Simple Summary
Androgen deprivation therapy plays a key role in the therapeutic management of patients with advanced prostate cancer. However, prediction of response before treatment initiation remains difficult. This study was undertaken to investigate whether 68Ga-PSMA-11 PET/CT imaging features extracted from different prostatic zones (zone-1, zone-2, and zone-3) might predict response to androgen deprivation therapy in patients with advanced prostate cancer. Seven radiomic features extracted from zone-1 were identified as significantly associated with treatment response. In addition, two radiomic features from zone-2 and two from zone-3 were able to distinguish between different treatment response groups. Our findings demonstrate the potential usefulness of radiomic features extracted from different prostatic zones in predicting treatment response prior to androgen deprivation therapy.
Abstract
Purpose: Prediction of treatment response to androgen deprivation therapy (ADT) prior to treatment initiation remains difficult. This study was undertaken to investigate whether 68Ga-PSMA-11 PET/CT features extracted from different radiomic zones within the prostate gland might predict response to ADT in patients with advanced prostate cancer (PCa). Methods: A total of 35 patients with prostate adenocarcinoma underwent two 68Ga-PSMA-11 PET/CT scans—termed PET-1 and PET-2—before and after 3 months of ADT, respectively. The prostate was divided into three radiomic zones, with zone-1 being the metabolic tumor zone, zone-2 the proximal peripheral tumor zone, and zone-3 the extended peripheral tumor zone. Patients in the response group were those who showed a reduction ratio > 30% for PET-derived parameters measured at PET-1 and PET-2. The remaining patients were classified as non-responders. Results: Seven features (glcm_idmn, glcm_idn, glcm_imc1, ngtdm_Contrast, glrlm_rln, gldm_dn, and shape_MeshVolume) from zone-1, two features (gldm_sdlgle and shape_MinorAxisLength) from zone-2, and two features (diagnostics_Mask-interpolated_Minimum and shape_Sphericity) from zone-3 successfully distinguished responders from non-responders to ADT. One predictive feature (shape_SurfaceVolumeRatio) was consistently identified in all of the three zones. Conclusions: this study demonstrates the potential usefulness of radiomic features extracted from different prostatic zones in distinguishing responders from non-responders prior to ADT initiation.
1. Introduction
Globally, prostate cancer (PCa) is the second most common cancer among men and the fifth leading cause of death [1]. The role of positron emission tomography (PET) imaging in the diagnosis, staging, and assessment of treatment response in patients with PCa is well-established [2]. 68Ga-labeled prostate-specific membrane antigen (PSMA)-targeted PET has recently emerged as a promising imaging modality for lesion detection [3,4] and monitoring of response to androgen deprivation therapy (ADT) in patients with metastatic PCa [5,6]. There is also evidence that the percentage variations of 68Ga-PSMA-11 PET/CT imaging parameters measured before and after three months of ADT are clinically useful in evaluating treatment response [7,8,9].
PCa is characterized by significant intratumor heterogeneity, which may in turn affect the biological aggressiveness, disease progression, and therapeutic resistance [10,11,12]. In recent years, radiomics—which can be defined as the high-throughput extraction of quantitative features from CT, MRI, or PET images—has been successfully used to predict clinical and treatment outcomes in patients with malignancies [13,14,15,16,17,18,19,20,21]. Image texture analysis—as a quantitative radiomics approach for the analysis of tumor heterogeneity—has been shown to correlate with established indices of glucose metabolic activity, including the standardized uptake value (SUV) [22,23,24,25]. Moreover, texture features may have a complementary role to metabolic parameters in the prediction of treatment response [26].
The ability of radiomics to comprehensively characterize PCa tissues from state-of-the-art PET imaging has attracted significant research interest [27,28,29,30,31,32]. On analyzing 18F-choline PET/CT scans of patients with high-risk PCa, Alongi et al. [30] identified three features (i.e., SUVmin, shape_Sphericity, and idmn_Correlation) that successfully predicted the occurrence of disease progression at follow-up. However, most studies in the field of PET radiomics have continued to rely on a cancer-centric model based on feature extraction from the whole tumor. More recently, the regions of interest have been expanded to include peripheral tumor areas [33,34,35]. In a previous study focusing on 11C-choline PET/MRI imaging, we have proposed dividing the prostate gland into three distinct radiomic zones, with zone-1 being the metabolic tumor zone, zone-2 the proximal peripheral tumor zone, and zone-3 the extended peripheral tumor zone [35]. Interestingly, these radiomic zones were found to have different predictive strengths in classifying risk groups in patients with PCa [35].
