Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systematic Review of Real-World Evidence
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Eligibility Criteria
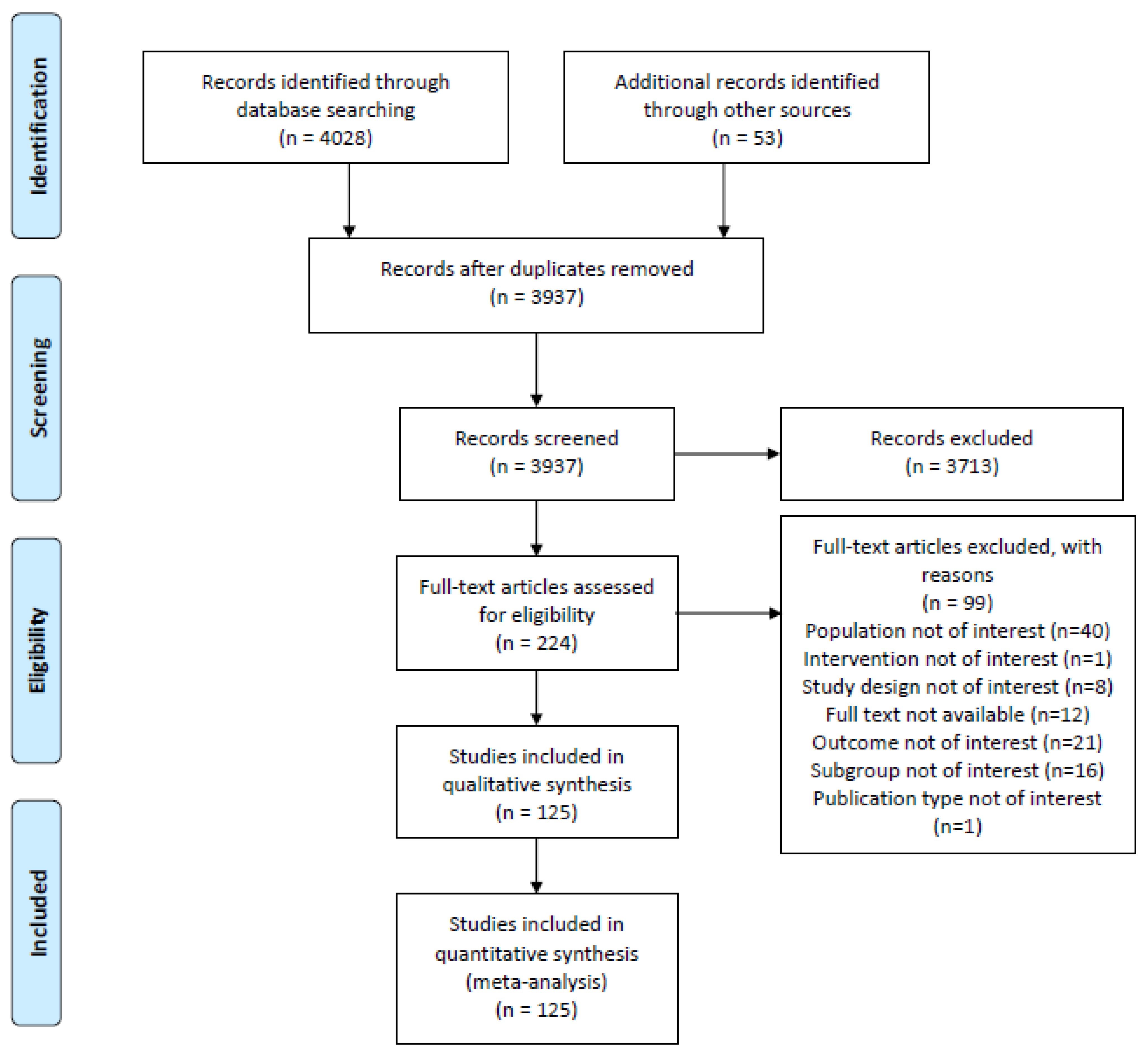
2.3. Study Selection
2.4. Data Extraction and Management
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. HCC Recurrence
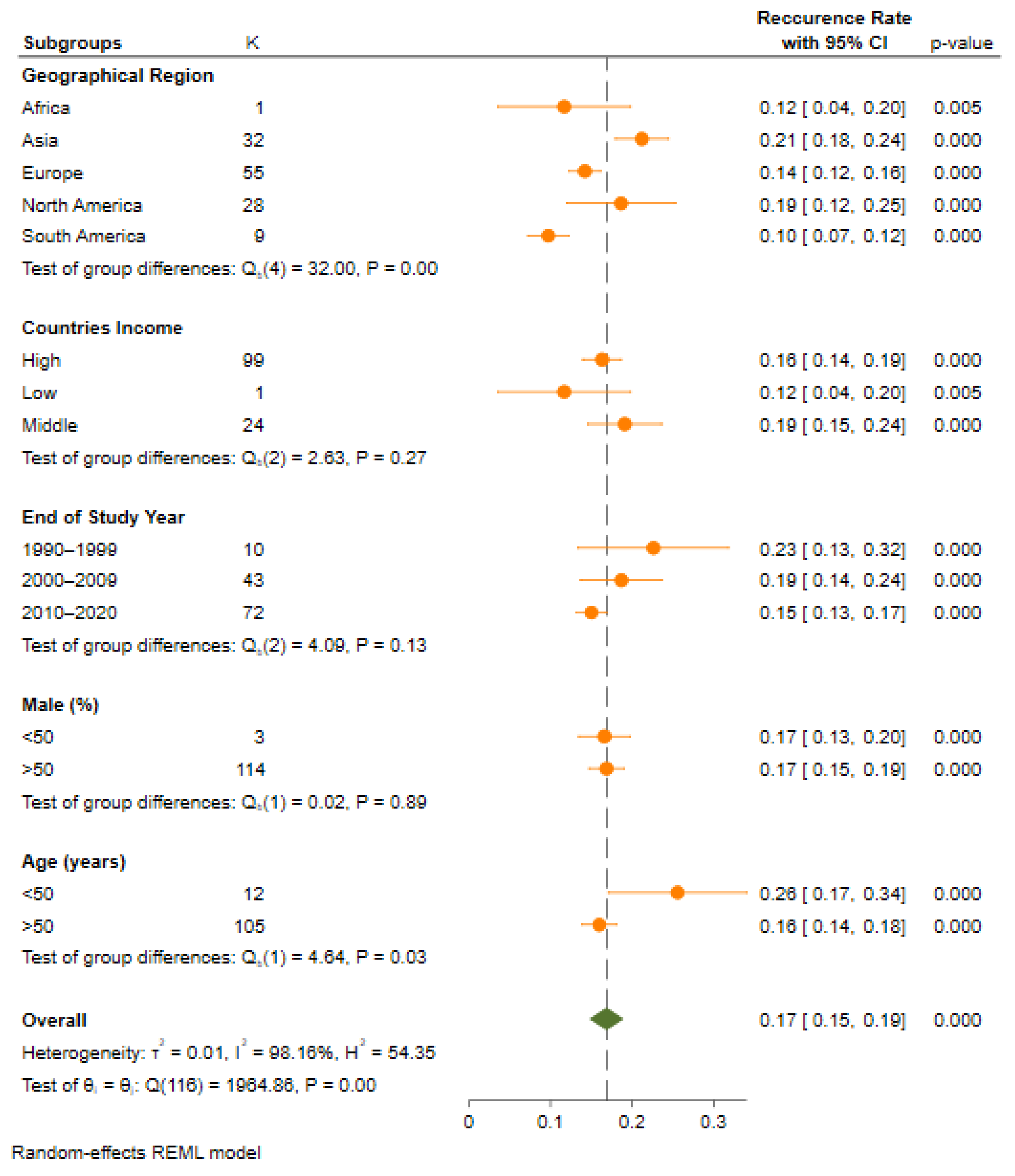
3.3. Recurrence Rate Based on Geographical Region
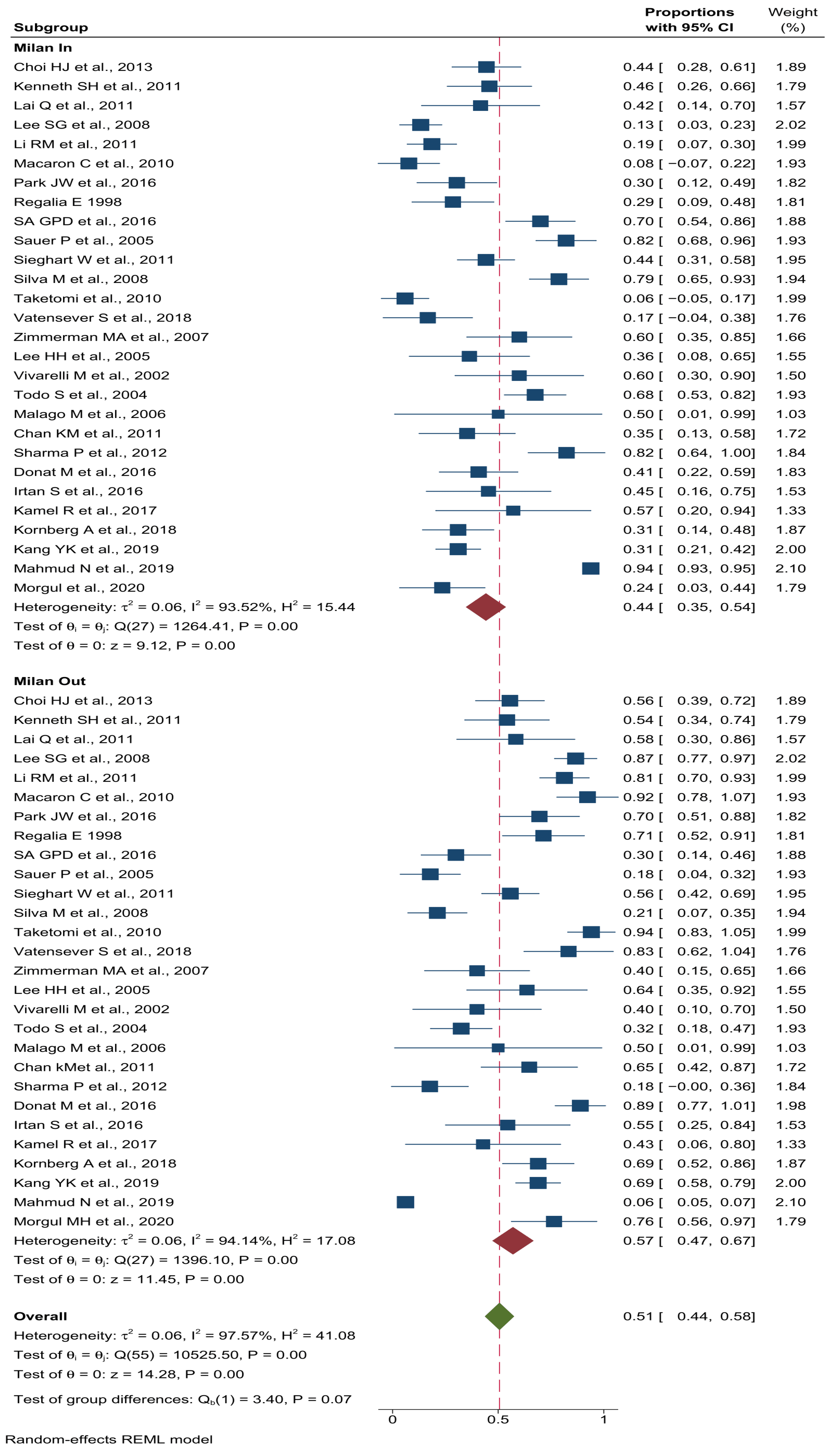
3.4. Recurrence Rate Based on Milan Criteria
3.5. Recurrence Rate Based on UCSF Criteria
3.6. Recurrence Rate Based on Type of Donor
3.7. Recurrence Rate Based on Countries Income
3.8. Recurrence Rate Based on Publication Year
3.9. Recurrence Rate Based on Population Age
3.10. Recurrence Rate Based on Pretransplant AFP
3.11. Mortality Rate in HCC Patients after LT
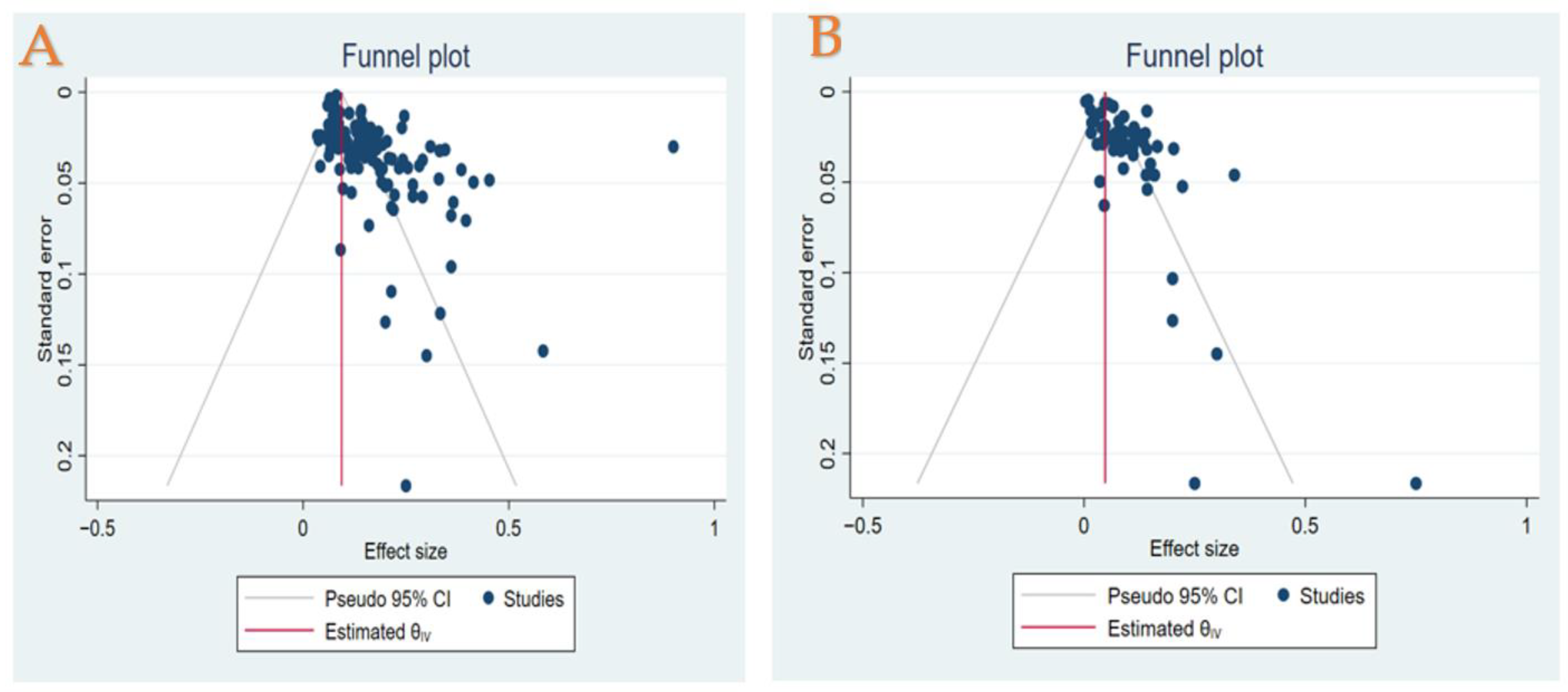
3.12. Publication Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, Y.; Fuchs, B.C.; Tanabe, K.K. Prevention of hepatocellular carcinoma: Potential targets, experimental models, and clinical challenges. Curr. Cancer Drug Targets 2012, 12, 1129–1159. [Google Scholar] [PubMed]
- Golabi, P.; Fazel, S.; Otgonsuren, M.; Sayiner, M.; Locklear, C.T.; Younossi, Z.M. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine 2017, 96, e5904. [Google Scholar] [CrossRef]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef] [PubMed]
- Santopaolo, F.; Lenci, I.; Milana, M.; Manzia, T.M.; Baiocchi, L. Liver transplantation for hepatocellular carcinoma: Where do we stand? World J. Gastroenterol. 2019, 25, 2591–2602. [Google Scholar] [CrossRef]
