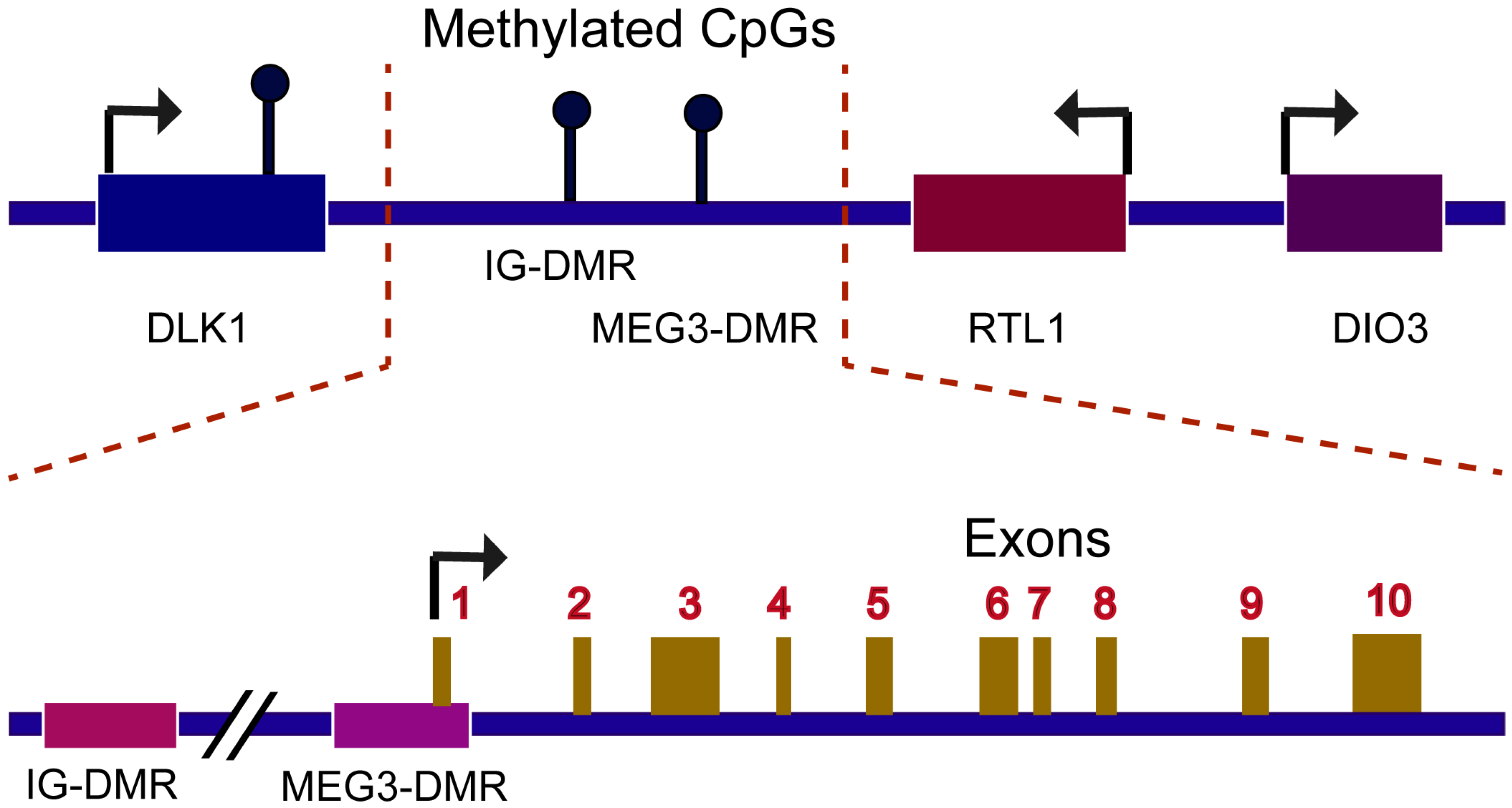
2. Mechanism of MEG3 Regulations
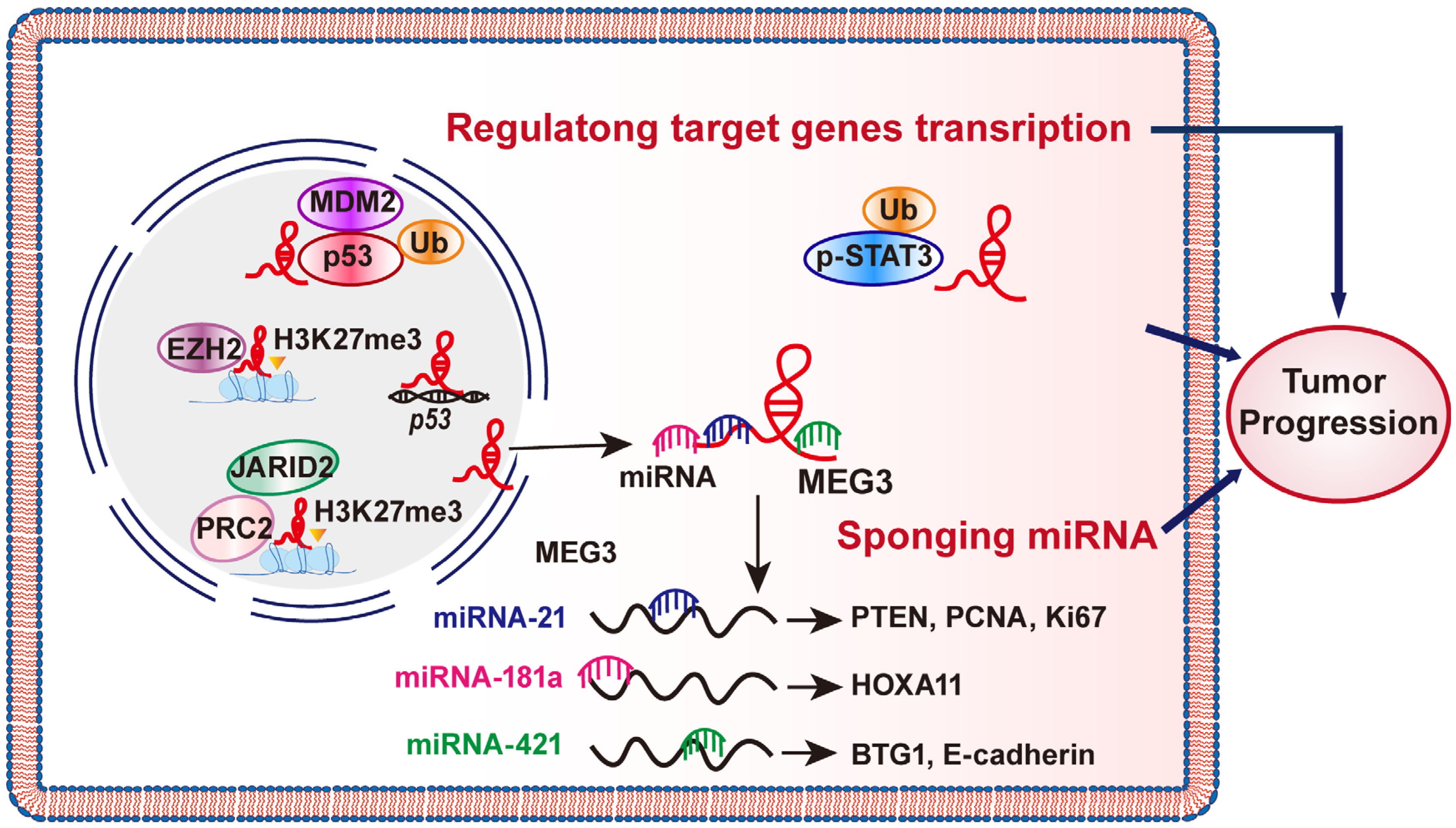
2.1. MEG3 Could Sponge miRNAs
2.2. MEG Regulations on Target Genes Transcription
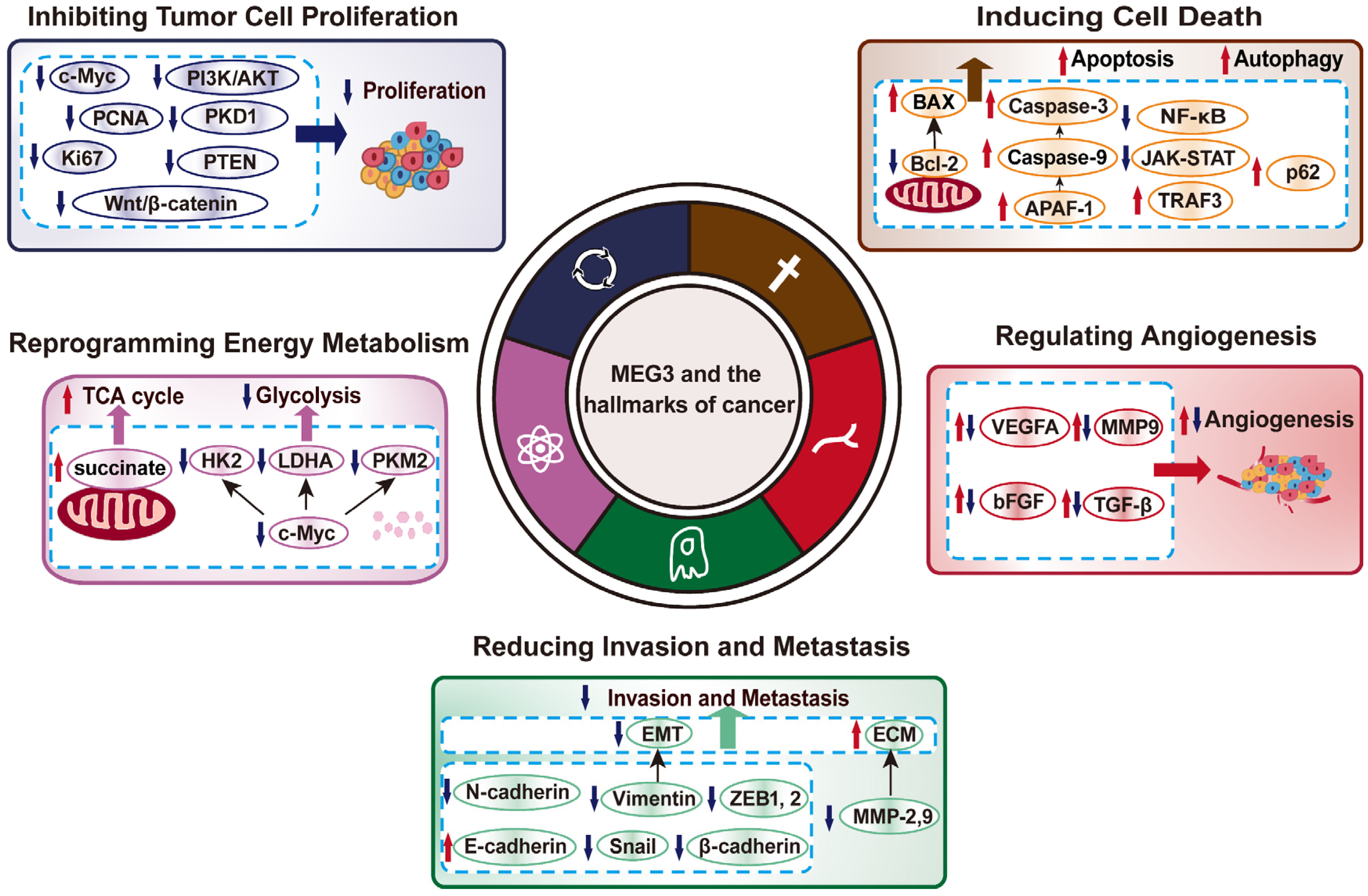
3. MEG3 Regulates Various Hallmarks of Cancer
3.1. MEG3 Inhibits Tumor Cell Proliferation
3.2. MEG3 Induces Cell Death
3.3. MEG3 Negatively Regulates Tumor Cells Invasion and Metastasis Potentials
3.4. MEG3 Regulation on Tumor Cells Metabolic Reprogramming
3.5. MEG3 Suppresses Tumor Angiogenesis
4. Clinical Significance of lncRNA MEG3
4.1. MEG3 Is a Potential Biomarker for Tumor Prognosis
4.2. MEG3 Is a Potential Target for Tumor Therapy
| Cancer Type | Expression | Target | Chemical-/Radioresistance | Refs |
|---|---|---|---|---|
| TNBC | Downregulated | NLRP3/caspase-1/GSDMD pathway | Cisplatin (DDP) | [134] |
| NSCLC | Downregulated | miR-21-5p/SOX7 | Cisplatin | [135] |
| T-cell lymphoblastic lymphoma | Downregulated | PI3K/mTOR signaling | Cisplatin and Cyclophosphamide | [136] |
| CRC | Downregulated | miR-141/PDCD4 | Oxaliplatin | [129] |
| AML | Downregulated | miR-21/MRP1, MDR1, and ABCG2 | Imatinib | [138] |
| Breast cancer | Downregulated | miR-4513/PBLD | Paclitaxel (PTX) | [137] |
| ACL | Downregulated | miR-155/ALG9 | Adriamycin and Vincristine | [139] |
| Thyroid carcinoma | Downregulated | miR-182 | 131I | [141] |
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Pitolli, C.; Wang, Y.; Mancini, M.; Shi, Y.; Melino, G.; Amelio, I. Do Mutations Turn p53 into an Oncogene? Int. J. Mol. Sci. 2019, 20, 6241. [Google Scholar] [CrossRef]
- Han, M.; Jia, L.; Lv, W.; Wang, L.; Cui, W. Epigenetic Enzyme Mutations: Role in Tumorigenesis and Molecular Inhibitors. Front. Oncol. 2019, 9, 194. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Lin, C.; Yang, L. Long Noncoding RNA in Cancer: Wiring Signaling Circuitry. Trends Cell Biol. 2018, 28, 287–301. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Ding, X.; Li, W. Identification of lncRNA/circRNA-miRNA-mRNA ceRNA Network as Biomarkers for Hepatocellular Carcinoma. Front. Genet. 2022, 13, 838869. [Google Scholar] [CrossRef]
- Yang, G.; Lu, X.; Yuan, L. LncRNA: A link between RNA and cancer. Biochim. Biophys. Acta 2014, 1839, 1097–1109. [Google Scholar] [CrossRef]
- Hartford, C.C.R.; Lal, A. When Long Noncoding Becomes Protein Coding. Mol. Cell. Biol. 2020, 40, e00528-19. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Abdi, E.; Latifi-Navid, S.; Latifi-Navid, H. Long noncoding RNA polymorphisms and colorectal cancer risk: Progression and future perspectives. Environ. Mol. Mutagen. 2022, 63, 98–112. [Google Scholar] [CrossRef]
- Tian, J.; Luo, B. Identification of Three Prognosis-Related Differentially Expressed lncRNAs Driven by Copy Number Variation in Thyroid Cancer. J. Immunol. Res. 2022, 2022, 9203796. [Google Scholar] [CrossRef] [PubMed]
- Zamaraev, A.V.; Volik, P.I.; Sukhikh, G.T.; Kopeina, G.S.; Zhivotovsky, B. Long non-coding RNAs: A view to kill ovarian cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188584. [Google Scholar] [CrossRef]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.; Klibanski, A. MEG3 noncoding RNA: A tumor suppressor. J. Mol. Endocrinol. 2012, 48, R45–R53. [Google Scholar] [CrossRef]
- Schuster-Gossler, K.; Bilinski, P.; Sado, T.; Ferguson-Smith, A.; Gossler, A. The mouse Gtl2 gene is differentially expressed during embryonic development, encodes multiple alternatively spliced transcripts, and may act as an RNA. Dev. Dyn. 1998, 212, 214–228. [Google Scholar] [CrossRef]
- Miyoshi, N.; Wagatsuma, H.; Wakana, S.; Shiroishi, T.; Nomura, M.; Aisaka, K.; Kohda, T.; Surani, M.A.; Kaneko-Ishino, T.; Ishino, F. Identification of an imprinted gene, Meg3/Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes Cells 2000, 5, 211–220. [Google Scholar] [CrossRef]
- Kagami, M.; O’Sullivan, M.J.; Green, A.J.; Watabe, Y.; Arisaka, O.; Masawa, N.; Matsuoka, K.; Fukami, M.; Matsubara, K.; Kato, F.; et al. The IG-DMR and the MEG3-DMR at human chromosome 14q32.2: Hierarchical interaction and distinct functional properties as imprinting control centers. PLoS Genet. 2010, 6, e1000992. [Google Scholar] [CrossRef]