While measurements of circulating prostate specific antigen (PSA) levels may have a role in predicting response to ADT when PCa has not spread to lymph nodes or skeletal sites [36], its clinical utility in patients with disseminated disease remains limited. In this scenario, there is pilot evidence supporting the potential utility of the reduction ratios of 68Ga-PSMA-11 PET/CT indices measured before and after 3 months of ADT [7,9]. By expanding our previous work [35], we designed this current study to investigate whether radiomic features from zone-1 may have clinical value for predicting response to ADT. We also examined whether features from peripheral areas (zone-2 and zone-3) could be useful to distinguish between different treatment response groups.
2. Materials and Methods
2.1. Study Patients
Thirty-five patients with advanced prostatic adenocarcinoma were included (Table 1). All participants were scheduled to undergo ADT for at least 6 months and have completed nearly 3 months (10–14 weeks) of ADT treatment. Two 68Ga-PSMA-11 PET/CT scans—termed PET-1 and PET-2—were obtained for each patient before and after 3 months of ADT, respectively. Ethics approval for this study was received from the Chang Gung Memorial Hospital institutional review board (reference number: 201801384A0). All participants provided written informed consent.

Table 1.
General characteristics of the 35 study patients with advanced prostate cancer.
2.2. PET/CT Imaging
The acquisition protocol for 68Ga-PSMA-11 PET/CT imaging has been previously described [9]. In brief, images were acquired on a GE Discovery MI PET/CT scanner (GE Healthcare, Milwaukee, WI, USA) 60 min after tracer injection (dose range: 103–182 MBq; median dose: 141 MBq). The following settings were applied for CT imaging: 120 kVp, automatic mA selection (ranging from 30 to 300 mA), 40 × 0.625 detector collimation, and 0.984 pitch. Transaxial PET images were acquired with the following parameters: field of view = 700 mm, matrix size = 256 × 256, and slice thickness = 5 mm. The acquisition time was 3 min per single-bed position, with the acquisition proceeding from the thigh to the skull. A Bayesian penalized likelihood algorithm (Q.Clear) was used for image reconstruction. Methods for image calibration included attenuation correction, the point spread function, and the QCHD-S technique [9].
2.3. PET-Derived Parameters
Region of interest (ROI)-based image segmentation was performed using the LIFEx software developed in Java [37]. A maximum standardized uptake value (SUV) threshold of 45% was used to delineate the primary prostate tumor and metastatic lymph nodes (Figure 1a,b) [38], whereas a fixed-absolute SUV threshold of 3.0 was applied for metastatic bone lesions (Figure 1c) [39].
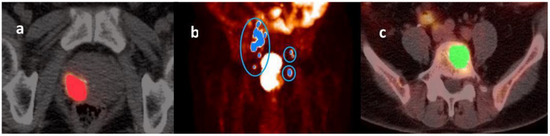
Figure 1.
Illustrative images of a primary prostate tumor (panel (a)), metastatic lymph nodes (panel (b)), and metastatic bone lesions (panel (c)) identified on a 68Ga-PSMA-11 PET scan performed before the start of androgen deprivation therapy (PET-1). A maximum standardized uptake value (SUV) threshold of 45% was used to delineate the primary prostate tumor (panel (a)) and metastatic lymph nodes (panel (b)), whereas a fixed-absolute SUV threshold of 3.0 was applied for metastatic skeletal lesions (panel (c)).
Traditional PET-derived parameters—including SUVmax, SUVmean, metabolic tumor volume (MTV), and total lesion (TL, calculated by multiplying SUVmean by the MTV)—were used for assessing treatment response [7,9,40]. Calculation of these indices for the primary prostate tumor, metastatic lymph nodes, and bone lesions was implemented on a patient basis using the LIFEx package. All parameters were calculated for both PET-1 and PET-2 images.
2.4. Analysis of Treatment Response
Patients who had undergone ADT treatment were classified as either responders or non-responders using the modified PET response criteria in solid tumors (mPERCIST) [41]. With this aim, the primary prostate tumor, metastatic lymph nodes, and bone lesions were taken into account. Patients in the response group were those who showed a reduction ratio (RR) > 30% for PET-derived parameters measured on PET-2 versus PET-1 [41]. The remaining patients were classified as non-responders. The RR was calculated with the following formula:
Parameters included SUVmax, SUVmean, MTV, or TL, respectively. Illustrative examples of tumor response and non-response are shown in Figure 2.
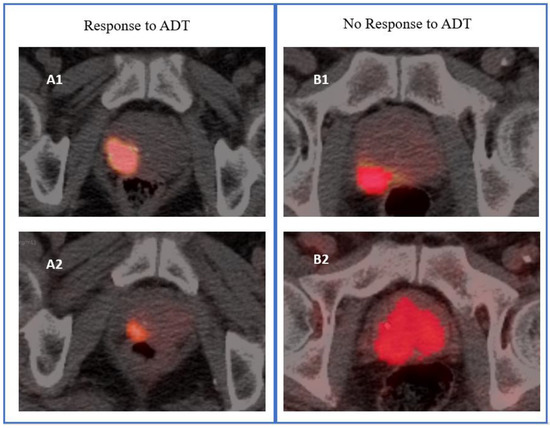
Figure 2.