- Muhammad, H.; Tehreem, A.; Ting, P.S.; Gurakar, M.; Li, S.Y.; Simsek, C.; Alqahtani, S.A.; Kim, A.K.; Kohli, R.; Gurakar, A. Hepatocellular Carcinoma and the Role of Liver Transplantation: A Review. J. Clin. Transl. Hepatol. 2021, 9, 738–748. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Fan, S.T.; Lo, C.M.; Liu, C.L.; Wong, J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: Implications for a strategy of salvage transplantation. Ann. Surg. 2002, 235, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.H.; Ruo, L.; Fong, Y.; Jarnagin, W.R.; Shia, J.; Blumgart, L.H.; DeMatteo, R.P. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann. Surg. 2003, 238, 315–323. [Google Scholar] [CrossRef]
- Chagas, A.L.; Felga, G.E.; Diniz, M.A.; Silva, R.F.; Mattos, A.A.; Silva, R.C.; Boin, I.F.; Garcia, J.H.; Lima, A.S.; Coelho, J.C.; et al. Hepatocellular carcinoma recurrence after liver transplantation in a Brazilian multicenter study: Clinical profile and prognostic factors of survival. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1148–1156. [Google Scholar] [CrossRef]
- Filgueira, N.A. Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation. World J. Hepatol. 2019, 11, 261–272. [Google Scholar] [CrossRef]
- de’Angelis, N.; Landi, F.; Carra, M.C.; Azoulay, D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J. Gastroenterol. 2015, 21, 11185–11198. [Google Scholar] [CrossRef] [PubMed]