- Sherpa, C.; Rausch, J.W.; Le Grice, S.F. Structural characterization of maternally expressed gene 3 RNA reveals conserved motifs and potential sites of interaction with polycomb repressive complex 2. Nucleic Acids Res. 2018, 46, 10432–10447. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fang, L.; Pu, Q.; Bu, H.; Zhu, P.; Chen, Z.; Yu, M.; Li, X.; Weiland, T.; Bansal, A.; et al. MEG3-4 is a miRNA decoy that regulates IL-1beta abundance to initiate and then limit inflammation to prevent sepsis during lung infection. Sci. Signal. 2018, 11, eaao2387. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Hou, S.; Zhu, B.; Wang, W.; Hao, T.; Bu, X.; Khan, M.; Lei, H. Nuclear retention element recruits U1 snRNP components to restrain spliced lncRNAs in the nucleus. RNA Biol. 2019, 16, 1001–1009. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Bai, X.; Lin, Y.; Li, Z.; Fu, J.; Li, M.; Zhao, T.; Yang, H.; Xu, R.; et al. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J. Pineal. Res. 2018, 64, e12449. [Google Scholar] [CrossRef]
- Sathishkumar, C.; Prabu, P.; Mohan, V.; Balasubramanyam, M. Linking a role of lncRNAs (long non-coding RNAs) with insulin resistance, accelerated senescence, and inflammation in patients with type 2 diabetes. Hum. Genom. 2018, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Rao, J.; Yuan, J.; Gao, L.; Huang, W.; Zhao, L.; Ren, J. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate ischemic neuronal death by targeting miR-21/PDCD4 signaling pathway. Cell Death Dis. 2017, 8, 3211. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Taheri, M. Maternally expressed gene 3 (MEG3): A tumor suppressor long non coding RNA. Biomed. Pharmacother. 2019, 118, 109129. [Google Scholar] [CrossRef]
- Benetatos, L.; Vartholomatos, G.; Hatzimichael, E. MEG3 imprinted gene contribution in tumorigenesis. Int. J. Cancer 2011, 129, 773–779. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Mehta, K.R.; Danila, D.C.; Scolavino, S.; Johnson, S.R.; Klibanski, A. A pituitary-derived MEG3 isoform functions as a growth suppressor in tumor cells. J. Clin. Endocrinol. Metab. 2003, 88, 5119–5126. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef]
- Moradi, M.T.; Fallahi, H.; Rahimi, Z. Interaction of long noncoding RNA MEG3 with miRNAs: A reciprocal regulation. J. Cell. Biochem. 2019, 120, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Karreth, F.A.; Pandolfi, P.P. ceRNA cross-talk in cancer: When ce-bling rivalries go awry. Cancer Discov. 2013, 3, 1113–1121. [Google Scholar] [CrossRef]
- Dan, J.; Wang, J.; Wang, Y.; Zhu, M.; Yang, X.; Peng, Z.; Jiang, H.; Chen, L. LncRNA-MEG3 inhibits proliferation and metastasis by regulating miRNA-21 in gastric cancer. Biomed. Pharmacother. 2018, 99, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, L.; Liu, X.; Nie, Z.; Luo, J. Long noncoding RNA MEG3 inhibits proliferation of chronic myeloid leukemia cells by sponging microRNA21. Biomed. Pharmacother. 2018, 104, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhu, L.; Li, Y.; Zheng, Z.; Lin, X.; Yang, C. LncRNA MEG3 promotes melanoma growth, metastasis and formation through modulating miR-21/E-cadherin axis. Cancer Cell Int. 2020, 20, 12. [Google Scholar] [CrossRef]
- Lin, L.; Liu, X.; Lv, B. Long non-coding RNA MEG3 promotes autophagy and apoptosis of nasopharyngeal carcinoma cells via PTEN up-regulation by binding to microRNA-21. J. Cell. Mol. Med. 2021, 25, 61–72. [Google Scholar] [CrossRef]
- Peng, W.; Si, S.; Zhang, Q.; Li, C.; Zhao, F.; Wang, F.; Yu, J.; Ma, R. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate gastric cancer progression. J. Exp. Clin. Cancer Res. 2015, 34, 79. [Google Scholar] [CrossRef]
- Shen, X.; Bai, H.; Zhu, H.; Yan, Q.; Yang, Y.; Yu, W.; Shi, Q.; Wang, J.; Li, J.; Chen, L. Long Non-Coding RNA MEG3 Functions as a Competing Endogenous RNA to Regulate HOXA11 Expression by Sponging miR-181a in Multiple Myeloma. Cell. Physiol. Biochem. 2018, 49, 87–100. [Google Scholar] [CrossRef]
- Ji, Y.; Feng, G.; Hou, Y.; Yu, Y.; Wang, R.; Yuan, H. Long noncoding RNA MEG3 decreases the growth of head and neck squamous cell carcinoma by regulating the expression of miR-421 and E-cadherin. Cancer Med. 2020, 9, 3954–3963. [Google Scholar] [CrossRef]
- Zhu, J.; Han, S. Lidocaine inhibits cervical cancer cell proliferation and induces cell apoptosis by modulating the lncRNA-MEG3/miR-421/BTG1 pathway. Am. J. Transl. Res. 2019, 11, 5404–5416. [Google Scholar]