Illustrative images of response and no response to androgen deprivation therapy (ADT) of a primary prostate tumor based on metabolic tumor volume. The (A1,B1) PET/CT images were from 68Ga-PSMA-11 PET scans performed before the start of ADT (PET-1), whereas (A2,B2) were the corresponding images from 68Ga-PSMA-11 PET scans performed after 3 months of ADT (PET-2). Response was defined as a reduction ratio > 30% for PET-derived parameters measured on PET-2 versus PET-1.
2.5. Radiomic Zones and Feature Extraction
Radiomic zones (zone-1, zone-2, and zone-3) of the prostate were defined in accordance with our previous study [35]. Specifically, zone-1 is the metabolic tumor region, zone-2 the proximal peripheral region surrounding zone-1, and zone-3 the expanded peripheral region reaching to the prostate boundary. In brief, SUV values for zone-1 and zone-2 were 45–100% and 20–45% of SUVmax, respectively. Zone-3 comprised the entire prostate with the exclusion of zone-1 (Figure 3). After segmentation of the three zones on PET-1 images, radiomics features were extracted through the open-source Python package PyRadiomics [42,43]. A total of 119 PyRadiomics features were examined across the following eight categories: first-order statistics (18 features), diagnosis (12), shape (14), gray level co-occurrence matrix (glcm) (24), gray level dependence matrix (gldm) (14), gray level run length matrix (glrlm) (16), gray level size zone matrix (glszm) (16), and neighboring gray tone difference matrix (ngtdm) (5) [43].
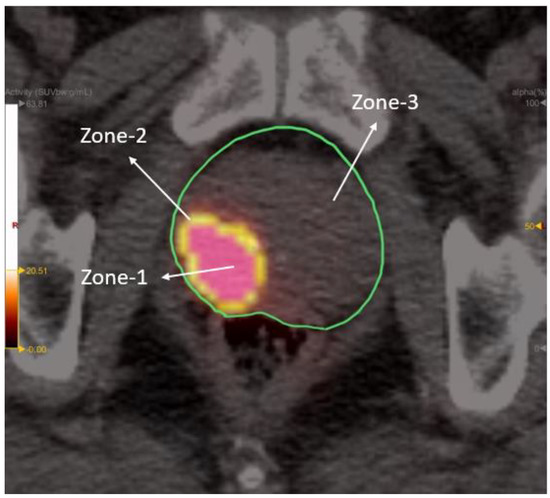
Figure 3.
Depiction of the three radiomic zones on a PET/CT image. Standardized uptake values for zone-1 and zone-2 were 45–100% and 20–45% of maximum standard uptake value (SUVmax), respectively. Zone-3 comprised the entire prostate with the exclusion of zone-1.
2.6. Statistical Analysis
We examined PyRadiomics features (n = 119) extracted from the three radiomic zones. Groups with different response to ADT were compared on normally distributed variables using independent Student’s t-tests and on skewed parameters with the Mann–Whitney U test. In each prostatic zone, a radiomic feature was considered useful when it successfully distinguished ADT response groups and showed an association with treatment outcomes on at least three of the following traditional PET parameters (i.e., SUVmax, SUVmean, MTV, and TL). All analyses were undertaken in SPSS, version 25.0 (IBM, Armonk, NY, USA), and statistical significance was determined by a two-tailed p value < 0.05.
3. Results
3.1. Response to Androgen Deprivation Therapy
Of the 35 patients with PCa who were staged with 68Ga-PSMA-11 PET imaging before ADT treatment, 16 had metastatic lymph nodes and 17 had bone metastases. Using the four traditional PET parameters, patients were classified into different treatment response groups (Table 2). On average, the percentage distribution of responders and non-responders for primary tumors (n = 35), metastatic lymph nodes (n = 16), and bone lesions (n = 17) was as follows: 73%/27%, 84%/16%, and 73%/27%, respectively.

Table 2.
Response to androgen deprivation therapy treatment: patient-based classification groups.