- López, F.; Rodrigo, J.P.; Silver, C.E.; Haigentz, M., Jr.; Bishop, J.A.; Strojan, P.; Hartl, D.M.; Bradley, P.J.; Mendenhall, W.M.; Suárez, C.; et al. Cervical lymph node metastases from remote primary tumor sites. Head Neck 2016, 38 (Suppl. S1), E2374–E2385. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chen, C.L.; Lin, C.C.; Wang, C.C.; Liu, Y.W.; Li, W.F.; Chen, Y.H. Efficacy and Safety of Lenvatinib in Hepatocellular Carcinoma Patients with Liver Transplantation: A Case-Control Study. Cancers 2021, 13, 4584. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.K.; Chawla, Y.K.; Dhiman, R.K.; Singh, V.; Duseja, A.; Taneja, S.; Kalra, N.; Gorsi, U. Rupture of Hepatocellular Carcinoma: A Review of Literature. J. Clin. Exp. Hepatol. 2019, 9, 245–256. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Marín-Martínez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef]
- Chao, S.D.; Roberts, J.P.; Farr, M.; Yao, F.Y. Short waitlist time does not adversely impact outcome following liver transplantation for hepatocellular carcinoma. Am. J. Transplant. 2007, 7, 1594–1600. [Google Scholar] [CrossRef]
- Wan, P.; Zhang, J.J.; Li, Q.G.; Xu, N.; Zhang, M.; Chen, X.S.; Han, L.Z.; Xia, Q. Living-donor or deceased-donor liver transplantation for hepatic carcinoma: A case-matched comparison. World J. Gastroenterol. 2014, 20, 4393–4400. [Google Scholar] [CrossRef] [PubMed]