- Zhang, W.; Shi, S.; Jiang, J.; Li, X.; Lu, H.; Ren, F. LncRNA MEG3 inhibits cell epithelial-mesenchymal transition by sponging miR-421 targeting E-cadherin in breast cancer. Biomed. Pharmacother. 2017, 91, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.J.; Tay, Y. Noncoding RNA:RNA Regulatory Networks in Cancer. Int. J. Mol. Sci. 2018, 19, 1310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rice, K.; Wang, Y.; Chen, W.; Zhong, Y.; Nakayama, Y.; Zhou, Y.; Klibanski, A. Maternally expressed gene 3 (MEG3) noncoding ribonucleic acid: Isoform structure, expression, and functions. Endocrinology 2010, 151, 939–947. [Google Scholar] [CrossRef]
- Uroda, T.; Anastasakou, E.; Rossi, A.; Teulon, J.M.; Pellequer, J.L.; Annibale, P.; Pessey, O.; Inga, A.; Chillon, I.; Marcia, M. Conserved Pseudoknots in lncRNA MEG3 Are Essential for Stimulation of the p53 Pathway. Mol. Cell. 2019, 75, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.F.; Tang, Y.L.; Shen, Z.L.; Yang, K.Y.; Gao, K. UXT, a novel DNMT3b-binding protein, promotes breast cancer progression via negatively modulating lncRNA MEG3/p53 axis. Mol. Ther. Oncolytics. 2022, 24, 497–506. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, P.; Gao, Y.; Ta, N.; Zhang, Y.; Cai, J.; Zhao, Y.; Liu, S.; Zheng, J. MEG3 Activated by Vitamin D Inhibits Colorectal Cancer Cells Proliferation and Migration via Regulating Clusterin. EBioMedicine 2018, 30, 148–157. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Y. Long non-coding RNA MEG3 inhibits cervical cancer cell growth by promoting degradation of P-STAT3 protein via ubiquitination. Cancer Cell Int. 2019, 19, 175. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Hushmandi, K.; Hashemi, F.; Zabolian, A.; Canadas, I.; Zarrabi, A.; Nabavi, N.; Aref, A.R.; Crea, F.; et al. The long and short non-coding RNAs modulating EZH2 signaling in cancer. J. Hematol. Oncol. 2022, 15, 18. [Google Scholar] [CrossRef]
- Trotman, J.B.; Braceros, K.C.A.; Cherney, R.E.; Murvin, M.M.; Calabrese, J.M. The control of polycomb repressive complexes by long noncoding RNAs. Wiley Interdiscip. Rev. RNA 2021, 12, e1657. [Google Scholar] [CrossRef]
- Terashima, M.; Tange, S.; Ishimura, A.; Suzuki, T. MEG3 Long Noncoding RNA Contributes to the Epigenetic Regulation of Epithelial-Mesenchymal Transition in Lung Cancer Cell Lines. J. Biol. Chem. 2017, 292, 82–99. [Google Scholar] [CrossRef]
- Mondal, T.; Subhash, S.; Vaid, R.; Enroth, S.; Uday, S.; Reinius, B.; Mitra, S.; Mohammed, A.; James, A.R.; Hoberg, E.; et al. MEG3 long noncoding RNA regulates the TGF-beta pathway genes through formation of RNA-DNA triplex structures. Nat. Commun. 2015, 6, 7743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, H.; Xia, W.; Cui, L.; Xu, R.; Lu, H.; Xue, D.; Tian, Z.; Ding, T.; Cao, Y.; et al. LncRNA MEG3 inhibits the progression of prostate cancer by facilitating H3K27 trimethylation of EN2 through binding to EZH2. J. Biochem. 2020, 167, 295–301. [Google Scholar] [CrossRef]
- Ye, M.; Gao, R.; Chen, S.; Wei, M.; Wang, J.; Zhang, B.; Wu, S.; Xu, Y.; Wu, P.; Chen, X.; et al. Downregulation of MEG3 and upregulation of EZH2 cooperatively promote neuroblastoma progression. J. Cell. Mol. Med. 2022, 26, 2377–2391. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 2020, 20, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, S.; Ye, F.; Shen, Y.; Tie, Y.; Zhu, J.; Wei, L.; Jin, Y.; Fu, H.; Wu, Y.; et al. Long Noncoding RNA MEG3 Interacts with p53 Protein and Regulates Partial p53 Target Genes in Hepatoma Cells. PLoS ONE 2015, 10, e0139790. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhong, Y.; Wang, Y.; Zhang, X.; Batista, D.L.; Gejman, R.; Ansell, P.J.; Zhao, J.; Weng, C.; Klibanski, A. Activation of p53 by MEG3 non-coding RNA. J. Biol. Chem. 2007, 282, 24731–24742. [Google Scholar] [CrossRef]
- Lu, K.H.; Li, W.; Liu, X.H.; Sun, M.; Zhang, M.L.; Wu, W.Q.; Xie, W.P.; Hou, Y.Y. Long non-coding RNA MEG3 inhibits NSCLC cells proliferation and induces apoptosis by affecting p53 expression. BMC Cancer 2013, 13, 461. [Google Scholar] [CrossRef]
- Zhu, X.; Lv, L.; Wang, M.; Fan, C.; Lu, X.; Jin, M.; Li, S.; Wang, F. DNMT1 facilitates growth of breast cancer by inducing MEG3 hyper-methylation. Cancer Cell Int. 2022, 22, 56. [Google Scholar] [CrossRef]
