Stereotactic Ablative Radiotherapy for Oligometastatic Hepatocellular Carcinoma: A Multi-Institutional Retrospective Study (KROG 20-04)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
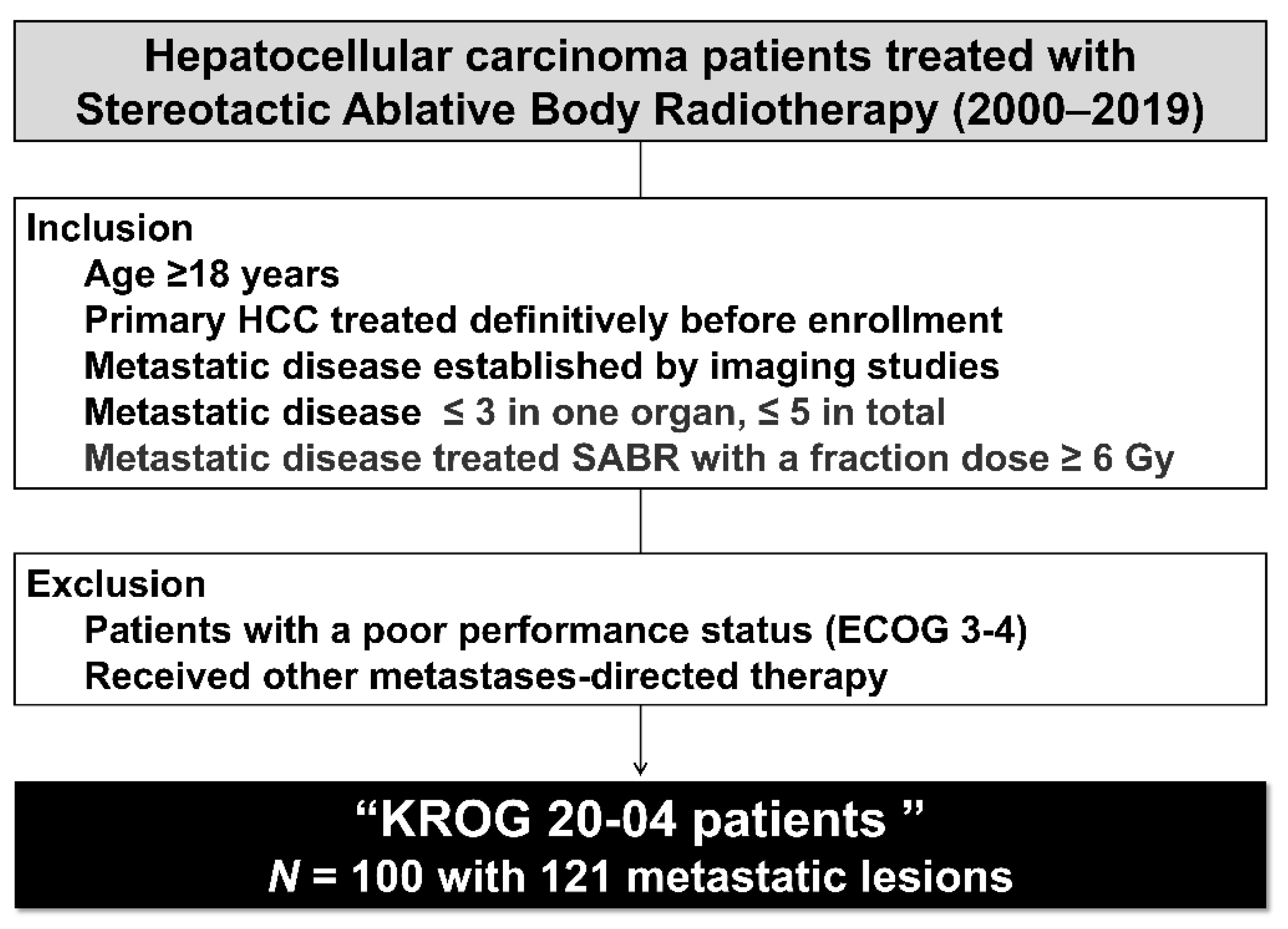
2.1. Study Population
2.2. Radiotherapy
2.3. Response Measurement and Statistical Analysis
3. Results
3.1. Patient
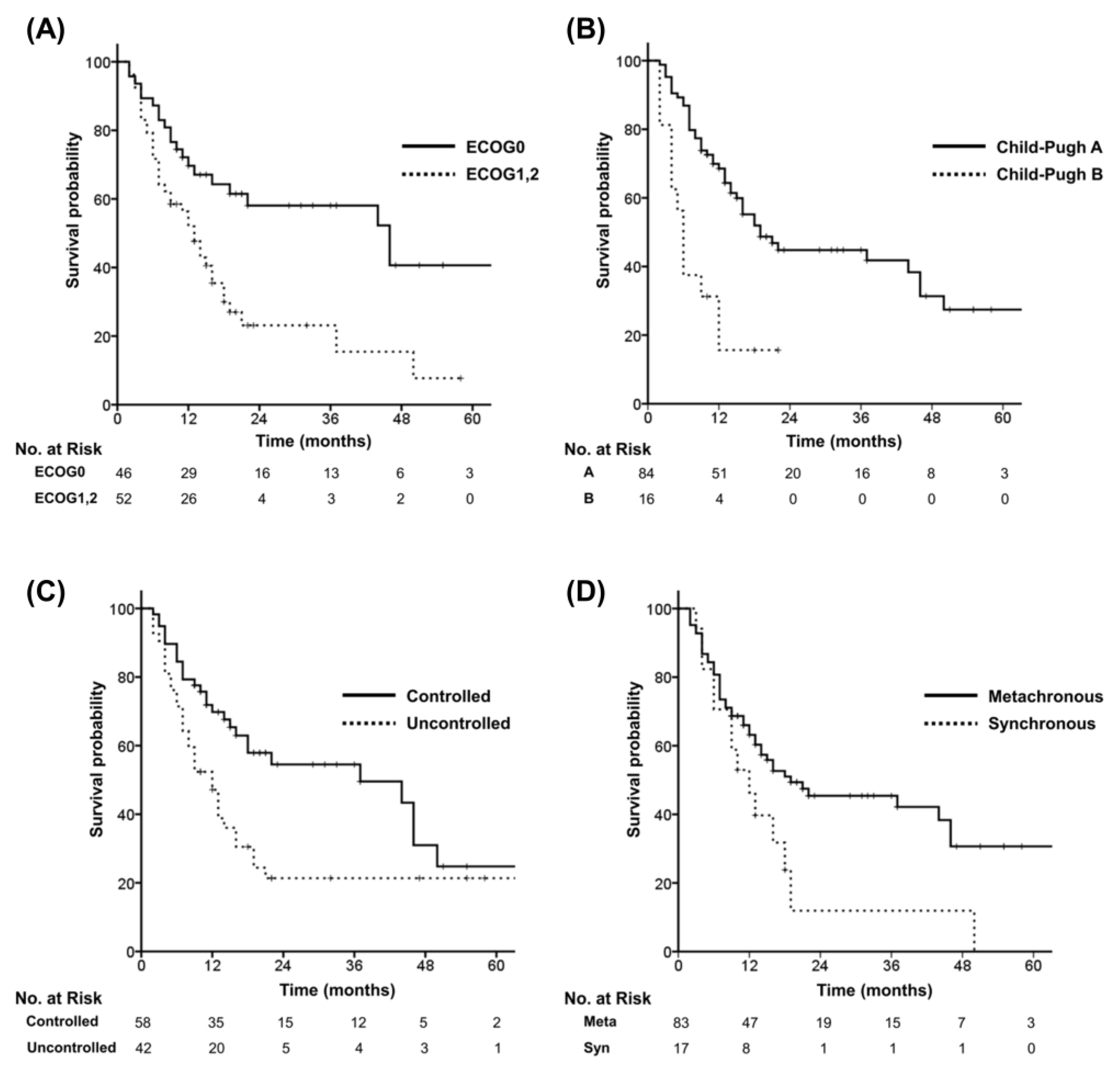
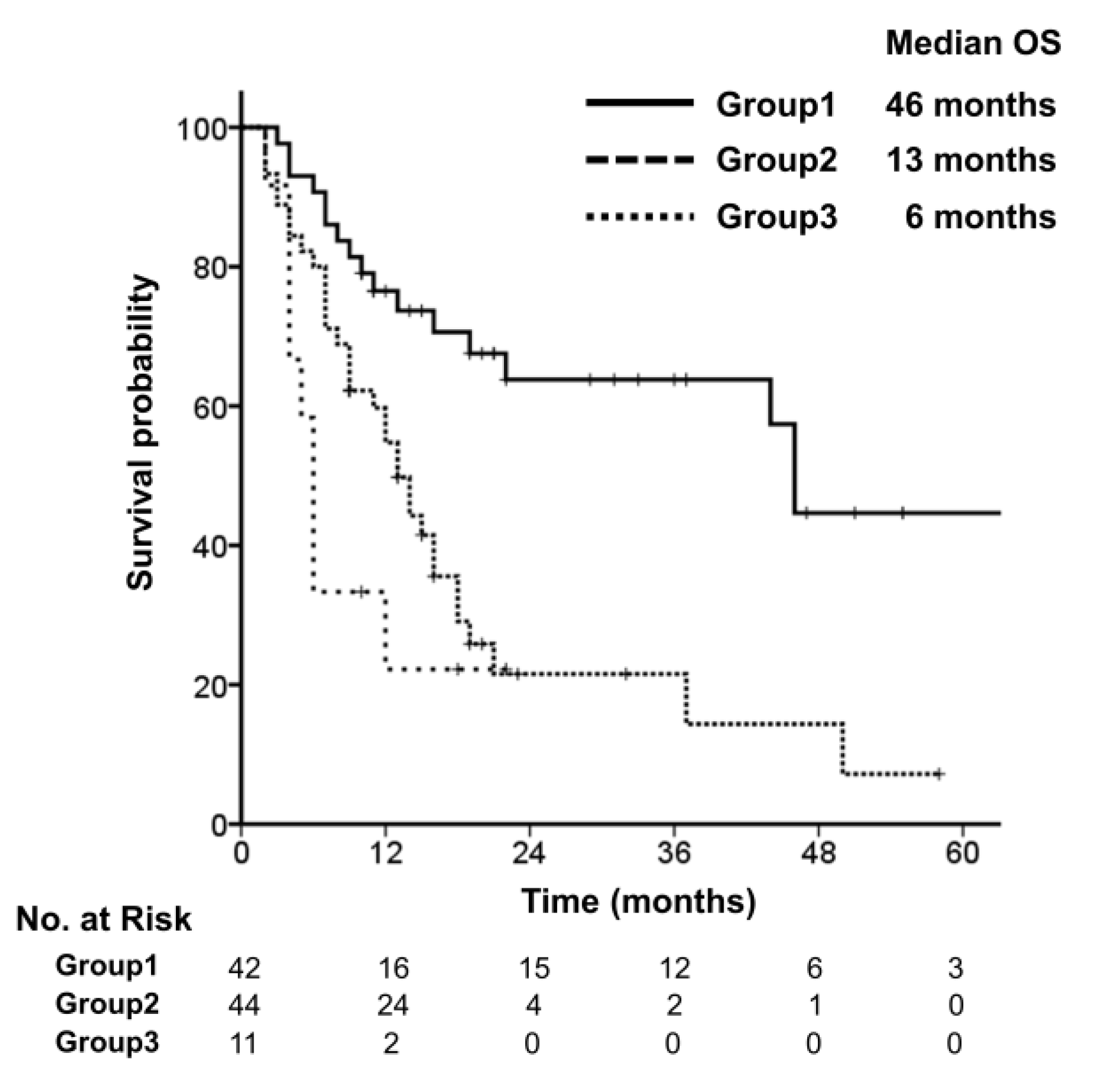
3.2. Survival
3.3. Patterns of Failure and Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Parikh, N.D.; Pillai, A. Recent advances in hepatocellular carcinoma treatment. Clin. Gastroenterol. Hepatol. 2021, 19, 2020–2024. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef]
- Bernstein, M.B.; Krishnan, S.; Hodge, J.W.; Chang, J.Y. Immunotherapy and stereotactic ablative radiotherapy (isabr): A curative approach? Nat. Rev. Clin. Oncol. 2016, 13, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (sabr-comet): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the sabr-comet phase ii randomized trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- National Cancer Institute; National Institutes of Health. Common Terminology Criteria for Adverse Events (Ctcae) Version 4.0; National Cancer Institute; National Institutes of Health: Bethesda, MD, USA, 2009.