- Balogh, J.; Victor, D., 3rd; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.J.; Wong, C.; Ng, C.H.; Poh, C.W.; Jain, S.R.; Huang, D.Q.; Muthiah, M.D. A Meta-Analysis on the Rate of Hepatocellular Carcinoma Recurrence after Liver Transplant and Associations to Etiology, Alpha-Fetoprotein, Income and Ethnicity. J. Clin. Med. 2021, 10, 238. [Google Scholar] [CrossRef]
- Frazzoni, L.; Sikandar, U.; Metelli, F.; Sadalla, S.; Mazzella, G.; Bazzoli, F.; Fuccio, L.; Azzaroli, F. Hepatocellular Carcinoma Recurrence after Hepatitis C Virus Therapy with Direct-Acting Antivirals. A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1694. [Google Scholar] [CrossRef] [PubMed]
- Jafri, W.; Kamran, M. Hepatocellular Carcinoma in Asia: A Challenging Situation. Euroasian J. Hepatogastroenterol. 2019, 9, 27–33. [Google Scholar] [CrossRef]
- Pelizzaro, F.; Gambato, M.; Gringeri, E.; Vitale, A.; Cillo, U.; Farinati, F.; Burra, P.; Russo, F.P. Management of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers 2021, 13, 4882. [Google Scholar] [CrossRef]
- Hakeem, A.; Young, R.; Marangoni, G.; Lodge, P.; Prasad, K. Systematic review: The prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2012, 35, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.H.; Tan, D.J.H.; Ong, Y.; Lim, W.H.; Ng, C.H.; Tay, P.W.L.; Yong, J.N.; Muthiah, M.D.; Tan, E.X.; Pang, N.Q.; et al. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: A meta-analysis of 18,421 patients. Hepatobiliary Surg. Nutr. 2022, 11, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Manzia, T.M.; Angelico, R.; Gazia, C.; Lenci, I.; Milana, M.; Ademoyero, O.T.; Pedini, D.; Toti, L.; Spada, M.; Tisone, G.; et al. De novo malignancies after liver transplantation: The effect of immunosuppression-personal data and review of literature. World J. Gastroenterol. 2019, 25, 5356–5375. [Google Scholar] [CrossRef]