- Gong, X.; Huang, M. Long non-coding RNA MEG3 promotes the proliferation of glioma cells through targeting Wnt/beta-catenin signal pathway. Cancer Gene Ther. 2017, 24, 381–385. [Google Scholar] [CrossRef]
- Fan, F.Y.; Deng, R.; Yi, H.; Sun, H.P.; Zeng, Y.; He, G.C.; Su, Y. The inhibitory effect of MEG3/miR-214/AIFM2 axis on the growth of T-cell lymphoblastic lymphoma. Int. J. Oncol. 2017, 51, 316–326. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Dai, J.; Zhuo, R.; Zhao, J.; Wang, H.; Sun, F.; Zhu, Y.; Xu, D. Study on the mechanism behind lncRNA MEG3 affecting clear cell renal cell carcinoma by regulating miR-7/RASL11B signaling. J. Cell. Physiol. 2018, 233, 9503–9515. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, J.; Ren, Y.; Zuo, Z.; Ni, S.; Cai, J. MEG3 can affect the proliferation and migration of colorectal cancer cells through regulating miR-376/PRKD1 axis. Am. J. Transl. Res. 2019, 11, 5740–5751. [Google Scholar]
- Zhang, Y.Y.; Feng, H.M. MEG3 Suppresses Human Pancreatic Neuroendocrine Tumor Cells Growth and Metastasis by Down-Regulation of Mir-183. Cell. Physiol. Biochem. 2017, 44, 345–356. [Google Scholar] [CrossRef]
- Tan, J.; Xiang, L.; Xu, G. LncRNA MEG3 suppresses migration and promotes apoptosis by sponging miR-548d-3p to modulate JAK-STAT pathway in oral squamous cell carcinoma. IUBMB Life 2019, 71, 882–890. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yang, L.; Liu, X.J.; Wang, X.Z.; Pan, Y.X.; Luo, J.M. The Long Noncoding RNA MEG3 and its Target miR-147 Regulate JAK/STAT Pathway in Advanced Chronic Myeloid Leukemia. EBioMedicine 2018, 34, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, T.; Wang, Y.; Yu, J.; Liu, Y.; Lin, Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol. Ther. 2016, 17, 104–113. [Google Scholar] [CrossRef]
- Wang, L.; Yu, M.; Zhao, S. lncRNA MEG3 modified epithelial-mesenchymal transition of ovarian cancer cells by sponging miR-219a-5p and regulating EGFR. J. Cell. Biochem. 2019, 120, 17709–17722. [Google Scholar] [CrossRef]
- Qin, N.; Tong, G.F.; Sun, L.W.; Xu, X.L. Long Noncoding RNA MEG3 Suppresses Glioma Cell Proliferation, Migration, and Invasion by Acting as a Competing Endogenous RNA of miR-19a. Oncol. Res. 2017, 25, 1471–1478. [Google Scholar] [CrossRef]
- Wu, J.; Pang, R.; Li, M.; Chen, B.; Huang, J.; Zhu, Y. m6A-Induced LncRNA MEG3 Suppresses the Proliferation, Migration and Invasion of Hepatocellular Carcinoma Cell Through miR-544b/BTG2 Signaling. Onco Targets Ther. 2021, 14, 3745–3755. [Google Scholar] [CrossRef]
- Huang, C.; Liao, X.; Jin, H.; Xie, F.; Zheng, F.; Li, J.; Zhou, C.; Jiang, G.; Wu, X.R.; Huang, C. MEG3, as a Competing Endogenous RNA, Binds with miR-27a to Promote PHLPP2 Protein Translation and Impairs Bladder Cancer Invasion. Mol. Ther. Nucleic Acids 2019, 16, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Wu, L.; Wang, Y.; Yuan, X. Long Non-coding RNA MEG3 Activated by Vitamin D Suppresses Glycolysis in Colorectal Cancer via Promoting c-Myc Degradation. Front. Oncol. 2020, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Sun, H.; Wang, X.; Yu, X.; Zhang, J.; Guo, B.; Hexige, S. Metabolic changes during malignant transformation in primary cells of oral lichen planus: Succinate accumulation and tumour suppression. J. Cell. Mol. Med. 2020, 24, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Yu, M.S.; Li, X.; Zhang, Z.; Han, C.R.; Yan, B. Overexpression of long non-coding RNA MEG3 suppresses breast cancer cell proliferation, invasion, and angiogenesis through AKT pathway. Tumour Biol. 2017, 39, 1010428317701311. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Lv, S.; Zhang, Y.; Zhang, C.; Lige, B.; Dan, S.; Sun, Y. Long noncoding RNA MEG3 plays a promoting role in the proliferation, invasion, and angiogenesis of lung adenocarcinoma cells through the AKT pathway. J. Cell. Biochem. 2019, 120, 16143–16152. [Google Scholar] [CrossRef] [PubMed]
- Buccarelli, M.; Lulli, V.; Giuliani, A.; Signore, M.; Martini, M.; D’Alessandris, Q.G.; Giannetti, S.; Novelli, A.; Ilari, R.; Giurato, G.; et al. Deregulated expression of the imprinted DLK1-DIO3 region in glioblastoma stemlike cells: Tumor suppressor role of lncRNA MEG3. Neuro Oncol. 2020, 22, 1771–1784. [Google Scholar] [CrossRef]