3.2. Prediction of Treatment Response Using Features from Radiomic Zone-1
Based on SUVmax, SUVmean, MTV, and TL, there were 80, 85, 14, and 28 features from radiomic zone-1 that were able to distinguish responders from non-responders to ADT (Figure 4), respectively. Interestingly, the glcm category included 20 features each for both SUVmax and SUVmean. In addition, the shape category comprised five and nine features that distinguished between different treatment response groups based on MTV and TL, respectively. Based on SUVmax or SUVmean, at least one feature from radiomic zone-1 effectively distinguished responders from non-responders. Importantly, we identified seven features extracted from at least three of the four traditional PET parameters that successfully predicted treatment response (Table 3). Of them, three—including glcm_idmn (p = 0.003, 0.002, 0.024, 0.014 for SUVmax, SUVmean, MTV, and TL, respectively), glcm_idn (p = 0.003/0.002/0.024/0.014 for SUVmax, SUVmean, MTV, and TL, respectively), and glrlm_rln (p = 0.003/0.001/0.013/1.97 × 10−4 for SUVmax, SUVmean, MTV, and TL, respectively)—were extracted from all four PET-derived parameters. The remaining four features were glcm_imc1 (p = 0.002/7.33 × 10−41/0.037 for SUVmax/SUVmean/MTV, respectively), ngtdm_Contrast (p = 0.004/0.002/0.037 for SUVmax/SUVmean/MTV, respectively), gldm_dn (p = 0.002/0.001/0.009 for SUVmax/SUVmean/TL, respectively), and shape_MeshVolume (p = 0.038/0.034/0.003 for SUVmax/SUVmean/TL, respectively).
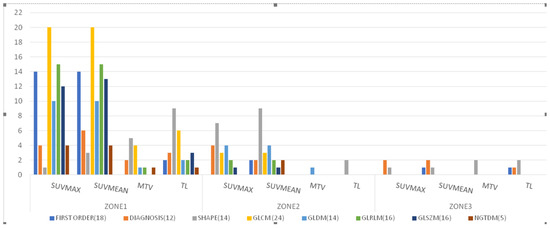
Figure 4.
Distribution of features extracted from the three radiomic zones for distinguishing between responders and non-responders to androgen deprivation therapy (p < 0.05; independent Student’s t-tests or Mann–Whitney U test). Features were examined across the following eight categories: first-order statistics (18 features), diagnosis (12), shape (14), gray level co-occurrence matrix (glcm) (24), gray level dependence matrix (gldm) (14), gray level run length matrix (glrlm) (16), gray level size zone matrix (glszm) (16), and neighboring gray tone difference matrix (ngtdm) (5). Abbreviations: SUVmax, maximum standardized uptake value; SUVmean, mean of standardized uptake value; MTV, metabolic total volume; TL, total lesion.

Table 3.
68Ga-PSMA-11 PET/CT features extracted from different radiomic zones in the prediction of response to androgen deprivation therapy in patients with advanced prostate cancer.
The glcm_idmn (inverse difference moment normalized) and glcm_idn (inverse difference normalized) features are measures of local homogeneity within ROIs. The glcm_imc1 (informational measure of correlation) feature summarizes the correlation between the probability distributions of texture complexity (complete independency, imc1 = 0; complete dependency, imc1 = −1). The ngtdm_Contrast feature is a measure of spatial intensity changes in each ROI. The glrlm_rln (run length non-uniformity) feature is a measure of the run length similarity throughout an image. Finally, the gldm_dn (dependence non-uniformity) feature expresses the similarity of dependency throughout an image [43].
3.3. Prediction of Treatment Response Using Features from Radiomic Zone-2
Based on SUVmax, SUVmean, MTV, and TL, there were 21, 25, 1, and 2 features from radiomic zone-2 that were able to distinguish responders from non-responders to ADT (Figure 4), respectively. The shape category comprised seven, nine, and two features that distinguished between different treatment response groups based on SUVmax, SUVmean, and TL, respectively. We identified only one feature (gldm) that effectively distinguished responders from non-responders based on MTV. Notably, there were two features extracted from at least three of the four traditional PET parameters that successfully predicted treatment response (Table 3). They included gldm_sdlgle (p = 0.034/0.027/0.045 for SUVmax, SUVmean, and MTV, respectively) and shape_MinorAxisLength (p = 0.018/0.005/0.015 for SUVmax, SUVmean, and TL, respectively). The gldm_sdlgle (small dependence low gray level emphasis) feature is a measure of joint distribution of small dependency with low-intensity SUV for radiomic zone-2. The shape_MinorAxisLength feature expresses the second-largest axis length of each ROI [43].
3.4. Prediction of Treatment Response Using Features from Radiomic Zone-3
Based on SUVmax, SUVmean, MTV, and TL, there were three, four, two, and four features from radiomic zone-3 that were able to distinguish responders from non-responders to ADT (Figure 4). The shape feature category comprised one, one, two, and two features that distinguished between different treatment response groups based on SUVmax, SUVmean, MTV, and TL, respectively. Moreover, the first-order, diagnosis, and glcm categories comprised one feature each that distinguished between different treatment response groups. We identified two features extracted from at least three of the four traditional PET parameters that successfully predicted treatment response (Table 3). They included diagnostics_Mask-interpolated_Minimum (p = 0.019/0.023/0.038 for SUVmax, SUVmean, and TL, respectively) and shape_Sphericity (p = 0.012/0.004/0.034 for SUVmax, SUVmean, and MTV, respectively). The diagnostics_Mask-interpolated_Minimum feature in radiomic zone-3 expresses the minimum SUV measured in the entire prostate gland, with the exclusion of tumor volume. The shape_Sphericity feature is a mathematical quantity that compares the morphology of an object to that of a perfect sphere. The shape_Sphericity of a perfect sphere is equal to one.