- Manzia, T.M.; Angelico, R.; Baiocchi, L.; Toti, L.; Ciano, P.; Palmieri, G.; Angelico, M.; Orlando, G.; Tisone, G. The Tor Vergata weaning of immunosuppression protocols in stable hepatitis C virus liver transplant patients: The 10-year follow-up. Transpl. Int. 2013, 26, 259–266. [Google Scholar] [CrossRef]
| Subgroups | No of Studies | Prevalence (%) (95%CI) | Test for Heterogeneity | Between Subgroup Differences | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tau2 | I2 (%) | p-Value | Q | df | p-Value | ||||
| HCC Recurrence | |||||||||
| Overall | 125 | 17 (15–19) | 0.01 | 98.1 | <0.001 | 116 | 1964.8 | 0.00 | |
| Milan Criteria * | Accord with | 29 | 44 (35–54) | 0.06 | 93.5 | <0.001 | 1 | 3.40 | 0.00 |
| Do not accord with | 29 | 57 (47–67) | 0.06 | 97.6 | <0.001 | ||||
| UCSF Criteria * | Accord with | 7 | 33 (18–48) | 0.03 | 83.6 | <0.001 | 13 | 35.4 | 0.00 |
| Do not accord with | 7 | 67 (51–82) | 0.03 | 83.6 | <0.001 | ||||
| Transplant type * | Living donor | 10 | 19 (12–26) | 0.00 | 0.00 | 0.97 | 9 | 167.6 | 0.00 |
| Deceased donor | 10 | 81 (77–85) | 0.00 | 0.00 | 0.78 | ||||
| Geographical Region | Africa | 1 | 12 (4–20) | 0 | 0.00 | - | 4 | 32.00 | 0.00 |
| Asia | 32 | 21 (18–24) | 0.006 | 86.7 | <0.001 | ||||
| Europe | 55 | 14 (13–16) | 0.004 | 89.2 | <0.001 | ||||
| North America | 28 | 19 (12–25) | 0.029 | 99.6 | <0.001 | ||||