- Dong, S.; Ma, M.; Li, M.; Guo, Y.; Zuo, X.; Gu, X.; Zhang, M.; Shi, Y. LncRNA MEG3 regulates breast cancer proliferation and apoptosis through miR-141-3p/RBMS3 axis. Genomics 2021, 113, 1689–1704. [Google Scholar] [CrossRef]
- Shan, G.; Tang, T.; Xia, Y.; Qian, H.J. MEG3 interacted with miR-494 to repress bladder cancer progression through targeting PTEN. J. Cell. Physiol. 2020, 235, 1120–1128. [Google Scholar] [CrossRef]
- Han, T.; Zhuo, M.; Yuan, C.; Xiao, X.; Cui, J.; Qin, G.; Wang, L.; Jiao, F. Coordinated silencing of the Sp1-mediated long noncoding RNA MEG3 by EZH2 and HDAC3 as a prognostic factor in pancreatic ductal adenocarcinoma. Cancer Biol. Med. 2020, 17, 953–969. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Li, C.; Liu, Y.; Kang, P.; Zhong, X.; Cui, Y. LncRNA-MEG3 inhibits cell proliferation and invasion by modulating Bmi1/RNF2 in cholangiocarcinoma. J. Cell. Physiol. 2019, 234, 22947–22959. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; He, Y.; Xia, Q.; Liu, S. LncRNA MEG3 impacts proliferation, invasion, and migration of ovarian cancer cells through regulating PTEN. Inflamm. Res. 2018, 67, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Lv, Y.; Zhang, C.; Guo, S. LncRNA meg3 suppresses hepatocellular carcinoma in vitro and vivo studies. Am. J. Transl. Res. 2019, 11, 4089–4099. [Google Scholar]
- Gong, A.; Zhao, X.; Pan, Y.; Qi, Y.; Li, S.; Huang, Y.; Guo, Y.; Qi, X.; Zheng, W.; Jia, L. The lncRNA MEG3 mediates renal cell cancer progression by regulating ST3Gal1 transcription and EGFR sialylation. J. Cell. Sci. 2020, 133, jcs244020. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, X.; Li, Y. Long non-coding RNA MEG3 inhibits cell growth of gliomas by targeting miR-93 and inactivating PI3K/AKT pathway. Oncol. Rep. 2017, 38, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wan, Y.; Qiu, M.; Wang, S.; Pan, C.; Wang, Y.; Ou, J. lncRNA MEG3 Suppresses the Tumorigenesis of Hemangioma by Sponging miR-494 and Regulating PTEN/ PI3K/AKT Pathway. Cell. Physiol. Biochem. 2018, 51, 2872–2886. [Google Scholar] [CrossRef] [PubMed]
- Peitzsch, C.; Tyutyunnykova, A.; Pantel, K.; Dubrovska, A. Cancer stem cells: The root of tumor recurrence and metastases. Semin. Cancer Biol. 2017, 44, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, Q.; Li, J.; Zhang, K.; Qin, J.; Zhao, J.; Sun, Q.; Wang, Z.; Wartmann, T.; Jauch, K.W.; et al. Targeting cancer stem cells and their niche: Perspectives for future therapeutic targets and strategies. Semin. Cancer Biol. 2018, 53, 139–155. [Google Scholar] [CrossRef]
- Chen, P.Y.; Hsieh, P.L.; Peng, C.Y.; Liao, Y.W.; Yu, C.H.; Yu, C.C. LncRNA MEG3 inhibits self-renewal and invasion abilities of oral cancer stem cells by sponging miR-421. J. Formos. Med. Assoc. 2021, 120, 1137–1142. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, W.W.; Wei, W.; Zhan, L.X.; Huang, X. Long noncoding RNA Meg3 sponges miR-708 to inhibit intestinal tumorigenesis via SOCS3-repressed cancer stem cells growth. Cell Death Dis. 2021, 13, 25. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Roberts, J.Z.; Crawford, N.; Longley, D.B. The role of Ubiquitination in Apoptosis and Necroptosis. Cell Death Differ. 2022, 29, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell. Dev. Biol. 1999, 15, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Wang, M.; Wu, X.; Tao, D.; Xiao, X.; Wang, L.; Min, F.; Zeng, F.; Jiang, G. Long Non-Coding RNA MEG3 Inhibits Cell Proliferation and Induces Apoptosis in Prostate Cancer. Cell. Physiol. Biochem. 2015, 37, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhou, N.; Hu, T.; Zhao, W.; Wu, D.; Wang, S. LncRNA MEG3 negatively modified osteosarcoma development through regulation of miR-361-5p and FoxM1. J. Cell. Physiol. 2019, 234, 13464–13480. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, X.; Zhou, J.; Cheng, X.; Ye, Z.; Ji, Z. Long non-coding RNA MEG3 suppresses the development of bladder urothelial carcinoma by regulating miR-96 and TPM1. Cancer Biol. Ther. 2018, 19, 1039–1056. [Google Scholar] [CrossRef]