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A european society for radiotherapy and oncology and european organisation for research and treatment of cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef]
- Klingenberg, S.; Jochumsen, M.R.; Ulhøi, B.P.; Fredsøe, J.; Sørensen, K.D.; Borre, M.; Bouchelouche, K. (68)ga-psma pet/ct for primary lymph node and distant metastasis nm staging of high-risk prostate cancer. J. Nucl. Med. 2021, 62, 214–220. [Google Scholar] [CrossRef]
- Ashworth, A.B.; Senan, S.; Palma, D.A.; Riquet, M.; Ahn, Y.C.; Ricardi, U.; Congedo, M.T.; Gomez, D.R.; Wright, G.M.; Melloni, G.; et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin. Lung Cancer 2014, 15, 346–355. [Google Scholar] [CrossRef]
- Lee, H.-W.; Sinn, D.H.; Kang, W.; Gwak, G.-Y.; Paik, Y.-H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W. Cause of mortality for hepatocellular carcinoma patients who were diagnosed within the milan criteria. J. Liver Cancer 2016, 16, 101–107. [Google Scholar] [CrossRef]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: The oriole phase 2 randomized clinical trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local consolidative therapy vs. Maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-term results of a multi-institutional, phase ii, randomized study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.; Pierie, J.E.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.A.; Mauer, M.; et al. Local treatment of unresectable colorectal liver metastases: Results of a randomized phase ii trial. J. Natl. Cancer Inst. 2017, 109, djx015. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, T.H.; Kim, T.H.; Seong, J. Efficacy of local therapy for oligometastatic hepatocellular carcinoma: A propensity score matched analysis. J. Hepatocell. Carcinoma 2021, 8, 35–44. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, S.; Rim, C.H.; Choi, C.; Seong, J. Improved oncologic outcomes by ablative radiotherapy in patients with bone metastasis from hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2021, 147, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Ohri, N.; Tomé, W.A.; Méndez Romero, A.; Miften, M.; Ten Haken, R.K.; Dawson, L.A.; Grimm, J.; Yorke, E.; Jackson, A. Local control after stereotactic body radiation therapy for liver tumors. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 188–195. [Google Scholar] [CrossRef]
- Kim, N.; Cheng, J.; Huang, W.Y.; Kimura, T.; Zeng, Z.C.; Lee, V.H.F.; Kay, C.S.; Seong, J. Dose-response relationship in stereotactic body radiation therapy for hepatocellular carcinoma: A pooled analysis of an asian liver radiation therapy group study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 464–473. [Google Scholar] [CrossRef]
- Byun, H.K.; Kim, N.; Park, S.; Seong, J. Acute severe lymphopenia by radiotherapy is associated with reduced overall survival in hepatocellular carcinoma. Strahlenther. Onkol. 2019, 195, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Byun, H.K.; Seong, J. Irradiation-related lymphopenia for bone metastasis from hepatocellular carcinoma. Liver Cancer 2019, 8, 468–479. [Google Scholar] [CrossRef]