| South America | 9 | 10 (7–12) | 0.001 | 74.4 | <0.001 | ||||
| Study End Date | 1991–1999 | 10 | 23 (15–32) | 0.017 | 91.2 | <0.001 | 2 | 4.09 | 0.13 |
| 2000–2009 | 43 | 18 (14–23) | 0.019 | 95.1 | <0.001 | ||||
| 2010–2019 | 66 | 15 (13–17) | 0.005 | 97.4 | <0.001 | ||||
| 2020–2022 | 6 | 14 (9–18) | 0.002 | 78.3 | <0.001 | ||||
| Countries Income | Low | 1 | 12 (4–20) | 0.000 | - | - | 2 | 2.63 | 0.27 |
| Middle | 25 | 19 (15–23) | 0.011 | 95.1 | <0.001 | ||||
| High | 99 | 16 (14–19) | 0.011 | 98.2 | <0.001 | ||||
| Alpha-Fetoprotein (ng/mL) | <50 | 25 | 14 (11–17) | 0.002 | 90.1 | <0.001 | 2 | 4.75 | 0.09 |
| ≥50 | 13 | 20 (14–26) | 0.001 | 94.3 | <0.001 | ||||
| Male (%) | <50 | 3 | 17 (13–20) | 0.000 | 0.01 | <0.001 | 1 | 0.02 | 0.89 |
| ≥50 | 113 | 17 (15–19) | 0.012 | 98.2 | <0.001 | ||||
| Age (years) | <50 | 11 | 24 (16–32) | 0.017 | 94.4 | <0.001 | 1 | 4.64 | 0.03 |
| ≥50 | 105 | 16 (14–18) | 0.010 | 97.8 | <0.001 | ||||
| Mortality in HCC Recurrence Patients | |||||||||
| Overall | 53 | 9 (8–11) | 0.00 | 88.2 | <0.001 | 52 | 402.7 | 0.00 | |
| Geographical Region | Asia | 12 | 10 (8–12) | 0.001 | 51.8 | <0.001 | 3 | 6.87 | 0.08 |
| Europe | 23 | 10 (7–12) | 0.003 | 88.4 | <0.001 | ||||
| North America | 11 | 11 (5–17) | 0.008 | 97.1 | <0.001 | ||||
| South America | 7 | 7 (5–9) | 0.000 | 38.9 | <0.001 | ||||
| Countries Income | Middle | 40 | 9 (7–11) | 0.001 | 71.2 | <0.001 | 1 | 0.07 | 0.79 |
| High | 13 | 9 (7–11) | 0.003 | 90.6 | <0.001 | ||||