3.5. Surface Volume Ratio in the Three Radiomic Zones
On analyzing the features that distinguished responders from non-responders to ADT, we found that shape_SurfaceVolumeRatio (SVR) was simultaneously present in all of the three radiomic zones. Specifically, SVR successfully predicted treatment response according to RR changes based on MTV (p = 0.017) and TL (p = 3.49 × 10−4) in radiomic zone-1; SUVmax (p = 0.017) and SUVmean (p = 0.018) in radiomic zone-2; and SUVmax (p = 0.01) and MTV (p = 0.027) in radiomic zone-3, respectively (Table 3).
4. Discussion
Prediction of response to ADT prior to treatment initiation is a difficult task. In this study, we were able to identify several features from prostate radiomic zones that were able to successfully predict response to ADT in patients with PCa. As expected, the highest number of predictive features was identified within radiomic zone-1 (i.e., the metabolic tumor zone; Figure 5). Specifically, seven features extracted from at least three of the four traditional PET parameters were significantly associated with ADT outcomes. Responders to ADT were more likely to have lower glcm_idmn, glcm_idn, glcm_imc1, glrlm_rln, gldm_dn, and shape_MeshVolume values as well as higher ngtdm_Contrast values (Table 4). Of note, five of these features (i.e., glcm_idmn, glcm_idn, glcm_imc1, ngtdm_Contrast, and glrlm_rln) were associated with the texture distribution properties of PET images [43] which are in turn strongly correlated with intra- and inter-tumor heterogeneity [44,45,46] and treatment response [26].
Figure 5.
Radar chart depicting the correlations between different features extracted from the three radiomic zones and response to androgen deprivation therapy in patients with prostate cancer.

Table 4.
Medians and interquartile ranges (IQRs) of predictive features identified within radiomic zone-1, zone-2, and zone-3 in responders and non-responders to androgen deprivation therapy.
A strength of this study is that the analysis of predictive radiomic features was not limited to the main metabolic tumor zone (i.e., zone-1). Accordingly, certain features from both zone-2 and zone-3 were also able to distinguish between treatment response groups. Findings from zone-2 suggested that responders to ADT were more likely to have lower shape_MinoAxisLength and higher gldm_sdlgle values, whereas data from zone-3 revealed that the ADT response group had lower diagnostics_Mask-interpolated_Minimum and higher shape_Sphericity values (Table 4). These results indicate that radiomic characteristics extracted from peripheral prostatic zones may also have value in the prediction of ADT response. We have previously shown that distinct radiomic zones are useful for classifying patients with PCa in different risk groups [35]. In another study, Rodrigues et al. [47] demonstrated that features extracted from tumor-surrounding regions are strongly associated with Gleason scores. By taking zone-2 and zone-3 into account, we extracted as much radiomics information as possible to assist prediction of ADT treatment outcomes during the pretreatment phase.
Within radiomic zone-2, two features (gldm_sdlgle and shape_MinorAxisLength) successfully predicted treatment response. This observation suggests that joint distribution of small dependency with low-intensity SUV (gldm_sdlgle) and the second-largest axis length (shape_MinorAxisLength) of each ROI in this zone are associated with treatment outcomes. This could reflect the paramount role played by the ring region surrounding the primary tumor volume in limiting cancer spread to both lymph nodes and distant sites. In general, radiomic zone-2 was characterized by lower SUV values and less heterogeneity compared to zone-1. Two features from radiomic zone-3 (diagnostics_Mask-interpolated_Minimum and shape_Sphericity) were also significantly associated with ADT outcomes. Previously, the same features extracted from 11C-choline PET images successfully differentiated between high- and low-risk PCa [30]. An interesting observation from our study is that SVR was the only feature identified as being associated with response to ADT in all of the three radiomic zones. Specifically, responders to ADT were more likely to show higher SVR values from both zone-1 and zone-2 and lower SVR values from zone-3 (Table 4). Notably, Cuocolo et al. [48] have recently demonstrated that SVR was the strongest independent predictor of clinically significant PCa among all of the MRI shape features taken into account.