- Zhu, D.; Xiao, Z.; Wang, Z.; Hu, B.; Duan, C.; Zhu, Z.; Gao, N.; Zhu, Y.; Wang, H. MEG3/MIR-376B-3P/HMGA2 axis is involved in pituitary tumor invasiveness. J. Neurosurg. 2020, 134, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.L.; Chen, R.P.; Zhou, X.T.; Zhan, H.L.; Hu, M.M.; Liu, B.; Wu, G.D.; Wu, L.F. Long non-coding RNA MEG3 induces cell apoptosis in esophageal cancer through endoplasmic reticulum stress. Oncol. Rep. 2017, 37, 3093–3099. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xie, F.; Cheng, X.; Chi, J.; Lei, Y.; Guo, R.; Han, J. LncRNA MEG3 promotes endoplasmic reticulum stress and suppresses proliferation and invasion of colorectal carcinoma cells through the MEG3/miR-103a-3p/PDHB ceRNA pathway. Neoplasma 2021, 68, 362–374. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, N.; Wang, J.; Li, Z. LncRNA MEG3 inhibits cell proliferation and induces apoptosis in laryngeal cancer via miR-23a/APAF-1 axis. J. Cell. Mol. Med. 2019, 23, 6708–6719. [Google Scholar] [CrossRef]
- Jin, L.; Cai, Q.; Wang, S.; Wang, S.; Mondal, T.; Wang, J.; Quan, Z. Long noncoding RNA MEG3 regulates LATS2 by promoting the ubiquitination of EZH2 and inhibits proliferation and invasion in gallbladder cancer. Cell Death Dis. 2018, 9, 1017. [Google Scholar] [CrossRef]
- Bao, D.; Yuan, R.X.; Zhang, Y. Effects of lncRNA MEG3 on proliferation and apoptosis of gallbladder cancer cells through regulating NF-kappaB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6632–6638. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.X. Long non-coding RNA MEG3 represses cholangiocarcinoma by regulating miR-361-5p/TRAF3 axis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7356–7368. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Kogel, D. Autophagy in Cancer Cell Death. Biology 2019, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Baehrecke, E.H. Autophagy, cell death, and cancer. Mol. Cell. Oncol. 2015, 2, e985913. [Google Scholar] [CrossRef]
- Das, G.; Shravage, B.V.; Baehrecke, E.H. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb. Perspect. Biol. 2012, 4, a008813. [Google Scholar] [CrossRef]
- Ye, M.; Lu, H.; Tang, W.; Jing, T.; Chen, S.; Wei, M.; Zhang, J.; Wang, J.; Ma, J.; Ma, D.; et al. Downregulation of MEG3 promotes neuroblastoma development through FOXO1-mediated autophagy and mTOR-mediated epithelial-mesenchymal transition. Int. J. Biol. Sci. 2020, 16, 3050–3061. [Google Scholar] [CrossRef]
- Meirson, T.; Gil-Henn, H.; Samson, A.O. Invasion and metastasis: The elusive hallmark of cancer. Oncogene 2020, 39, 2024–2026. [Google Scholar] [CrossRef]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Steps in metastasis: An updated review. Med. Oncol. 2021, 38, 3. [Google Scholar] [CrossRef]
- Xu, G.; Meng, L.; Yuan, D.; Li, K.; Zhang, Y.; Dang, C.; Zhu, K. MEG3/miR21 axis affects cell mobility by suppressing epithelialmesenchymal transition in gastric cancer. Oncol. Rep. 2018, 40, 39–48. [Google Scholar] [CrossRef]
- Tang, H.; Long, Q.; Zhuang, K.; Yan, Y.; Han, K.; Guo, H.; Lu, X. miR-665 promotes the progression of gastric adenocarcinoma via elevating FAK activation through targeting SOCS3 and is negatively regulated by lncRNA MEG3. J. Cell. Physiol. 2020, 235, 4709–4719. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Dong, P.; Xiong, Y.; Chen, R.; Konno, Y.; Ihira, K.; Yue, J.; Watari, H. PD-L1 Is a Tumor Suppressor in Aggressive Endometrial Cancer Cells and Its Expression Is Regulated by miR-216a and lncRNA MEG3. Front. Cell. Dev. Biol. 2020, 8, 598205. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Y.; Ding, L.; Yu, L.; Zhang, B.; Wei, D. LncRNA MEG3 suppressed the progression of ovarian cancer via sponging miR-30e-3p and regulating LAMA4 expression. Cancer Cell. Int. 2020, 20, 181. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liao, H.; Wu, J.; Chen, B.; Pang, R.; Huang, J.; Zhu, Y. Long noncoding RNA matrilineal expression gene 3 inhibits hepatocellular carcinoma progression by targeting microRNA-5195-3p and regulating the expression of forkhead box O1. Bioengineered 2021, 12, 12880–12890. [Google Scholar] [CrossRef]
- Li, M.K.; Liu, L.X.; Zhang, W.Y.; Zhan, H.L.; Chen, R.P.; Feng, J.L.; Wu, L.F. Long noncoding RNA MEG3 suppresses epithelialtomesenchymal transition by inhibiting the PSAT1dependent GSK3beta/Snail signaling pathway in esophageal squamous cell carcinoma. Oncol. Rep. 2020, 44, 2130–2142. [Google Scholar] [CrossRef]