| Variables | n |
|---|---|
| Age (in years) | |
| Median | 62 |
| Range | 40–83 |
| Sex | |
| Male | 82 |
| Female | 18 |
| Performance status | |
| ECOG0 | 47 |
| ECOG1 | 50 |
| ECOG2 | 3 |
| Etiology | |
| HBV | 66 |
| HCV | 11 |
| NBNC | 23 |
| Child-Pugh class | |
| A | 83 |
| B | 17 |
| Child-Pugh score | |
| CPS 5 | 73 |
| CPS 6 | 10 |
| CPS 7 | 17 |
| Primary HCC | |
| Controlled | 58 |
| Uncontrolled | 42 |
| Variables | n |
|---|---|
| Metastasis characteristics | |
| Time interval | |
| Synchronous oligometastasis | 17 |
| Metachronous oligometastasis | 83 |
| Number of metastasis | |
| 1 lesion | 85 |
| 2 lesions | 9 |
| 3 lesions | 6 |
| Site of metastases | |
| Bone | 40 |
| Lung | 38 |
| Abdominal lymph node | 11 |
| Adrenal gland | 5 |
| Others | 5 |
| Combined lung & bone | 1 |
| Details of treatment | |
| Systemic therapy | |
| No | 29 |
| Tyrosine kinase inhibitor | 59 |
| Chemotherapy | 12 |
| Timing of systemic therapy | |
| Pre-SABR | 27 |
| During-SABR | 12 |
| Post-SABR | 32 |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age (Continuous, year) | 1.012 | 0.989–1.035 | 0.306 | |||
| Sex (Female vs. Male) | 0.832 | 0.419–1.653 | 0.600 | |||
| Performance (ECOG0 vs.ECOG1&2) | 2.375 | 1.375–4.103 | 0.002 | 1.840 | 1.028–3.295 | 0.040 |
| Child-Pugh class (A vs. B) | 3.344 | 1.767–6.327 | <0.001 | 2.277 | 1.153–4.495 | 0.018 |
| Etiology (HBV vs. HCV) | 1.326 | 0.612–2.872 | 0.475 | |||
| Primary HCC (Controlled vs. Uncontrolled) | 1.957 | 1.171–3.268 | 0.010 | 1.437 | 0.831–2.484 | 0.195 |
| Time interval (Syn vs. Metachronous) | 0.525 | 0.287-0.962 | 0.037 | 0.761 | 0.405-1.430 | 0.396 |
| Number of metastases (1 vs. 2&3) | 1.220 | 0.632–2.357 | 0.553 | |||
| Systemic therapy (No vs.TKI) | 1.285 | 0.715–2.310 | 0.401 | |||
| Systemic therapy (No vs. chemotherapy) | 1.289 | 0.533–3.114 | 0.573 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.H.; Nam, T.-K.; Yoon, S.M.; Kim, T.H.; Choi, Y.M.; Seong, J. Stereotactic Ablative Radiotherapy for Oligometastatic Hepatocellular Carcinoma: A Multi-Institutional Retrospective Study (KROG 20-04). Cancers 2022, 14, 5848. https://doi.org/10.3390/cancers14235848
Kim TH, Nam T-K, Yoon SM, Kim TH, Choi YM, Seong J. Stereotactic Ablative Radiotherapy for Oligometastatic Hepatocellular Carcinoma: A Multi-Institutional Retrospective Study (KROG 20-04). Cancers. 2022; 14(23):5848. https://doi.org/10.3390/cancers14235848
Chicago/Turabian StyleKim, Tae Hyung, Taek-Keun Nam, Sang Min Yoon, Tae Hyun Kim, Young Min Choi, and Jinsil Seong. 2022. "Stereotactic Ablative Radiotherapy for Oligometastatic Hepatocellular Carcinoma: A Multi-Institutional Retrospective Study (KROG 20-04)" Cancers 14, no. 23: 5848. https://doi.org/10.3390/cancers14235848
APA StyleKim, T. H., Nam, T.-K., Yoon, S. M., Kim, T. H., Choi, Y. M., & Seong, J. (2022). Stereotactic Ablative Radiotherapy for Oligometastatic Hepatocellular Carcinoma: A Multi-Institutional Retrospective Study (KROG 20-04). Cancers, 14(23), 5848. https://doi.org/10.3390/cancers14235848