There are several limitations to our study. First, its single-center design may have limited the external validity of the results. Second, only 35 patients were included. A larger sample size might have improved the power of the study in terms of identifying between-group differences and, for that reason, larger prospective cohorts are needed. A longer follow-up is also necessary to confirm our findings and to evaluate whether the radiomic features identified in our study are correlated with clinical response to ADT.
5. Conclusions
Seven features extracted from radiomic zone-1 were significantly associated with ADT outcomes in patients with PCa. Two features from zone-2 and two from zone-3 were also able to distinguish between different treatment response groups. If independently validated in larger studies, feature analysis of different radiomic zones within the prostate gland could be useful to differentiate responders from non-responders before the initiation of ADT.
Author Contributions
Study concept and design: V.T.T., S.-J.T. and J.-R.T.; data collection, analysis, and interpretation: V.T.T., S.-J.T. and J.-R.T.; manuscript writing and critical revision for important intellectual content: V.T.T., S.-J.T. and J.-R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by a grant (CPRPVVM0011) from the Chang Gung Memorial Hospital.
Institutional Review Board Statement
Ethics approval for this study was received from the Chang Gung Memorial Hospital institutional review board (reference number: 201801384A0).
Informed Consent Statement
All participants provided written informed consent.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- de Wit-van der Veen, B.; Donswijk, M.L.; Slump, C.H.; Stokkel, M.P.M. Day-to-day variability of [68Ga] Ga-PSMA-11 accumulation in primary prostate cancer: Effects on tracer uptake and visual interpretation. EJNMMI Res. 2020, 10, 1–10. [Google Scholar]
- Ghosh, A.; Wang, X.; Klein, E.; Heston, W.D. Novel role of prostate-specific membrane antigen in suppressing prostate cancer invasiveness. Cancer Res. 2005, 65, 727–731. [Google Scholar] [CrossRef]
- Bois, F.; Noirot, C.; Dietemann, S.; Mainta, I.C.; Zilli, T.; Garibotto, V.; Walter, M. [68Ga] Ga-PSMA-11 in prostate cancer: A comprehensive review. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 349–374. [Google Scholar]
- Perlmutter, M.A.; Lepor, H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 2007, 9 (Suppl. 1), S3–S8. [Google Scholar] [PubMed]
- Li, J.-R.; Wang, S.-S.; Yang, C.-K.; Chen, C.-S.; Ho, H.-C.; Chiu, K.-Y.; Hung, C.-F.; Cheng, C.-L.; Yang, C.-R.; Chen, C.-C.; et al. First Line Androgen Deprivation Therapy Duration Is Associated with the Efficacy of Abiraterone Acetate Treated Metastatic Castration-Resistant Prostate Cancer after Docetaxel. Front. Pharmacol. 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, S.; von Klot, C.A.; Henkenberens, C.; Sohns, J.M.; Christiansen, H.; Wester, H.-J.; Ross, T.L.; Bengel, F.M.; Derlin, T. Initial experience with volumetric 68Ga-PSMA I&T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer. J. Nucl. Med. 2017, 58, 1962–1968. [Google Scholar] [PubMed]
- Manafi-Farid, R.; Ranjbar, S.; Jamshidi Araghi, Z.; Pilz, J.; Schweighofer-Zwink, G.; Pirich, C.; Beheshti, M. Molecular Imaging in Primary Staging of Prostate Cancer Patients: Current Aspects and Future Trends. Cancers 2021, 13, 5360. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.-R.; Chang, S.-H.; Wu, Y.-Y.; Fan, K.-H.; Yu, K.-J.; Yang, L.-Y.; Hsiao, I.-T.; Liu, F.-Y.; Pang, S.-T.J.C. Impact of Three-Month Androgen Deprivation Therapy on [68Ga] Ga-PSMA-11 PET/CT Indices in Men with Advanced Prostate Cancer—Results from a Pilot Prospective Study. Cancers 2022, 14, 1329. [Google Scholar] [CrossRef] [PubMed]