- Wang, C.; Yan, G.; Zhang, Y.; Jia, X.; Bu, P. Long non-coding RNA MEG3 suppresses migration and invasion of thyroid carcinoma by targeting of Rac1. Neoplasma 2015, 62, 541–549. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Burk, D.; Schade, A.L. On respiratory impairment in cancer cells. Science 1956, 124, 270–272. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhong, Z.; Shao, Y.; Yi, Y. Prognostic Value of MEG3 and Its Correlation With Immune Infiltrates in Gliomas. Front. Genet. 2021, 12, 679097. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Y.; Chen, F.; Liu, X.; Zhong, L.L.; Wang, H.Q.; Li, Q.C. LncRNA MEG3 acts a biomarker and regulates cell functions by targeting ADAR1 in colorectal cancer. World J. Gastroenterol. 2019, 25, 3972–3984. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, X.; Feng, X.; Li, X.; Pan, L.; Liu, J.; Wang, F.; Yuan, Z.; Yang, L.; Yu, J.; et al. Long non-coding RNA MEG3 regulates proliferation, apoptosis, and autophagy and is associated with prognosis in glioma. J. Neurooncol. 2018, 140, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, T.F.; Han, X.W.; Yuan, H.F. Downregulated MEG3 contributes to tumour progression and poor prognosis in oesophagal squamous cell carcinoma by interacting with miR-4261, downregulating DKK2 and activating the Wnt/beta-catenin signalling. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1513–1523. [Google Scholar] [CrossRef]
- Cui, X.; Yi, Q.; Jing, X.; Huang, Y.; Tian, J.; Long, C.; Xiang, Z.; Liu, J.; Zhang, C.; Tan, B.; et al. Mining Prognostic Significance of MEG3 in Human Breast Cancer Using Bioinformatics Analysis. Cell. Physiol. Biochem. 2018, 50, 41–51. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, P.; Zhang, J. Hypermethylation of MEG3 promoter correlates with inactivation of MEG3 and poor prognosis in patients with retinoblastoma. J. Transl. Med. 2017, 15, 268. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Zhang, L.; Yang, D. Overexpression of MEG3 sensitizes colorectal cancer cells to oxaliplatin through regulation of miR-141/PDCD4 axis. Biomed. Pharmacother. 2018, 106, 1607–1615. [Google Scholar] [CrossRef]
- Wang, C.; Tao, X.; Wei, J. Effects of LncRNA MEG3 on immunity and autophagy of non-small cell lung carcinoma through IDO signaling pathway. World J. Surg. Oncol. 2021, 19, 244. [Google Scholar] [CrossRef]
- Chen, B.; Dragomir, M.P.; Yang, C.; Li, Q.; Horst, D.; Calin, G.A. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct. Target. Ther. 2022, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wu, Z.X.; Assaraf, Y.G.; Chen, Z.S.; Wang, L. Overcoming anti-cancer drug resistance via restoration of tumor suppressor gene function. Drug Resist. Updat. 2021, 57, 100770. [Google Scholar] [CrossRef]
- Buttarelli, M.; De Donato, M.; Raspaglio, G.; Babini, G.; Ciucci, A.; Martinelli, E.; Baccaro, P.; Pasciuto, T.; Fagotti, A.; Scambia, G.; et al. Clinical Value of lncRNA MEG3 in High-Grade Serous Ovarian Cancer. Cancers 2020, 12, 966. [Google Scholar] [CrossRef]
- Yan, H.; Luo, B.; Wu, X.; Guan, F.; Yu, X.; Zhao, L.; Ke, X.; Wu, J.; Yuan, J. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer. Int. J. Biol. Sci. 2021, 17, 2606–2621. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, D.; Ma, H.; Li, Y. LncRNA MEG3 enhances cisplatin sensitivity in non-small cell lung cancer by regulating miR-21-5p/SOX7 axis. Onco. Targets Ther. 2017, 10, 5137–5149. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Fan, F.Y.; Yi, H.; Liu, F.; He, G.C.; Sun, H.P.; Su, Y. MEG3 affects the progression and chemoresistance of T-cell lymphoblastic lymphoma by suppressing epithelial-mesenchymal transition via the PI3K/mTOR pathway. J. Cell. Biochem. 2018, 120, 8144–8153. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, F.; Mi, H.; Li, L.; Wang, J.; Han, M.; Gu, Y. Long noncoding RNA MEG3 suppresses cell proliferation, migration and invasion, induces apoptosis and paclitaxel-resistance via miR-4513/PBLD axis in breast cancer cells. Cell Cycle 2020, 19, 3277–3288. [Google Scholar] [CrossRef]
- Zhou, X.; Yuan, P.; Liu, Q.; Liu, Z. LncRNA MEG3 Regulates Imatinib Resistance in Chronic Myeloid Leukemia via Suppressing MicroRNA-21. Biomol. Ther. 2017, 25, 490–496. [Google Scholar] [CrossRef]
- Yu, Y.; Kou, D.; Liu, B.; Huang, Y.; Li, S.; Qi, Y.; Guo, Y.; Huang, T.; Qi, X.; Jia, L. LncRNA MEG3 contributes to drug resistance in acute myeloid leukemia by positively regulating ALG9 through sponging miR-155. Int. J. Lab. Hematol. 2020, 42, 464–472. [Google Scholar] [CrossRef]