| Study End Date | 1991–1999 | 5 | 12 (−1–24) | 0.019 | 97.7 | <0.001 | 3 | 0.96 | 0.81 |
| 2000–2009 | 22 | 10 (8–12) | 0.002 | 70.7 | <0.001 | ||||
| 2010–2019 | 21 | 8 (7–10) | 0.001 | 84.7 | <0.001 | ||||
| 2020–2022 | 5 | 9 (6–13) | 0.001 | 61.3 | <0.001 | ||||
| Male (%) | <50 | 1 | 7 (0–13) | 0.000 | - | - | 1 | 0.66 | 0.42 |
| ≥50 | 45 | 9 (8–11) | 0.002 | 87.4 | <0.001 | ||||
| Age (years) | <50 | 4 | 18 (6–30) | 0.013 | 96.2 | <0.001 | 1 | 2.42 | 0.12 |
| ≥50 | 42 | 9 (7–10) | 0.002 | 84.3 | <0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bzeizi, K.I.; Abdullah, M.; Vidyasagar, K.; Alqahthani, S.A.; Broering, D. Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systematic Review of Real-World Evidence. Cancers 2022, 14, 5114. https://doi.org/10.3390/cancers14205114
Bzeizi KI, Abdullah M, Vidyasagar K, Alqahthani SA, Broering D. Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systematic Review of Real-World Evidence. Cancers. 2022; 14(20):5114. https://doi.org/10.3390/cancers14205114
Chicago/Turabian StyleBzeizi, Khalid I., Maheeba Abdullah, Kota Vidyasagar, Saleh A. Alqahthani, and Dieter Broering. 2022. "Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systematic Review of Real-World Evidence" Cancers 14, no. 20: 5114. https://doi.org/10.3390/cancers14205114
APA StyleBzeizi, K. I., Abdullah, M., Vidyasagar, K., Alqahthani, S. A., & Broering, D. (2022). Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systematic Review of Real-World Evidence. Cancers, 14(20), 5114. https://doi.org/10.3390/cancers14205114