- Cyll, K.; Ersvær, E.; Vlatkovic, L.; Pradhan, M.; Kildal, W.; Avranden Kjær, M.; Kleppe, A.; Hveem, T.S.; Carlsen, B.; Gill, S.; et al. Tumour heterogeneity poses a significant challenge to cancer biomarker research. Br. J. Cancer 2017, 117, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Carm, K.T.; Hoff, A.M.; Bakken, A.C.; Axcrona, U.; Axcrona, K.; Lothe, R.A.; Skotheim, R.I.; Løvf, M. Interfocal heterogeneity challenges the clinical usefulness of molecular classification of primary prostate cancer. Sci. Rep. 2019, 9, 13579. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Lee, S.; Ryu, D.; Park, S.; Park, W.Y.; Joung, J.G.; Jeong, J. Biomarkers Associated with Tumor Heterogeneity in Prostate Cancer. Transl. Oncol. 2019, 12, 43–48. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural features for image classification. IEEE Trans. Syst. Man Cybern. 1973, 610–621. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Di Dong, J.W.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M.; Tian, J.J.T. The applications of radiomics in precision diagnosis and treatment of oncology: Opportunities and challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Sirlin, C.B.; Ooi, C.; Adler, A.S.; Gollub, J.; Chen, X.; Chan, B.K.; Matcuk, G.R.; Barry, C.T.; Chang, H.Y.J. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat. Biotechnol. 2007, 25, 675–680. [Google Scholar] [CrossRef]
- Hawkins, S.H.; Korecki, J.N.; Balagurunathan, Y.; Gu, Y.; Kumar, V.; Basu, S.; Hall, L.O.; Goldgof, D.B.; Gatenby, R.A.; Gillies, R.J. Predicting outcomes of nonsmall cell lung cancer using CT image features. IEEE Access 2014, 2, 1418–1426. [Google Scholar] [CrossRef]
- Tu, S.-J.; Wang, C.-W.; Pan, K.-T.; Wu, Y.-C.; Wu, C.-T.J. Localized thin-section CT with radiomics feature extraction and machine learning to classify early-detected pulmonary nodules from lung cancer screening. Phys. Med. Biol. 2018, 63, 065005. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.J.N.C. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H.J.R. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Parmar, C.; Grossmann, P.; Bussink, J.; Lambin, P.; Aerts, H.J.W.L. Machine Learning methods for Quantitative Radiomic Biomarkers. Sci. Rep. 2015, 5, 13087. [Google Scholar] [CrossRef]
- Morland, D.; Triumbari, E.K.A.; Boldrini, L.; Gatta, R.; Pizzuto, D.; Annunziata, S. Radiomics in Oncological PET Imaging: A Systematic Review—Part 2, Infradiaphragmatic Cancers, Blood Malignancies, Melanoma and Musculoskeletal Cancers. Diagnostics 2022, 12, 1330. [Google Scholar] [CrossRef]
- Quartuccio, N.; Marrale, M.; Laudicella, R.; Alongi, P.; Siracusa, M.; Sturiale, L.; Arnone, G.; Cutaia, G.; Salvaggio, G.; Midiri, M. The role of PET radiomic features in prostate cancer: A systematic review. Clin. Transl. Imaging 2021, 9, 579–588. [Google Scholar] [CrossRef]
- Leijenaar, R.T.; Nalbantov, G.; Carvalho, S.; Van Elmpt, W.J.; Troost, E.G.; Boellaard, R.; Aerts, H.J.; Gillies, R.J.; Lambin, P. The effect of SUV discretization in quantitative FDG-PET Radiomics: The need for standardized methodology in tumor texture analysis. Sci. Rep. 2015, 5, 11075. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, R.; Takhar, M.; Tschudi, Y.; Ford, J.C.; Solórzano, G.; Erho, N.; Balagurunathan, Y.; Punnen, S.; Davicioni, E.; Gillies, R.J.; et al. Prostate cancer radiomics and the promise of radiogenomics. Transl. Cancer Res. 2016, 5, 432–447. [Google Scholar] [CrossRef]
- Feliciani, G.; Celli, M.; Ferroni, F.; Menghi, E.; Azzali, I.; Caroli, P.; Matteucci, F.; Barone, D.; Paganelli, G.; Sarnelli, A. Radiomics Analysis on [68Ga] Ga-PSMA-11 PET and MRI-ADC for the Prediction of Prostate Cancer ISUP Grades: Preliminary Results of the BIOPSTAGE Trial. Cancers 2022, 14, 1888. [Google Scholar] [CrossRef]
- Martin-Gonzalez, P.; Gómez de Mariscal, E.; Martino, M.-E.; Gordaliza, P.; Peligros, I.; Carreras Delgado, J.L.; Calvo, F.; Pascau, J.; Desco, M.; Muñoz-Barrutia, A. Association of visual and quantitative heterogeneity of 18F-FDG PET images with treatment response in locally advanced rectal cancer: A feasibility study. PLoS ONE 2020, 15, e0242597. [Google Scholar] [CrossRef]
- Cook, G.J.; Siddique, M.; Taylor, B.P.; Yip, C.; Chicklore, S.; Goh, V.J.C.; Imaging, T. Radiomics in PET: Principles and applications. Clin. Transl. Imaging 2014, 2, 269–276. [Google Scholar] [CrossRef]
- Naqa, I.E. The role of quantitative PET in predicting cancer treatment outcomes. Clin. Transl. Imaging 2014, 2, 305–320. [Google Scholar] [CrossRef]