- Ma, L.; Wang, F.; Du, C.; Zhang, Z.; Guo, H.; Xie, X.; Gao, H.; Zhuang, Y.; Kornmann, M.; Gao, H.; et al. Long non-coding RNA MEG3 functions as a tumour suppressor and has prognostic predictive value in human pancreatic cancer. Oncol. Rep. 2018, 39, 1132–1140. [Google Scholar] [CrossRef]
- Liu, Y.; Yue, P.; Zhou, T.; Zhang, F.; Wang, H.; Chen, X. LncRNA MEG3 enhances (131)I sensitivity in thyroid carcinoma via sponging miR-182. Biomed. Pharmacother. 2018, 105, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, W.; Jing, D.; Yang, L.; Guo, H.; Wang, L.; Zhang, W.; Pu, F.; Shao, Z. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J. Control. Release 2022, 343, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yue, P.; Miao, Y.; Gao, S.; Wang, B.; Leng, S.X.; Meng, X.; Zhang, H. The lncRNA MEG3/miR-16-5p/VGLL4 regulatory axis is involved in etoposide-induced senescence of tumor cells. J. Gene Med. 2021, 23, e3291. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | miRNA | Related Genes | Hallmarks | Refs |
|---|---|---|---|---|
| Breast cancer | miR-494-3p | OTUD4 | Growth inhibition | [59] |
| Glioma | / | Wnt/β-catenin | Cell cycle regulation | [60] |
| T-cell lymphoblastic lymphoma | miR-214 | AIFM2, Ki-67, PCNA | Growth inhibition | [61] |
| Clear cell renal cell carcinoma | miR-7 | RASL11B | Growth inhibition | [62] |
| CRC | miR-376 | PKD1 | Cell cycle regulation | [63] |
| Pancreatic neuroendocrine tumor | miR-183 | BRI3 | Growth inhibition | [64] |
| OSCC | miR-548d-3p | SOCS5, SOCS6 | Apoptosis induction | [65] |
| CML | miR-147 | JAK/STAT3 | Apoptosis induction | [66] |
| Cervical cancer | miR-21-5p | p53, caspase3 | Apoptosis induction | [67] |
| Breast cancer | miR-421 | E-cadherin | EMT inhibition | [42] |
| Ovarian cancer | miR-219a-5p | EGFR | EMT inhibition | [68] |
| Glioma | miR-19a | PTEN | Metastasis inhibition | [69] |
| HCC | miR-544b | BTG2 | Metastasis inhibition | [70] |
| Bladder cancer | miR-27a | PHLPP2, c-Myc | Metastasis inhibition | [71] |
| CRC | / | LDHA, PKM2, HK2 | Metabolic reprogramming | [72] |
| OSCC | miR-361-5p | succinate | Metabolic reprogramming | [73] |
| Breast cancer | / | VEGFA, PGF, bFGF, TGF-β1, MMP-9, AKT | Angiogenesis inhibition | [74] |
| Lung cancer | / | VEGFA, VEGFB, bFGF, SDF-1, TGF-β, angiogenin, MMP-9 | Angiogenesis promotion | [75] |
| Cancer Type | Expression | Relevant Clinical Characteristics | Refs |
|---|---|---|---|
| Glioma | Downregulated | Overall survival rates, Advanced WHO grade, Karnofsky performance score, IDH wild-type, tumor recurrence, progression-free survival | [125] |
| ESCC | Downregulated | Tumor size, lymph node metastasis, poor prognosis | [126] |
| NSCLC | Downregulated | Survival rate | [130] |
| Glioma | Downregulated | Tumor grade | [60] |
| CRC | Downregulated | Lymph node metastasis, TNM staging, Overall survival | [129] |
| Glioblastoma | Downregulated | Survival | [76] |
| Breast cancer | Downregulated | Overall survival, Relapse-free survival, Distant metastasis-free survival, Disease-specific survival | [127] |
| Retinoblastoma | Downregulated | Survival | [128] |
| Glioma | Downregulated | Overall survival, Progression-free survival | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhao, F.; Li, W.; Song, G.; Kasim, V.; Wu, S. The Biological Roles and Molecular Mechanisms of Long Non-Coding RNA MEG3 in the Hallmarks of Cancer. Cancers 2022, 14, 6032. https://doi.org/10.3390/cancers14246032
Zhang L, Zhao F, Li W, Song G, Kasim V, Wu S. The Biological Roles and Molecular Mechanisms of Long Non-Coding RNA MEG3 in the Hallmarks of Cancer. Cancers. 2022; 14(24):6032. https://doi.org/10.3390/cancers14246032
Chicago/Turabian StyleZhang, Lei, Fuqiang Zhao, Wenfang Li, Guanbin Song, Vivi Kasim, and Shourong Wu. 2022. "The Biological Roles and Molecular Mechanisms of Long Non-Coding RNA MEG3 in the Hallmarks of Cancer" Cancers 14, no. 24: 6032. https://doi.org/10.3390/cancers14246032
APA StyleZhang, L., Zhao, F., Li, W., Song, G., Kasim, V., & Wu, S. (2022). The Biological Roles and Molecular Mechanisms of Long Non-Coding RNA MEG3 in the Hallmarks of Cancer. Cancers, 14(24), 6032. https://doi.org/10.3390/cancers14246032