- Orlhac, F.; Soussan, M.; Maisonobe, J.-A.; Garcia, C.A.; Vanderlinden, B.; Buvat, I. Tumor texture analysis in 18F-FDG PET: Relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J. Nucl. Med. 2014, 55, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Alongi, P.; Stefano, A.; Comelli, A.; Laudicella, R.; Scalisi, S.; Arnone, G.; Barone, S.; Spada, M.; Purpura, P.; Bartolotta, T.; et al. Radiomics analysis of 18F-Choline PET/CT in the prediction of disease outcome in high-risk prostate cancer: An explorative study on machine learning feature classification in 94 patients. Eur. Radiol. 2021, 31, 4595–4605. [Google Scholar] [CrossRef]
- Zamboglou, C.; Carles, M.; Fechter, T.; Kiefer, S.; Reichel, K.; Fassbender, T.F.; Bronsert, P.; Koeber, G.; Schilling, O.; Ruf, J.J.T. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate-and high-risk prostate cancer-a comparison study with histology reference. Theranostics 2019, 9, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, P.; Marturano, F.; Bettinelli, A.; Gregianin, M.; Paiusco, M.; Evangelista, L. Additional Value of PET Radiomic Features for the Initial Staging of Prostate Cancer: A Systematic Review from the Literature. Cancers 2021, 13, 6026. [Google Scholar] [CrossRef] [PubMed]
- Beig, N.; Khorrami, M.; Alilou, M.; Prasanna, P.; Braman, N.; Orooji, M.; Rakshit, S.; Bera, K.; Rajiah, P.; Ginsberg, J.J.; et al. Perinodular and intranodular radiomic features on lung CT images distinguish adenocarcinomas from granulomas. Radiology 2019, 290, 783–792. [Google Scholar] [CrossRef]
- Wu, L.; Gao, C.; Xiang, P.; Zheng, S.; Pang, P.; Xu, M.J. CT-imaging based analysis of invasive lung adenocarcinoma presenting as ground glass nodules using peri-and intra-nodular radiomic features. Front. Oncol. 2020, 10, 838. [Google Scholar] [CrossRef]
- Tu, S.J.; Tran, V.T.; Teo, J.M.; Chong, W.C.; Tseng, J.R. Utility of radiomic zones for risk classification and clinical outcome predictions using supervised machine learning during simultaneous 11C-choline PET/MRI acquisition in prostate cancer patients. Med. Phys. 2021, 48, 5192–5201. [Google Scholar] [CrossRef] [PubMed]
- Onal, C.; Guler, O.C.; Torun, N.; Reyhan, M.; Yapar, A.F. The effect of androgen deprivation therapy on 68Ga-PSMA tracer uptake in non-metastatic prostate cancer patients. Eur. J. Pediatr. 2020, 47, 632–641. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Cordes, M.; Schmidt, D.; Bäuerle, T.; Goetz, T.I.; Beck, M.; Prante, O.; Cavallaro, A.; Uder, M.; Wullich, B.J.; et al. 68Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur. J. Pediatr. 2018, 45, 1862–1872. [Google Scholar] [CrossRef]
- Gafita, A.; Bieth, M.; Krönke, M.; Tetteh, G.; Navarro, F.; Wang, H.; Günther, E.; Menze, B.; Weber, W.A.; Eiber, M.J. qPSMA: Semiautomatic software for whole-body tumor burden assessment in prostate cancer using 68Ga-PSMA11 PET/CT. J. Nucl. Med. 2019, 60, 1277–1283. [Google Scholar] [CrossRef]
- Christensen, T.N.; Andersen, P.K.; Langer, S.W.; Fischer, B.M.B. Prognostic Value of 18F–FDG–PET Parameters in Patients with Small Cell Lung Cancer: A Meta-Analysis and Review of Current Literature. Diagnostics 2021, 11, 174. [Google Scholar] [CrossRef]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouvière, O.; Mottet, N.J.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. 2021, 48, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R. The image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Alic, L.; Niessen, W.J.; Veenland, J.F. Quantification of heterogeneity as a biomarker in tumor imaging: A systematic review. PLoS ONE 2014, 9, e110300. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dang, H.; Wang, X.W. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp. Mol. Med. 2018, 50, e416. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Rodrigues, A.; Santinha, J.; Galvão, B.; Matos, C.; Couto, F.M.; Papanikolaou, N. Prediction of Prostate Cancer Disease Aggressiveness Using Bi-Parametric Mri Radiomics. Cancers 2021, 13, 6065. [Google Scholar] [CrossRef] [PubMed]
- Cuocolo, R.; Stanzione, A.; Ponsiglione, A.; Romeo, V.; Verde, F.; Creta, M.; La Rocca, R.; Longo, N.; Pace, L.; Imbriaco, M. Clinically significant prostate cancer detection on MRI: A radiomic shape features study. Eur. J. Radiol. 2019, 116, 144–149. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).